Abstract
Objectives
Whether premature infants have lower gastric protein digestive capacity than term infants and the extent to which human milk proteases contribute to overall gastric digestion are unknown and were investigated in this study.
Methods
Human milk and infant gastric samples were collected from 16 preterm (24–32 wk gestational age (GA)) and 6 term (38–40 wk GA) mother-infant pairs within a range of 5–42 days postnatal age. For each pair, an aliquot of human milk was adjusted to pH 4.5 and incubated for 2 h at 37 °C to simulate the gastric conditions without pepsin (milkinc). Their gastric protein digestion capacity was measured as proteolysis (free N-terminals) and protease activities. Two-way ANOVA followed by Tukey’s post hoc test were applied to compare measurements between preterm and term infants as well as among human milk, milkinc and gastric samples.
Results
Measurements of gastric protein digestion were significantly lower in preterm infants than term infants. Overall milk protease activity did not differ between human milk samples from term- and preterm-delivering mothers. As protease activity did not increase with simulated gastric incubation, milk proteases likely contributed minimally to gastric digestion.
Conclusions
Preterm infants have lower gastric protein digestion capacity than term infants, which could impair nutrient acquisition. Human milk proteases contribute minimally to overall gastric digestion. The limited activity of milk proteases suggests that these enzymes cannot compensate for the premature infant’s overall lower gastric protein digestion.
Keywords: Term milk, preterm milk, postnatal age, enzyme, gastric acid
Introduction
Premature infants (<37 weeks of gestational age (GA)) often have poor growth outcomes (1). Exposure to extrauterine life prior to full maturation requires an abrupt shift from nourishment delivered in monomer form via the placenta to complex macronutrients that must be digested and absorbed in the infant gut. Though human milk is ideal for infant nutrition, preterm infants may not be ready to fully digest human milk components, particularly proteins (2). Optimal gastric digestion requires sufficient gastric acid and pepsin production. Preterm infants have higher pre-feeding gastric pH than term infants (3). The higher gastric pH of preterm infants, at least pre-feeding, may reduce pepsin activity, as pepsin hydrolyzes protein optimally at acidic pH (4). Indeed, pre-feeding gastric pepsin activity was suggested to be lower in preterm infants than term infants at birth (5). No work has examined differences in post-feeding pepsin activity between preterm and term infants (2).
Our previous peptidomics and enzyme substrate assay analyses reveal that numerous proteases are active in human milk (6–8). Our peptidomic analysis suggests that some milk proteases remain active within the infant stomach (9, 10). However, a comparison of neither the peptidomic profiles nor the active milk proteases of preterm and term infant gastric contents has been performed. No research has yet compared the overall protease activity between human milk from term- and preterm-delivering mothers via substrate assays. Whether differences in milk protease activity between preterm- and term-delivering mothers impact infant gastric digestion in term and preterm infants remains unknown. Our objective in this research was to determine differences in gastric protein digestion between term and preterm infants, and to determine the extent to which human milk proteases contribute to overall gastric digestion.
Methods
Participants and sample collection
This study was approved by the institutional review boards of the University of California, Davis (UC Davis) and Oregon State University (OSU). Inclusion criteria included inpatient admission to the neonatal intensive care unit (NICU), an indwelling nasogastric or orogastric feeding tube and tolerance of full enteral feeding. Most of the enrolled infants required a feeding tube because of uncoordinated or immature capacity to suck and swallow. Exclusion criteria were anatomic or functional gastrointestinal disorders. Samples were collected from 16 premature-delivering mother-infant pairs ranging in gestational age (GA) at birth from 24–32 weeks and 6 term-delivering mother-infant pairs ranging in GA at birth from 38–40 weeks (Table 1) from 5–42 days of postnatal age at the UC Davis Children’s Hospital NICU in Sacramento, California. Enrolled infants had a variety of medical conditions, including respiratory distress syndrome, chronic lung disease, apnea of prematurity and Dandy-Walker malformation in the premature infants and cleft palate, respiratory distress syndrome, hypoxic-ischemic encephalopathy and congenital diaphragmatic hernia in the term infants, but no overt gastrointestinal tract issues. Respiratory distress