Abstract
Serum levels of fibroblast growth factor 23 (FGF23) markedly increase with renal impairment, with FGF23 levels correlating with the presence of left ventricular hypertrophy (LVH) and mortality in patients with chronic kidney disease (CKD). FGF23 activates calcineurin/nuclear factor of activated T cell (NFAT) signaling and induces hypertrophy in murine cardiomyocytes. X-linked hypophosphatemia (XLH) is characterized by high circulating levels of FGF23 but, in contrast to CKD, is associated with hypophosphatemia. The cardiac effects of high circulating levels of FGF23 in XLH are not well defined. Thus, studies were undertaken to define the cardiac phenotype in the mouse model of XLH (Hyp mice). Echocardiographic and histological analyses demonstrated that Hyp left ventricles (LVs) are smaller than those of wild-type mice. Messenger RNA expression of cardiac hypertrophy markers was not altered in the LV or right ventricle of Hyp mice. However, the Hyp LVs had increased expression of the NFAT target genes NFATc1 and RCAN1. To determine whether phosphate alone can induce markers of hypertrophy, differentiated C2C12 myocytes were treated with phosphate. Phosphate treatment increased expression of cardiac hypertrophy markers, supporting a primary role for phosphate in inducing LVH. Although previous studies showed that increased circulating FGF23 and phosphate levels are associated with LVH, our results demonstrated that in XLH, high circulating levels of FGF23 in the setting of hypophosphatemia do not induce cardiac hypertrophy.
Analyses of hearts in the Hyp mouse model of XLH demonstrated that high serum levels of FGF23 in the setting of hypophosphatemia do not lead to left ventricular hypertrophy.
X-linked hypophosphatemia (XLH) is characterized by a mutation in PHEX, leading to rickets and osteomalacia (1, 2). Affected individuals have increased circulating levels of fibroblast growth factor 23 (FGF23) (2–4), a key regulator of phosphate homeostasis that is made predominantly by osteocytes and osteoblasts in bone (4, 5). It binds to fibroblast growth factor receptors (FGFRs) 1 and 4 with α-Klotho as a cofactor to decrease brush border sodium phosphate transporters 2a and 2c in the renal proximal tubule, suppress vitamin D 1-α-hydroxylase, and stimulate vitamin D 24-hydroxylase. This results in an increase in urinary phosphate excretion, a decrease in 1,25-dihydroxyvitamin D, and hypophosphatemia (6–10).
Cardiac left ventricular hypertrophy (LVH) is characterized by a thickening and/or lengthening of the left ventricle (LV) walls and can lead to congestive heart failure, arrhythmias, myocardial ischemia, and death from acute hemodynamic collapse (11–14). Health conditions such as hypertension and chronic kidney disease (CKD) increase the risk of LVH and its associated cardiovascular comorbidities (15–19). In particular, CKD, which affects approximately 13% of people in the United States, leads to multiple mineral ion and hormone abnormalities and increased risk for all-cause mortality (15, 16, 20–22). Circulating levels of FGF23 markedly increase with worsening renal function in CKD, with studies demonstrating an association between FGF23 levels, mortality, and LVH in patients with CKD (23, 24). FGF23 has also induced LVH in mice as well as activated calcineurin/nuclear factor of activated T cell (NFAT) signaling and increased cell size in cultured cardiomyocytes, suggesting that cardiomyocyte-specific FGF23 actions contribute to the pathogenesis of LVH in CKD (24, 25).
It has not been established whether the high levels of FGF23 in XLH are associated with LVH. Therefore, the current studies were undertaken to determine whether mice with XLH (Hyp) exhibit cardiac hypertrophy and activation of signaling pathways and genes that regulate cardiac remodeling. Distinct from other mouse models with elevated FGF23 levels (24–26), mice with X-linked hypophosphatemia (Hyp mice) do not have high phosphate levels or CKD (1, 27). Therefore, these investigations also addressed the cardiac effects of FGF23 in the absence of hyperphosphatemia.
