Abstract
Background
Phosphodiesterase type 4 (PDE4) inhibitors produce widespread anti-inflammatory effects and reduce ethanol consumption in several rodent models. These drugs are potential treatments for several diseases, including central nervous system disorders, but clinical use is limited by their emetic activity. Apremilast is a selective PDE4 inhibitor with fewer gastrointestinal side effects that is FDA approved for the treatment of psoriasis.
Methods
We measured the acute and chronic effects of apremilast on ethanol consumption in male and female C57BL/6J mice using the continuous and intermittent 24-h two-bottle choice drinking models. We also studied the effects of apremilast on preference for sucrose or saccharin, spontaneous locomotor activity, and blood ethanol clearance. Finally, apremilast levels in plasma, liver, and brain were measured one or two hours after injection.
Results
In the continuous and intermittent drinking tests, apremilast (15-50 mg/kg, p.o.) dose dependently reduced ethanol intake and preference in male and female mice. Higher doses of apremilast (30-50 mg/kg) also reduced total fluid intake in these mice. Chronic administration of apremilast (20 mg/kg) produced a stable reduction of ethanol consumption in both drinking tests with no effect on total fluid intake. The drinking effects were reversible after drug treatment was replaced with vehicle administration (saline) for 2-4 days. Six daily apremilast injections did not alter preference for saccharin or sucrose in male or female mice. Apremilast (20 mg/kg) transiently decreased spontaneous locomotor activity and did not alter blood ethanol clearance. The highest levels of apremilast were found in liver followed by plasma and brain.
Conclusions
Apremilast produces stable reductions in voluntary ethanol consumption and is rapidly distributed to plasma and tissues (including the brain), suggesting that it may be an improved PDE4 inhibitor for medication development and repurposing efforts to treat alcohol abuse.
Keywords: PDE4 inhibitor, two-bottle choice ethanol drinking, locomotor activity, apremilast biodistribution, C57BL/6J mice
Introduction
Alcohol misuse places a tremendous economic burden on our society (estimated cost of $249 billion/year in the U.S. in 2010) and is a leading risk factor for premature death and disability worldwide (niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics). Disulfiram, naltrexone, and acamprosate are the only FDA-approved drugs to treat alcohol use disorder (AUD). These drugs have limited clinical efficacy and are not routinely prescribed, and thus there is a critical need for improved treatment options. Behavioral and genetic evidence supports roles for immune/inflammatory signaling in alcohol abuse, including neuroimmune pathways (Mayfield et al., 2013, Crews and Vetreno, 2016, Warden et al., 2016, Montesinos et al., 2016). This area of research shows great potential to advance treatment strategies for AUD.
Phosphodiesterases (PDEs) regulate immune pathways, and PDE4 in particular has been implicated in alcohol and substance use disorders. PDEs are a superfamily of enzymes catalyzing the hydrolysis of 3′,5′-cyclic adenosine monophosphate (cAMP) and 3′,5′-cyclic guanosine monophosphate (cGMP) to their inactive 5′-AMP and 5′-GMP forms, respectively. Increased cAMP or cGMP, resulting from inhibition of PDE, reduces inflammatory signaling on a broad scale (Jin et al., 2012, Page and Spina, 2011). Cyclic nucleotides play pivotal roles in many cellular processes, including immune/inflammatory responses, cardiac function, smooth muscle relaxation, depression, and cognition. The anti-inflammatory actions of PDE inhibitors are beneficial in treating chronic obstructive pulmonary disease and asthma (Jin et al., 2012, Keravis and Lugnier, 2012). PDEs are encoded by 21 genes and are grouped into 11 families, according to their structural similarity. Each gene encodes multiple protein products generated by different promoters and alternative splicing events, resulting in more than 50 different PDE proteins that may be produced in mammalian cells (Bender and Beavo, 2006).
Our study of different classes of PDE inhibitors showed that only PDE4 inhibitors decreased ethanol drinking in mice (Blednov et al., 2014). The PDE4 inhibitor rolipram markedly reduced ethanol intake and preference in mice (Hu et al., 2011) and reduced ethanol seeking and consumption in alcohol-preferring fawn-hooded rats (Wen et al., 2012). In addition, rolipram diminished anxiety- and depression-like behaviors induced by abstinence from ethanol in different rodent models (Gong et al., 2017) and inhibited neuroimmune signaling in preclinical and clinical models (Zhu et al., 2001). The nonselective PDE inhibitor ibudilast (Gibson et al., 2006) also reduced ethanol consumption in three different rodent models (Bell et al., 2015). It did not alter primary measures of subjective responses to alcohol in a human laboratory trial but improved cue- and stress-induced changes in mood and decreased craving (Ray et al., 2017). Ibudilast did decrease the reward-related subjective effects of methamphetamine in dependent individuals (Worley et al., 2016) and decreased the subjective effects of oxycodone and drug cravings in opioid-dependent individuals who had undergone detoxification (Metz et al., 2017). In addition to drug addiction, PDE4 inhibitors have been investigated as potential therapeutics in diverse central nervous system (CNS) disorders such as depression, anxiety, schizophrenia, Parkinson's disease, and Alzheimer's disease (Halene and Siegel, 2007, Kanes et al., 2007, Smith et al., 2009).
Considering the widespread anti-inflammatory effects of PDE4 inhibitors and their ability to substantially reduce drinking in rodent models, they are strong candidates for treating AUD and other inflammatory disorders. However, improved prototypes with fewer side effects in humans are needed. We tested apremilast, a novel PDE4 inhibitor with diminished emetic activity that is currently used to treat psoriasis. Drug repurposing is an expedient and cost-efficient means of drug discovery, and prioritizing study of FDA-approved drugs to treat AUD is especially important considering the limited number of options available. We also note that it has been more than a decade since the latest drug for AUD was approved. In this study, we used two tests that can produce different levels of ethanol intake in mice and that model different patterns of drinking in humans. Continuous two-bottle choice (2BC) drinking is the most widely used test of voluntary ethanol consumption and is associated with other measures of ethanol reward, while the 2BC intermittent (every-other-day, EOD) test can increase ethanol consumption in C57BL/6J mice (Melendez, 2011). Apremilast significantly reduced ethanol intake in both the 2BC and 2BC-EOD drinking tests in male and female C57BL/6J mice.
Materials and Methods
Mice
C57BL/6J male and female mice were from a colony maintained at The University of Texas at Austin (original breeders were purchased from The Jackson Laboratory, Bar Harbor, ME). Mice were group-housed 4 or 5 to a cage, and food and water were available ad libitum. The vivarium was maintained on a 12-h light/dark cycle with lights on at 7:00 a.m.; however, for studies of ethanol and saccharin/sucrose intake, mice were housed on a reverse light cycle schedule. The temperature and humidity of the rooms were kept constant. Behavioral testing began when the mice were 2-3 months old. Experiments were conducted in isolated behavioral testing rooms in the Animal Resources Center at The University of Texas at Austin, and mice were allowed to adapt to the rooms for one week before testing began. Separate groups of mice were used for the different drinking and behavioral tests used in this study and that of our companion paper (Blednov et al., submitted), with the exception of locomotor activity testing in which the same group of mice are represented in both studies. Experiments were approved by the university's Institutional Animal Care and Use Committee.