syndrome and congenital diaphragmatic hernia can delay gastric emptying in preterm infants (11, 12); however, the other medical conditions in this cohort have not been associated with an effect on gastric emptying or digestion capacity. Hypoxic-ischemic encephalopathy may result in gastrointestinal tissue damage (13); however, the effects on gastric emptying and protein digestion are unknown. The infants with hypoxic ischemic encephalopathy were fasted and underwent whole body cooling for 72 hours starting shortly after birth, however this treatment was completed and the infants advanced to full enteral feeding prior to enrollment. The infant with congenital diaphragmatic hernia had the defect repaired and was advanced to full enteral feeding prior to enrollment. Gastroesophageal reflux is almost universal in this population; however, none of the infants sampled received medications known to affect gastric pH or gastric digestion capacity, including prokinetics, H2 blockers/antagonists or proton-pump inhibitors. The enrolled infants were clinically stable at the time of sample collection (not on mechanical ventilation, stable vital signs). Human milk samples were collected as described in our previous study (6). The preterm infants were fed their mother’s milk (not pasteurized) fortified with intact bovine milk protein-based fortifier (Similac Human Milk Fortifier Powder, Abbott Park, IL, USA). Term infants were fed their mother’s milk (not pasteurized) without fortifications. The human milk feedings were delivered via the nasogastric tubes over 30 min. Two hours after the initiation of feeding, 2 mL of each preterm and term infant’s gastric contents were collected in a syringe back through the feeding tube via suction. Gastric samples were aspirated at 2 h post-feeding to obtain the maximum feasible length of gastric digestion time based on gastric emptying times (14). Human milk and gastric samples were placed into sterile vials and stored at −20 °C and were transported to OSU on dry ice and stored at −80 °C.
TABLE 1.
Demographics of mother-infant pairs sampled for milk and gastric contents separated into preterm and term infants1
| Preterm infants | Term infants | |
|---|---|---|
| GA, weeks | 27 ± 3 (24-32) | 39 ± 1 (38-40) |
| Postnatal age, days | 22 ± 11 (5-42) | 24 ± 11 (14-28) |
| Birth weight, g | 1,062 ± 521 (620-2,210) | 3,556 ± 332 (3,050-3,837) |
| Mother’s age, years | 30 ± 6 (18-40) | 24 ± 10 (17-42) |
All values are mean ± SD (range), N = 16 for preterm, N = 6 for term infants. GA, gestational age.
Human milk incubated to simulate gastric conditions (milkinc)
After thawing milk samples at 4 °C, 300 μL of each sample were adjusted to pH 4.5 with 1 M hydrochloric acid and incubated for 2 h at 37 °C with shaking at 5.83 Hz to simulate gastric conditions. Samples were centrifuged at 4,226 × g for 10 min at 4 °C and the infranates were collected, separated into aliquots and stored at −80 °C.
General sample preparation
Human milk and gastric samples were thawed at 4 °C, pH was determined, and samples were delipidated and stored according to the above procedure.
Spectrophotometric and fluorometric assays
The assays were recorded with a microplate reader (Spectramax M2, Molecular Devices, Sunnyvale, CA, USA) using two replicates of blanks, standards and samples. For protease activity in human milk, the assay buffer was adjusted with 1 M hydrochloric acid to pH 6.5 to match milk pH. For protease and pepsin/cathepsin D activity in milkinc and gastric samples, the assay buffer was adjusted to pH 4.5 to match the average gastric pH of the present study.
Protein concentration
The protein concentration of all samples was measured with the BCA protein assay (Table, Supplemental Digital Content 1).
Proteolysis
The amount of proteolysis was determined by fluorescamine assay as described previously (15) (Table, Supplemental Digital Content 1) with a L-leucine standard curve, expressed as mM N-terminal. We also determined the N-terminal concentration in a 1 g/mL solution of fortifier in water (in triplicate).
General protease activity
General protease activity was determined based on the release of tyrosine and tryptophan from casein compared to a tyrosine standard curve (Table, Supplemental Digital Content 1). The results are expressed as units per milliliter, where one unit of protease activity is the amount of enzyme that generates 1 μmol of L-tyrosine per min.
Pepsin/cathepsin D activity
Pepsin/cathepsin D activity was determined with (7-methoxycoumarin-4-yl)acetyl (MCA)-Gly-Lys-Pro-Ile-Leu-Phe-|-Phe-Arg-Leu-Lys-dinitrophenol-D-Arg-NH2 (16) with an MCA standard curve (Table, Supplemental Digital Content 1). The results are expressed as enzyme activity in units per milliliter, where one unit of pepsin/cathepsin D activity is the amount of enzyme that generates 1 μmol of MCA per min.
