Abstract
Adrenal glucocorticoids (GCs) control a wide range of physiological processes, including metabolism, cardiovascular and pulmonary activities, immune and inflammatory responses, and various brain functions. During stress responses, GCs are secreted through activation of the hypothalamic–pituitary–adrenal axis, whereas circulating GC levels in unstressed states follow a robust circadian oscillation with a peak around the onset of the active period of a day. A recent advance in chronobiological research has revealed that multiple regulatory mechanisms, along with classical neuroendocrine regulation, underlie this GC circadian rhythm. The hierarchically organized circadian system, with a central pacemaker in the suprachiasmatic nucleus of the hypothalamus and local oscillators in peripheral tissues, including the adrenal gland, mediates periodicities in physiological processes in mammals. In this review, we primarily focus on our understanding of the circadian regulation of adrenal GC rhythm, with particular attention to the cooperative actions of the suprachiasmatic nucleus central and adrenal local clocks, and the clinical implications of this rhythm in human diseases.
Keywords: adrenal gland, circadian clock, circadian rhythm, glucocorticoid, hypothalamic-pituitary-adrenal axis
The present review mainly focuses on our understanding of the circadian regulation of adrenal glucocorticoids rhythm, particularly by the cooperative actions of the central and adrenal local clocks.
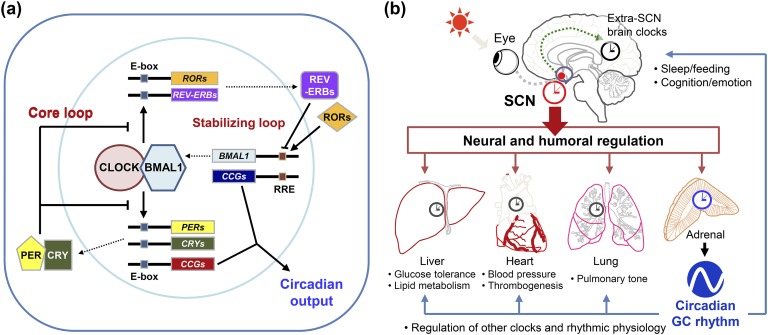
Circadian rhythm refers to evolutionarily conserved biological oscillations with a ~24-hour period. This type of daily rhythm is not a simple response to alternations of day and night; rather, it arises from a genetically operated timekeeping system called the “circadian clock.” This internal timekeeping system allows organisms to anticipate environmental cycling and thus optimize their physiology, metabolism, and behavior at the right time of day. The circadian clock is cell-autonomous and self-sustainable because of the cooperation of genetic components, but it can also be entrained by external time cues called “zeitgebers.” Most cells in multicellular organisms have their own cell-autonomous oscillators, which are hierarchically organized into a circadian timing system. The suprachiasmatic nucleus (SCN) of the hypothalamus, composed of densely packed neurons, generates self-sustaining rhythms by both genetic and neural mechanisms and is thus considered the central or master clock [1]. The SCN central clock receives environmental time information (primarily light) to adjust or entrain its phases. The SCN clock also orchestrates other oscillators in extra-SCN brain regions and peripheral tissues (referred to as local or peripheral clocks) to produce overt circadian rhythms, such as the rest–activity cycle, periodic daily variations in metabolism and body temperature, and rhythmic secretion of hormones [2].
Glucocorticoids (GCs)—primarily cortisol in primates and corticosterone in rodents—are steroid hormones secreted by the adrenocortical steroidogenic cells that constitute the stress-responsive neuroendocrine system [3–5]. Threatening stimuli from the environment cause an immediate increase in the levels of circulating GCs by activating the hypothalamic–pituitary–adrenal (HPA) neuroendocrine axis. Activation of stress-sensitive neural circuits in discrete brain regions leads to excitation of neurosecretory neurons in the paraventricular nucleus (PVN) of the hypothalamus, which in turn results in the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) into the hypothalamo–hypophyseal portal vessels. In turn, these secretagogues induce adrenocorticotropic hormone (ACTH) secretion from the pituitary corticotropes, which activates adrenal synthesis and the secretion of GCs. Finally, GCs interact with intracellular receptors in target cells and regulate diverse physiologic events by either genomic or nongenomic mechanisms to cope with stress. Notably, circulating GC levels oscillate robustly even in undisturbed states; that is, they exhibit clear circadian rhythm with a peak around the onset of the active period (night for nocturnal animals and day for diurnal animals) [3–5]. It is widely accepted that the daily GC rhythm is under the control of the circadian clock because the GC rhythmic profiles are completely eliminated by abolition of the SCN central clock or genetic components of the clock [6–9]. Recent studies on the adrenal clock imply a role of this local oscillator along with the SCN central pacemaker in regulating circadian GC oscillations [10, 11]. In view of these facts, the present review mainly focuses on our understanding of the circadian regulation of adrenal GC rhythm, particularly by the cooperative actions of the SCN central and adrenal clocks. Implications of adrenal GC rhythm with regard to human health and disease are also reviewed.
1. Molecular Clock and Circadian Timing System in Mammals
The intrinsic and self-sustainable nature of the circadian system is primarily attributed to the molecular oscillator driven by genetic components. The mammalian molecular clock is currently understood to be a molecular feedback loop formed by a group of clock proteins [2]. The clock proteins form two interlocked positive and negative transcription/translation feedback loops that promote periodic gene expression [Fig. 1(a)]. The foremost regulators of the core loop of the molecular oscillator belong to the basic helix-loop-helix–period (PER)-ARNT-SIM family of transcription factors and include circadian locomotor output cycle kaput (CLOCK) and brain muscle aryl hydrocarbon receptor nuclear translocator-like (BMAL1). CLOCK and BMAL1 form heterodimers, bind to E/E′-boxes (5′-CANNTG-3′) elements, and then induce transcription of the target genes through epigenetic activation of their promoters. These downstream genes include PERs (PER1–3) and cryptochromes (CRY) 1 and 2 constituting the negative limb of the feedback loop. Accumulated PER and CRY proteins form repressive protein complexes that inhibit the E-box–mediated transcription by directly binding with the CLOCK/BMAL1 complex [12–15]. In addition to the core feedback loop, the levels of CLOCK and BMAL1 proteins are also adjusted by an auxiliary or stabilizing feedback loop consisting of sets of the circadian nuclear receptors, such as retinoic acid receptor–related orphan nuclear receptors (ROR) α, β, and γ and REV-ERBα and β [16–18]. Taken together, these two interlocked feedback loops provide a molecular basis for self-sustaining circadian oscillations with a period of approximately 24 hours.
Figure 1.
Schematic representations of the mammalian circadian timing system. (a) Circadian molecular clock in mammals. The mammalian circadian oscillator is composed of two interlocking transcription/translation feedback loops, designated as core and auxiliary loops, to produce precise rhythms of cyclic gene expression. (b) Hierarchical organization of the mammalian circadian system. The SCN functions as the master pacemaker responsible for the coordination of multiple clock networks throughout the body. Local oscillators in extra-SCN brain regions and peripheral tissues, in turn, constitute tissue-specific physiological outputs. Of particular interest, circadian rhythm in adrenal GCs contributes to coordination of central and peripheral rhythms.