Apremilast Administration
Apremilast was purchased from Toronto Research Chemicals Inc. (Toronto, ON, Canada), freshly prepared as a suspension in saline with 3-4 drops of Tween-80, and administered daily in a volume of 0.05 ml/10 g of body weight, 1h before experiments. Control mice received the same injection volume of saline containing 3-4 drops of Tween-80. Single use, sterile gavage needles (27.5 gauge, Becton, Dickinson and Co., Franklin Lakes, NJ) were used. Saline or apremilast (5-50 mg/kg, p.o.) was administered daily for 6-11 days, depending on the drinking test. Drinking was then measured in the absence of apremilast for 2 or 4 days after saline injection. Doses were based on our initial dose-response studies.
Baseline Ethanol Drinking
Mice undergoing 2BC testing first consumed 15% (v/v) ethanol (Sigma-Aldrich, St. Louis, MO) for about 3 weeks, while baseline drinking in the 2BC-EOD group lasted about 5 weeks to provide a similar number of ethanol-drinking sessions in each group before beginning experiments. After these initial access periods, ethanol consumption was measured for at least 4 days to ensure stable levels of consumption (i.e., < 10% variation in average intake on days 1-2 and days 3-4). Ethanol intake was then measured after saline injection for 2 days, and mice were assigned to treatment and control groups based on similar levels of ethanol intake and preference. Ethanol and total fluid intake are shown as g/kg body weight/24h averaged for 2 drinking days. On drinking day 3, mice were injected once daily with either saline or apremilast for 6-11 days, depending on the drinking test. For dose-response measurements, saline or apremilast was injected daily for 2 days in the continuous 2BC test and for 3 days in the 2BC-EOD test.
Continuous Two-Bottle Choice Ethanol Drinking
The 24-h 2BC protocol, in which each mouse has access to a bottle of water and a bottle of ethanol, was carried out as previously described (Blednov et al., 2001). Bottles were weighed daily, and positions were alternated daily to control for side preferences. Food was available ad libitum, and mice were weighed every 4 days. Consumption of 15% (v/v) ethanol and water (g/kg/24h) was calculated for each mouse. Measurements of ethanol intake, preference, and total fluid intake were averaged over 2 days with different bottle positions. Each point in the line graphs (e.g., days 2, 4, 6, 8, 10) represents the average of 2 days of measurement. For example, day 2 is the average of days 1-2 and day 4 is the average of days 3-4.
Intermittent Two-Bottle Choice Ethanol Drinking
Intermittent access to ethanol induces high voluntary consumption in rats (Simms et al., 2008) and mice (Rosenwasser et al., 2013). We used this method to measure ethanol intake in mice with access to a bottle of 15% (v/v) ethanol and a bottle of water during 24-h drinking sessions offered every-other-day. The placement of the ethanol bottle was alternated with each drinking session to control for side preferences. Food was available ad libitum, and mice were weighed every 4 days. Consumption of 15% ethanol and water (g/kg/24h) was calculated for each mouse. Measurements of ethanol intake, preference, and total fluid intake were averaged over 3 days with different bottle positions.
Saccharin, Sucrose, and Water Intake
Saccharin (0.0165%, Sigma-Aldrich) and sucrose (1%, Sigma-Aldrich) intake experiments were carried out using the 2BC procedure. For both sweeteners, stable basal levels of consumption were established followed by two days of control measurements when all mice received saline or apremilast injections 1h before beginning the drinking session. Water intake was measured using one bottle of water.
Locomotor Activity
Locomotor activity was measured in standard mouse cages using the Opto-microvarimex animal activity meter (Columbus Instruments, Columbus, OH). Activity was monitored by six light beams spaced along the width of the cage at 2.5-cm intervals, 1.5 cm above the floor. Each cage had bedding and food and was covered by a heavy plastic lid with holes for ventilation. On the day of the experiment, mice were placed in individual experimental cages, and activity was monitored hourly for 22h. Saline or apremilast (20 mg/kg) was administered 1h before locomotor activity was monitored. For chronic drug treatment, apremilast (20 mg/kg) was administered daily for 6 days, and locomotor activity was measured on day 6, 1h after the last drug injection.
Blood Ethanol Concentration
The effect of saline or apremilast (20 mg/kg) on blood ethanol concentration (BEC) was measured over 4h in male and female mice after injection of ethanol (4 g/kg, i.p.). Retro-orbital blood samples (∼20 μl) were collected in capillary tubes and centrifuged for 6 min at 3100g using a Haematospin 1400 centrifuge (Analox Instruments, London, UK). Plasma samples were stored at −20°C until BECs were determined in 5-μl aliquots using an AM1 Alcohol Analyzer (Analox Instruments). The machine was calibrated every 15 samples using an industry standard, and BECs were determined using commercially available reagents according to the manufacturer's instructions. Samples were averaged from duplicate runs and expressed as mg/dl.
Biodistribution of Apremilast
Plasma, liver, and brain samples from male C57BL/6J mice were collected 1h or 2h after a single oral dose of apremilast (20 mg/kg). Plasma and tissue apremilast concentrations were measured using salting-out assisted liquid-liquid extraction (SALLE) and reversed phase high performance liquid chromatography (HPLC) with UV detection. Apremilast and the internal standard naproxen (MP Biomedicals, Santa Ana, CA) were quantitatively extracted from 100 μl of plasma, one hemisphere of brain (210-286 mg), or 200 ± 15 mg of liver tissue. Plasma samples were thawed, 20 μl of internal standard, 20 μl of 20 mM citric acid (Thermo Fisher Scientific, Waltham, MA), and 500 μl of acetonitrile Thermo Fisher Scientific) were added, with 30s of vortex-mixing, followed by the addition of 100 μl of 5 M magnesium chloride (Thermo Fisher Scientific) and 1 min of vortex-mixing. This mixture was centrifuged at 16900g for 5 min, and then the upper layer (acetonitrile) was transferred into an HPLC vial and evaporated under a stream of nitrogen at ∼30°C. The residue was reconstituted in 50 μl of (60:40) acetonitrile:10 mM citric acid solution (v/v) and 5 μl were injected into the HPLC for analysis.