Pepsin and cathepsin D have overlapping cleavage specificity (Leu/Phe (P1)) (17, 18). Our testing revealed that both pepsin and cathepsin D are highly active in cleaving the specific peptide substrate for cathepsin D. As pepsin is not present in milk, pepsin/cathepsin D activity in milk and milkinc are fully attributed to cathepsin D.
Estimation of pepsin contribution to gastric digestion
For proteolysis, general protease activity and pepsin/cathepsin D activity, the activity/contribution of pepsin to gastric digestion was estimated by subtracting the paired value in milkinc from the value in the gastric samples.
Statistical analysis
Two-way ANOVA followed by Tukey’s honest significant difference post hoc test (R software, version 3.2.1) was applied to compare human milk, milkinc and gastric samples as well as preterm versus term infant groups for all measurements. Student’s t-tests were used to compare estimated pepsin contribution between term and preterm groups. Linear regression models were applied to determine if the measurements changed across postnatal age. Differences were designated significant at P ≤ 0.05. The sample size of preterm (N = 16) and term (N = 6) paired milk and gastric samples was selected based on previous literature sample sizes and proved to be adequately powered to detect differences based on the results.
Results
Infant demographics
Demographic details for the mother-infant pairs are presented in Table 1.
Protein concentration
Protein concentration did not differ between the milks of preterm and term-delivering mothers (overall mean = 15.7 ± 0.5 mg/mL, P = 0.60) or between the gastric samples of term and preterm infants (overall mean = 16 ± 1 mg/mL, P = 0.51). Protein concentration did not change from milk to gastric contents for either preterm (P = 0.81) or term infants (P = 0.61). For preterm mother-infant pairs, protein concentration did not change with postnatal age in either human milk or gastric samples (Table, Supplemental Digital Content 2). However, for term mother-infant pairs, protein concentration decreased with increasing postnatal age in milk (P = 0.020), but not in the gastric contents (Table, Supplemental Digital Contents 3).
pH
Human milk pH from mothers who delivered prematurely was not significantly different from the pH of milk from mothers who delivered at term (overall mean = pH 6.35 ± 0.04, P = 0.91, Figure 1A). As expected, the pH of gastric contents from both term and preterm infants was lower than that of the paired human milk samples (P < 0.001). Gastric pH in preterm infants did not significantly differ from that in term infants (overall mean = 4.4 ± 0.2, P = 0.75). pH in human milk or gastric samples did not change significantly with postnatal age for either preterm or term infants (Table, Supplemental Digital Contents 2 and 3).
FIGURE 1.
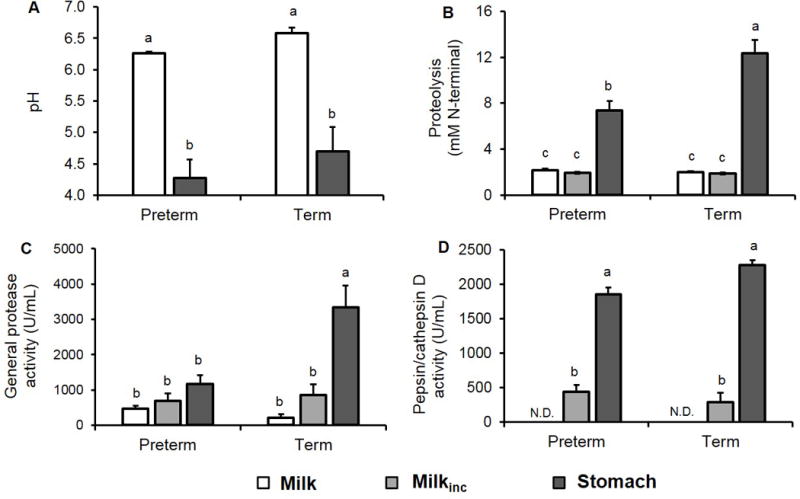
(A) pH, (B) proteolysis, (C) general protease activity and (D) pepsin/cathepsin D activity in human milk (white bars), human milk incubated to simulate gastric conditions (milkinc, gray bars) and infant gastric samples (black bars). Paired milk, milkinc and gastric samples were separated by gestational age (GA) at birth, either premature (N = 16, 24–32 wk GA, 5–42 days of postnatal age) or term (N = 6, 38–40 wk GA, 14–28 days of postnatal age). Values are mean ± SEM. Letters a, b and c show statistically significant differences between groups (P < 0.05) using two-way ANOVA followed by Tukey’s honest significant difference post hoc test for pH, proteolysis, general protease activity and pepsin/cathepsin D activity.