The hypothalamic SCN, considered to be the master clock that drives and organizes rhythmic physiology and behaviors, receives environmental information to adjust the circadian system to geophysical time [6, 19, 20]. Most cells in peripheral tissues and extra-SCN brain regions also contain their own oscillators that have similar molecular makeup to that found in the SCN pacemaker neurons. Therefore, the mammalian circadian system is organized in a hierarchical fashion consisting of the SCN central clock and various subsidiary local clocks. In the absence of synchronizing cues from the SCN, local clocks tend to lose phase coherence among cells and subsequently exhibit dampened rhythms [21, 22]. The SCN harmonizes and coordinates the local oscillators by continuously communicating with them through various humoral and neural synchronization signals [Fig. 1(b)] [2–5].
Adrenal GCs are one of the most potent humoral links between the SCN and the periphery as demonstrated by their clock-resetting effects on a variety of peripheral oscillators [23, 24] and profound effects on diverse physiological processes [5, 25]. GC signaling is a well-established extracellular stimulus that synchronizes and promotes the cyclic expression of clock gene messenger RNA (mRNA) transcripts in various types of cultured cells [23, 26]. Administration of synthetic GC has also been shown to promote phase shifts of circadian clock gene expression in various peripheral tissues in vivo [23, 27]. Alternatively, accumulating evidence suggests that there are stabilizing effects of GCs on established physiological rhythms in vivo; for example, GCs retard the daytime feeding-induced phase shift of peripheral oscillators [27]. Ablation of the entire adrenal gland or the adrenal clock facilitates re-entrainment of the SCN-driven behavioral rhythm to a shifted light–dark cycle in a zeitgeber time-dependent manner [28, 29]. Furthermore, the circadian GC rhythm may contribute to overt rhythms by directly producing the rhythmic physiological outputs from other tissues in a more direct fashion via either classical or nongenomic GC signaling. This notion is supported by findings that the rhythmic expression of many liver genes is more dependent on the adrenal gland and GC signaling than on the hepatic oscillator [30, 31]. The periodic clock gene expression in certain brain regions also requires rhythmic GC signaling, implying that even cognition and emotion can be influenced by the adrenal rhythm [32–34]. Overall, the circulating GC rhythm has a harmonizing role in the circadian rhythms of physiology, metabolism, and behavior by synchronizing local oscillators and/or directly driving rhythmic expression of wide spectrum of GC-responsive genes in the target tissues.
2. Adrenal Peripheral Clock and Steroid Biosynthesis
The adrenal gland is composed of the cortex and medulla, which differ in terms of cell types and secreted factors [4, 5]. The adrenal medulla contains chromaffin cells, which originate from neuronal ganglionic cells and secrete epinephrine and norepinephrine. In contrast, the adrenal cortex mainly produces steroid hormones and is divided into three separate zones [zona glomerulosa (ZG), zona fasiculata (ZF), and zona reticularis (ZR)]. Different sets of steroidogenic genes are expressed in each zone to produce different kinds of steroids from cholesterol, which is the common precursor of steroids. GCs are mainly produced by ACTH receptor–expressing ZF and ZR cells. Aldosterone, a major mineralocorticoid, and the adrenal androgens are synthesized in steroidogenic cells of the ZG and ZR, respectively.
The adrenal gland has its own functional circadian clockwork that is characterized by a cell-autonomous and self-sustaining nature [8, 10, 35–38]. The adrenal gland clock gene expression appears to be independent of the stress-responsive humoral inputs of the HPA axis [39], but it can be entrained by activation of the autonomic SCN–adrenal pathway [40]. Similar to other peripheral tissues, adrenal gland explant cultures maintain circadian oscillations in the murine Per2 promoter-driven luciferase reporter activities [41, 42]. More importantly, numerous adrenal genes constituting a variety of cellular pathways exhibit cyclic mRNA accumulation throughout the course of the day [36–38, 40]. Microarray analyses of the adrenal gland in two independent studies revealed that ∼4% to 7% of gene transcripts follow circadian oscillations in their expression [37, 38]. Comparison of these two studies, following re-evaluation by equivalent criteria using a nonparametric algorithm (JTK_CYCLE) to detect rhythmic components [43], yielded ~240 common genes that exhibit statistically significant circadian expression in both studies [Fig. 2(a) and 2(b); see Supplemental Table 1 for the full list of common rhythmic genes]. Many of the common RNA species with circadian expression are adrenal gland–enriched genes [Fig. 2(c)], implying possible crosstalk between circadian clockwork and cell type–specific transcriptional regulators in the adrenal gland, similarly to that in other tissues [38, 44, 45]. Subsequent gene enrichment analysis suggests that these circadian gene transcripts are significantly linked with several biological processes, such as circadian rhythm, steroid biosynthesis, concentration of cholesterol, protein folding, and quantity of monoamine [Fig. 2(d); Supplemental Table 2].
Figure 2.
Genome-wide circadian rhythmicity in the adrenal gland. Circadian gene expression profiles in the adrenal gland from two independent microarray studies [37, 38] are compared. Raw data are available from the Gene Expression Omnibus database (accession nos. GSE4238 and GSE54650, respectively). (a) Venn diagram for circadian genes identified using JTK_CYCLE [43]. Rhythmic gene transcripts are defined as follows: adjusted P < 0.05 for rhythmicity and amplitude >10% of mean expression level of a given gene as calculated by the JTK_CYCLE algorithm. (b) Top 30 circadian genes according to P value and their expression profiles are expressed as a heat map. (c) Tissue enrichment analysis of 240 common circadian genes using the BioGPS database (http://biogps.org). The commonly rhythmic genes overlap with 6.12% of the adrenal-enriched genes with statistical significance (P < 0.05 for overlapping). (d) Top 10 biological functions annotated by gene set enrichment analysis using Ingenuity Pathway Analysis (https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/) for the commonly rhythmic genes (upper). A heat map representation for cyclic expression profiles of the genes involved in synthesis of steroid is shown (lower). Raw data from GSE54650 [38] were used to construct heat maps in (b) and (d).
Among adrenal genes involved in steroidogenic processes, steroidogenic acute regulatory protein (StAR) is worthy of being highlighted. Delivery of free cholesterol to the inner mitochondrial membrane, the first and rate-limiting step of GC biosynthesis, is mediated by StAR and its expression and activity are correlated with steroid biosynthesis [46]. Induced StAR expression by tropic hormones such as ACTH enhances adrenal steroidogenesis, whereas defects in StAR expression result in a dramatic decrease in steroid production [46–49]. The diurnal rhythm of adrenal StAR gene expression is quite controversial. Several reports, including our previous studies, demonstrated rhythmic StAR expression in rodent models [10, 35, 50–52]. In contrast, some microarray datasets, including examples in Fig. 2, failed to show significant rhythmicity of adrenal StAR mRNA expression [37, 38, 53]. This discrepancy may originate from the relatively modest rhythmicity in adrenal StAR expression compared with that of canonical clock genes. Nevertheless, the direct link between StAR gene transcription and the circadian molecular clock has been strongly supported by a number of independent studies. Adrenal StAR expression is reduced in mouse models with global or adrenal-specific ablation of BMAL1 expression [9, 10, 41]. In a previous study, we clearly demonstrated that StAR gene expression is transcriptionally regulated by the binding of the CLOCK/BMAL1 heterodimer to the E-box elements on its distal promoter and, more importantly, StAR gene expression mediates molecular clock–evoked steroid production in cultured adrenocortical cells [10]. The transcriptional mechanism of StAR gene expression by clock components appears to be evolutionarily conserved in both avian and mammalian reproductive systems [54, 55], supporting the role of StAR as a key molecular link between the circadian clock and steroidogenic pathway.