Materials and Methods
Animal studies
Murine studies were approved by the institutional animal care and use committee and were conducted according to accepted standards of humane animal care. All mice were in the C57BL/6J background. They were maintained in a virus- and parasite-free barrier facility, exposed to a 12-hour light/12-hour dark cycle, weaned on day 18 onto house chow (1% calcium, 0.6% phosphate; 3003219-249; PMI Nutrition International, LLC, St. Louis, MO), and housed up to five mice per cage. Wild-type (WT) and Hyp male mice were examined at 30 weeks of age.
Histology
Hearts dissected from mice were horizontally transected. The superior half of the heart was fixed in 10% formalin and processed for paraffin sectioning. For picro‒Sirius Red staining, sections were rehydrated, stained with hematoxylin, and then immersed in picro‒Sirius Red solution [1% Sirius Red (Sigma, St. Louis, MO) in 1.3% picric acid (Sigma)] for 1 hour. Sections were washed in acidified water and dehydrated in 100% alcohol. LVs and right ventricles (RVs) were isolated from the apex of each heart and frozen at −80°C for RNA analyses.
Noninvasive measurement of systolic blood pressure
Systolic blood pressure (SBP) was measured with a noninvasive blood pressure system (CODA; Kent Scientific, Torrington, CT) in awake WT and Hyp mice as previously described (28). Briefly, the mouse was initially placed in a restrainer (Kent Scientific) for 1 minute and then maintained in the restrainer for longer times to allow it to acclimate to the device, as judged by the absence of agitation. After a few days of these acclimation sessions, the mouse remained comfortable for prolonged periods, and SBP was measured and recorded.
Echocardiography
Transthoracic echocardiography was performed with a commercially available echocardiography system (Vivid 7; GE Medical, Milwaukee, WI) using a 13-MHz linear ultrasonography probe as previously described (29, 30). Anterior wall thickness, posterior wall thickness, LV end-diastolic diameter, LV end-systolic diameter, and fractional shortening were obtained from M-mode tracings at the level of the papillary muscles (30). LV ejection fraction was calculated from the two-dimensional parasternal long-axis view using the prolate ellipsoid method (31).
Cardiac RNA analyses
LVs or RVs were homogenized in Trizol (Thermo Fisher Scientific, Waltham, MA). Total RNA was precipitated using 100% ethanol and purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA was reverse transcribed with PrimeScriptTM (Takara-Clontech, Mountain View, CA), and quantitative real-time polymerase chain reaction (PCR) was performed using the QuantiTect SYBR Green Real-Time PCR Kit (Qiagen) on the DNA Engine Opticon (MJ Research, Waltham, MA). Gene expression was normalized to that of WT for each sample, using the methods of Livak and Schmittgen (32).
Immunohistochemistry and antibody
Phospho–extracellular signal–regulated kinase (ERK) 1/2 (9101; Cell Signaling, Danvers, MA) immunohistochemistry was performed on paraffin sections as previously described (33, 34). The following antibody was used: p44/42 MAP Kinase (Research Resource Identifier: AB_331646; Cell Signaling).
Cell culture
Undifferentiated C2C12 myoblasts (35) were allowed to proliferate in growth medium (20% fetal bovine serum, 5% penicillin-streptomycin in Dulbecco’s modified Eagle medium; Gibco, Waltham, MA). Upon reaching 50% to 60% confluence, they were then maintained in differentiation medium (2% donor equine serum, 5% penicillin-streptomycin, and 1 μM of insulin in Dulbecco’s modified Eagle medium) for 72 hours. Differentiated C2C12 cells were serum starved overnight and treated with 7 mM of sodium sulfate (control) or sodium phosphate for 12 hours. RNA was isolated from treated cells, reverse transcribed, and subjected to real-time quantitative PCR analyses as described.
Statistical Analysis
All data shown are reported as mean ± standard deviation. One-way analysis of variance followed by Fisher least significant difference test was used to analyze significance between all groups. Significance was defined as P < 0.05.
