Abstract
Aims
To assess the use of downstream coronary angiography (ICA) and short-term safety of frontline coronary CT angiography (CTA) with selective CT-derived fractional flow reserve (FFRCT) testing in stable patients with typical angina pectoris.
Methods and results
Between 1 January 2016 and 30 June 2016 all patients (N = 774) referred to non-emergent ICA or coronary CTA at Aarhus University Hospital on a suspicion of CAD had frontline CTA performed. Downstream testing and treatment within 3 months and adverse events ≥90 days were registered. Patients were divided into two groups according to the presence of typical angina pectoris, which according to local practice would have resulted in referral to ICA, (low-intermediate-risk, n = 593 [76%]; high-risk, n = 181 [24%]) with mean pre-test probability of CAD of 31 ± 16% and 67 ± 16%, respectively. Coronary CTA was performed in 745 (96%) patients in whom FFRCT was prescribed in 212 (28%) patients. In the high- vs. low-intermediate-risk group, ICA was cancelled in 75% vs. 91%. Coronary revascularization was performed more frequently in high-risk than in low-intermediate-risk patients, 76% vs. 52% (P = 0.03). Mean follow-up time was 157 ± 50 days. Serious clinical events occurred in four patients, but not in any patients with cancelled ICA by coronary CTA with selective FFRCT testing.
Conclusion
Frontline coronary CTA with selective FFRCT testing in stable patients with typical angina pectoris in real-world practice is associated with a high rate of safe cancellation of planned ICAs.
Keywords: stable angina, diagnostic testing, coronary ct angiography, fractional flow reserve
Introduction
In patients suspected of stable coronary artery disease (CAD) with low-intermediate pre-test risk of significant coronary stenosis guidelines recommend non-invasive ischaemia testing as gatekeeper to invasive coronary angiography (ICA), whereas patients with high pre-test probability may be referred directly to ICA.1 Numerous studies have shown that non-invasive ischaemia testing has a low accuracy in identifying patients with obstructive CAD resulting in low diagnostic yield of ICA.2–5
Coronary computed tomography angiography (CTA) is accurate in excluding CAD,6 and absence of CAD by coronary CTA is associated with an excellent prognosis.7–9 Guidelines recommend coronary CTA as an alternative to standard non-invasive ischaemia testing in symptomatic stable patients with low-intermediate pre-test probability of obstructive CAD.1 However, coronary CTA is of modest value in quantifying stenosis severity, especially in the presence of coronary calcification,10 and the correlation of stenosis to downstream ischaemia is poor.11 Therefore, in symptomatic patients with suspected obstructive CAD determined by coronary CTA, guidelines recommend additional ischaemia testing before referral of patients to ICA.1
Fractional flow reserve (FFR) derived by standard acquired coronary CTA (FFRCT) enables computational assessment of coronary blood flow and pressure.12 FFRCT has high diagnostic performance,13–15 also in patients with high calcific burden,16 and, since the FFRCT model incorporates the microcirculatory resistance,12 in patients at high risk of having microvascular disease.17 Recently, the technology has advanced beyond diagnostic validation into clinical utility.18–20 Although promising, the real-world clinical utility of coronary CTA and FFRCT testing in stable patients with high risk of obstructive CAD is unknown.
The purpose of this study was two-fold: In stable patients with typical angina pectoris to assess (i) Influence on the use of downstream ICA, and (ii) short-term safety of frontline coronary CTA with selective FFRCT testing.
Methods
Study population
All symptomatic patients referred to non-emergent frontline ICA or coronary CTA testing on a suspicion of stable CAD at Aarhus University Hospital between 1 January 2016 and 30 June 2016 were included. Patients were referred from our outpatient clinic, two community hospitals, and two private cardiologist practices. In this institution, coronary CTA is the frontline diagnostic test in symptomatic patients with low-intermediate-risk of CAD,19 whereas patients with typical angina pectoris are considered high-risk and preferably referred directly to ICA. In this study we changed our diagnostic workflow to frontline coronary CTA with selective FFRCT testing in all symptomatic patients suspected of stable CAD referred for coronary assessment. Patients <18 years old, with known CAD, severe renal impairment, pregnancy, or with low probability of obtaining a conclusive coronary CTA result were excluded.21 All patients underwent a 12-lead electrocardiogram, routine biochemistry, echocardiography, and clinical evaluation before referral to frontline coronary CTA assessment. Data were obtained from patient files and registries. The study complies with the Declaration of Helsinki and was approved by the Danish Data Protection Agency (1-16-02-54-16) and the Danish Health Authority (3-3013-1641/1/).