Proteolysis
Proteolysis (based on the fluorescamine assay) did not differ between term- and preterm-delivering mothers’ human milk (overall mean = 2.1 ± 0.1 mM N-terminals, P = 0.99, Figure 1B). Contrary to our expectations, the amount of free N-terminals did not increase from milk to milkinc for either preterm- or term-delivering mothers’ samples (P = 0.99 for both term and preterm). However, proteolysis did increase from milk/milkinc to gastric contents for both preterm and term infants (by 2.4- and 5.2-fold, respectively, P < 0.001). Proteolysis in the gastric contents of preterm infants was 67.6% lower than in term infants (P < 0.001). The N-terminal concentration of the 1 g/mL fortifier solution was 14.7 mM N-terminals. Based on normal dilution of the fortifier in milk (0.9 g of fortifier in 25 mL or 50 mL of milk), we calculated that added fortifier could be contributing an additional 0.26–0.53 mM N-terminals to the measured values for preterm infant gastric samples. Proteolysis in human milk, milkinc and gastric samples did not change with postnatal age for preterm or term infants (Table, Supplemental Digital Contents 2 and 3).
General protease activity
General protease activity did not differ between milks from mothers who delivered prematurely and at term (overall mean = 394 ± 77 U/mL, P = 0.99). Neither preterm nor term-delivering mothers’ milks showed significantly increased general protease activity from breast milk to milkinc (P = 0.97 and P = 0.78, respectively). General protease activity in milkinc samples also did not differ between term- and preterm-delivering mothers (overall mean = 728 ± 178 U/mL, P = 0.99). In term infant samples, general protease activity in the gastric samples was 14.3- and 2.9-fold higher than in milk and milkinc samples, respectively (P < 0.001, Figure 1C). However, in preterm infant samples, general protease activity in the gastric contents was not significantly higher than in milk (P = 0.18) or milkinc (P = 0.58). Gastric protease activity was 1.9-fold lower in preterm infants than term infants (P < 0.001). General protease activity in human milk, milkinc or gastric samples did not change with postnatal age for preterm or term infants (Table, Supplemental Digital Contents 2 and 3).
Pepsin/cathepsin D activity
Pepsin/cathepsin D activity was not detectable in human milk, which is not surprising as pepsin is not present in human milk, and cathepsin D is not active at pH 6.5 (6). The detected activity of pepsin/cathepsin D in milkinc represents only cathepsin D as pepsin is first introduced in the stomach. Cathepsin D was active in milkinc but was not significantly different between mothers who delivered prematurely and at term (overall mean = 402 ± 76 U/mL, P = 0.54). Pepsin/cathepsin D activity increased 3.2- and 6.9-fold from milkinc to the stomach in preterm and term infants, respectively (Figure 1D, P < 0.001). The increase in pepsin/cathepsin D activity between milkinc and the gastric contents is assigned to pepsin. Pepsin/cathepsin D activity in gastric contents was 22.8% lower in preterm infants than in term infants (Figure 1D, P = 0.055). Pepsin/cathepsin D activity in milkinc or gastric samples did not change with postnatal age for preterm or term infants (Table, Supplemental Digital Contents 2 and 3).
Estimation of pepsin contribution to gastric digestion
Based on subtraction of milkinc from the gastric sample values, we estimated that pepsin activity was 92.6% (P = 0.003), 294% (P = 0.004) and 37.5% (P = 0.079) lower in preterm infants than term infants for proteolysis, general protease activity and pepsin/cathepsin activity assays, respectively (Figure 2A-C).
FIGURE 2.
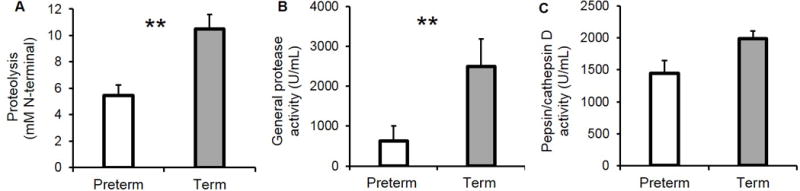
Activity of pepsin estimated by subtracting the paired values from human milk incubated to simulate gastric conditions (milkinc) from those in the stomach for (A) proteolysis, (B) general protease activity and (C) pepsin/cathepsin D activity. Paired milkinc and gastric samples were separated by gestational age (GA), premature (N = 16, 24–32 wk GA, 5–42 days of postnatal age) and term (N = 6, 38–40 wk GA, 14–28 days of postnatal age). Values are mean ± SEM. Asterisks show statistically significant differences between variables (**, P < 0.01; * P < 0.05) using t-tests between preterm (white bars) and term (gray bars) infants.
