Abstract
Background and Purpose
Intracerebral hemorrhage (ICH) is a devastating disease with a 30-day mortality of approximately 50%. There are no effective therapies for ICH. ICH results in brain damage in two major ways; through the mechanical forces of extravasated blood and then through toxicity of the intraparenchymal blood components including hemoglobin/iron. Lactoferrin (LTF) is an iron-binding protein, uniquely abundant in polymorphonuclear neutrophils (PMNs). After ICH, circulating blood PMNs enter the ICH-afflicted brain where they release LTF. By virtue of sequestrating iron, LTF may contribute to hematoma detoxification.
Methods
ICH in mice was produced using intra-striatal autologous blood injection. PMNs were depleted with intraperitoneal administration of anti-Ly-6G antibody. Treatment of mouse brain cell-cultures with lysed RBC or iron was used as in-vitro model of ICH.
Results
LTF mRNA was undetectable in the mouse brain, even after ICH. Unlike mRNA, LTF protein increased in ICH-affected hemispheres by 6h, peaked at 24–72h, and remained elevated for at least a week after ICH. At the single cell level, LTF was detected in PMNs in the hematoma-affected brain at all timepoints after ICH. We also found elevated LTF in the plasma after ICH, with a temporal profile similar to LTF changes in the brain. Importantly, recombinant mouse LTF (mrLTF) reduced the cytotoxicity of lysed RBC and FeCl3 to brain cells in culture. Ultimately, in an ICH model, systemic administration of mrLTF (at 3h, 24h and 48h after ICH) reduced brain edema and ameliorated neurological deficits caused by ICH. mrLTF retained the benefit in reducing behavioral deficit even with 24h treatment delay. Interestingly, systemic depletion of PMNs at 24h after ICH worsened neurological deficits, suggesting that PMN infiltration into the brain at later stages after ICH could be a beneficial response.
Conclusions
LTF delivered to the ICH-affected brain by infiltrating PMNs may assist in hematoma detoxification and represent a powerful potential target for the treatment of ICH.
Keywords: Intracerebral Hemorrhage, Neutrophil, Lactoferrin, Iron toxicity, Inflammation
INTRODUCTION
Intracerebral hemorrhage (ICH) is a devastating form of stroke with 30–67% mortality and poor prognosis for which no effective therapy is available.1, 2 Rapid deposition of blood within the brain parenchyma causes increased intracranial pressure, resulting in primary “mechanical” brain damage. Subsequently, various toxic blood components and hemolysis products within the hematoma trigger irreversible injury to the brain. Iron toxicity associated with oxidative stress,3–5 or ferroptosis,6 or HIF-1a,7 could play a central role in this damage and ICH–associated inflammation.8–12
After ICH, masses of polymorphonuclear neutrophils (PMNs) infiltrate into the ICH-affected brain parenchyma where they release various granule components during degranulation.13–15 Lactoferrin (LTF) is an iron-binding protein found in the secondary granules of PMNs.16 Normally, LTF released by PMNs acts as a first-line defense protein against microbial pathogens through the chelation of iron. Structurally, LTF is a single-chain glycoprotein that folds into two lobes, and each lobe can tightly bind and sequester one ferric iron with high affinity (Kd~10−20M).17, 18 Importantly, unlike other iron binding proteins, LTF can retain ferric ions-(Fe3+) even at the low pH associated with inflamed tissue. This property can effectively prevent ferrous ions-(Fe2+) engagement in Fenton’s reaction, a source of oxidative stress and inflammation.19, 20 This is particularly important in ICH pathogenesis because after ICH, the erythrocytes (RBCs) in the hematoma undergo progressive hemolysis, generating large quantities of hemoglobin (Hb), heme, and iron, leading to damage to brain cells and neurovasculature.3, 10, 21–24 Thus, LTF delivered from infiltrated PMNs could mediate neutralization of toxic iron and heme, which could be of particular clinical relevance to ICH.15
Here we measured the temporal and spatial changes of LTF protein in the ICH-affected brain and explored its potential therapeutic role in rodents after ICH.
MATERIAL AND METHODS
The data that support the findings of this study are available from the authors upon reasonable request.
All animal studies followed the guidelines outlined in Guide for the Care and Use of Laboratory Animals from the NIH and ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments) and were approved by the AWC of the UTHSC at Houston. All studies were performed using a randomization (coin toss) approach. All analyses were performed by investigators blinded to treatment assignments.
ICH in mouse
ICH in male 3-mo-old mouse was induced by intra-striatal injection of 15μL of autologous blood as described previously.24–26
Animal perfusion and tissue collection
Animals were anesthetized with chloral hydrate (0.5 g/kg; i.p.) and intracardially perfused with ice-cold PBS. For histology/biochemical analyses, the brains or the hematoma-affected striata were frozen in −80°C 2-methylbutane and stored in −80°C prior to cryosectioning, RNA isolation, or protein extraction.