Coronary CT angiography and FFRCT assessment
Coronary CTA was performed on Siemens SOMATOM Definition Flash and SOMATOM Force scanners (Siemens, Forchheim, Germany). The strategy of coronary CTA acquisition in this institution has previously been described.19 In brief, all patients received 0.8 mg sublingual nitrates and oral/intravenous beta-blockers or oral ivabradine targeting a heart rate <60 beats per minute. An initial 120 kV non-enhanced high-pitch spiral acquisition was performed for calcium scoring. Coronary CTA was performed with prospective electrocardiographic triggering. Experienced cardiologists evaluated vessels ≥2 mm in diameter using axial images and multi-planar reconstructions.19 Local recommendations for downstream management of patients after coronary CTA and FFRCT testing are shown in Table 1. FFRCT analysis was based on standard coronary CTA datasets (HeartFlow Inc., Redwood City, CA, USA).15 The principles underlying FFRCT computation have been described previously.12 FFRCT values distally in the major epicardial coronary arteries (including side branches) ≥2 mm in diameter were registered and FFRCT values ≤0.8 were considered diagnostic of lesion-specific ischaemia.19 For clinical decision-making on FFRCTTable 1 specifies local recommendations.
Table 1.
Local recommendation for diagnostic work-up in patients referred for coronary CTA or ICA between 1 January 2016 and 30 June 2016
| Frontline coronary CTA | Test outcome | Diagnostic consequence |
|---|---|---|
| Conclusive | High risk anatomya | OMT and ICA (±FFRCTd) |
| Intermediate risk anatomyb | OMT and FFRCTe | |
| Low risk anatomyc | OMT | |
| Inconclusive | OMT, MPI or ICA | |
| FFRCT | Test outcome | Clinical recommendation |
| Conclusive | >0.8 | OMT |
| 0.75–0.8 | OMT and follow-upf | |
| <0.75 diffuse diseaseg | OMT and follow-upf | |
| <0.75 focal stenosish | OMT and ICA | |
| Inconclusive | OMT or ICA | |
ICA, invasive coronary angiography; FFRCT, coronary computed tomography angiography (CTA) derived fractional flow reserve; OMT, optimal medical treatment; MPI, myocardial perfusion imaging.
Patients with left main, three vessel disease or high-grade proximal left anterior descending coronary artery stenosis.
Patients with ≥1 intermediate coronary stenosis (30–70%).
Patients without coronary artery disease or maximum 30% coronary artery stenosis.
Selective FFRCT possible (i.e. for functional assessment of lesions which did not directly lead to ICA).
FFRCT as a gatekeeper to ICA.
Preferably OMT and follow-up within 2 months: ICA recommended in the event of ongoing chest pain.19
FFRCT with gradual decline or distally located stenosis.
FFRCT result indicating significant mid-proximal focal stenosis. Supplementary material online, Figure S1 gives examples of the four modes of FFRCT test outcome.
Invasive coronary angiography and FFR
ICA was performed according to standard practice by experienced interventional cardiologists who had access to all clinical information, including available coronary CTA, FFRCT, and myocardial perfusion imaging (MPI) results. Additional FFR testing was performed at the discretion of the interventional cardiologist. FFR measurements were performed using the Verrata (Volcano Therapeutics, Cordova, CA, USA) or Aeris (St. Jude Medical, St. Paul, MN, USA) pressure wire and standard practice protocols. Instantaneous wave-free ratio (iFR) was measured in few patients.19 Decision on revascularization was at the discretion of the interventional cardiologist and experienced cardio-thoracic surgeons.
Radiation exposure
Radiation exposure is reported in mSv using conversion factors of 0.014 mSv/mGy-cm for coronary CTA (including calcium scans), 0.18 mSv/(Gy cm2) for ICA and 0.00126 MBq for MPI.19
Follow-up
Within 90 days of follow-up from the coronary CTA investigation, diagnostic procedures (FFRCT, MPI, ICA, FFR/iFR) and treatment [percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)] planned on the basis of the initial coronary CTA were registered. Within a minimum of 90 days of follow-up patients with cardiac or death from any cause, myocardial infarction, hospitalization or ambulatory visits due to chest pain were registered as adverse clinical events. Death and myocardial infarction were categorized as serious adverse clinical events.
Statistical analysis
Categorical data are presented as numbers and proportions and comparisons were done using χ2 tests. Non-categorical data are presented as means (standard deviation, SD) and compared by t-test and Mann–Whitney U test as appropriate. A P-value ≤0.05 was considered statistically significant. SPSS version 21 (©Copyright IBM Corporation and other(s) 1989, 2012) was used for analyses.