3. Integrative Mechanisms Controlling the Circadian GC Rhythm
A robust daily rhythm in the unstressed state and a prompt response to stress are key characteristics of GCs. Recent advances in chronobiology reveal that multiple regulatory mechanisms involved in both SCN central and adrenal-intrinsic clocks cooperate to generate the robust circadian GC rhythm in circulation (Fig. 3).
Figure 3.
Cooperative actions of central and adrenal clocks underlying circadian GC rhythm. The robust daily variations in circulating GC levels are achieved by multiple regulatory mechanisms. The SCN central clock regulates the adrenal rhythm by modulating the HPA axis as well as through autonomic neural inputs [autonomic nervous system (ANS)] into the gland. In addition to the central mechanisms, adrenal-intrinsic mechanisms involving the adrenal local clock also underlie the circadian GC rhythm. The adrenal peripheral clock gates adrenal sensitivity to ACTH and controls the GC biosynthesis through coordinated transcriptional regulation of a subset of adrenal steroidogenic genes.
A. Regulation of the HPA Axis Activity by the SCN Central Clock
Circadian GC secretion has been primarily attributed to the SCN control of the HPA axis [56]. Ablation of the SCN completely eliminates the daily rhythms of both ACTH and GCs in circulation [7, 19]. Notably, SCN graft transplantation into SCN-lesioned hamsters restores circadian rhythmicity in locomotion, but not GC rhythm, indicating that synaptic connectivity may underlie SCN control of the HPA axis [57]. From a neuroanatomical perspective, SCN pacemaker neurons appear to indirectly control ACTH secretagogues by innervating neighboring neurons in the subparaventricular zone and the dorsomedial hypothalamus nucleus, which are in turn connected to the CRH/AVP neurons in the PVN [58, 59]. Given that trough corticosterone levels have been reported to increase after the SCN lesion in rats, the regulation of basal GC release by the SCN seems to be inhibitory [7]. The AVP produced by a subset of SCN neurons is considered one of the main neurotransmitters mediating this inhibition [60, 61]. Another possibility involves the presence of local clockworks in the PVN and pituitary [35, 62]. The local rhythms in ACTH secretagogue-producing neurons in the PVN and pituitary corticotropes may have a certain role in the adrenal GC rhythm.
However, several lines of evidence suggest relatively restricted roles for the upstream hormonal regulators of the HPA axis and the involvement of multiple inputs into the adrenal gland. First, circulating ACTH shows a circadian profile similar to that of circulating GC rhythm but usually has relatively lower amplitude than that of the GC rhythm [10, 63–65]. Furthermore, the circadian rhythm of CRH does not account for rhythms observed in its downstream targets [35, 63, 66], suggesting that additional regulatory activity is required for the robust adrenal rhythm. Second, the rhythmic release of CRH/ACTH may not be essential for circadian GC rhythm; the plasma GC rhythm persists even in hypophysectomized rats with implanted ACTH pellets [67, 68]. Similarly, a constant infusion of CRH was sufficient to rescue diurnal GC rhythm in CRH knockout mice [69]. Also note that even in the absence of SCN inhibitory signals, plasma GC levels did not reach a peak level, and there was an apparent discrepancy in rats between the decrease in inhibitory regulation of the SCN and the afternoon increase in circulating GC levels [7, 70]. Based on a series of disinhibition experiments, Buijs and colleagues [70] suggested a requirement for additional stimulatory signals, with a delay of several hours after the major inhibitory signal but preceding the peak GC level.
B. Regulation of Daily GC Rhythm by the SCN-Autonomic System
Circadian GC rhythm is maintained in hypophysectomized animals with a constant supply of ACTH, whereas adrenal rhythm is completely abolished in hypophysectomized rats after denervation of the adrenal glands, showing the involvement of a neural mechanism [68]. In this regard, several studies suggest the impact of the SCN central pacemaker via splanchnic nerve innervation of the adrenal gland [40, 61, 71–73]. SCN-derived autonomic nervous system signals can be transmitted through preautonomic PVN neurons and sympathetic preganglionic intermediolateral neurons of the spinal cord to reach the adrenal gland [61]. One possible explanation for autonomic control of the daily GC rhythm involves the regulation of adrenal sensitivity to ACTH. The adrenal responsiveness to ACTH follows a diurnal rhythm, with a higher sensitivity during the active phase in rodents [74], and such daily variation depends on an intact SCN and splanchnic innervation of the adrenal glands [71, 72, 75]. Another significant feature of the SCN-driven neural pathway is that sympathetic innervation provides a rapid photic input to the gland, leading to increased GC release in mice during subjective night regardless of the state of HPA axis activation [40]. The light pulses activate the sympathetic nervous system in an SCN-dependent fashion, and adrenal denervation abolishes the light-induced GC release [40, 76]. Release of GCs in response to a brief light pulse occurs in a delayed fashion compared with stress-evoked GC secretion, supporting the involvement of a pathway other than the HPA axis [40], which is independent of GC synthesis and of the local clock in the adrenocortical GC-producing cells [11]. Medullary–cortical signaling pathways have been proposed as mediators of sympathetic nerve-dependent adrenal activation via catecholamines, neuropeptides, and intra-adrenal blood flow [77].
We have recently shown that photic signal-evoked GC secretion is associated with decreased adrenal GC content, suggesting neural mechanisms acutely release the steroids from the stored pool [11]. It has long been thought that steroid hormone secretion is primarily controlled by the regulation of steroidogenesis through concentration gradient–dependent diffusion. However, several studies observed the retention of steroids against a concentration gradient at intracellular sites proximal to the plasma membrane, and they proposed a possible steroid transport mechanism involving organic anion transport [78, 79]. More recently, it has been suggested that acute release of stored GC can be induced by stress, and this release may be mediated by the paracrine actions of prostaglandins and subsequent production of nitric oxide [80]. Ultrastructural analysis revealed more microcytotic vesicles and filopodia-like structures of the cell membrane in adrenocortical cells exposed to stress than in unstressed cells. In activated adrenocortical cells, invaginations in close contact with mitochondria, lipid droplets, and additional microcytotic vesicles are also observed [80]. It is plausible that medullary–cortical interactions and accompanying intracellular signals leading to the rapid release of GC may mediate the SCN–autonomic system-dependent increase in circulating GC levels.