LTF ELISA
Blood plasma or PMN culture-medium LTF was measured with a mouse LTF ELISA kit (LS-F4352/LSBio).
Blood neutrophil isolation
PMNs were purified from heart puncture-drawn blood, using Ficoll-Paque gradient.15 PMNs were further purified using Neutrophil Enrichment kit/(STEMCELLTM Technologies).
LTF Degranulation Assay
Fresh PMNs in RPMI1640/10% mouse-serum at 2×105-cells/ml were cultured in the CO2 incubator for 2h before being subjected to 15min oxygen-glucose-deprivation.27 At 2h after reperfusion, or incubation with lysed-RBC, or 1μM A23187 (calcium-ionophore), culture medium was assessed for LTF with ELISA.
LTF administration
Mouse-recombinant LTF (mrLTF) was injected i.v. at 10mg/kg in 250μl of saline (or saline alone/control), starting 3h after ICH, plus 5mg/kg, p.o. at 24h and 48h after ICH. For cell culture study, the mrLTF was added to the culture medium at 15min before the insult.
Neurological/functional deficits (NDS) and edema measurement
An individual test score and a combination test score (grand neurological deficit score/NDS) assumed an equal weight of each of the tests (Footfault, Forelimb Placing, Postural Flexing, Wire and Corner test).24, 28 Brain edema was assessed with wet/dry method29.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
The dissected hematoma-affected striata were processed with Trizol-Reagents for mRNA extraction. LTF primers:(5′-cgaagcacgaatgacaaaga/3′-atcacacttgcgcttctcct) and GAPDH primers (5′-tgttcctacccccaatgtgt/3′-tgtgagggagatgctcagtg) were used for RT-PCR.24, 30
Western blot
LTF and GAPDH in the naïve and ICH-affected striatum were determined as described.24 Rabbit anti-LTF/(bs-5810R/Bioss), or chicken anti-GAPDH/(AB2302/Millipore) immunopositive bands were visualized using goat anti-rabbit IgG-HRP/(Invitrogen) or goat anti-chicken IgG-HRP/(Invitrogen) and ECL/(Pierce).
Immunofluorescence and cell counting
The immunohistochemistry for LTF and LTF/Neutrophil double labeling on 10μm-thick cryosections was performed as described earlier.24 Rabbit anti-LTF/(L3262/Sigma) and/or rat anti-mouse neutrophil antibody/(ab53457/Abcam) were employed to visualize LTF and PMNs. The nuclei were visualized with DAPI.
For PMNs counting, cryosections were generated at the level of needle insertion (site of blood injection). The total number of LTF+-PMNs on the digitized images representing whole striatum or PMNs on smears (from tail blood) was counted with CellSens/(Olympus).
PMN depletion in mice after ICH
PMNs were depleted with rat monoclonal α-Ly-6G/(BioXCell/BE0075) 500μg/mouse i.p. at 24h and 48h after ICH, according to the validated protocol.15, 31 A rat IgG2a isotype antibody (BioXCell/BE00892) served as the control. PMNs’ depletion was verified with flow cytometry and microscopic morphology.
For flow cytometry, blood was collected via cardiac puncture. We stained cells with the following fluorophores (Tonbo-Bioscience): CD45-vf450, CD11b-APC-Cy7, Ly6G-Pe-Cy7, Ly6C-APC. Data was acquired on a Cytoflex S cytometer (Beckmann Coulter) and analyzed by FlowJo Software (Tree Star). CD45+/CD11b+/Ly6C-Intermediate/SSC-High was used to identify neutrophils. Gating strategy is described in Supplemental Fig-I. We additionally performed a direct blood leukocyte classification and counting on Giemsa-stained tail blood smears using microscopic morphological features.32
Primary cortical neuron-glial and cortical neuron cultures
The primary neuron-glial co-cultures from E18–20 embryos from C57/BJ6 mice was prepared as we described.29 The cortical neuron cultures from E-18 mouse embryos in Neurobasal medium with B27 were prepared as we described.27
ICH-like Injury in vitro
We exposed the 12d-old cortical neuron-glial co-cultures to RBC-lysate (1μl lysates/100μl medium) in the presence/absence of mrLTF. 20h later, cell viability was assessed with MTT reagent/(G4000/Premega).
To simulate iron-induced neurotoxicity, we added FeCl3 (1–100μM) to the 12d-old cortical neurons in culture. This produced a dose-dependent neuronal death, with 50μM FeCl3 causing 95% neuronal death within 24h. To determine the rLTF role in detoxifying iron, we incubated neurons with 10μM FeCl3 for 6h with or without 20μg/ml of mrLTF. The LDH assay kit (G1780/Promega) was used to measure injury.