Discussion
Though postprandial gastric protease activity was shown to be lower in preterm infants than adults (19) and postprandial histalog-stimulated gastric pepsin output was lower in term infants than adults (20), no previous study has compared post-feeding gastric protease activity between term and preterm infants (2).
Neither proteolysis (cumulative release of N-terminals) nor general protease activity (instantaneous protease activity) differed between term and preterm mother’s milk. This result differed from previous work showing that preterm milk had higher milk plasmin activity than term milk (21), and our previous peptidomic analysis, which showed that preterm milk had more released peptides than term milk (22).
Though previous reports demonstrated a higher pre-feeding gastric pH in preterm infants than term infants (3), gastric pH of preterm and term infants 2 h post-feeding were not different. Gastric pH was lower than milk pH as preterm and term infants are able to secrete some gastric acid in response to milk ingestion (23). The average gastric pH measured matches previous literature for both term (24, 25) and preterm infants (19, 25–30).
For both preterm and term milks, general protease activity and proteolysis stayed constant during incubation simulating gastric conditions, suggesting that milk proteases have minimal contribution to gastric digestion. However, pepsin/cathepsin D activity increased from human milk to milkinc. This increase is due to cathepsin D, as there is no pepsin in human milk. Cathepsin D is inactive at pH 6.5 in human milk (6), but the acidic conditions of the infant stomach lead to its auto-activation (31, 32). The lack of change in proteolysis and general protease activity in milkinc, however, suggests that the contribution of milk proteases to gastric milk protein digestion is small.
We demonstrated for the first time that proteolysis and protease activity increased from human milk to the stomach for both term and preterm infants, but the increase was lesser for preterm infants, mostly due to differences in pepsin activity.
Our measurement of general protease activity in the premature infant stomach matched well with that of Armand et al. (19) who found 75 U/mL/kg body weight of protease activity (estimated from figure 3A in Armand et al.) in the stomach of preterm infants (26–32 wk GA, 1–13 wk postnatal age) 50 min after human milk feeding (19, 33). Conversion of our measurements to the units used by Armand (values divided by infant body weight in kilograms and converting to 0.1 μM/min tyrosine per unit) yielded values in the same range: 118 ± 36 U/mL/kg of body weight.
No paper has previously compared term and preterm infant gastric proteolysis. However, Henderson et al. (34) found that up to 13.3% of human milk protein is partially hydrolyzed in the preterm stomach by 50 min, based on acid precipitation followed by Lowry assay. Our results in N-terminal release were not comparable to the previous studies. The present study is the first to demonstrate that gastric proteolysis is lower in preterm infants than in term infants.
Preterm and term milk had lower pH values (pH 6.35 ± 0.04) than previous studies (e.g., pH 7.0-7.5 across lactation (37)). This lower milk pH likely stems from the initial milk storage at −20 °C in home freezers and within the NICU, thawing at 4 °C prior to feeding and then elongated storage at −80 °C whereas previous studies analyzed fresh milk or milk stored immediately at −80 °C. Storage at 4 °C and −20 °C and thawing reduces milk pH (38) likely due to lipolytic release of free fatty acids (39). Within milk, this post-storage decreased pH could not affect proteolysis (cumulative measurement) or cathepsin D/pepsin activity (not active in milk); however, it could slightly decrease general protease activity (as most milk proteases have a slightly higher pH optima). As we expect this pH decrease to affect both term and preterm milks similarly, the lack of difference between term and preterm milk protease activity would likely be present with or without sample storage.
As the study utilized infants whose health conditions required feeding tubes, the results may not be generalizable to healthy infants; however, we excluded any conditions known to affect digestion. Another potential limitation of the study is the larger postnatal age range of preterm infants compared with term infants. However, there were no significant changes across postnatal age in human milk or gastric samples within the preterm infants for any measurement, which suggests that the larger postnatal age range did not affect our outcomes. Our results could be influenced by the different diets (milk alone for term infants and milk plus fortifier for preterm infants). However, this design was selected to best represent the reality of feeding within the NICU. We also examined protein digestion at a single timepoint (2 h) which does not allow for comparison of the relative effects of gastric emptying time on protein digestion in preterm and term infants. As preterm infants often have delayed gastric emptying (in part due to the addition of fortifier (40)), this elongated time in the stomach could compensate for the initially low degree of protein digestion observed herein.