Results
Hyp hearts did not exhibit hypertrophy
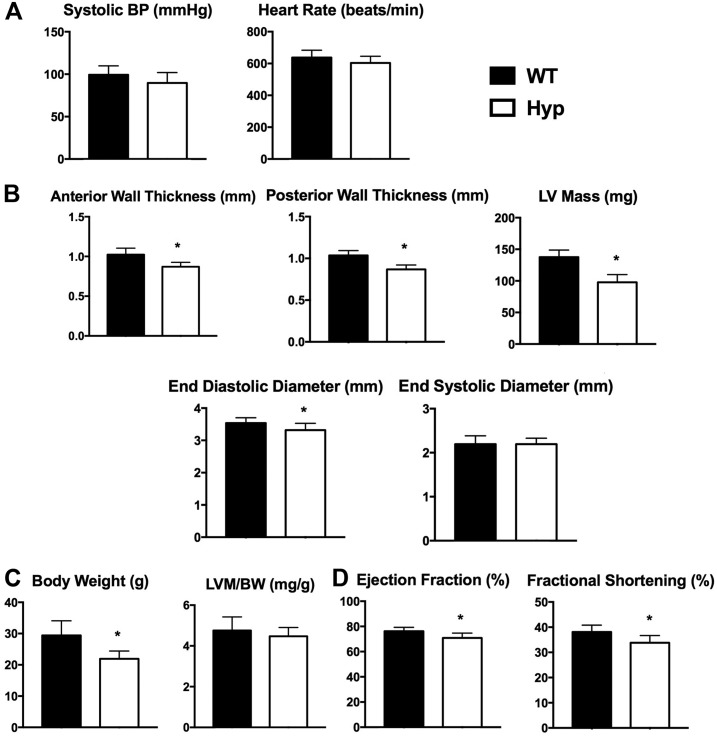
Previous studies demonstrated that FGF23 increased blood pressure by enhancing membrane abundance of the sodium chloride (Na+/Cl−) cotransporter and thus renal sodium reabsorption in the renal distal tubule (36). Although Hyp mice have elevated circulating levels of FGF23, tail cuff measurements did not reveal any alterations in SBP (Fig. 1A). Because FGF23 induces cardiomyocyte hypertrophy (24), transthoracic echocardiography and histological analyses were performed to evaluate whether Hyp hearts exhibit hypertrophic changes. Hyp mice had a normal heart rate as shown by M-mode echocardiograms (Fig. 1A). Transthoracic echocardiography analyses also demonstrated that the LVs of Hyp mice were smaller than those of WT mice, with a significant decrease in anterior and posterior wall thickness, LV mass, and end-diastolic diameter. However, no change in end-systolic diameter was observed (Fig. 1B). Because hypophosphatemia leads to growth retardation and rickets in Hyp mice (37), resulting in significantly decreased body weights (Fig. 1C), LV mass was normalized to body weight. These analyses confirmed the absence of LVH in Hyp mice (Fig. 1C). Ejection fraction and fractional shortening were decreased in Hyp mice (Fig. 1D).
Figure 1.
Hyp hearts were smaller than WT hearts. (A) Systolic blood pressure (BP) measured by noninvasive tail cuff and heart rate measured by echocardiography in 30-week-old mice. Data are representative of those obtained from 10 mice per genotype. (B) Measurements of cardiac parameters in WT and Hyp mice obtained by transthoracic echocardiography (anterior wall thickness, posterior wall thickness, LV mass, end-diastolic diameter, and end-systolic diameter). (C) Body weight (BW) and normalization of LV mass (LVM; quantitated from echocardiography analyses) to BW. (D) Ejection fraction and fractional shortening as measured by echocardiography. *P value <0.05. Data are represented as mean ± standard deviation.