Statistical analyses
We used GraphPad and InStat programs for statistical analyses. One-way analysis of variance followed by Newman-Keuls post-test was used for multiple comparisons. Paired t-test was used when two groups were compared. Sample size was established based on our previous experience with this model.
RESULTS
LTF increases in brain after ICH
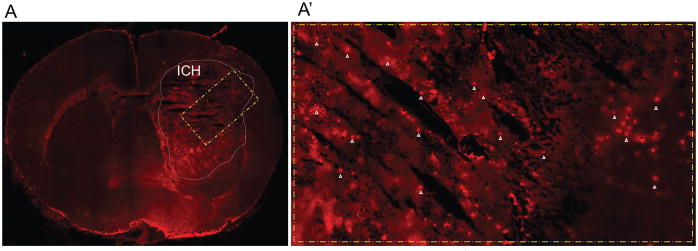
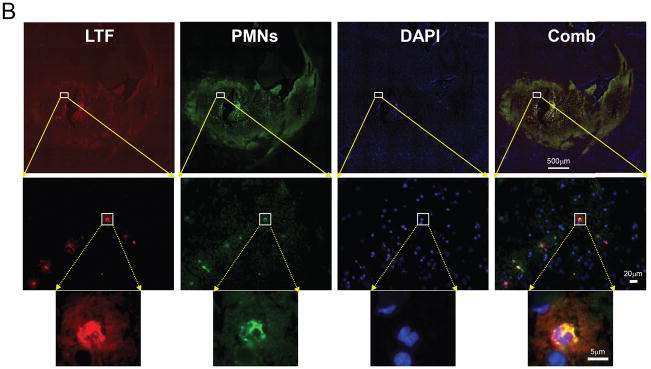
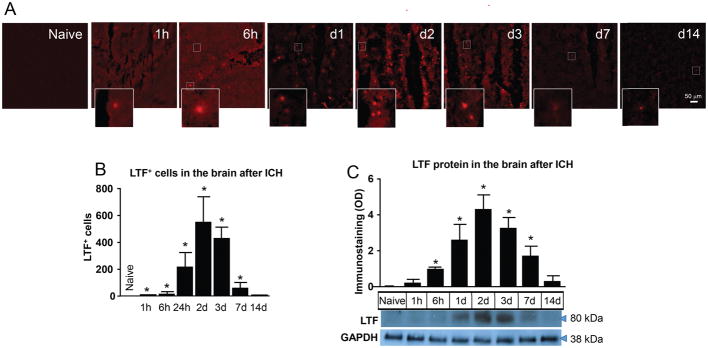
Using immunofluorescence to label LTF+-cells, we detected rare LTF+-cells in the naïve mouse brain or in the contralateral brain hemisphere of ICH-affected brain up to 14d after ICH (data not shown). However, we detected a robust increase in the number of LTF+-cells in the peri-hematoma/hematoma territory after ICH (Fig-1A/A′). Using double immunofluorescence staining for LTF and PMNs, we found that almost all of the LTF+-cells found in the brain are PMNs (Fig-1B). The increase in LTF+-cells became apparent at 6h after ICH, peaked at d2, and slowly subsided over 14d (Fig-2A and -2B). Corresponding to these immunohistochemical findings, Western blot showed LTF levels in the ICH-affected brain hemisphere increase robustly, showing many-folds increase in brain LTF at d2 after ICH, compared to naïve (Fig-2C).
Fig-1. LTF+ PMNs in brain after ICH in mouse.
(A)-LTF immunofluorescence (red) in brain coronal section at 48h after ICH. A′ is a highlighted area in A. The arrowheads in A′ indicate the LTF+-cells. (B)-Double immunofluorescence of LTF-(red) and neutrophil-(green) in the brain at 24h after ICH. The nuclei are stained with DAPI-(blue). A combination-(Comb) of LTF and PMN staining shows the 100% co-localization.
Fig-2. ICH transiently increases LTF in the ICH-affected brain.
(A)-Representative images of LTF immunofluorescence in striatum of naïve mouse and mice at 1h-14d after ICH in the hematoma-affected sub-cortical striatum. The scale bar=50 μm. (B)-Bar graph quantitating presence of LTF+-cells in brain at 1–14days after ICH. Data are expressed as mean±SEM (n=5). *P≤0.05, vs. naïve group. (C)-Representative LTF and GAPDH Western blot in naïve striatum and in hematoma-affected striatum at 1h-14d after ICH. Data are expressed as mean±SEM (n=3). *P≤0.05, vs. naïve group.