Results
A total of 795 consecutive patients were referred for ICA or coronary CTA testing during the study period. Twenty-one patients did not enter the study due to atrial fibrillation (n = 14), high BMI (n = 5), and renal insufficiency (n = 2). Thus, the study cohort comprised 774 patients of whom 181 (24%) had typical angina pectoris and thus constituted the high-risk group. Table 2 shows baseline and Table 3 coronary CTA acquisition characteristics according to risk group categorization. Patients in the high-risk group were older, had higher prevalence of hypertension, hyperlipidaemia, diabetes, and higher Diamond-Forrester risk than the low-intermediate-risk group. Patients in the high-risk group had higher prevalence of CAD, higher Agatston score, and lower FFRCT values than the low-intermediate-risk group (Table 4).
Table 2.
Patient characteristics
| All (N = 774) | Risk group |
P-valuea | |||
|---|---|---|---|---|---|
| Low-intermediate (n = 593) | High (n = 181) | ||||
| Mean (SD) age, years | 59 (11) | 58 (11) | 62 (11) | <0.001 | |
| Male gender | 401 (52) | 297 (51) | 104 (57) | 0.09 | |
| Hypertension | 288 (37) | 202 (34) | 86 (48) | 0.001 | |
| Hyperlipidaemia | 248 (32) | 170 (29) | 78 (43) | 0.001 | |
| Diabetes | 69 (9) | 41 (7) | 28 (15) | 0.001 | |
| Tobacco | 456 (59) | 353 (60) | 103 (57) | 0.59 | |
| Family history of CAD | 352 (47) | 273 (46) | 79 (44) | 0.85 | |
| Mean (SD) BMI, kg/m2 | 26 (5) | 26 (5) | 27 (5) | 0.02 | |
| Mean (SD) serum creatinine, µmol/L | 78 (31) | 77 (31) | 81 (29) | 0.06 | |
| Typical angina | 181 (23) | 0 | 181 (100) | <0.001 | |
| Symptoms | Atypical angina | 449 (58) | 449 (76) | 0 | <0.001 |
| Non-anginal chest pain | 144 (19) | 144 (24) | 0 | <0.001 | |
| Mean (SD) Updated Diamond-Forrester risk score, % | 40 (22) | 31 (16) | 67 (16) | <0.001 | |
| Exercise-ECGb prior to inclusion | 67 (9) | 47 (8) | 20 (11) | 0.23 | |
Values are numbers (%) if not stated otherwise.
CAD, coronary artery disease; BMI, body mass index; ECG, electrocardiogram.
Comparison between low-intermediate- and high-risk.
Exercise-ECG testing was used only in patients referred to coronary assessment from private cardiologist practices.
Table 3.
Coronary CTA acquisition characteristics
| All (N = 774) | Risk group |
P-valuea | ||
|---|---|---|---|---|
| Low-intermediate (n = 593) | High (n = 181) | |||
| Mean (SD) heart rate, beats/min | 58 (11) | 58 (11) | 58 (10) | 0.53 |
| Sinus rhythm at CTA | 717 (96) | 574 (97) | 143 (93) | 0.06 |
| Pre-CTA betablockers | 618 (83) | 493 (83) | 125 (82) | 0.63 |
| Pre-CTA nitroglycerine | 736 (99) | 585 (99) | 151 (99) | 1.0 |
| Mean (SD) CTA radiation dose, mSv | 3.8 (2.1) | 3.7 (2) | 3.9 (2.4) | 0.99 |
| Mean (SD) cumulative radiation dose, mSv | 4.2 (2.8) | 4.1 (2.7) | 4.7 (3.1) | 0.10 |
Values are numbers (%) if not stated otherwise.
CTA, computed tomography angiography.
Comparison between low-intermediate- and high-risk.
Table 4.
Patient characteristics according to conclusive coronary CTA and FFRCT
| All (N = 721) | Risk group |
P-valuea | ||
|---|---|---|---|---|
| Low-intermediate (n = 574) | High (n = 147) | |||
| Coronary artery stenosis >50% | 176 (24) | 109 (19) | 67 (46) | <0.001 |
| Coronary high risk anatomyb | 62 (9) | 35 (6) | 27 (18) | <0.001 |
| Mean (SD, range) Agatstonc | 185 (543, 0–6085) | 150 (491, 0–5743) | 320 (695, 0–6085) | <0.001 |
| Agatston score ≥400 | 95 (13) | 61 (10) | 34 (22) | <0.001 |
| Mean (SD) FFRCTd | 0.78 (0.13) | 0.81 (0.10) | 0.75 (0.16) | 0.005 |
| FFRCT <0.80 | 93 (44) | 53 (39) | 40 (56) | 0.03 |
Values are numbers (%) if not stated otherwise. FFRCT, coronary computed tomography angiography (CTA) derived fractional flow reserve.
