Abstract
Background
Few studies have investigated the changes in weight that may occur over time among adults with the progression of chronic kidney disease (CKD). Whether such weight changes are independently associated with death after the onset of end-stage renal disease (ESRD) has also not been rigorously examined.
Study Design
Prospective cohort study.
Setting, and Participants
We studied 3933 participants of the Chronic Renal Insufficiency Cohort (CRIC) Study, a longitudinal cohort of patients with CKD. We also performed similar analyses among 1067 participants of the African American Study of Kidney Disease and Hypertension (AASK).
Predictors
Estimated glomerular filtration rate (eGFR) and weight change during CKD.
Outcome
Weight and all-cause mortality after dialysis initiation.
Results
During median follow-up of 5.7 years in CRIC, weight change was not linear. Weight was stable until cystatin C–based eGFR (eGFRcys) fell below 35 mL/min/1.73m2; thereafter, weight declined at a mean rate of 1.45 (95% CI, 1.19–1.70) kg for every 10 mL/min/1.73m2 decline in eGFRcys. Among the 770 CRIC participants who began hemodialysis or peritoneal dialysis during follow-up, a greater than 5% annualized weight loss after eGFR fell below 35 mL/min/1.73m2 was associated with a 54% higher risk of death after dialysis initiation (95% CI, 1.17–2.03) compared to those with more stable weight (annualized weight changes within 5% of baseline) in adjusted analysis. Similar findings were observed in the AASK.
Limitations
Inclusion of research participants only; inability to distinguish intentional versus unintentional weight loss.
Conclusions
Significant weight loss began relatively early during the course of CKD and was associated with a substantially higher risk of death after dialysis initiation. Further studies are needed to determine whether interventions to optimize weight and nutritional status before the initiation of dialysis will improve outcomes after ESRD.
Keywords: weight, nutrition, mortality, body mass index (BMI), weight change, mortality, chronic kidney disease (CKD), CKD progression, dialysis initiation, end-stage renal disease (ESRD), risk of death
Patients with end-stage renal disease (ESRD) undergoing maintenance dialysis experience substantial morbidity and mortality.1, 2 Recent studies have emphasized that clinical events and medical management during the pre-ESRD phase of chronic kidney disease (CKD) could impact outcomes after onset of ESRD.3–5 Suboptimal delivery of care during the transition to ESRD may contribute to the increased morbidity and mortality of dialysis patients,3 whereas appropriate treatment of dyslipidemia and hypertension before onset of kidney failure has been associated with improved outcomes after the onset of ESRD.6–8
Although anorexia and weight loss accompany the progression of CKD,9, 10 few studies have documented the time course, frequency, and magnitude of weight changes that occur with the progression of CKD. These patterns of weight change are important, as they may provide insights into the appropriate timing of nutritional interventions to achieve the greatest impact on outcomes of patients with CKD.
In the current study, we first determined the longitudinal patterns of weight changes during the progression of CKD among enrollees of the Chronic Renal Insufficiency Cohort (CRIC) Study. We then examined the association between these patterns of weight changes and risk of all-cause mortality after dialysis initiation. Similar analyses were performed in a second cohort of CKD patients without diabetes, the African American Study of Kidney Disease and Hypertension (AASK) to confirm our findings in CRIC Study.
Methods
CRIC Study
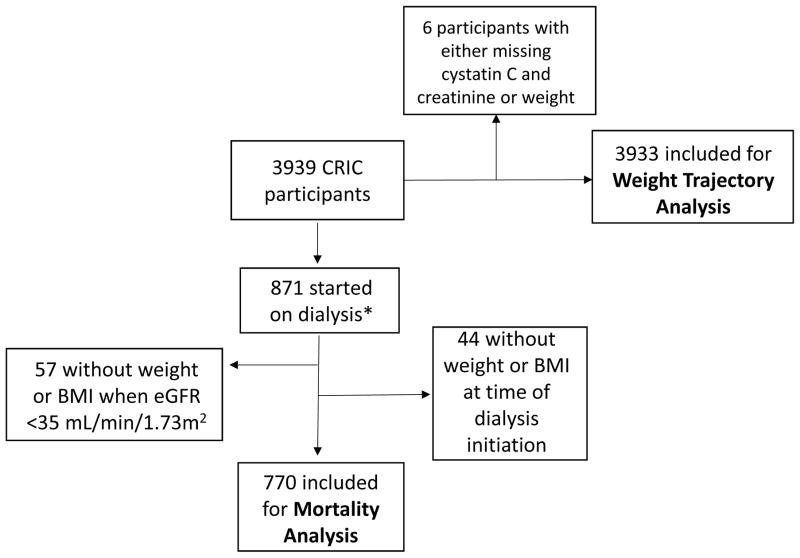
The CRIC Study is a multicenter prospective cohort study of adults with CKD.11–13 Participants with estimated glomerular filtration rate (eGFR) between 20–70 mL/min/1.73 m2 based on the 4-variable IDMS-traceable Modification of Diet in Renal Disease (MDRD) Study equation at the screening visit were recruited for study between June 2003 and September 2008. The inclusion and exclusion criteria have been previously described.11 Data used for our primary analysis, including weight, height, serum creatinine, and cystatin C were derived from the baseline and annual study visits. We included 3933 (out of 3939 total) CRIC participants with at least one eGFR based on cystatin C (eGFRcys), serum creatinine (eGFRcre), and one weight measurement to contribute to our analysis (Figure 1).