We emphasize that there is little detectable LTF mRNA in the naïve mouse brain or in the ICH-affected brains at any time point after ICH, despite the ICH-induced increase in LTF protein (data not included). Furthermore, there is no detectable LTF mRNA found in purified peripheral blood PMNs (data not included). This is in agreement with the existing data that in contrast to bone marrow-derived developing PMNs, mature blood neutrophils primarily transport but do not transcribe LTF.33–37 Taken together, these results suggest that the abundant amount of LTF found in the brain after ICH is delivered by mature, bone-marrow-derived PMNs.
LTF is present in peripheral PMNs and is released by PMNs upon exposure to RBC
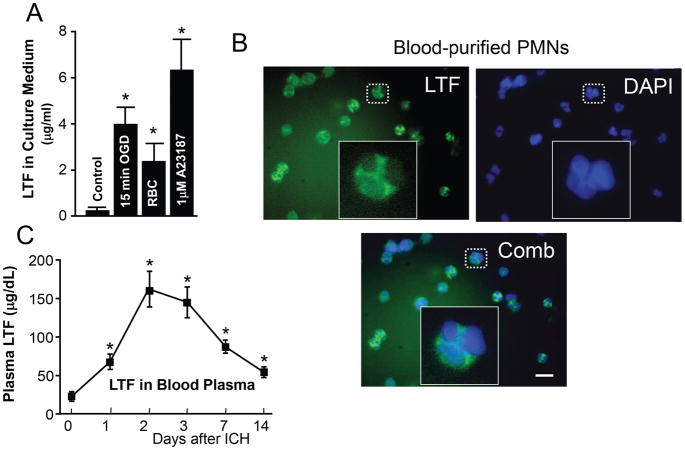
Using fresh blood-purified mature PMNs, we demonstrated that LTF (normally packaged in the specific granules of PMNs) can be quickly released into the media upon PMNs activation with RBCs or brief sublethal exposure to oxygen-glucose-deprivation (Fig-3A). Exposure to A23187, a calcium ionophore (capable of inducing degranulation), served as a positive control. This data suggest that RBCs and/or hypo-perfusion (which often occurs secondary to ICH) can trigger degranulation and the release of LTF. However, the precise mechanism underlying this process is unknown.
Fig-3. LTF in Peripheral Blood PMNs.
(A)- Quantification (using ELISA) of LTF released into the culture medium by purified blood PMN exposed to 15min oxygen-glucose-deprivation, lysed-RBC, or calcium inophore (A23187; 1μM). The data are mean±SEM (n=3). *p≤0.05, vs. control. (B)-LTF immunofluorescence-(green) in purified mouse blood PMNs. The LTF+-cell has a lobular nucleus, which is typical morphology of a mature neutrophil. Bar=20μm. (C)-X-Y Plot of blood plasma LTF levels at indicated time point after ICH. The data are mean±SEM (n=5). *p≤0.05, vs. d0/prior to ICH.
It is intriguing to note that as a molecule that is normally stored in blood PMNs’ (Fig-3B), LTF after ICH was not only increased in the ICH-affected brain (site of PMNs infiltration), but also in the blood plasma (Fig-3C) and that the temporal profile of LTF increase in the blood and brain were very similar (Fig-2B and -C). This suggests that ICH may also induce degranulation of circulating PMNs.
PMN-depletion after ICH aggravates neurological deficits
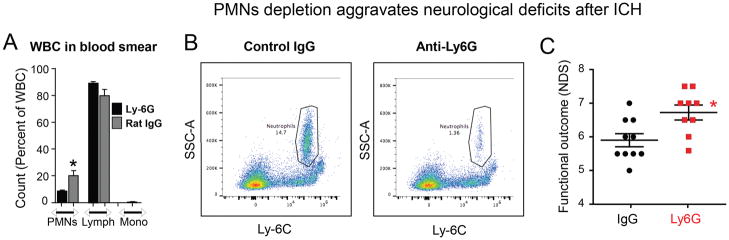
Although earlier studies reported that PMN depletion prior to ICH is protective toward ICH-mediated damage,38 the role of PMNs entering the brain at later stages after ICH is not known. In light of our recent studies demonstrating that the LTF levels in PMNs gradually increase after ICH,15 we hypothesized that PMNs may exert beneficial effects in the later stages of ICH. To test this, we used a well-validated technique employing systemic injection of anti-mouse Ly-6G neutralizing antibody to deplete systemic PMNs in mice. The isotype matched antibody served as a control.15, 39 Mice who received this neutralizing antibody at 24h and 48h after ICH became neutropenic, showing a robust 50–90% (depending on the method) depletion of peripheral PMNs (Fig-4A and -B), as measures at 72h after ICH. These neutropenic mice showed 62% reduction in the circulating LTF level. Ultimately, we demonstrated that this delayed post-ICH depletion of PMNs worsens functional/neurological deficits as measured at d3 after ICH (Fig-4C), suggesting that PMNs at later stages could play some beneficial role, e.g. through delivery of LTF.