Comparison between low-intermediate- and high-risk.
Coronary high risk anatomy: left main, three vessel disease or high-grade proximal left anterior descending coronary artery stenosis.
Agatston: n = 592 + 153 in low-intermediate- and high-risk groups, respectively.
FFRCT: lowest per patient value, i = 137 + 72 in low-intermediate- and high-risk groups, respectively.
In the high-risk group, 28 (15%) patients were referred directly to ICA. Baseline characteristics of this group are presented in Supplementary data online, Table S1. Coronary CTA was performed in 745 (96%) patients. The coronary CTA result was conclusive in 147 (96%) and 574 (97%) in the high- and low-intermediate-risk groups, respectively.
Downstream test utilization
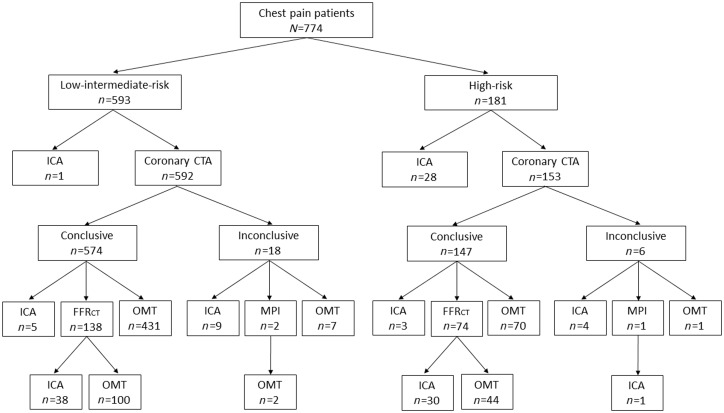
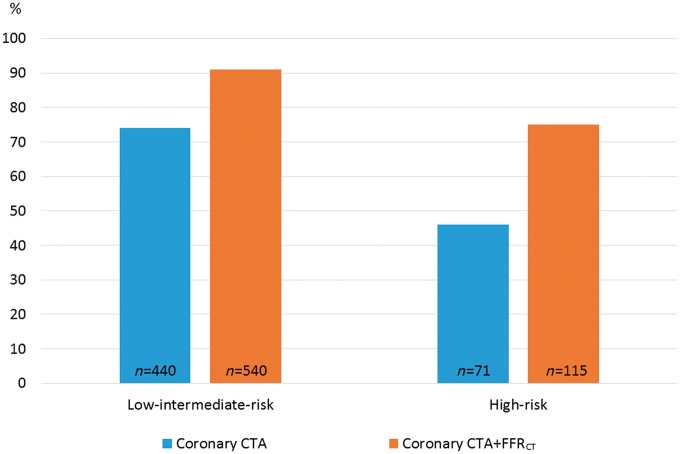
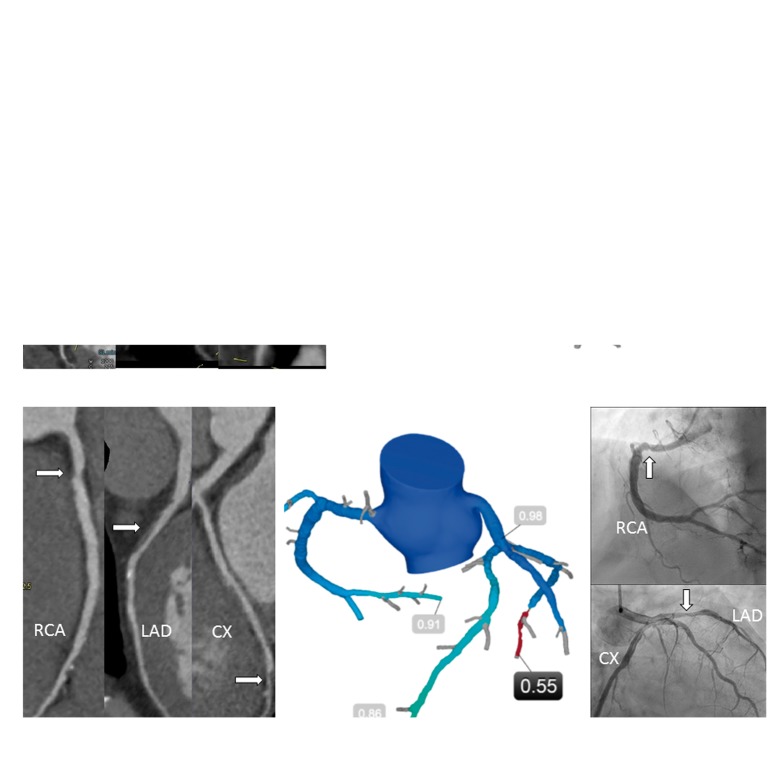
Figure 1 shows the diagnostic flow in the two groups. 212 (28%) patients were prescribed FFRCT testing with a conclusive test result obtained in 72 (97%) and 137 (99%) in the high- and low-intermediate-risk patients, respectively. FFRCT testing was more frequent, and more patients were referred to ICA in high- than in low-intermediate-risk patients, 41% (74/181) vs. 23% (138/593) (P < 0.001), and 36% (66/181) vs. 9% (53/593) (P < 0.001), respectively. During the study period, seven patients underwent downstream MPI due to an inconclusive coronary CTA result (n = 3), as supplement to ICA (n = 3) or a positive FFRCT result (n = 1). The impact of coronary CTA and FFRCT testing on cancellation of ICA is shown in Figure 2. Overall, in high-risk patients having coronary CTA performed, ICA was cancelled in 75% (115/153). Overall the effect of FFRCT testing on cancellation of ICA was higher in high- as compared with low-intermediate-risk patients. FFRCT was