Figure 1.
Derivation of CRIC cohort for weight trajectory and death analysis.
*Dialysis includes peritoneal dialysis and hemodialysis
Weight trajectory with progressive CKD
To determine the pattern of weight change (and body mass index, BMI) with progressive CKD, we examined the association between repeated determinations of eGFR and repeated measures of weight (in kg) (or BMI in kg/m2) in CRIC participants during follow-up. We determined eGFR using the CKD-EPI cystatin C equation instead of the serum creatinine-based estimating equation in our primary analysis, as the former is less likely to be influenced by changes in body composition.14
We first used unadjusted splines to flexibly model the relation between eGFRcys and repeated measures of weight and BMI separately. Our primary outcome of interest was weight (because of the greater ease in interpreting weight rather than BMI changes). We specified a priori that our primary predictor would be eGFRcys represented as a restricted cubic spline with knots at 15, 30, 45, and 60 mL/min/1.73m2; this spline was used as the predictor of repeated measures of weight or BMI in mixed models. After we observed qualitatively an acute decline in weight and BMI when eGFR fell below 35 mL/min/1.73m2, we tested for differences in the coefficient for the slope terms above and below an eGFR of 35 mL/min/1.73m2 using segmented, mixed effects models (i.e., a linear spline with a single knot at 35 mL/min/1.73 m2). These analyses were conducted in unadjusted models, as our primary interest was changes within the same individual over time. Because prior literature suggested differences between men and women,15 we also stratified these models by sex to explore whether there were differences in weight trajectory between men and women.
In sensitivity analysis, we used a serum creatinine-based CKD-EPI equation (eGFRcre) as the primary predictor of repeated measures of weight and BMI as this is much more commonly available in clinical practice.14 In addition, in this analysis, we were also able to include in the mixed model both serum creatinine and weight values at the time of dialysis initiation obtained from a secondary data source, the Centers for Medicare and Medicaid (CMS)-2728 form.
To support that the weight loss we observed with progressive CKD was associated with loss of muscle mass or worsening nutritional status, we also examined changes in 24-hour urinary creatinine excretion, fat free mass (measured from bioimpedance analysis as previously described),16 serum albumin concentration, and low-density lipoprotein (LDL) cholesterol level with declines in kidney function (eGFRcys). This analysis was performed in the subset of CRIC participants with at least one measurement for each parameter of interest (24-hour urine creatinine, n=3886; fat free mass, n=3865; serum albumin, n=3927; and LDL, n=3928) using unadjusted spline-based mixed models similar to our weight models as described above. We tested for differences in the trajectory of these parameters using the same eGFR inflection point of 35 mL/min/1.73 m2 based on our weight trajectory data in mixed model analyses.
Weight change and risk of death after dialysis initiation
We next examined the relation between weight change before ESRD and risk of death after initiation of dialysis. Based on results of our weight trajectory analysis above, we included only those participants who had an observed eGFR less than 35 mL/min/1.73 m2 at a CRIC visit. If weight (or BMI) was not available at the time of dialysis initiation (n=220), the weight or BMI determined at the most recent CRIC visit within one year of dialysis initiation was carried forward. In cases where weight at dialysis initiation was unknown, the study participant was excluded from analysis (n=44). A total of 770 CRIC participants met this criteria (Figure 1) and were treated with either peritoneal dialysis (n=96) or hemodialysis (n=674). We compared the baseline characteristics of participants who were included and excluded from this analysis.
The main predictor of interest was weight change before initiating dialysis, defined as the absolute difference between the weight at the visit when eGFR was first documented less than 35 mL/min/1.73m2 and the weight at the time of dialysis initiation. We then converted this change in weight into an annualized percent weight change, accounting for the time period between these two measurements. We categorized this annualized weight change as weight change within 5% loss or gain per year, weight loss >5% per year, or weight gain >5% per year based on definitions of clinically significant involuntary weight loss.17, 18 We performed a sensitivity analysis using an annualized absolute weight change-based definition: ± 1 kg per year (stable weight), weight loss >1 kg per year, or weight gain >1 kg per year.
The main outcome was death after initiation of maintenance dialysis. ESRD onset among CRIC study participants was ascertained by participants’ self-report or by report of their named contact, and supplemented with cross-linkage with US Renal Data System (USRDS). Deaths were identified through report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the Social Security Death Index (SSDI). If vital status was missing or unknown (n=30), data from study participants were censored using either the time of last known follow-up visit or March 2013, the administrative censoring date for this study.