Brain and liver samples were thawed and a total of 50 μl of internal standard, 50 μl of 20 mM citric acid, and 400 μl of acetonitrile were added with 30s of vortex-mixing, followed by centrifugation at 16900g for 1 min. Tissue was homogenized (ProScientific 200 homogenizer, Pro Scientific, Oxford, CT) for 1 min. An additional 750-μl aliquot of acetonitrile was added to the homogenate, and the sample was vortex-mixed for 30s, followed by the addition of 300 μl of a mixture of 5 M sodium chloride (BDH Chemicals, West Chester, PA) and 2.5 M ammonium sulfate (Thermo Fisher Scientific), and 1 min of vortex-mixing. This mixture was centrifuged at 16900g for 15 min. For brain tissue, 1 ml of the upper layer (acetonitrile) was filtered through 0.2 μm polytetrafluoroethylene (Corning Inc., Corning, NY) into an HPLC vial and evaporated under a stream of nitrogen at ∼30°C. The residue was reconstituted in 50 μl of acetonitrile, and 5 μl were injected into the HPLC for analysis. For liver tissue, 1 ml of the upper layer (acetonitrile) was transferred to a centrifuge tube, then 300 μl of 5 M magnesium chloride was added, followed by 1 min of vortex-mixing. This mixture was centrifuged at 16900g for 15 min, and 900 μl of the upper layer (acetonitrile) was filtered through 0.2 μm polytetrafluoroethylene into an HPLC vial and evaporated under a stream of nitrogen at ∼30°C. The residue was reconstituted in 50 μl of acetonitrile and 5 μl were injected into the HPLC as described below.
Calibration curves were prepared by spiking apremilast into blank mouse plasma (0.01-10 μg/ml), brain (0.025-1.25 μg/ml), and liver tissue (0.06-125 μg/ml), in triplicate across a minimum of six levels of apremilast. Method selectivity was verified by extracting and analyzing unspiked blank samples. The chromatographic assay was performed with a Dionex Ultimate 3000 HPLC with UV detector (Dionex Corp., Sunnyvale, CA) using gradient elution on an Eclipse Plus C18 column (3.0 × 150 mm, 3.5 μm) with an EC-18 guard column (3.0 × 5.0 mm, 2.7 μm) (Agilent Technologies, Santa Clara, CA), 30°C column temperature, 230 nm, and a run time of 15 min. A ternary gradient separation was performed using 0.1% phosphoric acid (EMD Chemicals, Gibbstown, NJ) in water (v/v), acetonitrile, and methanol as the mobile phases, and a flow rate of 0.6 ml/min.
Statistical Analysis
Data are reported as mean ± S.E.M values. The statistics software program Prism (GraphPad Software, Inc., La Jolla, CA) was used to perform one-way or two-way repeated measures ANOVA, Bonferroni post-hoc tests, and Student's t-tests.
Results
Apremilast Reduces Voluntary Ethanol Consumption in the Two-Bottle Choice Test
In the continuous 2BC test, single injections of apremilast dose dependently reduced 15% ethanol intake (F4,31 = 12.2, p < 0.001), preference for ethanol (F4,31 = 5.2, p < 0.01), and total fluid intake (F4,31 = 6.1, p < 0.01) in C57BL/6J male mice (Fig. 1A-C). The data shown represent the differences between two-day drinking averages after drug injection (days 3, 4 after apremilast) and the first two days of saline (control) injections (days 1, 2). Two-day averages account for the effects of different bottle positions on drinking behavior and provide a more accurate measurement of ethanol intake and drug effects. The raw drinking data in male mice are shown in Supplemental Fig. 1A-C. Post-hoc analyses showed significant decreases in all drinking parameters at doses of 30-50 mg/kg, while 15 mg/kg of apremilast reduced the amount of ethanol consumed without changing total fluid intake. There was a trend for 15 mg/kg to reduce preference for ethanol, but this did not reach statistical significance. In female mice, apremilast also dose dependently reduced ethanol intake (F4,27 = 15.8, p < 0.001), preference for ethanol (F4,27 = 9.4, p < 0.001), and total fluid intake (F4,27 = 15.5, p < 0.001; Fig. 1D-F). The raw drinking data in female mice are shown in Supplemental Fig. 1D-F. Post-hoc analyses showed that 15-50 mg/kg apremilast significantly decreased the amount of ethanol consumed and preference for ethanol, and 30-50 mg/kg apremilast also reduced total fluid intake. The lowest dose of apremilast tested (5 mg/kg) did not significantly alter ethanol consumption in male or female mice. Based on the dose-response effects, a 20 mg/kg dose was chosen for chronic drug treatment experiments.
Figure 1. Apremilast dose dependently decreases 15% ethanol intake in the continuous 2BC test.
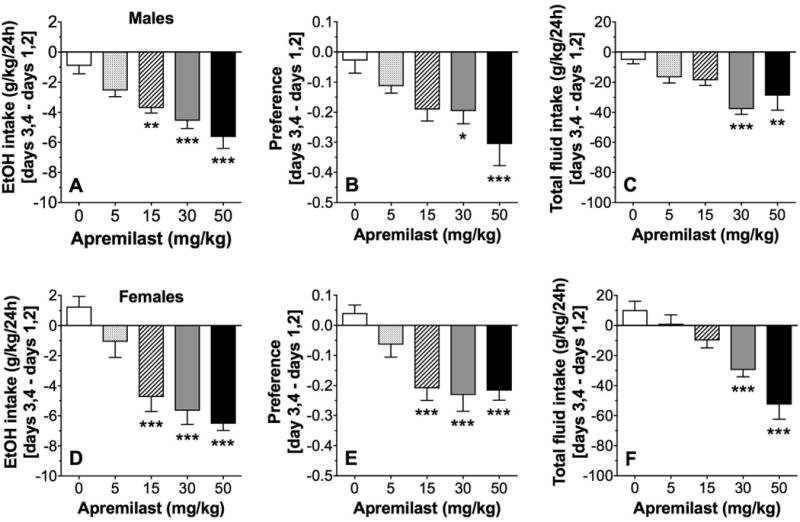
Effects of apremilast (5-50 mg/kg) on ethanol (EtOH) intake (A), preference for EtOH (B), and total fluid intake (C) in male C57BL/6J mice; n= 7-8 per group. Effects of apremilast (5-50 mg/kg) on EtOH intake (D), preference for EtOH (E), and total fluid intake (F) in female C57BL/6J mice; n= 6-7 per group. Data are presented as the differences between two-day drinking averages after drug injection (days 3, 4 after apremilast) and the first two days of saline (control) injections (days 1, 2). Data were analyzed by one-way ANOVA with Bonferroni post-hoc tests (*p < 0.05, **p < 0.01, ***p < 0.001 compared with control).
Six daily injections of apremilast (20 mg/kg) significantly reduced ethanol consumption (F1,14 = 12.8, p < 0.001, effect of treatment; F2,28 = 3.8, p < 0.05, effect of time) and preference for ethanol (F1,14 = 9.9, p < 0.001, effect of treatment) but did not alter total fluid consumption in male mice (Fig. 2A-C). During the washout phase when injection of apremilast was replaced with saline, mice previously treated with apremilast showed no differences in ethanol consumption or preference compared with control mice. Similar to male mice, 6 daily injections of apremilast (20 mg/kg) in female mice reduced ethanol consumption (F1,16 = 16.4, p < 0.001, effect of treatment) and preference for ethanol (F1,16 = 4.6, p < 0.05, effect of treatment; F2,32 = 4.4, p < 0.05, effect of time) but did not change total fluid consumption (Fig. 2D-F). During the washout phase, female mice previously treated with apremilast showed no differences in ethanol intake or preference compared with control mice.