used as supplement to planned ICA in 35 patients. In 34 of these patients FFRCT analysis was conclusive with positive or negative FFRCT result in 28 and 6 patients, respectively. In these patients FFR/iFR interrogations were performed in 21% (6/28) and 67% (4/6) (P < 0.05), respectively. Figure 3 shows clinical examples of the impact of FFRCT testing on the referral to ICA.
Figure 1.
Diagnostic work-up of patients. N = numbers of patients. Numbers refer to planned diagnostic activities within 3 months after the initial coronary CTA. Inconclusive coronary CTAs were due to obesity (n = 2), irregular heart rhythm (n = 6), high Agatston (n = 0), lack of cooperation (n = 4), or combinations hereof (n = 12). CTA, CT angiography; ICA, invasive coronary angiography; OMT, optimal medical treatment; FFRCT, prescribed coronary CTA derived fractional flow reserve; MPI, myocardial perfusion imaging.
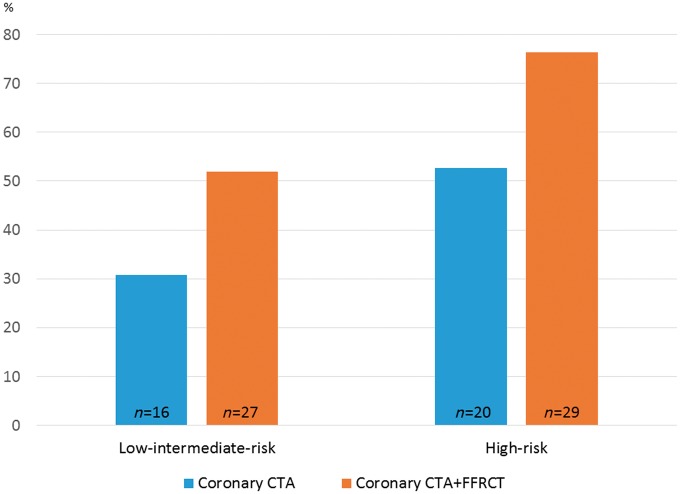
Figure 2.
Effect of FFRCT testing on cancellation of ICA. The Y-axis shows percentage in each risk group. CTA, CT angiography; FFRCT, prescribed coronary CTA derived fractional flow reserve.
Figure 3.
(A) 66-year-old female. Typical angina. Left: Coronary CTA, moderate stenosis in RCA and LAD (arrows). Right: FFRCT 0.85 (RCA) and 0.90 (LAD). OMT was recommended. (B) 61-year-old male. Typical angina. Left: Coronary CTA, moderate stenosis in RCA, LAD, and CX (arrows). Centre: FFRCT 0.91 (RCA), 0.86 (LAD), 0.55 (CX). The patient was referred to ICA (Right): CX was stented and FFR was measured: 0.84 (LAD) and 0.90 (RCA). CTA, CT angiography; ICA, invasive coronary angiography; OMT, optimal medical treatment; FFRCT, prescribed coronary CTA derived fractional flow reserve; RCA, right coronary artery; LAD, left anterior descending coronary artery; CX, circumflex coronary artery.