We used Cox regression to examine the association between categories of weight change and risk of death in unadjusted analysis. Subsequently, we adjusted for age at dialysis initiation, sex, race/ethnicity, diabetes, self-reported annual income (baseline value), hypertension, any cardiovascular disease (ascertained by self-report at baseline), and smoking status. We considered our adjusted models to be the primary analyses.
We tested for the presence of an interaction between BMI at study entry and weight change category in order to determine whether weight changes would potentially have differing associations with the risk of death based on entry weight status (for example, weight loss may be less detrimental among those who were obese at the time of study entry).
Analyses in AASK
Details of the AASK trial design and results have been previously published.19–21 Between 1995 and 2001, 1094 African-American participants 18–70 years of age without diabetes mellitus and with serum creatinine-based eGFR 20–65 mL/min/1.73m2 were randomly assigned to either a mean arterial pressure (MAP) ≤92 mmHg or 102–107 mmHg. At trial closure, 691 participants (87% of eligible participants who had not developed ESRD or died) were enrolled in a cohort study from April 2002 to June 2007.22–24 To extend ascertainment of ESRD and death beyond the cohort phase, we linked identifying information from AASK participants with the USRDS and SSDI through June 30, 2012 as previously described.25 Information on ESRD and death outcomes could not be obtained for 27 (2%) of the original 1094 AASK participants as a result of missing patient health identifiers, so that 1067 participants were included for analysis (Figure S1).
During the first six months of AASK trial, weight was measured monthly, and serum creatinine measured every three months. Thereafter, weight was measured every two months during the trial and every three months during the cohort phase, while thereafter serum creatinine was measured every six months during both the trial and cohort phase. Among the 1067 participants included in our weight trajectory analysis, 333 started dialysis and had at least one serum creatinine, BMI, and weight measurement after eGFRcre fell below 35 mL/min/1.73m2 (Figure S1).
We repeated our cubic and linear spline models in AASK participants using eGFRcre as a predictor of weight or BMI (repeated measures of cystatin C were not available in AASK). We also used Cox regression to examine the association between weight changes during CKD and death after dialysis initiation. These models were adjusted for age at dialysis initiation, sex, self-reported income, diabetes, cardiovascular disease, and smoking status ascertained via a combination of data collection from the CMS-2728 form and the AASK trial and cohort phases.
All statistical analyses were performed using Stata 14 and subsequently verified independently in SAS by a separate analyst. Informed consent was obtained from all participants of CRIC study at all sites. The Institutional Review Boards of all participating CRIC sites approved this study.