Fig-4. PMNs-depletion aggravates Neurological deficits.
To deplete PMNs, mice were injected with anti-Ly-6G, a neutrophil neutralizing antibody or isotype IgG at 500μg/mouse at 24h and 48h after ICH (i.p.)(n=10 mice/group). The Ly-6G-mediated PMNs depletion at d3 resulted in 50–70% reduction of PMNs while having negligible effect on lymphocytes (Lymph) or monocytes (mono), as determined on tail blood smears using microscopic morphological features (A); and about 90% depletion as measured with flow cytometry using CD45+/CD11b+/Ly6C-Intermediate/SSC-High as markers for gating on cardiac puncture-collected blood (B). The neurological deficit scores (NDS) were quantified with Postural Flexing and Forward Placing at 3d after ICH (C). The data are mean±SEM. *p≤0.05, vs. control.
LTF is cytoprotective in an ICH-like injury model in vitro
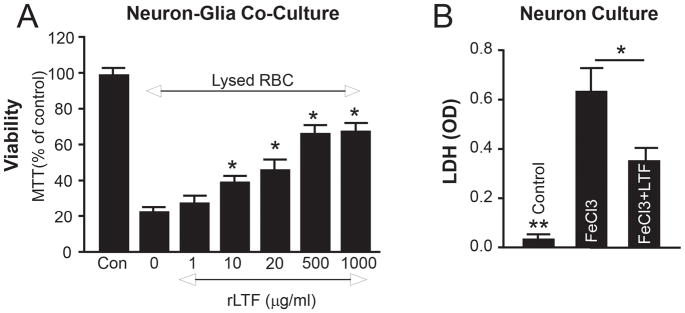
The neurotoxicity of products of hemolysis, including Hb, heme and iron is an important component of ICH-mediated brain damage. 40, 41 To clarify the role of LTF in RBC-induced cerebral toxicity, we added RBC-lysate to the primary neuronal-glial co-cultures29 (an in vitro “ICH-like” injury model) containing mrLTF. As anticipated, we observed that RBC-lysates caused severe neuronal damage, as showed using MTT assay and that exogenously added mrLTF in a dose-dependent manner preserved the viability of cells exposed to RBC-lysates (Fig-5A).
Fig-5. mrLTF protects brain cells from ICH-like Injury.
(A)-MTT/(viability index) in the mouse neuron-glia co-culture at 24h after exposure to lysed RBC in presence of 0–100μg/ml of mrLTF. The data are mean±SEM (n=3). *p≤0.05, vs. control without rLTF (“0”). The “Con” is the naïve control. (B)-LDH in cortical neuron culture at 6h after exposure to 10μM FeCl3, with or without 20μg/ml of mrLTF. The data are mean±SEM (n=3). *p≤0.05; **p≤0.01 vs. other two groups.
To further explore the protective nature of LTF, we tested primary cultured neurons that were exposed to FeCl3. First, we established that FeCl3 at 5–100μM induced dose-dependent neuronal injury, which was assessed by LDH release assay (data not included). We then incubated cortical neurons with 10μM FeCl3 (a dose which produces about 80% neuronal loss) in the presence or absence of 20μM mrLTF, demonstrating that LTF can robustly protect neurons from iron-induced injury (Fig-5B).
rLTF protects mice from damage caused by ICH
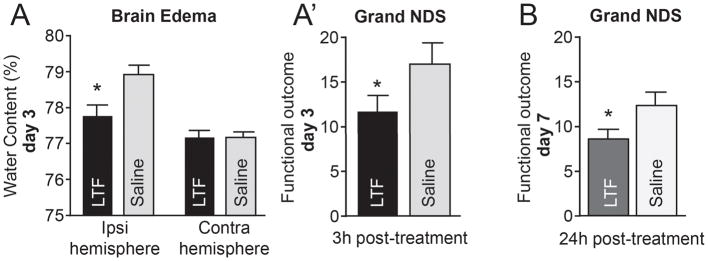
Encouraged by the neuroprotective capacities of mrLTF in the in vitro testing, we next examined whether mrLTF treatment conferred therapeutic benefit in a mouse model of ICH. We subjected mice to ICH and 3h later treated the mice with mrLTF (10mg/kg, iv, at 3h after ICH, plus 5mg/kg, orally on d1 and d2). We found that rLTF provided a robust benefit after ICH, reducing brain edema (Fig-6A) and neurological deficit - grand NDS (a composite neurological deficit score from 5 individual behavioral tests: Postural Flexing, Forward Placing, Footfault, Wire, and Circling) by 35.2% (11.4 vs. 17.6; p≤0.05) on d3 after ICH (Fig-6A′). Ultimately, we showed that the 24h delayed administration of mrLTF (10mg/kg, orally on d1–7) was effective in reducing the neurological deficit (12.2±0.7 vs. 8.4±0.5; p<0.05), as assessed on d7 after ICH (Fig-6B).