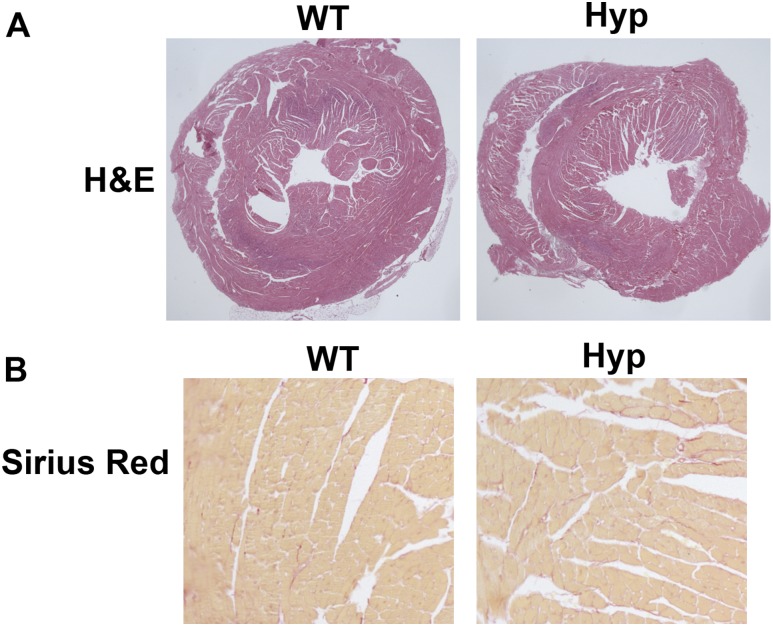
On hematoxylin and eosin staining, neither the RV nor the LV of Hyp hearts appeared different from those of WT hearts (Fig. 2A). Consistent with the lack of hypertrophy, no increase in fibrosis was observed in the Hyp hearts on picro‒Sirius Red stain (Fig. 2B).
Figure 2.
Hyp mice did not have cardiac hypertrophy. (A) Hematoxylin and eosin (H&E) and (B) picro‒Sirius Red stain of cardiac sections of 30-week-old mice. Data are representative of those obtained from five mice per genotype.
Calcineurin signaling was increased in the LV of Hyp mice
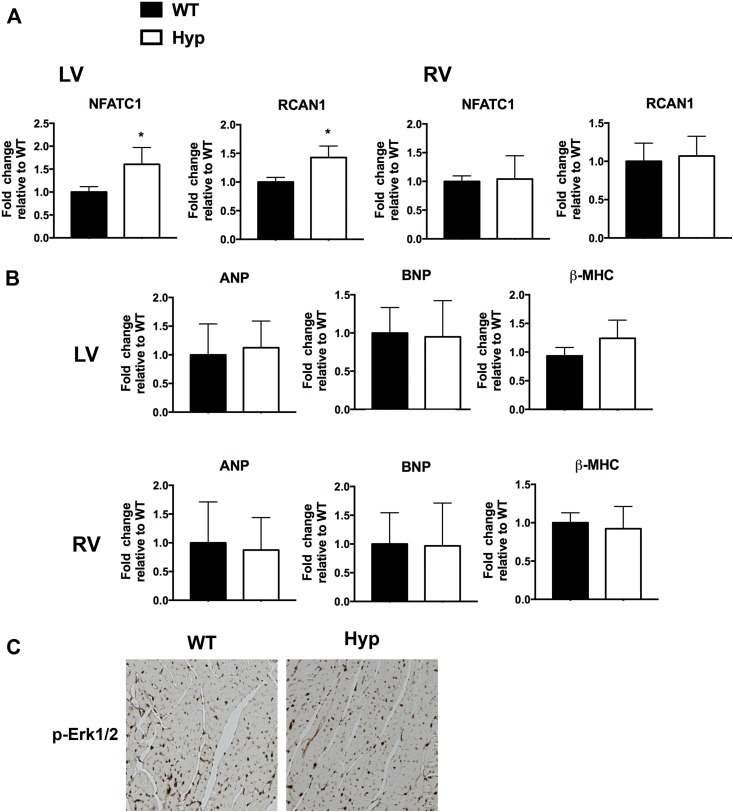
FGF23 activates FGFR4 in cardiomyocytes to induce calcineurin/NFAT signaling, leading to induction of genes that regulate cardiac remodeling and myocyte hypertrophy (24, 25). Therefore, the messenger RNA expression of the NFAT target genes NFATc1 and RCAN1 (38, 39) was quantitated in the LVs and RVs of WT and Hyp hearts. Although RV gene expression was unchanged in Hyp mice, LV gene expression of NFATc1 and RCAN1 was significantly increased (Fig. 3A). Consistent with the lack of hypertrophy on echocardiographic and histological analyses, LV and RV expression levels of markers of cardiac hypertrophy, including atrial natriuretic peptide, brain natriuretic peptide, and β-myosin heavy chain, were similar in Hyp and WT mice (Fig. 3B) (40, 41).
Figure 3.