Coronary revascularization
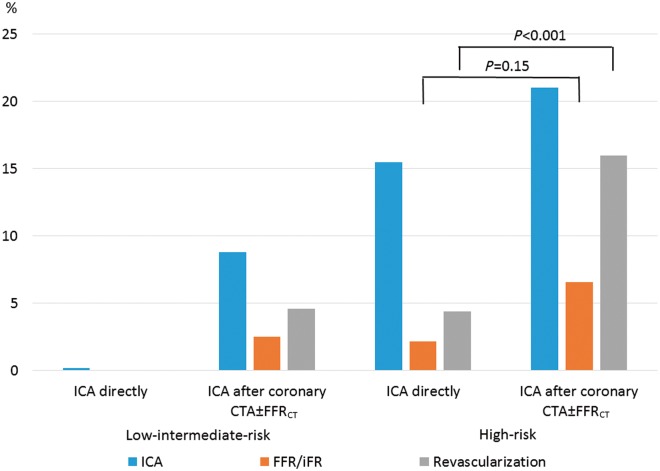
Figures 4 and 5 provide overviews of the effect of FFRCT testing on the downstream use of planned ICA, FFR/iFR, and revascularization. Coronary revascularization was performed in 54% (64/119) of the patients (PCI, 61%; CABG, 39%). In patients with typical angina pectoris the rate of revascularization was higher in patients referred for coronary CTA as compared with patients being referred directly to ICA. Figure 6 shows the impact of coronary CTA and FFRCT testing on rates of subsequent revascularization. Overall, the effect of FFRCT testing was lower in high- as compared with low-intermediate-risk patients.
Figure 4.
Use of downstream diagnostic testing and revascularization. N = numbers of patients. Numbers in parenthesis refer to numbers of positive FFRCT results and numbers in brackets refer to the numbers of patients having fractional flow reserve (FFR) and/or instantaneous wave-free ratio (iFR) performed. FFR was performed in 26 (84%), iFR in two (6%), and FFR + iFR in three (10%) patients, respectively. Rev, revascularization; CTA, CT angiography; ICA, invasive coronary angiography; OMT, optimal medical treatment; FFRCT, prescribed coronary CTA derived fractional flow reserve.
Figure 5.
Use of FFR and revascularization following ICA. Numbers refer to percentage in each risk group. In patients referred to coronary CTA revascularization was performed more frequently in high-risk as compared with low-intermediate risk (P = 0.03) without significant difference in use of FFR/iFR (P = 0.78). Of the 56 patients in whom coronary revascularization was performed after coronary CTA with optional FFRCT, 59% (33/56) had FFRCT performed, 21% (12/56) had FFRCT plus FFR and/or iFR, and 20% (11/56) had neither performed. ICA, invasive coronary angiography; CTA, CT angiography; FFRCT, coronary CTA derived fractional flow reserve; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio.
Figure 6.
Effect of FFRCT testing on use of revascularization. The Y-axis shows percentage of patients in each risk group. CTA, CT angiography; FFRCT, prescribed coronary CTA derived fractional flow reserve.
Clinical adverse events
Mean (SD) follow-up time was 157 (50) days. Clinical adverse events are presented in Table 5. Serious clinical events occurred in four patients. None of the patients where ICA was cancelled due to the results of coronary CTA with selective FFRCT testing experienced serious clinical events.
Table 5.
Clinical adverse events after a minimum of 90 days follow-up
| Clinical event | All (N = 774) | Low-intermediate-risk (n = 593) | High-risk (n = 181) |
|
|---|---|---|---|---|
| ICA (n = 66) | ICA cancelled (n = 115) | |||
| Hospitalization | 8 (1.0) | 6 (1.0) | 1 (1.5) | 1 (0.9) |
| Ambulatory referral | 3 (0.4) | 2 (0.3) | 1 (1.5) | |
| Cardiac death | 1 (0.1) | 1 (1.5) | ||
| Non-cardiac death | 2 (0.3) | 2 (0.3) | ||
| Total | 14 (1.8) | 10 (1.7) | 3 (4.5) | 1 (0.9) |
Numbers of adverse events (%). In the low-intermediate-risk group one non-ST-elevation myocardial infarction and two non-cardiac deaths occurred (known terminal cancer before referral and trauma, respectively). In the high-risk group one cardiac death occurred (refractory cardiac arrest within 24 h after coronary artery bypass surgery). The remaining 10 clinical adverse events did not result in testing or treatment.
Discussion
In this single-centre all-comer cohort study, frontline coronary CTA with selective FFRCT testing in stable, symptomatic patients with typical angina pectoris was feasible, and associated with a high rate of cancellation of scheduled ICAs. No serious adverse clinical events occurred in high-risk patients in whom ICA was cancelled within 90 days of follow-up.