C. Adrenal-Intrinsic Mechanisms: The Role of the Adrenal Local Oscillator
In addition to the SCN-driven central mechanisms, growing evidence suggests the presence of adrenal-intrinsic mechanisms, particularly involving the adrenal local oscillator. The circadian GC rhythm is often more severely attenuated than other rhythmic physiology and behavior patterns in clock gene-deficient mice. Mice bearing a defective Per1 or Per2 allele exhibit normal daily cycles in locomotor activity under light–dark conditions but significantly altered daily GC rhythms [81, 82]. Restricted daytime feeding of nocturnal rodents supports the importance of the adrenal clock. The daytime feeding regimen is known to dissociate the phases of the SCN central pacemaker and other peripheral oscillators, including the adrenal clock, presumably by the action of food-entrainable oscillators in the dorsomedial hypothalamus [11, 83, 84]. Under this condition, the daily GC profile has two distinct peaks per day, strongly suggesting that the circadian GC rhythm is produced by the integrated activity of feeding-independent SCN central clock and food-entrainable local oscillators [11, 85, 86].
Based on the rhythmic expression of genes controlling ACTH receptor signaling and evidence obtained from well-designed transplantation experiments, Oster et al. [8, 37] proposed that the adrenal clock is involved in the circadian GC rhythm by gating adrenal sensitivity to ACTH. Splanchnic innervation entrains the adrenal local clock, and the autonomic SCN–adrenal pathway is involved in the daily variation in adrenal receptivity to ACTH [40, 59], suggesting that the adrenal clock might link the SCN neural inputs to the rhythmicity of the gland’s responsiveness. However, there remains the important issue of paracrine interaction between the adrenal cortex and medulla [77]. There is a need to clarify which peripheral clock (in the adrenal cortex or medulla) plays the key role in modulating adrenal sensitivity to ACTH. Studies with mutant mice models provide some insight into this issue. Adrenal GC secretion in response to ACTH is drastically impaired in global Bmal1-null mice [9]. However, stress-evoked expression of ACTH and secretion of corticosterone are not significantly attenuated in mice models with impaired Bmal1 expression in adrenocortical steroidogenic cells [10, 41]. These findings collectively indicate that the local clock in GC-producing cells may have a marginal role in controlling adrenal responsiveness to the tropic hormone.
Several lines of evidence imply a more direct role of the adrenocortical clock in the circadian regulation of GC production. For example, pioneering studies demonstrated the rhythmic nature of adrenal GC biosynthesis and secretion in isolated adrenal explants without any external cues [87, 88]. We also reported that cyclic steroid production from a cultured adrenocortical cell line was induced by a brief serum treatment, which evoked and synchronized clock gene oscillations in these cells [10]. Several rhythmic steroidogenic genes controlled by adrenal local clock, such as Star, Stard4, Ldlr, and Por, strongly suggest an adrenal-autonomous mechanism involving intrinsic GC biosynthesis [9, 10, 37]. Indeed, the levels of both adrenal and circulating GC display robust daily oscillations with similar phases to each other in vivo [10].
Our previous studies to dissect the adrenal and circulating levels of GC have unraveled important roles of the adrenal local clock in circadian GC rhythm. Suppression of BMAL1 expression selectively in the ACTH receptor–expressing adrenocortical cells in transgenic mice flattens the daily oscillation in adrenal GC content, whereas attenuated, but significant, circadian rhythm of GC in circulation persists [10]. Interestingly, daytime-restricted feeding that shifts the phase of the adrenal clock from the SCN in nocturnal mice produces two split peaks of circulating GC levels as mentioned above, but only shifts the peak in adrenal GC content to phases similar to those of adrenal clock genes, which is in accord with the daytime feeding-specific GC peak in circulation [11]. More importantly, the daytime feeding-specific peaks in adrenal and plasma GC can be produced even in SCN-ablated mice. This result supports adrenal local clock- and steroidogenesis-dependent regulation [11]. Nevertheless, the robust oscillation of the adrenal peripheral clock is primarily maintained by synchronizing actions of the SCN-autonomic system [40]. Furthermore, it took several days in constant darkness for the adrenal clock–suppressed mice to exhibit attenuated GC rhythms, indicating that environmental cycles may be capable of compensating defects in the adrenal peripheral clock [10]. It is therefore reasonable that under normal 24-hour light–dark cycle conditions, rhythmic GC production by the adrenal local oscillator has a supporting role, which contributes to the robust daily rhythm in the circulating GC levels by maintaining a releasable pool of the steroid hormone in a circadian fashion.
4. Deregulation of Circadian GC Rhythm Related to Various Human Diseases
Dysregulated GC secretion is related to various pathological conditions due to its impacts on a wide range of biological processes [4, 5]. Abnormalities in circadian GC rhythms are associated with human diseases, including Cushing syndrome, adrenal insufficiency, metabolic and cardiovascular diseases, and neuropsychiatric disorders [89–91]. It is often not clear whether the disrupted GC rhythm is causal to or a consequence of these diseases. Nevertheless, the importance of the GC rhythm has been well recognized in the course of therapeutic applications. For example, a tonic application with a constant dose of GC in the treatment of adrenal insufficiency does not effectively alleviate symptoms as expected, but often exacerbates cardiovascular, metabolic, and psychiatric disturbances [92]. In this section, we focus on several human diseases closely related to dysregulation of circadian GC rhythm.
A. Cushing Syndrome and Adrenal Insufficiency
Disrupted circadian rhythmicity in circulating GC is frequently found in pathological conditions associated with either GC excess or insufficiency. Cushing syndrome is a prevalent form of hypercortisolism that can be caused by either an ACTH-dependent or -independent mechanism. The long-term consequences of severe hypercortisolism include diabetes mellitus, hypertension, dyslipidemia, osteoporosis, bone fractures, recurrent infections, sleep disorder, and psychiatric abnormalities, and even mild hypercortisolism has harmful effects on long-term health resulting from decreased insulin sensitivity and altered glucose tolerance [89, 93, 94]. Cushing syndrome patients tend to exhibit increased trough levels, an altered diurnal pattern, and an increase in circulating levels of cortisol [95]. In this context, late-night salivary cortisol measurement has been proposed as a diagnostic test for Cushing syndrome [89, 96].
The opposite condition, adrenal insufficiency, can arise from Addison disease, congenital adrenal hyperplasia with defective adrenal steroidogenesis, or certain pituitary diseases. Adrenal insufficiency is commonly characterized by vulnerability to stress, hypoglycemia, hypotension, hyperactivity in the immune/inflammatory systems, and some psychiatric symptoms [97, 98]. Although rhythmic levels of circulating cortisol are often observed in patients with adrenal insufficiency, the amplitude of the rhythms is highly attenuated by the reduction in peak GC levels [92, 98]. Both hypercortisolism and hypocortisolism are linked with disruptions of the circadian timing system, such as sleep disturbance, fatigue in the active period, affective disorders, and cognitive defects [90, 91, 99]. These common features strongly suggest that an attenuated circadian GC rhythm by itself may mediate some pathological outcomes of GC excess or insufficiency.