Results
Weight trajectory with advancing CKD
CRIC
Baseline characteristics of CRIC and AASK participants have been previously described and are shown in Table S1.12, 21, 24 In general, CRIC participants were older than AASK participants, and less than half of CRIC participants (42%) were non-Hispanic black.12, 24 Approximately 48% of CRIC participants had diabetes at the time of enrollment. Mean BMI (in kg/m2) was higher in CRIC than AASK.
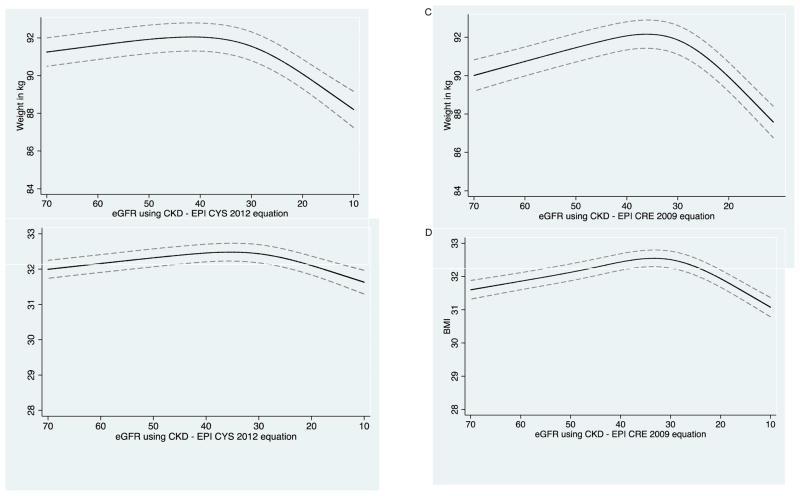
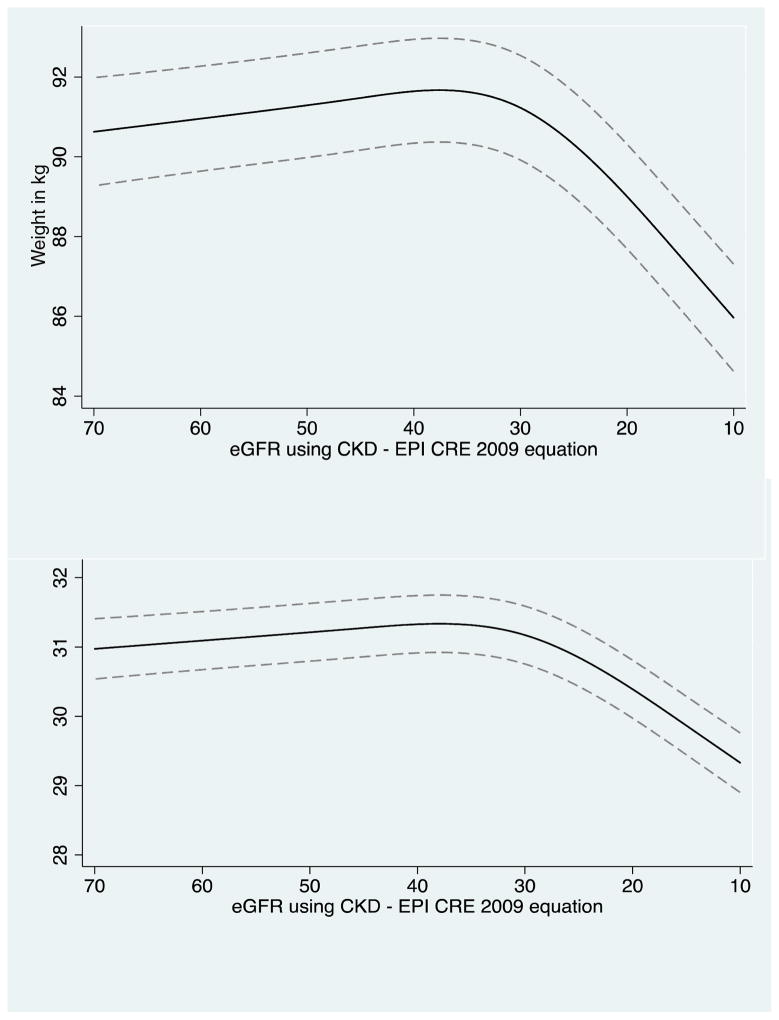
CRIC participants had a mean of 5.7 weight measurements available for trajectory analysis. After eGFRcys reached 35 mL/min/1.73m2 (Figure 2A–2B), every 10 mL/min/1.73m2 decline in eGFRcys was associated with a mean 1.45 (95% CI, 1.19–1.70) kg decrease in weight and a mean 0.31 (95% CI, 0.21–0.40) kg/m2 decline in BMI. Results were similar using eGFRcre (Figure 2C–2D). Similar trends were observed between men and women (Figure S2).
Figure 2.
Repeated measures of weight and body mass index with advancing CKD.
A. Longitudinal repeated measures of weight with repeated measures of eGFR by cystatin C in CRIC.
B. Longitudinal repeated measures of BMI with repeated measures of eGFR by cystatin C in CRIC.
C. Longitudinal repeated measures of weight with repeated measures of eGFR by creatinine in CRIC.
D. Longitudinal repeated measures of BMI with repeated measures of eGFR by creatinine in CRIC.
E. Longitudinal repeated measures of weight with repeated measures of eGFR by creatinine in AASK.
F. Longitudinal repeated measures of BMI with repeated measures of eGFR by creatinine in AASK.
---- 95% CI
**The figure shows repeated measurements of weight (or BMI) with repeated measures of eGFR among the same participants over time. In general, weight (or BMI) is noted to be stable until around eGFR of 35 mL/min/1.73 m2, when steeper declines in weight (or BMI) are noted to occur.
Using the same inflection point as our weight analysis, we also noted a decline in 24-hour urine creatinine, serum albumin, fat free mass (FFM), and serum LDL cholesterol after eGFRcys fell below 35 mL/min/1.73m2 (Figure S3–S6). Daily urine creatinine excretion, serum albumin, serum LDL, and FFM all declined after eGFRcys fell below 35 mL/min/1.73m2 (p<0.001) based on our mixed model analyses (Figures S3–S6).
AASK
The mean number of weight measurements included for trajectory analysis was 16.4 in AASK participants. The trajectory of weight change in AASK was similar to that of CRIC participants, with a significant difference in weight trajectory that occurred around eGFR of 35 mL/min/1.73m2 (Figure 2E–F). After eGFR reached 35 mL/min/1.73m2, every 10 mL/min/1.73m2 decline in eGFRcre was associated with a mean 2.3 (95% CI, 2.1–2.4) kg decline in weight and a mean 0.79 (95% CI, 0.73–0.86) kg/m2 decline in BMI.