Fig-6. mrLTF protects mouse from ICH-injury.
(A)-Brain edema (water content in the ipsilateral- and contralateral-striatum) on d3 after ICH. The data are mean±SEM (n=7). *p≤0.05, vs. saline control. (A)-Grand NDS (A combination score of sensory-motor behavioral tests) on d3 after ICH. The data are mean±SEM (n=12/group). *p≤0.05, vs. saline control. (B)-Grand NDS on d7 after ICH in animals treated with mrLTF starting 24h after ICH. (n=5/group) *p≤0.05, vs. saline control
DISCUSSION
In this study, we have demonstrated that the iron binding protein LTF is normally present in the brain at very low levels and that ICH results in a robust and transient increase in brain LTF content. LTF detected in the brain was primarily identified in infiltrating PMNs that were seen in the brain areas directly affected by the ICH. Using in vitro models, we have demonstrated that ICH-like environments could induce PMNs to release LTF and that LTF added exogenously to the culture medium could protect brain cells in culture from toxicity induced by the product of hemolysis and iron. We also showed that the systemic depletion of PMNs using Ly-6G antibody 24 hours after ICH could exacerbate ICH outcome, suggesting that PMNs at later stages of ICH could contribute to the positive outcome, possibly in part by delivering cytoprotective LTF. Finally, we demonstrated that the recombinant mouse LTF used as therapy for ICH was effective in reducing ICH-mediated damage when injected 3 hours after the ictus.
LTF is a glycoprotein and a member from the transferrin family. LTF is normally synthesized and released by mucosal tissues and PMNs, and PMNs specifically are known to be the key contributor to plasma LTF levels.42 The initial objective of this study was to examine the time-course and source of LTF in the brain after ICH. We found that LTF increases robustly within the initial 24h after ICH, reaching its highest level on d2 and slowly declining within the next 2 weeks. Interestingly, this time-profile is identical to the time profile of neutrophil infiltration to the brain after ICH, as we reported previously in a similar ICH model.14 Thus, in conjunction with the present findings showing that all of the LTF-positive cells in ICH-affected brain are neutrophils, we believe that the primary source of LTF in the brain after ICH are neutrophils. However, as LTF is also increased in the peripheral blood after ICH, it is likely that some of the LTF present in the ICH-affected brain originates from the blood circulation. The origin of increased levels of LTF in peripheral blood after ICH was not investigated in this study; nevertheless, it is known that LTF can be readily released from circulating blood PMNs during any environmental insult, including trauma.43 Indeed, experimentally-induced neutropenia in this study, reduced ICH-induced LTF increase. It has to be emphasized that in this study and in our earlier work,15 LTF mRNA was very low both in the naïve brain as well as in the brain after ICH, even when PMNs counts peaked in the brain. This suggests that local brain cells and mature infiltrating neutrophils have limited abilities to transcribe LTF and that LTF found in the brain is synthesized at other remote locations. This is in agreement with the notion that LTF synthesis and packaging into granules occurs during PMN maturation in bone marrow.33–37
It is well accepted that LTF, besides its effect on iron sequestration, acts as a pleiotropic agent playing an important role of immune sensor, which directs specific immune responses and maintains immune homeostasis.43 The level of LTF in blood is normally low (0.2–0.6μg/ml), with a transient 100-fold increase upon insult-induced activation of neutrophils.44
After ICH, PMN infiltration into the ICH-affected brain has been proposed to augment local damage.13, 31, 45 Indeed, the systemic depletion of neutrophils prior to ICH was shown to ameliorate ICH-mediated damage,31 suggesting that the early infiltration of PMNs is deleterious. In contrast to the pre-ICH depletion paradigm, we found that late (24h) post-ICH PMN depletion is detrimental, suggesting the presence of some beneficial function of PMNs at this sub-acute stage of injury. One possibility, suggested by this study and our earlier findings, is that the PMNs can deliver cytoprotective LTF to the ICH-affected brain, neutralizing iron and blocking its toxicity. We have recently found that in response to ICH, microglia activated by this insult produce and release IL-27. Furthermore, IL-27 is elevated in the cerebra-spinal fluid (CSF) and in peripheral blood after ICH, which could amplify production of LTF and haptoglobin and reduce expression of iNOS and MMP in the maturing PMNs in bone marrow.15 Thus, ICH can alter the phenotype of PMNs in a way that the PMNs entering brain at the later stages could have acquired augmented beneficial effects, including increased blood detoxification efficacy.