Figure 2. Apremilast produces stable reduction of 15% ethanol intake in the continuous 2BC test.
Effects of apremilast (20 mg/kg) on ethanol (EtOH) intake (A), preference for EtOH (B), and total fluid intake (C) in male C57BL/6J mice; n= 8 per group. Effects of apremilast (20 mg/kg) on EtOH intake (D), preference for EtOH (E), and total fluid intake (F) in female C57BL/6J mice; n= 9 per group. Each data point represents the average of two days of drinking. S/S represents two-day drinking averages after saline injections for both groups. S/Drug represents the two-day averages after saline or apremilast injections (6 days total). The second S/S period shows the extinction (4 days total) of effects on drinking in mice previously treated with apremilast. Data were analyzed by two-way repeated measures ANOVA.
Apremilast Reduces Voluntary Ethanol Consumption in the Two-Bottle Choice Intermittent Drinking Test
Compared with 2BC continuous drinking, intermittent access usually produces higher ethanol intake in mice and can be used to model a different pattern of drinking in humans. In the 2BC-EOD test, single injections of apremilast dose dependently reduced 15% ethanol intake (F4,35 = 8.2, p < 0.001) and total fluid intake (F4,35 = 4.5, p < 0.01) but did not alter preference for ethanol in C57BL/6J male mice (Fig. 3A-C). Figure 3 shows the differences between two-day drinking averages after drug injection (days 3, 4 after apremilast) and the first two days of saline (control) injections (days 1, 2). The raw drinking data in male mice are shown in Supplemental Fig. 2A-C. Post-hoc analyses showed that all doses of apremilast significantly reduced the amount of ethanol consumed. Apremilast (30-50 mg/kg) reduced total fluid intake, and 50 mg/kg decreased preference for ethanol. In female mice, apremilast dose dependently reduced ethanol intake (F4,50 = 7.3, p < 0.001), preference for ethanol (F4,50 = 6.2, p < 0.001), and total fluid intake (F4,50 = 3.3, p < 0.05; Fig. 3D-F). The raw drinking data in female mice are shown in Supplemental Fig. 2D-F. Post-hoc analyses revealed significant reductions in ethanol intake and preference by 30-50 mg/kg of apremilast, and only the 50 mg/kg dose significantly reduced total fluid intake.
Figure 3. Apremilast dose dependently decreases 15% ethanol intake in the intermittent 2BC-EOD test.
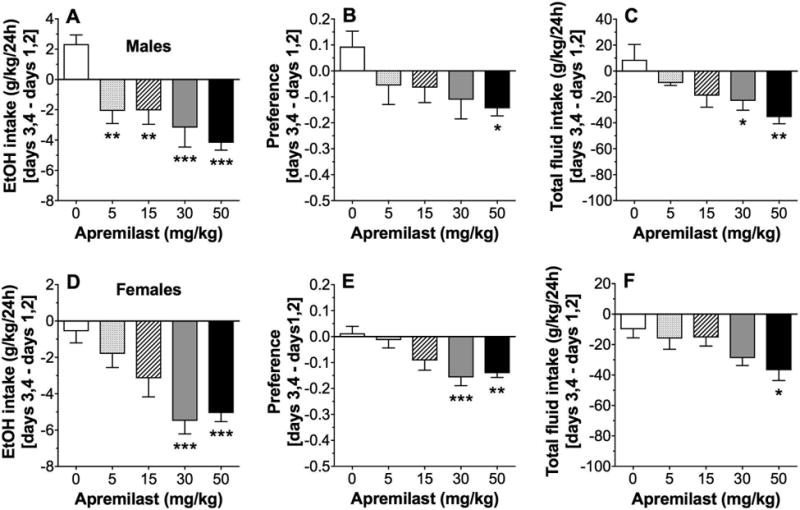
Effects of apremilast (5-50 mg/kg) on ethanol (EtOH) intake (A), preference for EtOH (B), and total fluid intake (C) in male C57BL/6J mice; n= 8 per group. Effects of apremilast (5-50 mg/kg) on EtOH intake (D), preference for EtOH (E), and total fluid intake (F) in female C57BL/6J mice; n= 11 per group. Data are presented as the differences between two-day drinking averages after drug injection (days 3, 4 after apremilast) and the first two days of saline (control) injections (days 1, 2). Data were analyzed by one-way ANOVA with Bonferroni post-hoc tests (*p < 0.05, **p < 0.01, ***p < 0.001 compared with control).
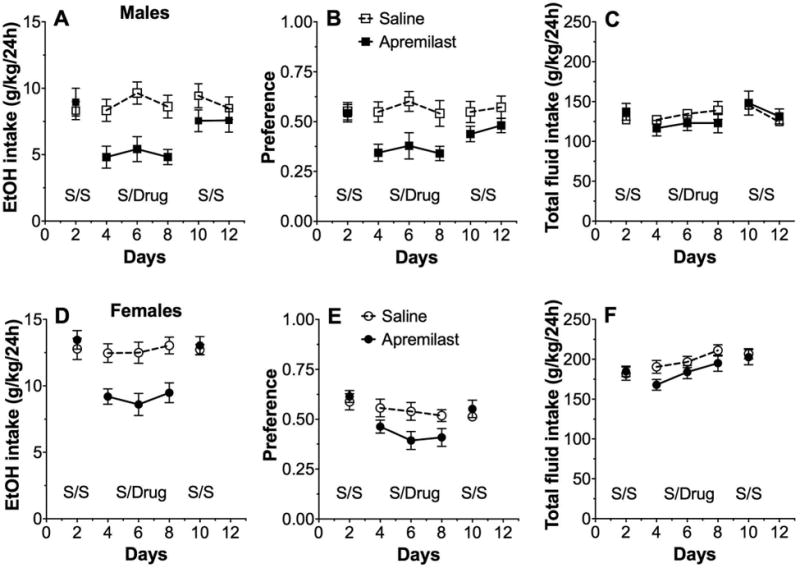
In the 2BC-EOD test, 11 daily injections of apremilast (20 mg/kg, administered on the 6 drinking days and on intervening days) reduced the amount of ethanol consumed (F1,360 = 89.1, p < 0.001, effect of treatment; F2,36 = 4.6, p < 0.05, effect of time), preference for ethanol (F1,36 = 28.4, p < 0.001, effect of treatment), and total fluid intake (F1,36 = 5.2, p < 0.05, effect of treatment; F2,36 = 7.1, p < 0.05, treatment × time interaction) in male mice (Fig. 4A-C). During the washout phase, mice previously treated with apremilast showed no differences from control mice. In female mice, 11 daily injections of apremilast (20 mg/kg) significantly reduced ethanol intake (F1,18 = 4.6, p < 0.05, effect of treatment) but did not alter preference for ethanol or total fluid intake (Fig. 4D-F).
Figure 4. Apremilast produces stable reduction of 15% ethanol intake in the intermittent 2BC-EOD test.