B. Environmental Disruption of Circadian GC Rhythm
Prolonged disturbance of intrinsic circadian rhythms by environmental factors, such as shift work, jet lag, sleep disturbance, and mistimed eating, evokes a misalignment of internal circadian clocks and external time and can have hazardous health consequences. For example, shift work is known to have long-term effects on the likelihood of onset of obesity, insulin resistance, hyperlipidemia, ischemic heart disease, and even cancer [100, 101]. Chronic jet lag may influence cognitive impairment associated with reduced temporal lobe activity of the brain [102]. It is noteworthy that the symptoms related to such chronic circadian misalignment share many features with those of human diseases associated with GC dysregulation [90, 91, 97]. Indeed, chronic disturbance of circadian rhythms results in an attenuated GC rhythm, particularly by elevating the nadir levels in both humans and rodents [103, 104], and increased cortisol levels are closely related to cognitive deficits in human subjects repeatedly exposed to jet lag [102]. It is therefore plausible that chronic disruption of circadian GC rhythm impacts the capacity to adapt to environmental disruption of the circadian system, thereby mediating pathological consequences.
C. Circadian Rhythms in the Adrenal Gland and Metabolic Diseases
One of the most important functions of circadian GC secretion appears to be a proactive regulation of energy balance for the activity phase. In agreement with this notion, an increase in the GC level usually precedes the beginning of the activity period in both diurnal and nocturnal species. Elevated circulating GC levels stimulate metabolic processes to supply an immediately available energy source [4, 5]. The importance of GCs in maintaining metabolic functions is strongly supported by symptoms of pathological GC excess and insufficiency as described above. Additionally, both experimental and clinical evidence implies that attenuated circadian GC rhythms are linked with metabolic disorders such as obesity, type 2 diabetes, dyslipidemia, and atherosclerosis [90, 105–107]. The findings strongly suggest that dampening of the GC rhythm may have a key role in the onset or progression of metabolic dysfunctions presumably by impacting metabolic tissues such as liver, skeletal muscle, adipocytes, and pancreas.
The coordinated control of hepatic carbohydrate metabolism by GC signaling and the hepatic clock has been demonstrated. Genome-wide gene expression studies clearly show that a number of the hepatic genes involved in carbohydrate metabolism exhibit diurnal variation in their expression [30, 31, 38]. Interestingly, liver-specific Bmal1-ablated mice exhibit metabolic abnormalities distinct from those of global Bmal1−/− mice, suggesting that hepatic metabolism is under the control of multiple regulatory mechanisms involving the hepatic local clockwork as well as the systemic inputs [108]. Rhythmic expression of some hepatic genes with pivotal roles in metabolic functions, such as phosphoenolpyruvate carboxykinase 1 and carnitine palmitoyltransferase 1, is more dependent on an intact adrenal gland and/or GC signaling than on the peripheral circadian oscillator [30, 31]. Similarly, a large number of genes with circadian expression in muscular tissue were also identified as GC-responsive genes [109]. Furthermore, cyclic expression of canonical circadian clock genes in visceral adipose tissue is drastically attenuated in adrenalectomized rats, suggesting that normal molecular rhythms in adipocytes are highly dependent on circulating GC rhythm [110]. It can thus be postulated that the circulating GC rhythm may contribute to the circadian control of metabolic processes by acting on metabolic tissues either directly or indirectly through local clockworks in those tissues.
D. Circadian GC Rhythm and Psychiatric Illness
The brain is also a target of circadian GC signaling, and dysfunctions of both the circadian system and adrenal GC secretion are reported to be key risk factors for the onset of various psychiatric illnesses such as sleep disturbances, affective disorders, and cognitive impairment [42, 111]. Hyperactivity of the HPA axis by impaired negative feedback regulation is one of the well-established characteristics of patients suffering from major depressive disorder. Major depressive disorder patients usually exhibit higher circulating cortisol levels with reduced rhythm amplitude [112]. Hypercortisolism in depressed patients appears to be causal to some symptoms as indicated by a report that the unipolar depression is found in 50% to 80% of Cushing disease patients [94]. Hypocortisolism and reinforced negative feedback in the HPA axis are also associated with other forms of depression and anxiety-related disorders such as posttraumatic stress disorder [113] and chronic fatigue syndrome (CFS) [114]. Considering crucial roles of adrenal GC in processing aversive memory, dysregulated GC secretion during formation or extinction of conditioned fear could be linked with the onset and/or symptoms of posttraumatic stress disorder [115, 116]. Although it is still debatable whether disturbed HPA function is a cause or a consequence of CFS, some phenotypes of our transgenic mice with dampened GC rhythms due to adrenal-specific abolition of BMAL1 expression are comparable to symptoms observed in CFS patients [117]; that is, they exhibited hypolocomotion but a normal temperature rhythm during the activity period [10]. Alternatively, the altered circadian phase of the GC rhythm is often linked with other forms of psychiatric disorders, such as seasonal affective disorder, bipolar disorder, and schizophrenia. For example, phase-delayed cortisol and body temperature rhythms are found in seasonal affective disorder patients [118], whereas phase advances in salivary cortisol profiles as well as clock gene expression in oral mucosa are accompanied by the onset of manic episode of bipolar disorder [119]. Taken together, these findings suggest the importance of circadian GC rhythm in proper control of emotional and cognitive functions, presumably by interactions with circadian rhythms in neurotransmitter and neuromodulator systems in the brain.
5. Conclusion
In conclusion, the current understanding of the circadian control of the adrenal GC and human diseases related to disruptions of this temporal regulation indicate that GC secretion and biosynthesis are tightly controlled by coincident multimodal mechanisms. The central rhythm of the SCN directly drives the circadian GC rhythm by modulating the HPA axis and sympathetic innervation of the adrenal gland. The oscillating adrenal clock plays additional roles in maintaining the rhythm by controlling the adrenal capacity for steroidogenesis and the responsiveness to ACTH. The next question regarding circadian GC rhythm may be its relevance in physiology and pathophysiology. Although the importance of rhythmic GC profiles has been well recognized for a long time, the precise roles and modes of actions of the rhythm, particularly in human health and diseases, still remain to be further elucidated. Recent advances in our knowledge of circadian GC rhythm may provide valuable insights into its clinical applications for prognostic, diagnostic, and therapeutic purposes.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the Ministry of Science and ICT and the Ministry of Education through the National Research Foundation of Korea (Grants NRF-2015M3A9E7029176 and NRF-2016M3C7A1904340 to G.H.S., NRF-2014R1A6A3A04054863 to S.C., and NRF-20171A2A1A05001351 to K.K.). G.H.S. and K.K. were supported by a Korea University research grant and Daegu Gyeongbuk Institute of Science and Technology start-up research fund (20180136), respectively.
Disclosure Summary:
The authors have nothing to disclose.