LTF is stored in the specific granules of PMNs and is normally released into the extracellular environment via degranulation in order to sequester hematoma-derived iron. Using PMNs harvested from blood, we demonstrated that PMNs release LTF upon in vitro exposure to RBCs (the main component of an ICH hematoma). In addition, we showed that low, sub-injurious oxygen-glucose-deprivation (mimicking the moderate deficit in blood perfusion seen in the peri-hematoma area after ICH) can also augment LTF release from PMNs. Taken together, these results suggest that PMNs entering the ICH-affected brain may detect similar stimuli (blood products and mildly ischemic tissue), causing them to degranulate and release LTF.
Guided by the fact that PMN-delivered LTF could be beneficial to ICH pathobiology and our recent studies showing that LTF limits ICH-mediated injury,15 we have now shown that rLTF blocks the toxicity of hemolytic products in mixed glia-neuron culture and the toxicity of iron in primary neuronal culture. Similar to the human rLTF used in our earlier studies, mouse rLTF injected 3h after ICH could effectively limit ICH-mediated damage, and specifically reduce brain edema and neurological deficit.
In conclusion, our present study demonstrates that LTF that normally has a limited presence in the brain, but is delivered and released to the site of ICH injury by neutrophils. Moreover, LTF has potent capacity to limit iron-mediated cytotoxicity and protects the brain from injury caused by ICH.
Supplementary Material
Acknowledgments
Source of Funding: Supported by NIH-NINDS, grants RO1NS096308 and R42NS090650.
Footnotes
Disclosure: NONE
References
- 1.Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. Lancet neurology. 2005;4:662–672. doi: 10.1016/S1474-4422(05)70195-2. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: Role in cerebral hemorrhage. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain research. 2005;1039:30–36. doi: 10.1016/j.brainres.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Nunez MT, Urrutia P, Mena N, Aguirre P, Tapia V, Salazar J. Iron toxicity in neurodegeneration. Biometals. 2012;25:761–776. doi: 10.1007/s10534-012-9523-0. [DOI] [PubMed] [Google Scholar]
- 6.Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke; a journal of cerebral circulation. 2017;48:1033–1043. doi: 10.1161/STROKEAHA.116.015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karuppagounder SS, Alim I, Khim SJ, Bourassa MW, Sleiman SF, John R, et al. Therapeutic targeting of oxygen-sensing prolyl hydroxylases abrogates atf4-dependent neuronal death and improves outcomes after brain hemorrhage in several rodent models. Science translational medicine. 2016;8:328ra329. doi: 10.1126/scitranslmed.aac6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: Experience from preclinical studies. Neurological research. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Dore S. Inflammation after intracerebral hemorrhage. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 10.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke; a journal of cerebral circulation. 2011;42:1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Sun G, Ting SM, Song S, Zhang J, Edwards NJ, et al. Cleaning up after ich: The role of nrf2 in modulating microglia function and hematoma clearance. Journal of neurochemistry. 2015;133:144–152. doi: 10.1111/jnc.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappab and cell death after experimental intracerebral hemorrhage in rats. Stroke; a journal of cerebral circulation. 1999;30:2472–2477. doi: 10.1161/01.str.30.11.2472. discussion 2477–2478. [DOI] [PubMed] [Google Scholar]
- 13.Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain research. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, Sun G, Zhang H, Ting SM, Song S, Gonzales N, et al. Polymorphonuclear neutrophil in brain parenchyma after experimental intracerebral hemorrhage. Translational stroke research. 2014;5:554–561. doi: 10.1007/s12975-014-0341-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Ting SM, Liu CH, Sun G, Kruzel M, Roy-O’Reilly M, et al. Neutrophil polarization by il-27 as a therapeutic target for intracerebral hemorrhage. Nature communications. 2017;8:602. doi: 10.1038/s41467-017-00770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baggiolini M, De Duve C, Masson PL, Heremans JF. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med. 1970;131:559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Current pharmaceutical design. 2009;15:1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonnerdal B, Iyer S. Lactoferrin: Molecular structure and biological function. Annual review of nutrition. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 19.Baker HM, Baker EN. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals. 2004;17:209–216. doi: 10.1023/b:biom.0000027694.40260.70. [DOI] [PubMed] [Google Scholar]