Effects of apremilast (20 mg/kg) on ethanol (EtOH) intake (A), preference for EtOH (B), and total fluid intake (C) in male C57BL/6J mice; n= 10 per group. Effects of apremilast (20 mg/kg) on EtOH intake (D), preference for EtOH (E), and total fluid intake (F) in female C57BL/6J mice; n= 10 per group. Each data point represents the average of two days of drinking. S/S represents two-day drinking averages after saline injections for both groups. S/Drug represents the two-day averages after saline or apremilast injections (11 days total). The second S/S period shows the extinction (2 days total) of effects on drinking in mice previously treated with apremilast. Data were analyzed by two-way repeated measures ANOVA with Bonferroni post-hoc tests (**p < 0.01 compared with corresponding control time point).
Effects of Apremilast on Saccharin, Sucrose, and Water Consumption
In the 2BC test (continuous 24-h access to saccharin and water) in male mice, apremilast (20 mg/kg) did not alter preference for saccharin or total fluid (saccharin plus water) intake (Fig. 5A,B). There was no treatment effect on water intake measured in a separate experiment (Fig. 5C). Apremilast also did not change preference for saccharin or total fluid intake in female mice (Fig. 5D,E). There was a time x treatment interaction effect on water intake (F5,50 = 3.8, p < 0.01; Fig. 5F); post-hoc analysis showed that apremilast treatment significantly reduced water intake in female mice only during the first two days of treatment.
Figure 5. Apremilast does not alter preference for saccharin or water intake.
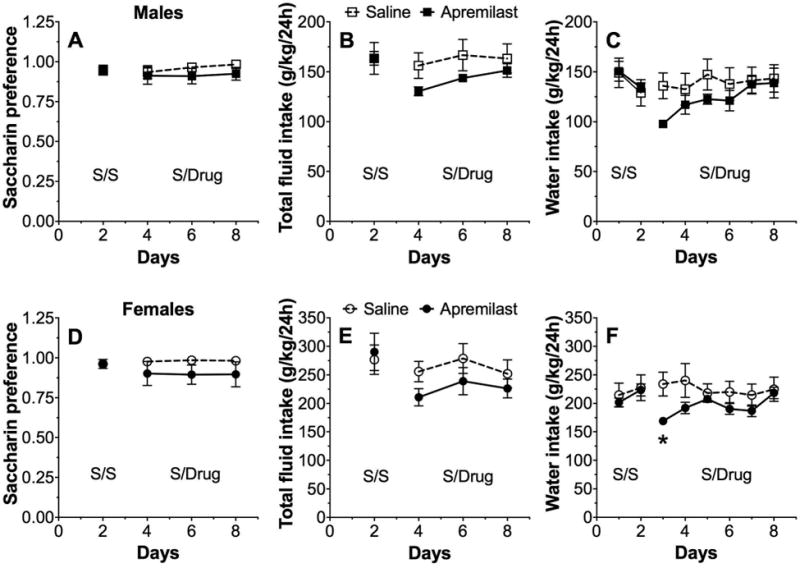
Effects of apremilast (20 mg/kg) on preference for saccharin (A), total fluid intake (B), and water intake (C) in male C57BL/6J mice; n= 6 per group for the saccharin experiment and n= 5-6 per group for the water experiment. Effects of apremilast (20 mg/kg) on preference for saccharin (D), total fluid intake (E), and water intake (F) in female C57BL/6J mice; n= 6 per group. For 2BC saccharin drinking, data represent the average of two days of drinking. For water intake using one bottle of water, data are presented as daily intake. S/S represents two days of saline injections for both groups. S/Drug represents two days of saline or apremilast injections (6 days total for saccharin experiment and 8 days total for water experiment). Data were analyzed by two-way repeated measures ANOVA with Bonferroni post-hoc tests (*p < 0.05 compared with corresponding control time point).
There was also no effect of apremilast on preference for sucrose or total fluid intake in male (Fig. 6A,B) or female (Fig. 6C,D) mice.
Figure 6. Apremilast does not alter preference for sucrose.
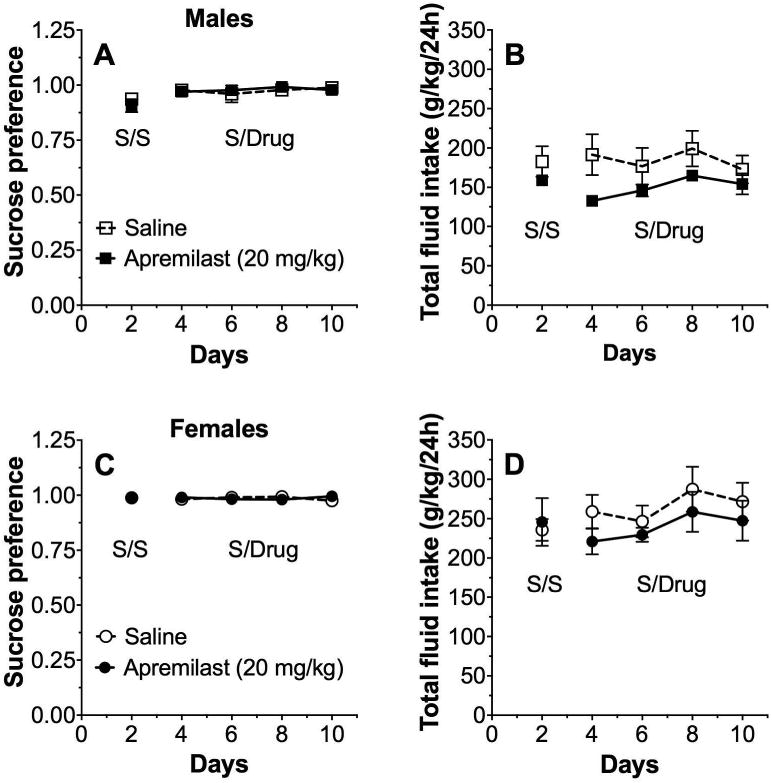
Effects of apremilast (20 mg/kg) on preference for sucrose (A) and total fluid intake (B) in male C57BL/6J mice; n= 6 per group. Effects of apremilast (20 mg/kg) on preference for sucrose (A) and total fluid intake (B) in female C57BL/6J mice; n= 6 per group. Data are presented as two-day drinking averages. S/S represents two days of saline injections for both groups. S/Drug represents two days of saline or apremilast injections (6 days total). Data were analyzed by two-way repeated measures ANOVA with Bonferroni post-hoc tests, but no significant group differences were found.
Locomotor Activity
We studied the acute effect of apremilast (20 mg/kg) on spontaneous locomotor activity measured hourly for 22h (Fig. 7). The main effect of apremilast treatment was not significant in male mice, but there was a significant time x treatment interaction (F21,630 = 1.7, p < 0.05; Fig. 7A), although post-hoc tests did not show significant differences at any time point. In contrast to male mice, an overall reduction in motor activity was observed in females treated with apremilast (F1,30 = 10.7, p < 0.01, effect of treatment; F21,630 = 50.5, p < 0.001, effect of time; F21,630 = 3.2, p < 0.001, time × treatment interaction; Fig. 7C). The apremilast effect on motor activity was also evaluated by comparing the areas under the motor activity curves, which showed a reduction of motor activity in female (p < 0.01) but not in male mice (Fig. 7B,D).