Glossary
Abbreviations:
- ACTH
adrenocorticotropic hormone
- AVP
arginine vasopressin
- BMAL1
brain muscle aryl hydrocarbon receptor nuclear translocator-like
- CFS
chronic fatigue syndrome
- CLOCK
circadian locomotor output cycle kaput
- CRH
corticotropin-releasing hormone
- CRY
cryptochrome
- GC
glucocorticoid
- HPA
hypothalamic–pituitary–adrenal
- mRNA
messenger RNA
- PER
period
- PVN
paraventricular nucleus
- ROR
retinoic acid receptor-related orphan nuclear receptor
- SCN
suprachiasmatic nucleus
- StAR
steroidogenic acute regulatory protein
- ZF
zona fasiculata
- ZG
zona glomerulosa
- ZR
zona reticularis
Contributor Information
Gi Hoon Son, Email: songh@korea.ac.kr.
Kyungjin Kim, Email: kyungjin@dgist.ac.kr.
References and Notes
- 1. Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8(10):790–802. [DOI] [PubMed] [Google Scholar]
- 2. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72(1):517–549. [DOI] [PubMed] [Google Scholar]
- 3. Son GH, Chung S, Kim K. The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front Neuroendocrinol. 2011;32(4):451–465. [DOI] [PubMed] [Google Scholar]
- 4. Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta. 2011;1812(5):581–591. [DOI] [PubMed] [Google Scholar]
- 5. Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. 2017;38(1):3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–206. [DOI] [PubMed] [Google Scholar]
- 7. Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264(6 Pt 2):R1186–R1192. [DOI] [PubMed] [Google Scholar]
- 8. Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4(2):163–173. [DOI] [PubMed] [Google Scholar]
- 9. Leliavski A, Shostak A, Husse J, Oster H. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology. 2014;155(1):133–142. [DOI] [PubMed] [Google Scholar]
- 10. Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, Lee HW, Choi S, Sun W, Kim H, Cho S, Lee KH, Kim K. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci USA. 2008;105(52):20970–20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung S, Lee EJ, Cha HK, Kim J, Kim D, Son GH, Kim K. Cooperative roles of the suprachiasmatic nucleus central clock and the adrenal clock in controlling circadian glucocorticoid rhythm. Sci Rep. 2017;7:46404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280(5369):1564–1569. [DOI] [PubMed] [Google Scholar]
- 13. Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix–loop–helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA. 1998;95(10):5474–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98(2):193–205. [DOI] [PubMed] [Google Scholar]
- 15. Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol. 2000;20(17):6269–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S. A transcription factor response element for gene expression during circadian night. Nature. 2002;418(6897):534–539. [DOI] [PubMed] [Google Scholar]
- 17. Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–260. [DOI] [PubMed] [Google Scholar]
- 18. Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43(4):527–537. [DOI] [PubMed] [Google Scholar]
- 19. Abe K, Kroning J, Greer MA, Critchlow V. Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology. 1979;29(2):119–131. [DOI] [PubMed] [Google Scholar]
- 20. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. [DOI] [PubMed] [Google Scholar]
- 21. Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119(5):693–705. [DOI] [PubMed] [Google Scholar]
- 22. Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14(24):2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. [DOI] [PubMed] [Google Scholar]
- 24. Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21(6):494–506. [DOI] [PubMed] [Google Scholar]
- 25. Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol. 2006;147(Suppl 1):S258–S268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Izumo M, Sato TR, Straume M, Johnson CH. Quantitative analyses of circadian gene expression in mammalian cell cultures. PLOS Comput Biol. 2006;2(10):e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sujino M, Furukawa K, Koinuma S, Fujioka A, Nagano M, Iigo M, Shigeyoshi Y. Differential entrainment of peripheral clocks in the rat by glucocorticoid and feeding. Endocrinology. 2012;153(5):2277–2286. [DOI] [PubMed] [Google Scholar]
- 28. Sage D, Ganem J, Guillaumond F, Laforge-Anglade G, François-Bellan AM, Bosler O, Becquet D. Influence of the corticosterone rhythm on photic entrainment of locomotor activity in rats. J Biol Rhythms. 2004;19(2):144–156. [DOI] [PubMed] [Google Scholar]
- 29. Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120(7):2600–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oishi K, Amagai N, Shirai H, Kadota K, Ohkura N, Ishida N. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res. 2005;12(3):191–202. [DOI] [PubMed] [Google Scholar]
- 31. Reddy AB, Maywood ES, Karp NA, King VM, Inoue Y, Gonzalez FJ, Lilley KS, Kyriacou CP, Hastings MH. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45(6):1478–1488. [DOI] [PubMed] [Google Scholar]
- 32. Malek ZS, Sage D, Pévet P, Raison S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology. 2007;148(11):5165–5172. [DOI] [PubMed] [Google Scholar]
- 33. Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA. 2005;102(11):4180–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gilhooley MJ, Pinnock SB, Herbert J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: implications for neurogenesis. Neurosci Lett. 2011;489(3):177–181. [DOI] [PubMed] [Google Scholar]
- 35. Girotti M, Weinberg MS, Spencer RL. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab. 2009;296(4):E888–E897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lemos DR, Downs JL, Urbanski HF. Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol Endocrinol. 2006;20(5):1164–1176. [DOI] [PubMed] [Google Scholar]
- 37. Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms. 2006;21(5):350–361. [DOI] [PubMed] [Google Scholar]
- 38. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fahrenkrug J, Hannibal J, Georg B. Diurnal rhythmicity of the canonical clock genes Per1, Per2 and Bmal1 in the rat adrenal gland is unaltered after hypophysectomy. J Neuroendocrinol. 2008;20(3):323–329. [DOI] [PubMed] [Google Scholar]
- 40. Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2(5):297–307. [DOI] [PubMed] [Google Scholar]
- 41. Dumbell R, Leliavski A, Matveeva O, Blaum C, Tsang AH, Oster H. Dissociation of molecular and endocrine circadian rhythms in male mice lacking Bmal1 in the adrenal cortex. Endocrinology. 2016;157(11):4222–4233. [DOI] [PubMed] [Google Scholar]
- 42. Leliavski A, Dumbell R, Ott V, Oster H. Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J Biol Rhythms. 2015;30(1):20–34. [DOI] [PubMed] [Google Scholar]
- 43. Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms. 2010;25(5):372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, Kim KS, Dluzen DE, Lee I, Hwang O, Son GH, Kim K. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell. 2014;157(4):858–868. [DOI] [PubMed] [Google Scholar]