Comparison of proteolytic capacity between the earlier (24–27 wk of GA) and later (29–32 wk of GA) gestational age preterm infants and their mother’s milk revealed no differences in proteolysis, general protease activity or pepsin/cathepsin D activity (Supplemental Digital Contents 4-6); however, gastric pH of the later preterm infants was slightly higher than the earlier preterm infants.
Our study revealed that preterm infants have lower gastric digestive capacity than term infants, which may limit their ability to break down milk proteins and absorb amino acids, thus limiting growth and development. Milk proteases are active in the infant stomach but are not major contributors to overall gastric digestion in either preterm or term infants; therefore, the milk enzymes in preterm-delivering mothers’ milk cannot compensate for the infant’s overall lower gastric protein digestion. As the pH optima of most milk proteases are around neutral, the contribution of milk proteases to digestion may increase as pH increases in the small intestine due to pancreatic secretions.
Supplementary Material
What is known
Preterm infants have higher pre-feeding gastric pH than term infants.
Pre-feeding gastric pepsin activity could be lower in preterm infants than term infants at birth.
Human milk contains an array of active proteases, as well as protease precursors, inhibitors and activators.
What is new
Preterm infants have lower gastric protein digestion capacity than term infants at 2 h post-feeding.
Milk proteases from mothers who delivered prematurely and at term contributed minimally to gastric digestion.
Acknowledgments
Statement of authors’ contributions to manuscript. The authors thank C. J. Dillard for editing the manuscript. V. D. M. conducted the research and analyzed data. Y. Q. conducted the statistical analysis. M. A. U. and R. B. provided milk samples. V. D. M. and D. C. D. designed the study and wrote the manuscript. V. D. M. and D. C. D. had primary responsibility for the final content. All authors read and approved the final manuscript.
Supported by the K99/R00 Pathway to Independence Career Award, Eunice Kennedy Shriver Institute of Child Health & Development of the National Institutes of Health (R00HD079561) (DCD).
Footnotes
Author disclosures: V. Demers-Mathieu, Y. Qu, M. A. Underwood, R. Borghese and D. C. Dallas have no conflicts of interest
References
- 1.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 2.Dallas DC, Underwood MA, Zivkovic AM, German JB. Digestion of protein in premature and term infants. J Nutr Disord Ther. 2012;2:112–21. doi: 10.4172/2161-0509.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamosh M, Scanlon JW, Ganot D, Likel M, Scanlon KB, Hamosh P. Fat digestion in the newborn: characterization of lipase in gastric aspirates of premature and term infants. J Clin Invest. 1981;67:838–46. doi: 10.1172/JCI110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henschel M, Newport M. Parmar V. Gastric proteases in the human infant. Neonatology. 1987;52:268–72. doi: 10.1159/000242719. [DOI] [PubMed] [Google Scholar]
- 5.Adamson I, Esangbedo A, Okolo A, Omene J. Pepsin and its multiple forms in early life. Neonatology. 1988;53:267–73. doi: 10.1159/000242801. [DOI] [PubMed] [Google Scholar]
- 6.Demers-Mathieu V, Nielsen SD, Underwood MA, Borghese R, Dallas DC. Analysis of milk from mothers who delivered prematurely reveals few changes in proteases and protease inhibitors across gestational age at birth and infant postnatal age. J Nutr. 2017:jn244798. doi: 10.3945/jn.116.244798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallas DC, Murray NM, Gan J. Proteolytic systems in milk: perspectives on the evolutionary function within the mammary gland and the infant. J Mammary Gland Biol Neoplasia. 2015;20:133–47. doi: 10.1007/s10911-015-9334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallas DC, German JB. Enzymes in human milk. In: Isolauri E, Sherman P, Walker W, editors. Intestinal Microbiome: Functional Aspects in Health and Disease. Basel, Switzerland: Karger Publishers; 2017. pp. 129–36. [Google Scholar]