Figure 7. Acute administration of apremilast (20 mg/kg) decreases locomotor activity in female mice.
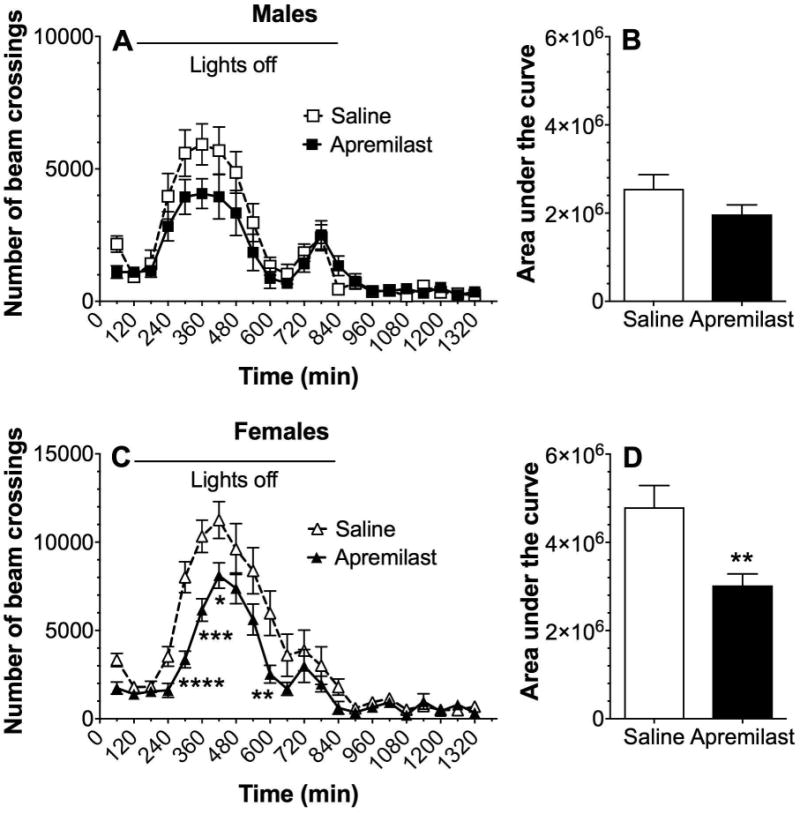
Spontaneous locomotion recorded every hour (A) and area under the curve (B) in male C57BL/6J mice (n= 16 per group). Spontaneous locomotion (C) and area under the curve (D) in female C57BL/6J mice (n= 16 per group). Spontaneous locomotor data were analyzed by two-way repeated measures ANOVA with Bonferroni post-hoc tests (*p < 0.05, **p< 0.01, ***p<0.001, ****p<0.0001 compared with corresponding control time point). Area under the curve data were analyzed using Student's t-tests (**p < 0.01 compared with saline control).
We also measured locomotor activity in male and female mice after repeated (6 daily injections) administration of apremilast (20 mg/kg) since we had used repeated dosing in our drinking studies. Motor activity was significantly reduced in males (F1,30 = 8.5, p < 0.01, effect of treatment; F21,630 = 41.4, p < 0.001, effect of time; F21,630 = 3.5, p <0.001, time x treatment interaction; Fig. 8A) but not in females (Fig. 8C). There was also significant reduction in the area under the curve in male (p < 0.01) but not in female mice after repeated injections (Fig. 8B,D).
Figure 8. Chronic administration of apremilast (20 mg/kg) decreases locomotor activity in male mice.

Apremilast was given daily for 6 days. Spontaneous locomotion recorded every hour (A) and area under the curve (B) in male C57BL/6J mice (n= 16 per group). Spontaneous locomotion (C) and area under the curve (D) in female C57BL/6J mice (n= 16 per group). Spontaneous locomotor data were analyzed by two-way repeated measures ANOVA with Bonferroni post-hoc tests (*p < 0.05, **p< 0.01, ****p<0.0001 compared with corresponding control time point). Area under the curve data were analyzed using Student's t-tests (**p < 0.01 compared with saline control).
Blood Ethanol Clearance
Pretreatment with apremilast did not affect blood ethanol clearance in male or female mice (Fig. 9A,B). Comparison of the slopes of the regression lines showed no effect of apremilast in male (-68 ± 3 for saline and -71 ± 3 for apremilast pretreatment) or female mice (-52 ± 3 for saline and -49 ± 3 for apremilast pretreatment).
Figure 9. Apremilast does not alter clearance of blood ethanol.
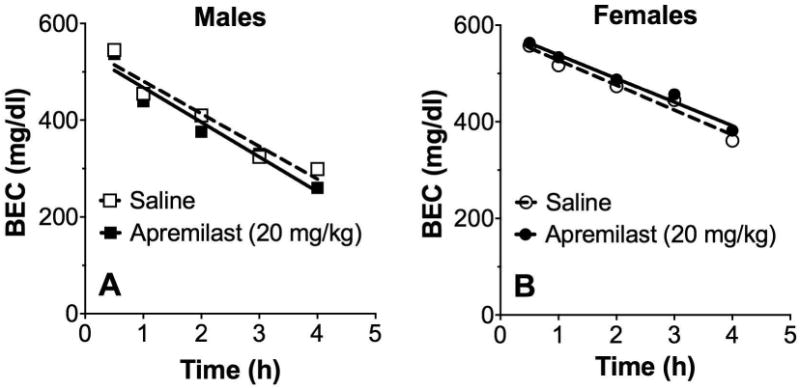
Effect of saline or apremilast (20 mg/kg) on blood ethanol concentration (BEC) measured over 4h in male (A) and female (B) C57BL/6J mice after i.p. injection of 4 g/kg ethanol (n= 12 per group).
Biodistribution of Apremilast
Maximum plasma levels in mice and humans were found ∼2h after a single oral dose of apremilast (https://www.tga.gov.au/sites/default/files/auspar-apremilast-151022.pdf). We thus collected plasma, liver, and brain samples from male mice 1h or 2h after a single treatment with apremilast (20 mg/kg). The highest levels of apremilast were found in liver followed by plasma and brain (Table 1).
Table 1. Plasma and tissue levels of apremilast.