Weight change before ESRD and risk of death after dialysis
CRIC
Baseline characteristics of CRIC participants at the first follow-up visit after eGFRcre fell below 35 mL/min/1.73m2 and who started dialysis as their first modality of renal replacement therapy (n=770) are shown in Table 1. Differences in the baseline characteristics of those who were included and excluded from our mortality analyses are shown in panel a of Table S2. In CRIC, those who were excluded from our mortality analysis tended to be younger, and had a higher prevalence of COPD. Median follow-up time after the onset of ESRD was 3.3 (IQR, 1.5–4.8) years, and median time to death among those who died after ESRD was 1.9 (IQR, 0.6–3.6) years.
Table 1.
Characteristics when eGFR was first <35 mL/min/1.73m2 among CRIC and AASK participants who eventually required renal replacement therapy (hemodialysis or peritoneal dialysis).
| Characteristics during CKD | CRIC (N=770) | AASK (N=333) |
|---|---|---|
|
| ||
| Mean age (yrs) | 57.2 ± 11.0 | 51.7 ± 11.3 |
|
| ||
| Men | 455 (59.1) | 199 (59.8) |
|
| ||
| Black | 418 (54.3) | 333 (100%) |
|
| ||
| Annual household income | ||
| lowa | 337 (43.8) | 165 (49.6) |
| mediumb | 179 (23.2) | 117 (35.1) |
| high c | 135 (17.5) | 51 (15.3) |
| Declined to answer | 119 (15.5) | -- |
|
| ||
| Positive smoking history** | 449 (58.3%) | 179 (53.8%) |
|
| ||
| Diabetes | 523 (67.9%) | 17 (5.1%)* |
|
| ||
| Hypertension | 745 (96.8%) | 333 (100%) |
|
| ||
| Prevalent CVD | 340 (44.2%)1 | 168 (50.5%) 2 |
|
| ||
| Mean serum albumin (g/dL) | 3.7 ± 0.53 | 4.1 ± 0.44 |
|
| ||
| Cancer | 51 (6.6%) | 9 (2.6%) |
|
| ||
| Systolic BP (mm Hg) | 139.4 ± 24.13 | 147.0 ± 24.0 |
|
| ||
| Diastolic BP (mm Hg) | 72.9 ± 13.93 | 91.5 ± 16.3 |
|
| ||
| Median hemoglobin A1C (%) | 6.9 [5.9–8.1] | NA |
|
| ||
| COPD | 28 (3.7%)3 | NA |
|
| ||
| Fat free mass, kg | 63.4 ± 16.23 | NA |
Continuous variables given as mean ± SD or median [interquartile range]; categorical variables as count (percentage).
Defined as MI, stroke, CHF, or PVD based on self-report.
Heart disease was determined at baseline based on a combination of self-report, chart review, or baseline electrocardiogram reading
Missing serum albumin in n=5; missing BP in n=2; missing COPD in n=13; missing hemoglobin A1C in n=84; missing FFM in n=123 in CRIC
Missing serum albumin in n=14
at baseline enrollment
for CRIC, smoked > 100 cigarettes over lifetime; for AASK, current or past smoker determined at time of baseline enrollment
≤$20,000 for CRIC; <$15,000 (determined at baseline enrollment) for AASK
$20,001–$50,000 for CRIC; $15,000–39,999 (determined at baseline enrollment) for AASK
≥$50,001 for CRIC; >$40,000 (determined at baseline enrollment) for AASK
CRIC Chronic Renal Insufficiency Cohort; AASK African American Study of Kidney Disease and Hypertension; BP blood pressure; CKD chronic kidney disease; COPD chronic obstructive pulmonary disease; NA, not available
Among the 770 CRIC participants who started dialysis, 155 (20.1%) participants met our definition for weight loss, and 45 (5.8%) participants met that for weight gain. The distribution of participants who experienced significant weight changes by BMI category at baseline and at ESRD are shown in Table S3. A large proportion of patients who were obese when they had non-dialysis-dependent CKD lost weight (102 out of 456 participants), such that the number of patients who were obese (defined as BMI ≥ 30) decreased from 59% to 47% by the time of dialysis initiation.
Participants who experienced weight loss (>5% annualized decline) before dialysis had a 54% higher adjusted risk of all-cause death (HR, 1.54; 95% CI, 1.17–2.03) after starting dialysis compared to those who maintained their weight to within ±5% (Table 2). In contrast, there was no significant difference in the risk of death in CRIC participants after initiating dialysis among those who gained weight compared to those who maintained weight to within ±5% before dialysis initiation. No interactions were noted between BMI at the time of study entry and weight loss category (all p values>0.05).
Table 2.