- 9.Holton TA, Vijayakumar V, Dallas DC, Guerrero A, Borghese RA, Lebrilla CB, German JB, Barile D, Underwood MA, Shields DC, et al. Following the digestion of milk proteins from mother to baby. J Proteome Res. 2014;13:5777–83. doi: 10.1021/pr5006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallas DC, Guerrero A, Khaldi N, Borghese R, Bhandari A, Underwood MA, Lebrilla CB, German JB, Barile D. A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J Nutr. 2014;144:815–20. doi: 10.3945/jn.113.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victor Y. Effect of body position on gastric emptying in the neonate. Archives of Disease in Childhood. 1975;50:500–4. doi: 10.1136/adc.50.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arena F, Romeo C, Baldari S, Arena S, Antonuccio P, Campennì A, Zuccarello B, Romeo G. Gastrointestinal sequelae in survivors of congenital diaphragmatic hernia. Pediatrics International. 2008;50:76–80. doi: 10.1111/j.1442-200X.2007.02527.x. [DOI] [PubMed] [Google Scholar]
- 13.Faingold R, Cassia G, Prempunpong C, Morneault L, Sant’Anna GM. Intestinal ultrasonography in infants with moderate or severe hypoxic-ischemic encephalopathy receiving hypothermia. Pediatric radiology. 2016;46:87–95. doi: 10.1007/s00247-015-3447-0. [DOI] [PubMed] [Google Scholar]
- 14.Siegel M, Lebenthal E, Topper W, Krantz B, Li P. Gastric emptying in prematures of isocaloric feedings with differing osmolalities. Pediatr Res. 1982;16:141–7. doi: 10.1203/00006450-198202000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Larsen LB, Rasmussen MD, Bjerring M, Nielsen JH. Proteases and protein degradation in milk from cows infected with Streptococcus uberis. Int Dairy J. 2004;14:899–907. [Google Scholar]
- 16.Yasuda Y, Kageyama T, Akamine A, Shibata M, Kominami E, Uchiyama Y, Yamamoto K. Characterization of new fluorogenic substrates for the rapid and sensitive assay of cathepsin E and cathepsin D. J Biochem. 1999;125:1137–43. doi: 10.1093/oxfordjournals.jbchem.a022396. [DOI] [PubMed] [Google Scholar]
- 17.Sun H, Lou X, Shan Q, Zhang J, Zhu X, Zhang J, Wang Y, Xie Y, Xu N, Liu S. Proteolytic characteristics of cathepsin D related to the recognition and cleavage of its target proteins. PloS one. 2013;8:e65733. doi: 10.1371/journal.pone.0065733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn J, Cao M-J, Yu YQ, Engen JR. Accessing the reproducibility and specificity of pepsin and other aspartic proteases. Biochim Biophys Acta. 2013;1834:1222–9. doi: 10.1016/j.bbapap.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armand M, Hamosh M, Mehta NR, Angelus PA, Philpott JR, Henderson TR, Dwyer NK, Lairon D, Hamosh P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res. 1996;40:429–37. doi: 10.1203/00006450-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Agunod M, Yamaguchi N, Lopez R, Luhby A, Glass G. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Dig Dis Sci. 1969;14:400–14. doi: 10.1007/BF02239360. [DOI] [PubMed] [Google Scholar]
- 21.Armaforte E, Curran E, Huppertz T, Ryan CA, Caboni MF, O’Connor PM, Ross RP, Hirtz C, Sommerer N, Chevalier F. Proteins and proteolysis in pre-term and term human milk and possible implications for infant formulae. Int Dairy J. 2010;20:715–23. [Google Scholar]
- 22.Dallas DC, Smink CJ, Robinson RC, Tian T, Guerrero A, Parker EA, Smilowitz JT, Hettinga KA, Underwood MA, Lebrilla CB, et al. Endogenous human milk peptide release is greater after preterm birth than term birth. J Nutr. 2015;145:425–33. doi: 10.3945/jn.114.203646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly EJ, Newell SJ, Brownlee KG, Primrose JN, Dear PR. Gastric acid secretion in preterm infants. Early Hum Dev. 1993;35:215–20. doi: 10.1016/0378-3782(93)90108-7. [DOI] [PubMed] [Google Scholar]