Apremilast levels were measured in C57BL/6J male mice 1h or 2h after a single oral treatment with apremilast (20 mg/kg; n= 3 per time period). Values represent mean ± S.E.M.
| Tissue | 1 h | 2 h |
|---|---|---|
| Liver (ng/mg) | 20.4 ± 1.2 | 16.4 ± 1.3 |
| Plasma (ng/μl) | 2.1 ± 0.5 | 2.0 ± 0.2 |
| Brain (ng/mg) | 0.15 ± 0.01 | 0.14 ± 0.02 |
Discussion
The PDE4 inhibitor rolipram decreased ethanol intake and preference in mice (Blednov et al., 2014, Hu et al., 2011), as well as ethanol seeking and consumption in rats (Wen et al., 2012). In this study, we showed that apremilast, another selective inhibitor of PDE4, also reduced ethanol intake and preference in the 2BC and 2BC-EOD tests. Unlike rolipram, which transiently reduced ethanol drinking, apremilast produced long lasting decreases. Apremilast was effective within a similar range of doses in male and female mice, but its overall effectiveness in reducing ethanol intake was greater in male compared with female mice. Only the higher doses (30-50 mg/kg) reduced total fluid intake. This effect may have been due to the sedative effect of PDE4 inhibitors (Hu et al., 2011). Although 20 mg/kg of apremilast reduced spontaneous locomotor activity, this was not likely a factor in reducing ethanol consumption because this dose did not affect total fluid consumption. In rodents, there is a positive correlation between ethanol intake and consumption of sweet solutions, as shown in studies of inbred strains, hybrid mice, and taste-deficient mutants (Bachmanov et al., 1996, Blednov et al., 2008, Yoneyama et al., 2008). However, apremilast did not alter consumption of a sweet solution of saccharin or a sweet and highly caloric solution of sucrose.
Despite being introduced in the late 1980s, the clinical use of PDE inhibitors has been hampered because of lack of efficacy and gastrointestinal side effects such as nausea and vomiting (Giembycz, 2008). One approach to mitigating these adverse effects is to seek agents with selectivity for PDE4 isoforms not associated with toxic effects. PDE4 isozymes are encoded by four genes (A,B,C,D), and inhibition of PDE4D is thought to promote emesis (Robichaud et al., 2002), whereas selective inhibition of PDE4A or PDE4B in proinflammatory and immune cells is thought to produce the desired anti-inflammatory effects (Giembycz, 2008, Jin et al., 2012). Apremilast is a partial competitive inhibitor of PDE4 but does not demonstrate marked PDE4 subfamily (A-D) selectivity (Schafer et al., 2010). In comparison, cilomilast is 10-fold more selective for PDE4D (Giembycz, 2001), the isozyme associated with emesis. The lack of PDE4D selectivity of apremilast may in part explain its improved therapeutic index compared with cilomilast in preclinical models (see Schett et al., 2010 for review).
The anti-inflammatory effects of PDE4 inhibitors may be important in their ability to decrease ethanol drinking. Lipopolysaccharide (LPS), proinflammatory cytokines, and ethanol all increase the activity of PDE4 in several cell types (microglia, astrocytes, bronchial epithelial), and rolipram reversed these effects (Buttini et al., 1997, Forget et al., 2003, Ghosh et al., 2012). Rolipram dose dependently decreased LPS-induced production of TNF-α in rat brain and microglia (Buttini et al., 1997, Yoshikawa et al., 1999). Apremilast is also known to regulate immune signaling. For example, it inhibited production of TNF-α (Man et al., 2009), INF-α, and cytokines (Schafer et al., 2014). Overall, the pharmacological effects of apremilast are consistent with those of a selective PDE4 inhibitor with fewer side effects that also inhibits innate immune responses. Considering the accumulating evidence for immune/inflammatory mechanisms in AUD (Mayfield and Harris, 2017), apremilast may decrease drinking by targeting these peripheral and central neuroimmune pathways. One approach to test this mechanism would be to determine if apremilast inhibits changes in neuroimmune gene expression induced by ethanol (McCarthy et al., 2017, Osterndorff-Kahanek et al., 2013). To determine if the effects of apremilast are related to decreased production of TNF-α or INF-α, another approach would be to compare its effects on drinking with a more specific antagonist for these receptors.
In mice and humans, the time to reach maximum plasma concentrations after a single oral dose of apremilast was similar (∼2h) (https://www.tga.gov.au/sites/default/files/auspar-apremilast-151022.pdf), suggesting rapid absorption; thus, the 1h and 2h time points that we measured should represent near peak plasma levels. It is of interest to compare the concentrations of apremilast found in our study with those needed to produce anti-inflammatory actions. The plasma level of 2.1 ng/μl (Table 1) corresponds to 4.6 μM of total drug. However free drug concentrations are more reflective of activity (Muller and Milton, 2012), and apremilast is 89% bound in mouse plasma (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003746/WC500182629.pdf), giving a free concentration of 0.5 μM. The concentrations required to inhibit cytokine production are similar or lower (IC50 values in μM: INF-γ, 0.013; TNF-α, 0.11; MCP-1, 1.3; (Schafer et al., 2010). Although brain levels of apremilast were much lower than those detected in plasma and liver, the drug did reach the brain 1-2h after a single oral treatment at levels similar to the calculated free concentration in plasma. This evidence, along with the effects on ethanol drinking that we observed after systemic treatment, indicate that apremilast can act centrally, further supporting the feasibility of using PDE4 inhibitors to treat CNS diseases. In this regard, it is interesting to compare clinical concentrations with those in our study. Human doses of 100 mg/day (roughly equivalent to the mouse doses of 20 mg/kg used in our study (Nair and Jacob, 2016) resulted in peak total plasma levels of 0.99 ng/μl (https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205437Orig1s000ClinPharmR.pdf). The drug is 68% bound in human plasma (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003746/WC500182629.pdf), resulting in a free drug concentration of 0.7 μM. This is somewhat higher than found in mice, again supporting the feasibility of clinical testing of apremilast to treat AUD.
As discussed above, we and others showed that PDE4 inhibitors such as rolipram reduce voluntary ethanol consumption in different rodent models. Rolipram was initially developed as an antidepressant but gastrointestinal side effects caused clinical use to be discontinued. Our current findings suggest that apremilast may be a more promising PDE4 inhibitor for clinical testing and repurposing to treat AUD. Our companion article examines the effects of apremilast on other ethanol-related behaviors that can impact ethanol consumption (Blednov et al.). Subtype-selective inhibitors targeting PDE4B are of interest given the role for PDE4B in immune function versus the association of PDE4D with nausea and vomiting. In addition to its function in immune signaling, PDE4B may be of particular interest in AUD because it is localized in brain reward pathways (Cherry and Davis, 1999), its expression is upregulated in mice with a genetic predisposition for high ethanol consumption (Mulligan et al., 2006), and a large genome-wide association study linked PDE4B with ethanol consumption in humans (Clarke et al., 2017). In addition, Pde4b expression is critical for ethanol-induced neuroinflammatory responses (Avila, 2015, Avila et al., 2017). Continued drug discovery efforts to identify well tolerated PDE4 inhibitors with improved ability to reach the brain may help advance the treatment outlook for many inflammatory and immune-related CNS disorders.
Supplementary Material
Acknowledgments
The authors thank Dr. Jody Mayfield for help writing, editing, and preparing figures. We also thank Dr. Esther Maier at the Drug Dynamics Institute (College of Pharmacy, The University of Texas at Austin) for assistance with the biodistribution study of apremilast in mouse tissue. This research was supported by the National Institute on Alcohol Abuse and Alcoholism (grants AA013520/INIA Project and AA006399).
Footnotes
The authors declare no conflicts of interest.