Risk of death after dialysis initiation according to weight change (between eGFR of 35 mL/min/1.73 m2 and ESRD) in CRIC and AASK.
| Hazard ratio (95% CI) | ||
|---|---|---|
| Unadjusted | Adjusteda | |
| CRIC | ||
| Annualized percent weight change (N=770) | ||
| Lost weight (> 5%/y) (n=155) | 1.47 (1.12–1.94) | 1.54 (1.17–2.03) |
| Weight change ± 5% (n=570) | 1.00 (reference) | 1.00 (reference) |
| Gained (>5%/y) (n=45) | 1.31 (0.80–2.13) | 1.09 (0.66–1.79) |
| Absolute weight change (N=770) | ||
| Weight loss > 1 kg/y (n=431) | 1.64 (1.19–2.25) | 1.46 (1.06–2.02) |
| Stable weight ± 1 kg/y (n=214) | 1.00 (reference) | 1.00 (reference) |
| Gained > 1 kg/y (n=125) | 1.20 (0.79–1.84) | 1.08 (0.70–1.66) |
| AASK | ||
| Annualized percent weight change (N=333) | ||
| Lost weight (> 5%/y) (n=43) | 1.40 (0.96–2.06) | 1.56 (1.06–2.30) |
| Modest weight change (± 5%) (n=280) | 1.00 (reference) | 1.00 (reference) |
| Gained (> 5%/y) (n=10) | 1.79 (0.91–3.52) | 2.15 (1.07–4.34) |
For CRIC, adjusts for age at dialysis initiation, sex, race, diabetes, income category, hypertension, cardiovascular disease, smoking status ascertained at last CRIC visit available before dialysis initiation or from the 2728 form if available. For AASK, adjusts for age at dialysis initiation, sex, diabetes, income category, cardiovascular disease, and smoking status ascertained via a combination of CMS-2728 form and AASK trial and cohort data.
CRIC Chronic Renal Insufficiency Cohort; eGFR estimated glomerular filtration rate
AASK
Baseline characteristics of AASK participants at the visit when eGFRcre was first less than 35 mL/min/1.73m2 and were treated with hemodialysis or peritoneal dialysis (n=333) are shown in Table 1. In AASK, those who were excluded from our mortality analysis had higher baseline annual household income and diastolic BP (panel b of Table S2). The distribution of participants who experienced significant weight changes by BMI category at baseline entry and at ESRD are shown in Table S3. A smaller proportion of participants in AASK (27 out of 192, 14.1%) than in the CRIC Study who were obese before starting dialysis lost significant weight, such that the proportion of patients who were obese decreased from 58% to 41% by the time of dialysis initiation (Table S3).
We confirmed our CRIC findings in the AASK during an average follow-up of 5.6 years. We noted that participants who had a >5% annualized weight loss (after eGFRcre fell below 35 mL/min/1.73m2) had a 56% higher risk of death after dialysis initiation [HR, 1.56; 95% CI, 1.06–2.30] compared to those with weight within ±5% in our adjusted models (Table 2). However, a higher risk of death was also noted in AASK participants who gained weight compared to those with stable weight before dialysis initiation (although there were only 10 participants in this category). No statistically significant interaction was noted between BMI category when eGFRcre was first lower than 35 mL/min/1.73 m2 and weight loss category (all p values >0.10).
Discussion
There have been limited data regarding weight changes that occur with CKD progression, and how these weight changes may affect the risk of death after dialysis initiation. Previously, it has been reported that CKD patients exhibit a spontaneous decrease in dietary protein and energy intake as they progress towards ESRD.10 In a cross-sectional study from the Modification of Diet in Renal Disease (MDRD) Study group, men lost weight below an eGFR of 40 mL/min/1.73 m2, but weight was stable in women.15 A limitation of this prior study was its cross-sectional design and exclusion of persons with evidence of malnutrition (since the MDRD Study investigated protein limitation), which may have resulted in a “floor effect” (i.e. patients with more advanced CKD and who already lost weight were selectively not enrolled). We found that a significant decline in weight (and hence BMI) occurred after eGFR fell below 35 mL/min/1.73m2 in two ethnically and racially diverse cohorts of persons with CKD.15 Furthermore, we found that an annualized weight loss greater than 5% before dialysis initiation was associated with a 55–60% higher adjusted rate of death after starting dialysis in both cohorts. Our study provides important insights into the natural history of weight change with progressive CKD and subsequent risk of death in patients who start dialysis. Our findings are strengthened by the remarkably similar results in CRIC and AASK, studies separated by almost a decade.
Current guidelines recommend monitoring nutritional status every 1–3 months if eGFR is <30 mL/min/1.73m2, and every 6–12 months if eGFR is between 30–60 mL/min/1.73m2.26 In addition, recommendations for minimal caloric intake are made for those with eGFR below 25 mL/min/1.73m2.26 However, studies to support these recommendations are primarily cross-sectional,15 and did not consider weight change with progressive CKD. We observed that weight loss begins when serum creatinine may be in the range of 1.5–2.0 mg/dL and earlier than may be expected. An average 5.1 kg weight loss was noted to occur between CKD stage 3b (and potentially earlier) and the onset of dialysis in CRIC, which is substantial. Our study results suggest that recognition of early weight loss as a poor prognostic factor may be warranted.