- 24.Mason S. Some aspects of gastric function in the newborn. Arch Dis Child. 1962;37:387–91. doi: 10.1136/adc.37.194.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamosh M, Sivasubramanian K, Salzman-Mann C, Hamosh P. Fat digestion in the stomach of premature infants: I. Characteristics of lipase activity. J Pediatr. 1978;93:674–9. doi: 10.1016/s0022-3476(78)80915-9. [DOI] [PubMed] [Google Scholar]
- 26.Weisselberg B, Yahav J, Reichman B, Jonas A. Basal and meal-stimulated pepsinogen secretion in preterm infants: a longitudinal study. J Pediatr Gastroenterol Nutr. 1992;15:58–62. doi: 10.1097/00005176-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Smith L, Kaminsky S, D’souza S. Neonatal fat digestion and lingual lipase. Acta Paediatrica. 1986;75:913–8. doi: 10.1111/j.1651-2227.1986.tb10316.x. [DOI] [PubMed] [Google Scholar]
- 28.Roman C, Carriere F, Villeneuve P, Pina M, Millet V, Simeoni U, Sarles J. Quantitative and qualitative study of gastric lipolysis in premature infants: do MCT-enriched infant formulas improve fat digestion? Pediatr Res. 2007;61:83–8. doi: 10.1203/01.pdr.0000250199.24107.fb. [DOI] [PubMed] [Google Scholar]
- 29.Yahav J, Carrion V, Lee PC, Lebenthal E. Meal-stimulated pepsinogen secretion in premature infants. J Pediatr. 1987;110:949–51. doi: 10.1016/s0022-3476(87)80421-3. [DOI] [PubMed] [Google Scholar]
- 30.Harries J, Fraser A. The acidity of the gastric contents of premature babies during the first fourteen days of life. Neonatology. 1968;12:186–93. doi: 10.1159/000240105. [DOI] [PubMed] [Google Scholar]
- 31.Larsen LB, Boisen A, Petersen TE. Procathepsin D cannot autoactivate to cathepsin D at acid pH. FEBS Lett. 1993;319:54–8. doi: 10.1016/0014-5793(93)80036-t. [DOI] [PubMed] [Google Scholar]
- 32.Briozzo P, Morisset M, Capony F, Rougeot C, Rochefort H. In vitro degradation of extracellular matrix with Mr 52,000 cathepsin D secreted by breast cancer cells. Cancer Res. 1988;48:3688–92. [PubMed] [Google Scholar]
- 33.DiPalma J, Kirk C, Hamosh M, Colon A, Benjamin S, Hamosh P. Lipase and pepsin activity in the gastric mucosa of infants, children, and adults. Gastroenterology. 1991;101:116–21. doi: 10.1016/0016-5085(91)90467-y. [DOI] [PubMed] [Google Scholar]
- 34.Henderson TR, Hamosh M, Armand M, Mehta NR, Hamosh P. Gastric proteolysis in the newborn infant: Protein digestion is limited and is not affected by diet, human milk or formula. FASEB J. 1998;12:A971. [Google Scholar]
- 35.Weber A, Loui A, Jochum F, Bührer C, Obladen M. Breast milk from mothers of very low birthweight infants: variability in fat and protein content. Acta Paediatrica. 2001;90:772–5. [PubMed] [Google Scholar]
- 36.Gross SJ, Geller J, Tomarelli R. Composition of breast milk from mothers of preterm infants. Pediatrics. 1981;68:490–3. [PubMed] [Google Scholar]
- 37.Morriss FH, Brewer ED, Spedale SB, Riddle L, Temple DM, Caprioli RM, West MS. Relationship of human milk pH during course of lactation to concentrations of citrate and fatty acids. Pediatrics. 1986;78:458–64. [PubMed] [Google Scholar]
- 38.Ogundele MO. Effects of storage on the physicochemical and antibacterial properties of human milk. British journal of biomedical science. 2002;59:205–11. doi: 10.1080/09674845.2002.11783661. [DOI] [PubMed] [Google Scholar]
- 39.Bitman J, Wood LD, Mehta NR, Hamosh P, Hamosh M. Lipolysis of triglycerides of human milk during storage at low temperatures: a note of caution. J Pediatr Gastroenterol Nutr. 1983;2:521–4. doi: 10.1097/00005176-198302030-00021. [DOI] [PubMed] [Google Scholar]
- 40.van Wijk MP, Benninga MA, Dent J, Lontis R, Goodchild L, McCall LM, Haslam R, Davidson GP, Omari T. Effect of body position changes on postprandial gastroesophageal reflux and gastric emptying in the healthy premature neonate. The journal of pediatrics. 2007;151:585–90.e2. doi: 10.1016/j.jpeds.2007.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