References
- Avila D, Gobejishvili L, Myers S, Zhang J, Barker D, Whittemore S, McClain C, Barve S. Pathogenic role of phosphodiesterase 4B (PDE4B) in alcohol-induced neuro-inflammation. Faseb J. 2015;29:771–718. [Google Scholar]
- Avila DV, Myers SA, Zhang J, Kharebava G, McClain CJ, Kim HY, Whittemore SR, Gobejishvili L, Barve S. Phosphodiesterase 4b expression plays a major role in alcohol-induced neuro-inflammation. Neuropharmacology. 2017;125:376–385. doi: 10.1016/j.neuropharm.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, Becker HC. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 2015;20:38–42. doi: 10.1111/adb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Harris RA. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci. 2014;8:129. doi: 10.3389/fnins.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Da Costa AJ, Ponomareva O, Harris RA, Messing RM. Apremilast alters behavioral responses to ethanol in mice: II. Increased sedation, intoxication and reduced acute functional tolerance. Alcohol Clin Exp Res submitted. doi: 10.1111/acer.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. The Journal of pharmacology and experimental therapeutics. 2001;298:521–530. [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Mir A, Appel K, Wiederhold KH, Limonta S, Gebicke-Haerter PJ, Boddeke HW. Lipopolysaccharide induces expression of tumour necrosis factor alpha in rat brain: inhibition by methylprednisolone and by rolipram. British journal of pharmacology. 1997;122:1483–1489. doi: 10.1038/sj.bjp.0701502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;407:287–301. [PubMed] [Google Scholar]
- Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, Murray AD, Smith BH, Campbell A, Hayward C, Porteous DJ, Deary IJ, McIntosh AM. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117) Mol Psychiatry. 2017;22:1376–1384. doi: 10.1038/mp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl) 2016;233:1543–1557. doi: 10.1007/s00213-015-3906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget MA, Sisson JH, Spurzem JR, Wyatt TA. Ethanol increases phosphodiesterase 4 activity in bovine bronchial epithelial cells. Alcohol. 2003;31:31–38. doi: 10.1016/j.alcohol.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Garcia-Castillo D, Aguirre V, Golshani R, Atkins CM, Bramlett HM, Dietrich WD, Pearse DD. Proinflammatory cytokine regulation of cyclic AMP-phosphodiesterase 4 signaling in microglia in vitro and following CNS injury. Glia. 2012;60:1839–1859. doi: 10.1002/glia.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LC, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, Mackenzie FL, Nagasawa M, Stevens PA, Mackenzie SJ. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. Cilomilast: a second generation phosphodiesterase 4 inhibitor for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2001;10:1361–1379. doi: 10.1517/13543784.10.7.1361. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. Can the anti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking? Br J Pharmacol. 2008;155:288–290. doi: 10.1038/bjp.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong MF, Wen RT, Xu Y, Pan JC, Fei N, Zhou YM, Xu JP, Liang JH, Zhang HT. Attenuation of ethanol abstinence-induced anxiety- and depressive-like behavior by the phosphodiesterase-4 inhibitor rolipram in rodents. Psychopharmacology (Berl) 2017:27. doi: 10.1007/s00213-017-4697-3. 2017 Jul. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Halene TB, Siegel SJ. PDE inhibitors in psychiatry--future options for dementia, depression and schizophrenia? Drug Discov Today. 2007;12:870–878. doi: 10.1016/j.drudis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang HT. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology (Berl) 2011;218:331–339. doi: 10.1007/s00213-011-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SL, Ding SL, Lin SC. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Chang Gung Med J. 2012;35:197–210. doi: 10.4103/2319-4170.106152. [DOI] [PubMed] [Google Scholar]
- Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience. 2007;144:239–246. doi: 10.1016/j.neuroscience.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. 2012;165:1288–1305. doi: 10.1111/j.1476-5381.2011.01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HW, Schafer P, Wong LM, Patterson RT, Corral LG, Raymon H, Blease K, Leisten J, Shirley MA, Tang Y, Babusis DM, Chen R, Stirling D, Muller GW. Discovery of (S)-N-[2-[1-(3-ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2,3-dihy dro-1H-isoindol-4-yl] acetamide (apremilast), a potent and orally active phosphodiesterase 4 and tumor necrosis factor-alpha inhibitor. J Med Chem. 2009;52:1522–1524. doi: 10.1021/jm900210d. [DOI] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013;23:513–520. doi: 10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Harris RA. The neuroimmune basis of excessive alcohol consumption. Neuropsychopharmacology. 2017;42:376. doi: 10.1038/npp.2016.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy GM, Farris SP, Blednov YA, Harris RA, Mayfield RD. Microglial-specific transcriptome changes following chronic alcohol consumption. Neuropharmacology. 2017;128:416–424. doi: 10.1016/j.neuropharm.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz VE, Jones JD, Manubay J, Sullivan MA, Mogali S, Segoshi A, Madera G, Johnson KW, Comer SD. Effects of Ibudilast on the Subjective, Reinforcing, and Analgesic Effects of Oxycodone in Recently Detoxified Adults with Opioid Dependence. Neuropsychopharmacology. 2017;42:1825–1832. doi: 10.1038/npp.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S, Guerri C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res. 2016;40:2260–2270. doi: 10.1111/acer.13208. [DOI] [PubMed] [Google Scholar]
- Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. 2012;11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterndorff-Kahanek E, Ponomarev I, Blednov YA, Harris RA. Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation. PLoS One. 2013;8:e59870. doi: 10.1371/journal.pone.0059870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page CP, Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol. 2011:391–414. doi: 10.1007/978-3-642-17969-3_17. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, Roche DJ, Heinzerling K, Miotto K. Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: A randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology. 2017;42:1776–1788. doi: 10.1038/npp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, Liu S, Huang Z, Conti M, Chan CC. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S. Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol. 2013;18:496–507. doi: 10.1111/j.1369-1600.2012.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer PH, Parton A, Capone L, Cedzik D, Brady H, Evans JF, Man HW, Muller GW, Stirling DI, Chopra R. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016–2029. doi: 10.1016/j.cellsig.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, Bartlett JB, Loveland MA, Gilhar A, Cheung YF, Baillie GS, Houslay MD, Man HW, Muller GW, Stirling DI. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842–855. doi: 10.1111/j.1476-5381.2009.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism, clinical and experimental research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci U S A. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden A, Erickson E, Robinson G, Harris RA, Mayfield RD. The neuroimmune transcriptome and alcohol dependence: potential for targeted therapies. Pharmacogenomics. 2016;17:2081–2096. doi: 10.2217/pgs-2016-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, Liu Q, Wang WP, Lawrence AJ, Zhang HT, Liang JH. The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol Clin Exp Res. 2012;36:2157–2167. doi: 10.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Heinzerling KG, Roche DJ, Shoptaw S. Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study. Drug Alcohol Depend. 2016;162:245–250. doi: 10.1016/j.drugalcdep.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M. Effects of phosphodiesterase inhibitors on cytokine production by microglia. Multiple sclerosis. 1999;5:126–133. doi: 10.1177/135245859900500210. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 2001;7:387–398. doi: 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