In order to assess whether the weight loss observed in our study correlated with declines in lean body mass, we also observed a similar decline in urinary creatinine excretion and fat free mass (as a reflection of lean body mass) between the period when the level of kidney function was less than 35 mL/min/1.73 m2 and ESRD. In support of the presence of a decline in nutritional status with progressive CKD, we also found simultaneous declines in serum albumin and serum LDL cholesterol concentrations that corresponded with the weight loss observed with advancing CKD. In fact, our spline models suggest that 24-hour urinary creatinine excretion, serum albumin, and FFM began to decline earlier than weight.
The strengths of our study include the inclusion of two large, well-characterized cohorts with research protocol-driven measurements of weight during follow-up, detailed ascertainment of ESRD and mortality outcomes, and the similarities of our findings in two separate cohorts. Although BMI may be an imperfect marker of lean body mass and nutritional status in patients with CKD,27, 28 BMI currently remains the most convenient clinical marker of weight status and has been shown to be strongly associated with mortality risk in multiple large CKD and ESRD epidemiologic studies.29–33 In addition, our results are strengthened by the availability of repeated measures of 24-hour urinary creatinine and fat free mass by bioimpedance analysis as a reflection of muscle or lean body mass.
Limitations to our study include the inability to delineate definitively the contribution of fluid and diuretic use to our weight assessments, which could lead to an underestimation or overestimation of the degree of non-fluid weight changes that occurred. However, CRIC excluded patients on active immunosuppression for glomerular disease, and the 90th percentile for baseline urinary protein-creatinine ratio was 2.8 g/g. Therefore, few patients with massive proteinuria and nephrotic syndrome were included. We are less confident in our ability to address weight loss after ESRD onset due to increased missing data, but the novelty of this study lies in its focus on pre-ESRD weight change. We also have limited data on cause of death. Due to a lack of repeated measures of C-reactive protein or PTH, we are unable to explore potential biological pathways that have been hypothesized to contribute to weight loss among patients with CKD.34–37 Residual confounding by the presence of non-renal co-morbidities that may be independently associated with both weight changes and mortality risk over time is possible. Finally, we cannot distinguish between intentional and unintentional weight loss.
In conclusion, significant weight loss begins in stage 3 CKD and is associated with higher mortality after dialysis initiation. Awareness of the potential for early weight loss to occur with the progression of CKD is warranted, even in patients with moderate kidney disease. Evidence from randomized controlled trials are needed to determine whether interventions to optimize weight and nutritional status before the initiation of dialysis will improve outcomes after dialysis initiation.
Supplementary Material
Derivation of AASK cohort for weight trajectory and death analysis.
Stratified analysis of weight trajectory by sex in CRIC participants.
Repeated measures of 24-hour urine creatinine during longitudinal prospective follow-up of CRIC participants.
Repeated measures of serum albumin during longitudinal prospective follow-up of CRIC participants.
Repeated measures of LDL during longitudinal prospective follow-up of CRIC participants.
Repeated measures of fat-free mass during longitudinal prospective follow-up of CRIC participants.
Characteristics at first visit with serum creatinine, cystatin C, and weight available in CRIC and AASK.
Baseline characteristics of those included and excluded from mortality analysis in CRIC and AASK.
Distribution of participants by BMI at CKD, BMI at time of dialysis initiation, and weight change category in CRIC and AASK participants.
Acknowledgments
Support: This work was supported by the National Kidney Foundation Satellite Dialysis Clinical Investigator Grant to EK, NIDDK K24 DK92291 to CYH, and National Institutes of Health K24 DK085153 to KLJ. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. The funders of this study did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. NHLBI
Footnotes
Authors’ Contributions: research idea and study design: EK, KJ, JK, CEM; AG; LH; JH; JWK; SN; AR; CYH; data acquisition: all authors; data analysis/interpretation: all authors; statistical analysis: EK, DX, FL, CEM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: The authors declare that they have no other financial interests.