- 45. Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, Armour SM, Remsberg JR, Jager J, Soccio RE, Steger DJ, Lazar MA. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science. 2015;348(6242):1488–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod. 2009;15(6):321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spiga F, Liu Y, Aguilera G, Lightman SL. Temporal effect of adrenocorticotrophic hormone on adrenal glucocorticoid steroidogenesis: involvement of the transducer of regulated cyclic AMP-response element-binding protein activity. J Neuroendocrinol. 2011;23(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishii T, Hasegawa T, Pai CI, Yvgi-Ohana N, Timberg R, Zhao L, Majdic G, Chung BC, Orly J, Parker KL. The roles of circulating high-density lipoproteins and trophic hormones in the phenotype of knockout mice lacking the steroidogenic acute regulatory protein. Mol Endocrinol. 2002;16(10):2297–2309. [DOI] [PubMed] [Google Scholar]
- 49. Zaidi SK, Shen WJ, Bittner S, Bittner A, McLean MP, Han J, Davis RJ, Kraemer FB, Azhar S. p38 MAPK regulates steroidogenesis through transcriptional repression of STAR gene. J Mol Endocrinol. 2014;53(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kil IS, Lee SK, Ryu KW, Woo HA, Hu MC, Bae SH, Rhee SG. Feedback control of adrenal steroidogenesis via H2O2-dependent, reversible inactivation of peroxiredoxin III in mitochondria. Mol Cell. 2012;46(5):584–594. [DOI] [PubMed] [Google Scholar]
- 51. Fahrenkrug J, Georg B, Hannibal J, Jørgensen HL. Altered rhythm of adrenal clock genes, StAR and serum corticosterone in VIP receptor 2-deficient mice. J Mol Neurosci. 2012;48(3):584–596. [DOI] [PubMed] [Google Scholar]
- 52. Park SY, Walker JJ, Johnson NW, Zhao Z, Lightman SL, Spiga F. Constant light disrupts the circadian rhythm of steroidogenic proteins in the rat adrenal gland. Mol Cell Endocrinol. 2013;371(1-2):114–123. [DOI] [PubMed] [Google Scholar]
- 53. Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock–deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16(1):67–74. [DOI] [PubMed] [Google Scholar]
- 54. Nakao N, Yasuo S, Nishimura A, Yamamura T, Watanabe T, Anraku T, Okano T, Fukada Y, Sharp PJ, Ebihara S, Yoshimura T. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology. 2007;148(7):3031–3038. [DOI] [PubMed] [Google Scholar]
- 55. Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2(7):521–526. [DOI] [PubMed] [Google Scholar]
- 57. Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140(1):207–218. [DOI] [PubMed] [Google Scholar]
- 58. Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464(1):36–48. [DOI] [PubMed] [Google Scholar]
- 59. Engeland WC, Arnhold MM. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine. 2005;28(3):325–332. [DOI] [PubMed] [Google Scholar]
- 60. Kalsbeek A, Buijs RM, van Heerikhuize JJ, Arts M, van der Woude TP. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992;580(1–2):62–67. [DOI] [PubMed] [Google Scholar]
- 61. Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11(5):1535–1544. [DOI] [PubMed] [Google Scholar]
- 62. Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, Yoo OJ, Yamazaki S, Lee C, Takahashi JS. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci USA. 2005;102(7):2608–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140(9):4015–4023. [DOI] [PubMed] [Google Scholar]
- 64. Dalm S, Enthoven L, Meijer OC, van der Mark MH, Karssen AM, de Kloet ER, Oitzl MS. Age-related changes in hypothalamic-pituitary-adrenal axis activity of male C57BL/6J mice. Neuroendocrinology. 2005;81(6):372–380. [DOI] [PubMed] [Google Scholar]
- 65. Chen A, Zorrilla E, Smith S, Rousso D, Levy C, Vaughan J, Donaldson C, Roberts A, Lee KF, Vale W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus–pituitary–adrenal axis and depressive-like behavior. J Neurosci. 2006;26(20):5500–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Watts AG, Tanimura S, Sanchez-Watts G. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinology. 2004;145(2):529–540. [DOI] [PubMed] [Google Scholar]
- 67. Meier AH. Daily variation in concentration of plasma corticosteroid in hypophysectomized rats. Endocrinology. 1976;98(6):1475–1479. [DOI] [PubMed] [Google Scholar]
- 68. Ottenweller JE, Meier AH. Adrenal innervation may be an extrapituitary mechanism able to regulate adrenocortical rhythmicity in rats. Endocrinology. 1982;111(4):1334–1338. [DOI] [PubMed] [Google Scholar]
- 69. Muglia LJ, Jacobson L, Weninger SC, Luedke CE, Bae DS, Jeong KH, Majzoub JA. Impaired diurnal adrenal rhythmicity restored by constant infusion of corticotropin-releasing hormone in corticotropin-releasing hormone-deficient mice. J Clin Invest. 1997;99(12):2923–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo–pituitary–adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16(17):5555–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jasper MS, Engeland WC. Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am J Physiol. 1997;273(2 Pt 1):E363–E368. [DOI] [PubMed] [Google Scholar]
- 72. Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R1128–R1135. [DOI] [PubMed] [Google Scholar]
- 73. Wotus C, Lilley TR, Neal AS, Suleiman NL, Schmuck SC, Smarr BL, Fischer BJ, de la Iglesia HO. Forced desynchrony reveals independent contributions of suprachiasmatic oscillators to the daily plasma corticosterone rhythm in male rats. PLoS One. 2013;8(7):e68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaneko M, Kaneko K, Shinsako J, Dallman MF. Adrenal sensitivity to adrenocorticotropin varies diurnally. Endocrinology. 1981;109(1):70–75. [DOI] [PubMed] [Google Scholar]
- 75. Sage D, Maurel D, Bosler O. Corticosterone-dependent driving influence of the suprachiasmatic nucleus on adrenal sensitivity to ACTH. Am J Physiol Endocrinol Metab. 2002;282(2):E458–E465. [DOI] [PubMed] [Google Scholar]
- 76. Niijima A, Nagai K, Nagai N, Akagawa H. Effects of light stimulation on the activity of the autonomic nerves in anesthetized rats. Physiol Behav. 1993;54(3):555–561. [DOI] [PubMed] [Google Scholar]
- 77. Engeland WC. Sensitization of endocrine organs to anterior pituitary hormones by the autonomic nervous system. Handb Clin Neurol. 2013;117:37–44. [DOI] [PubMed] [Google Scholar]
- 78. Steffgen J, Rohrbach S, Beery E, Ersoy D, Jarry H, Metten M, Bornstein SR, Müller GA, Burckhardt G. Demonstration of a probenecid-inhibitable anion exchanger involved in the release of cortisol and cAMP and in the uptake of p-aminohippurate in bovine adrenocortical cells. Cell Physiol Biochem. 1999;9(2):72–80. [DOI] [PubMed] [Google Scholar]
- 79. Beéry E, Middel P, Bahn A, Willenberg HS, Hagos Y, Koepsell H, Bornstein SR, Müller GA, Burckhardt G, Steffgen J. Molecular evidence of organic ion transporters in the rat adrenal cortex with adrenocorticotropin-regulated zonal expression. Endocrinology. 2003;144(10):4519–4526. [DOI] [PubMed] [Google Scholar]
- 80. Mohn CE, Fernandez-Solari J, De Laurentiis A, Prestifilippo JP, de la Cal C, Funk R, Bornstein SR, McCann SM, Rettori V. The rapid release of corticosterone from the adrenal induced by ACTH is mediated by nitric oxide acting by prostaglandin E2. Proc Natl Acad Sci USA. 2005;102(17):6213–6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dallmann R, Touma C, Palme R, Albrecht U, Steinlechner S. Impaired daily glucocorticoid rhythm in Per1Brd mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192(7):769–775. [DOI] [PubMed] [Google Scholar]