- 20.Haney EF, Nazmi K, Bolscher JG, Vogel HJ. Structural and biophysical characterization of an antimicrobial peptide chimera comprised of lactoferricin and lactoferrampin. Biochim Biophys Acta. 2012;1818:762–775. doi: 10.1016/j.bbamem.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Koeppen AH. The history of iron in the brain. J Neurol Sci. 1995;134(Suppl):1–9. doi: 10.1016/0022-510x(95)00202-d. [DOI] [PubMed] [Google Scholar]
- 22.Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, et al. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29:2580–2586. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 23.Wagner KR, Dwyer BE. Hematoma removal, heme, and heme oxygenase following hemorrhagic stroke. Annals of the New York Academy of Sciences. 2004;1012:237–251. doi: 10.1196/annals.1306.020. [DOI] [PubMed] [Google Scholar]
- 24.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Annals of neurology. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 25.Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, et al. Cell death in experimental intracerebral hemorrhage: The “black hole” model of hemorrhagic damage. Annals of neurology. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, et al. Transcription factor nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, et al. Neuronal ppargamma deficiency increases susceptibility to brain damage after cerebral ischemia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:6186–6195. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:15819–15827. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Sun G, Zhang J, Ting SM, Gonzales N, Aronowski J. Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving nrf2. Stroke. 2015 doi: 10.1161/STROKEAHA.115.009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sansing LH, Harris TH, Kasner SE, Hunter CA, Kariko K. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta neurochirurgica Supplement. 2011;111:173–178. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gautam A. Classification of white blood cells based on morphological features. IEEE Xplore Digital Libarory. 2014 978-1-4799-3080-7/14:2363-2368. [Google Scholar]
- 33.Rado TA, Bollekens J, St Laurent G, Parker L, Benz EJ., Jr Lactoferrin biosynthesis during granulocytopoiesis. Blood. 1984;64:1103–1109. [PubMed] [Google Scholar]
- 34.Rado TA, Wei XP, Benz EJ., Jr Isolation of lactoferrin cdna from a human myeloid library and expression of mrna during normal and leukemic myelopoiesis. Blood. 1987;70:989–993. [PubMed] [Google Scholar]
- 35.Itoh K, Okubo K, Utiyama H, Hirano T, Yoshii J, Matsubara K. Expression profile of active genes in granulocytes. Blood. 1998;92:1432–1441. [PubMed] [Google Scholar]
- 36.Nagaoka I, Hirata M, Sugimoto K, Tsutsumi-Ishii Y, Someya A, Saionji K, et al. Evaluation of the expression of human cap18 gene during neutrophil maturation in the bone marrow. Journal of leukocyte biology. 1998;64:845–852. doi: 10.1002/jlb.64.6.845. [DOI] [PubMed] [Google Scholar]
- 37.Cowland JB, Borregaard N. The individual regulation of granule protein mrna levels during neutrophil maturation explains the heterogeneity of neutrophil granules. Journal of leukocyte biology. 1999;66:989–995. doi: 10.1002/jlb.66.6.989. [DOI] [PubMed] [Google Scholar]
- 38.Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol. 2011;70:218–235. doi: 10.1097/NEN.0b013e31820d94a5. [DOI] [PubMed] [Google Scholar]
- 39.Johnson HL, Chen Y, Jin F, Hanson LM, Gamez JD, Pirko I, et al. Cd8 t cell-initiated blood-brain barrier disruption is independent of neutrophil support. J Immunol. 2012;189:1937–1945. doi: 10.4049/jimmunol.1200658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riopelle RJ, Kennedy JC. Some aspects of porphyrin neurotoxicity in vitro. Can J Physiol Pharmacol. 1982;60:707–714. doi: 10.1139/y82-096. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Mori T, Sumii T, Lo EH. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: Caspase activation and oxidative stress. Stroke; a journal of cerebral circulation. 2002;33:1882–1888. doi: 10.1161/01.str.0000020121.41527.5d. [DOI] [PubMed] [Google Scholar]
- 42.Hansen NE, Malmquist J, Thorell J. Plasma myeloperoxidase and lactoferrin measured by radioimmunoassay: Relations to neutrophil kinetics. Acta medica Scandinavica. 1975;198:437–443. doi: 10.1111/j.0954-6820.1975.tb19572.x. [DOI] [PubMed] [Google Scholar]
- 43.Kruzel ML, Actor JK, Boldogh I, Zimecki M. Lactoferrin in health and disease. Postepy higieny i medycyny doswiadczalnej. 2007;61:261–267. [PubMed] [Google Scholar]
- 44.Erga KS, Peen E, Tenstad O, Reed RK. Lactoferrin and anti-lactoferrin antibodies: Effects of ironloading of lactoferrin on albumin extravasation in different tissues in rats. Acta physiologica Scandinavica. 2000;170:11–19. doi: 10.1046/j.1365-201x.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- 45.Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: Time course of inflammation and cell death. Neuroscience letters. 2000;283:230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.