Disclaimer: The data from the AASK trial reported here were supplied in part by the NIDDK Central Repositories. This manuscript does not necessarily reflect the opinions or views of the AASK trial and cohort studies, the NIDDK Central Repositories, or the NIDDK grants MD000182, UL1TR000124 and P30AG021684. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. The New England journal of medicine. 1983;308:1553–1558. doi: 10.1056/NEJM198306303082602. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3.Chang TI, Zheng Y, Montez-Rath ME, Winkelmayer WC. Antihypertensive Medication Use in Older Patients Transitioning from Chronic Kidney Disease to End-Stage Renal Disease on Dialysis. Clinical journal of the American Society of Nephrology: CJASN. 2016;11:1401–1412. doi: 10.2215/CJN.10611015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green JA, Boulware LE. Patient Education and Support During CKD Transitions: When the Possible Becomes Probable. Advances in chronic kidney disease. 2016;23:231–239. doi: 10.1053/j.ackd.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Hsu RK, Chai B, Roy JA, et al. Abrupt Decline in Kidney Function Before Initiating Hemodialysis and All-Cause Mortality: The Chronic Renal Insufficiency Cohort (CRIC) Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2016;68:193–202. doi: 10.1053/j.ajkd.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku E, Glidden DV, Johansen KL, et al. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney international. 2015;87:1055–1060. doi: 10.1038/ki.2014.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku E, Gassman J, Appel LJ, et al. BP Control and Long-Term Risk of ESRD and Mortality. Journal of the American Society of Nephrology: JASN. 2017;28:671–677. doi: 10.1681/ASN.2016030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chazot C. Why are chronic kidney disease patients anorexic and what can be done about it? Seminars in nephrology. 2009;29:15–23. doi: 10.1016/j.semnephrol.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Ikizler TA, Greene JH, Wingard RL, Parker RA, Hakim RM. Spontaneous dietary protein intake during progression of chronic renal failure. Journal of the American Society of Nephrology: JASN. 1995;6:1386–1391. doi: 10.1681/ASN.V651386. [DOI] [PubMed] [Google Scholar]
- 11.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. Journal of the American Society of Nephrology: JASN. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 12.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clinical journal of the American Society of Nephrology: CJASN. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney international. 2000;57:1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson FP, Xie D, Anderson AH, et al. Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: the CRIC study. Clinical journal of the American Society of Nephrology: CJASN. 2014;9:2095–2103. doi: 10.2215/CJN.03790414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stack S, Chertow GM, Johansen KL, Si Y, Tamura MK. Pre-ESRD changes in body weight and survival in nursing home residents starting dialysis. Clinical journal of the American Society of Nephrology: CJASN. 2013;8:1734–1740. doi: 10.2215/CJN.01410213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong CJ. Involuntary weight loss. The Medical clinics of North America. 2014;98:625–643. doi: 10.1016/j.mcna.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. Jama. 2001;285:2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 20.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK) Journal of the American Society of Nephrology: JASN. 2003;14:S154–165. doi: 10.1097/01.asn.0000070080.21680.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. Jama. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 22.Appel LJ, Middleton J, Miller ER, 3rd, et al. The rationale and design of the AASK cohort study. Journal of the American Society of Nephrology: JASN. 2003;14:S166–172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 23.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sika M, Lewis J, Douglas J, et al. Baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Clinical Trial and Cohort Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2007;50:78–89. 89.e71. doi: 10.1053/j.ajkd.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Ku E, Lipkowitz MS, Appel LJ, et al. Strict blood pressure control associates with decreased mortality risk by APOL1 genotype. Kidney international. 2017;91:443–450. doi: 10.1016/j.kint.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2001;37:S66–70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 27.Kovesdy CP, Czira ME, Rudas A, et al. Body mass index, waist circumference and mortality in kidney transplant recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:2644–2651. doi: 10.1111/j.1600-6143.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- 28.Stenvinkel P, Zoccali C, Ikizler TA. Obesity in CKD--what should nephrologists know? Journal of the American Society of Nephrology: JASN. 2013;24:1727–1736. doi: 10.1681/ASN.2013040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vashistha T, Mehrotra R, Park J, et al. Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014;63:612–622. doi: 10.1053/j.ajkd.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall YN, Xu P, Chertow GM. Relationship of body size and mortality among US Asians and Pacific Islanders on dialysis. Ethnicity & disease. 2011;21:40–46. [PMC free article] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clinic proceedings. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16:2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 33.Ricks J, Molnar MZ, Kovesdy CP, et al. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2011;58:574–582. doi: 10.1053/j.ajkd.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kir S, Komaba H, Garcia AP, et al. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell metabolism. 2016;23:315–323. doi: 10.1016/j.cmet.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney international. 1999;55:1945–1951. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2001;38:1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 37.Kaysen GA, Dubin JA, Muller HG, Rosales L, Levin NW, Mitch WE. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney international. 2004;65:1408–1415. doi: 10.1111/j.1523-1755.2004.00520.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Derivation of AASK cohort for weight trajectory and death analysis.
Stratified analysis of weight trajectory by sex in CRIC participants.
Repeated measures of 24-hour urine creatinine during longitudinal prospective follow-up of CRIC participants.
Repeated measures of serum albumin during longitudinal prospective follow-up of CRIC participants.
Repeated measures of LDL during longitudinal prospective follow-up of CRIC participants.
Repeated measures of fat-free mass during longitudinal prospective follow-up of CRIC participants.
Characteristics at first visit with serum creatinine, cystatin C, and weight available in CRIC and AASK.
Baseline characteristics of those included and excluded from mortality analysis in CRIC and AASK.
Distribution of participants by BMI at CKD, BMI at time of dialysis initiation, and weight change category in CRIC and AASK participants.