- 82. Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150(5):2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320(5879):1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Holmes MC, French KL, Seckl JR. Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids. J Neurosci. 1997;17(11):4056–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Le Minh N, Damiola F, Tronche F, Schütz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20(24):7128–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Andrews RV, Folk GE Jr. Circadian metabolic patterns in cultured hamster adrenal glands. Comp Biochem Physiol. 1964;11(4):393–409. [DOI] [PubMed] [Google Scholar]
- 88. Lehoux JG, Lefebvre A. De novo synthesis of corticosteroids in hamster adrenal glands. J Steroid Biochem. 1980;12:479–485. [DOI] [PubMed] [Google Scholar]
- 89. Carroll T, Raff H, Findling JW. Late-night salivary cortisol measurement in the diagnosis of Cushing’s syndrome. Nat Clin Pract Endocrinol Metab. 2008;4(6):344–350. [DOI] [PubMed] [Google Scholar]
- 90. Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci. 2006;1083(1):111–128. [DOI] [PubMed] [Google Scholar]
- 91. Landgraf D, McCarthy MJ, Welsh DK. Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Curr Psychiatry Rep. 2014;16(10):483. [DOI] [PubMed] [Google Scholar]
- 92. Kalafatakis K, Russell GM, Zarros A, Lightman SL. Temporal control of glucocorticoid neurodynamics and its relevance for brain homeostasis, neuropathology and glucocorticoid-based therapeutics. Neurosci Biobehav Rev. 2016;61:12–25. [DOI] [PubMed] [Google Scholar]
- 93. Lindholm J, Juul S, Jørgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jørgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J. Incidence and late prognosis of Cushing’s syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86(1):117–123. [DOI] [PubMed] [Google Scholar]
- 94. Pivonello R, Simeoli C, De Martino MC, Cozzolino A, De Leo M, Iacuaniello D, Pivonello C, Negri M, Pellecchia MT, Iasevoli F, Colao A. Neuropsychiatric disorders in Cushing’s syndrome. Front Neurosci. 2015;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van den Berg G, Frölich M, Veldhuis JD, Roelfsema F. Combined amplification of the pulsatile and basal modes of adrenocorticotropin and cortisol secretion in patients with Cushing’s disease: evidence for decreased responsiveness of the adrenal glands. J Clin Endocrinol Metab. 1995;80(12):3750–3757. [DOI] [PubMed] [Google Scholar]
- 96. Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83(8):2681–2686. [DOI] [PubMed] [Google Scholar]
- 97. Anglin RE, Rosebush PI, Mazurek MF. The neuropsychiatric profile of Addison’s disease: revisiting a forgotten phenomenon. J Neuropsychiatry Clin Neurosci. 2006;18(4):450–459. [DOI] [PubMed] [Google Scholar]
- 98. Giebels V, Repping-Wuts H, Bleijenberg G, Kroese JM, Stikkelbroeck N, Hermus A. Severe fatigue in patients with adrenal insufficiency: physical, psychosocial and endocrine determinants. J Endocrinol Invest. 2014;37(3):293–301. [DOI] [PubMed] [Google Scholar]
- 99. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids. Mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179(1):19–40. [DOI] [PubMed] [Google Scholar]
- 100. Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17(4):489–500. [DOI] [PubMed] [Google Scholar]
- 101. Faraut B, Bayon V, Léger D. Neuroendocrine, immune and oxidative stress in shift workers. Sleep Med Rev. 2013;17(6):433–444. [DOI] [PubMed] [Google Scholar]
- 102. Cho K. Chronic “jet lag” produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4(6):567–568. [DOI] [PubMed] [Google Scholar]
- 103. Touitou Y, Motohashi Y, Reinberg A, Touitou C, Bourdeleau P, Bogdan A, Auzéby A. Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. Eur J Appl Physiol Occup Physiol. 1990;60(4):288–292. [DOI] [PubMed] [Google Scholar]
- 104. Salgado-Delgado R, Angeles-Castellanos M, Buijs MR, Escobar C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience. 2008;154(3):922–931. [DOI] [PubMed] [Google Scholar]
- 105. Lederbogen F, Hummel J, Fademrecht C, Krumm B, Kühner C, Deuschle M, Ladwig KH, Meisinger C, Wichmann HE, Lutz H, Breivogel B. Flattened circadian cortisol rhythm in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119(9):573–575. [DOI] [PubMed] [Google Scholar]
- 106. Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med. 2000;247(2):198–204. [DOI] [PubMed] [Google Scholar]
- 107. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–421. [DOI] [PubMed] [Google Scholar]
- 108. Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105(39):15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Almon RR, Yang E, Lai W, Androulakis IP, Ghimbovschi S, Hoffman EP, Jusko WJ, Dubois DC. Relationships between circadian rhythms and modulation of gene expression by glucocorticoids in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;295(4):R1031–R1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Soták M, Bryndová J, Ergang P, Vagnerová K, Kvapilová P, Vodička M, Pácha J, Sumová A. Peripheral circadian clocks are diversely affected by adrenalectomy. Chronobiol Int. 2016;33(5):520–529. [DOI] [PubMed] [Google Scholar]
- 111. Jagannath A, Peirson SN, Foster RG. Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr Opin Neurobiol. 2013;23(5):888–894. [DOI] [PubMed] [Google Scholar]
- 112. Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol Psychol. 2013;93(1):150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocr Rev. 2003;24(2):236–252. [DOI] [PubMed] [Google Scholar]
- 115. Woodruff ER, Greenwood BN, Chun LE, Fardi S, Hinds LR, Spencer RL. Adrenal-dependent diurnal modulation of conditioned fear extinction learning. Behav Brain Res. 2015;286:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee EJ, Son GH, Chung S, Lee S, Kim J, Choi S, Kim K. Impairment of fear memory consolidation in maternally stressed male mouse offspring: evidence for nongenomic glucocorticoid action on the amygdala. J Neurosci. 2011;31(19):7131–7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hamilos DL, Nutter D, Gershtenson J, Redmond DP, Clementi JD, Schmaling KB, Make BJ, Jones JF. Core body temperature is normal in chronic fatigue syndrome. Biol Psychiatry. 1998;43(4):293–302. [DOI] [PubMed] [Google Scholar]
- 118. Avery DH, Dahl K, Savage MV, Brengelmann GL, Larsen LH, Kenny MA, Eder DN, Vitiello MV, Prinz PN. Circadian temperature and cortisol rhythms during a constant routine are phase-delayed in hypersomnic winter depression. Biol Psychiatry. 1997;41(11):1109–1123. [DOI] [PubMed] [Google Scholar]
- 119. Moon JH, Cho CH, Son GH, Geum D, Chung S, Kim H, Kang SG, Park YM, Yoon HK, Kim L, Jee HJ, An H, Kripke DF, Lee HJ. Advanced circadian phase in mania and delayed circadian phase in mixed mania and depression returned to normal after treatment of bipolar disorder. EBioMedicine. 2016;11:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.