An effective non-invasive test in stable CAD identifies patients who benefit from ICA, and provides guidance for subsequent patient care. Coronary CTA is increasingly used in patients suspected of stable CAD. However, the inverse relationship between increasing pre-test probability of CAD and the diagnostic specificity of coronary CTA should be acknowledged.22 Moreover, the diagnostic specificity of coronary CTA declines with increasing calcium scores.10 Consequently, guidelines do not recommend coronary CTA testing in patients with high pre-test probability of CAD or in the event of a high coronary calcium score.1
In prospective multi-centre trials including patients with low-intermediate pre-test probability of stable CAD with blinded comparison to FFR, FFRCT showed high and, when compared with anatomical interpretation by coronary CTA alone, superior diagnostic specificity.13–15 Moreover, FFRCT has improved diagnostic specificity beyond coronary CTA alone in patients with high coronary calcification.16 In the PLATFORM study, in patients with planned ICA, FFRCT testing resulted in safe cancellation of ICA in 61% of the patients when compared with standard practice.18 The present study extends previous findings by demonstrating that in a real-world large consecutive cohort of symptomatic patients with suspected CAD, evaluation with coronary CTA and selective FFRCT testing results in a high rate of cancellation of planned ICA irrespective of level of risk of CAD. Notably, pre-test risk of CAD by the Diamond-Forrester algorithm in our high-risk group was 67% vs. 51% in the PLATFORM ‘invasive’ cohort. In addition, we observed that the ICA cancellation rate based on coronary CTA alone was higher in the low-intermediate than in the high-risk group (74% vs. 46%). These findings are in accordance with a recent study of patients suspected of CAD and low-intermediate pre-test risk (45%) of significant CAD where ICA safely could be cancelled in 75% of patients based on the coronary CTA result alone.9 In a study by Dewey et al.23 of patients referred to ICA, 86% had ICA safely cancelled based on frontline coronary CTA testing. However, the latter study cohort was relative low risk with a low proportion of patients having typical angina and a pre-test risk score of 34%. The present study adds to previous findings by demonstrating the limited value of coronary CTA as a gatekeeper test to ICA in patients with high risk of CAD. Accordingly, following a strategy of coronary CTA with selective FFRCT testing, the relative proportion of cancellations of planned ICAs was 63% in the high- vs. 23% in the low-intermediate-risk group. In addition, a strategy of coronary CTA with selective FFRCT testing in the high- vs. low-intermediate-risk group was associated with an increased diagnostic yield of ICA by demonstrating revascularization rates of 76% and 52%, respectively. The present non-invasive diagnostic strategy in patients with classical angina pectoris was supported by a short-term low rate of adverse events in patients where ICA was cancelled. The overall low adverse event rate in this study is in accord with recent large scale studies.7,8,18
Guidelines for coronary revascularization support anatomic stenosis evaluation by ICA with FFR or conventional ischaemia testing.1 In current practice less than two-thirds of patients undergo non-invasive ischaemia testing before ICA and the majority of patients have no angiographic evidence of obstructive CAD.2,4 Opposite to conventional ischaemia testing, FFR is a validated tool for the functional assessment on a per-lesion basis, which is needed to guide revascularization. However, the adoption of FFR in clinical practice is limited.24 In the present study, the majority of revascularizations were performed with available information on lesion-specific ischaemia. Of note, the majority of functional tests were FFRCT, which in this institution has been adopted into clinical practice for revascularization guidance.19 These findings are in accordance with the PLATFORM study in which data regarding the functional significance of coronary stenosis at the time of revascularization were available in 95% in the FFRCT guidance group compared with 49% in the usual care group.18
In this institution FFRCT is used as a diagnostic gatekeeper to ICA or as a supplemental test in patients with multiple lesions in whom referral to ICA has been decided. Interestingly, a supplemental negative FFRCT result prompted use of FFR/iFR more frequently than when a positive FFRCT was present. This probably relates to the severity of CAD in these patients. Thus, in patients with positive FFRCT results the severity of CAD was high and the need of additional per-lesion functional information for guidance of revascularization was less than in patients with normal dichotomized FFRCT results.
Limitations
This is an observational and single-centre study and thus with inherent limitations. Specifically, it should be acknowledged that the criteria used in this study to define high risk anatomy and clinical recommendations following FFRCT testing have not been tested in randomized studies. However, our study included a large consecutive cohort of patients with limited exclusion criteria, and represent patients encountered in clinical practice. An extended follow-up including systematic registration of angina symptoms, which is the major target of PCI, would have added valuable information. High-risk was defined according to local practice as typical angina. This strategy was legitimized by previous findings showing that the presence of typical angina is a very strong predictor of finding obstructive CAD at ICA.5 Moreover, the findings of a high baseline risk profile, high Diamond-Forrester score (67% vs. 31%), high calcium score (320 vs. 120) and a high revascularization rate (76% vs. 52%) in patients with vs. those without typical angina support this strategy. Local recommendations were not followed in all patients, thus 29 (4%) patients went directly to ICA and out of 511 patients who were prescribed OMT, 5 (1%) had coronary stenosis >50%. We did not record plaque composition, hence the potential value of this in terms of risk reclassification could not be elucidated.25
Conclusion
Frontline coronary CTA with selective FFRCT testing in stable, symptomatic patients with typical angina pectoris is associated with a high rate of cancellation of planned ICAs. The results obtained in this study need confirmation in larger randomized studies with longer follow-up.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Conflict of interest: J.M.J. has received speaker’s honorarium from Bracco Imaging. J.L. has received personal fees from Circl CVI, Philips, Samsung, and HeartFlow. B.L.N. has received unrestricted research grants from HeartFlow and Siemens. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplementary Material
References
- 1. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV. et al. Low diagnostic yield of elective coronary angiography. New Engl J Med 2010;362:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Douglas PS, Patel MR, Bailey SR, Dai D, Kaltenbach L, Brindis RG. et al. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol 2011;58:801–9. [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Dai D, Hernandez AF, Douglas PS, Messenger J, Garratt KN. et al. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J 2014;167:846–52. [DOI] [PubMed] [Google Scholar]
- 5. Vavalle JP, Shen L, Broderick S, Shaw LK, Douglas PS.. Effect of the presence and type of angina on cardiovascular events in patients without known coronary artery disease referred for elective coronary angiography. JAMA Cardiol 2016;1:232–4. [DOI] [PubMed] [Google Scholar]
- 6. Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA. et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44. [DOI] [PubMed] [Google Scholar]
- 7. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 8. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B. et al. Outcomes of anatomical versus functional testing for coronary artery disease. New Engl J Med 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lubbers M, Dedic A, Coenen A, Galema T, Akkerhuis J, Bruning T. et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J 2016;37:1232–43. [DOI] [PubMed] [Google Scholar]
- 10. Alkadhi H, Scheffel H, Desbiolles L, Gaemperli O, Stolzmann P, Plass A. et al. Dual-source computed tomography coronary angiography: influence of obesity, calcium load, and heart rate on diagnostic accuracy. Eur Heart J 2008;29:766–76. [DOI] [PubMed] [Google Scholar]
- 11. Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR. et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol 2008;52:636–43. [DOI] [PubMed] [Google Scholar]
- 12. Taylor CA, Fonte TA, Min JK.. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–41. [DOI] [PubMed] [Google Scholar]
- 13. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS. et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989–97. [DOI] [PubMed] [Google Scholar]
- 14. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C. et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H. et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145–55. [DOI] [PubMed] [Google Scholar]
- 16. Norgaard BL, Gaur S, Leipsic J, Ito H, Miyoshi T, Park SJ. et al. Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT trial. JACC Cardiovasc Imaging 2015;8:1045–55. [DOI] [PubMed] [Google Scholar]
- 17. Eftekhari A, Min J, Achenbach S, Marwan M, Budoff M, Leipsic J. et al. Fractional flow reserve derived from coronary computed tomography angiography: diagnostic performance in hypertensive and diabetic patients. Eur Heart J Cardiovasc Imaging 2017;18:1351–60. [DOI] [PubMed] [Google Scholar]
- 18. Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA. et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J 2015;36:3359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norgaard BL, Hjort J, Gaur S, Hansson N, Botker HE, Leipsic J. et al. Clinical use of coronary CTA-derived FFR for decision-making in stable CAD. JACC Cardiovasc Imaging 2016. doi:10.1016/j.jcmg.2015.11.025 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20. Packard RR, Li D, Budoff MJ, Karlsberg RP.. Fractional flow reserve by computerized tomography and subsequent coronary revascularization. Eur Heart J Cardiovasc Imaging 2017;18:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK. et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10:435–49. [DOI] [PubMed] [Google Scholar]
- 22. Meijboom WB, van Mieghem CA, Mollet NR, Pugliese F, Weustink AC, van Pelt N. et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol 2007;50:1469–75. [DOI] [PubMed] [Google Scholar]
- 23. Dewey M, Rief M, Martus P, Kendziora B, Feger S, Dreger H. et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial. BMJ 2016;355:i5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park SJ, Ahn JM, Park GM, Cho YR, Lee JY, Kim WJ. et al. Trends in the outcomes of percutaneous coronary intervention with the routine incorporation of fractional flow reserve in real practice. Eur Heart J 2013;34:3353–61. [DOI] [PubMed] [Google Scholar]
- 25. Gaur S, Ovrehus KA, Dey D, Leipsic J, Botker HE, Jensen JM. et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J 2016;37:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.