Hyp LVs had increased expression of calcineurin/NFAT targets. (A) Messenger RNA (mRNA) expression of NFATc1 and RCAN1 in WT and Hyp LVs and RVs. (B) mRNA expression of markers of cardiac hypertrophy (ANP, BNP, β-MHC) in WT and Hyp LVs and RVs. Gene expression is normalized to that of WT. Data are representative of those obtained from five mice per genotype. *P value <0.05. Data are represented as mean ± standard deviation. (C) Immunohistochemistry for p-ERK1/2 in WT and Hyp LVs. ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; p-ERK1/2, phospho–extracellular signal–regulated kinase 1/2; β-MHC, β-myosin heavy chain.
FGFR signaling has been shown to activate the mitogen-activated protein kinase (MAPK) signaling cascade in cardiomyocytes, leading to phosphorylation of ERK1/2 and activation of genes that promote cardiac hypertrophy (24). Despite the increased circulating levels of FGF23 in Hyp mice, Hyp cardiomyocytes did not exhibit increased phospho-ERK1/2 immunoreactivity (Fig. 3C).
Phosphate increased expression of cardiac hypertrophy markers in differentiated myocytes
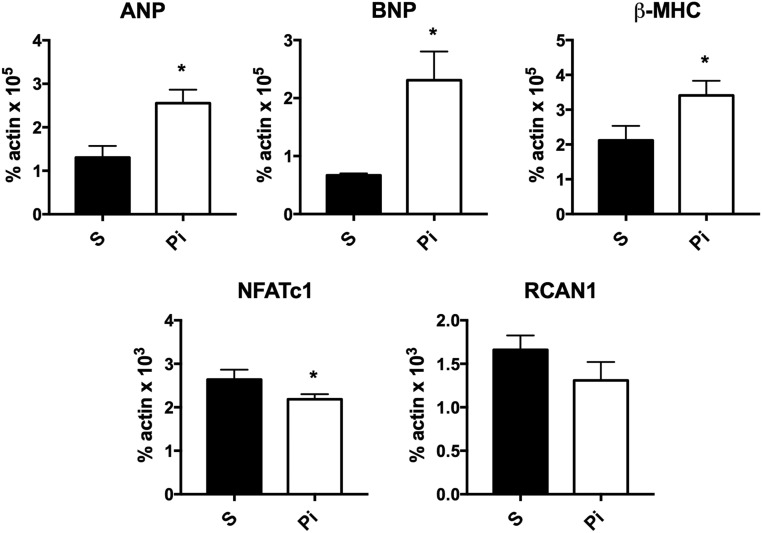
Previous murine studies demonstrating an association of high FGF23 levels with LV hypertrophy were performed in models that also had high serum phosphate levels (24–26). To address the hypothesis that phosphate alone can induce the molecular signature of cardiac hypertrophy, differentiated C2C12 myocytes were treated with 7 mM of sodium sulfate (control) or sodium phosphate. Phosphate treatment led to a significant induction in messenger RNA expression of markers of cardiac hypertrophy, including atrial natriuretic peptide, brain natriuretic peptide, and β-myosin heavy chain. However, expression of NFAT-signaling target genes was not enhanced. Rather, phosphate decreased NFATc1 expression in C2C12 myocytes and did not alter RCAN1 expression (Fig. 4). These results suggest that cooperative interactions between phosphate and FGF23 promote cardiac remodeling in the setting of CKD.
Figure 4.
Phosphate increased expression of cardiac hypertrophy markers in myocytes. C2C12 myocytes were differentiated for 72 hours and treated with 7 mM of sodium sulfate (S) or sodium phosphate (Pi) for 12 hours. Expression of cardiac hypertrophy markers (ANP, BNP, β-MHC) and targets of calcineurin/NFAT signaling (NFATc1, RCAN1) were quantitated. Data normalized for actin in each sample represent RNA obtained from three independent experiments. *P value <0.05. Data are represented as mean ± standard deviation. ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; β-MHC, β-myosin heavy chain.
Discussion
The current studies demonstrate that despite increased circulating levels of FGF23, Hyp mice did not exhibit cardiac hypertrophy. These results are consistent with clinical studies reporting unaltered LV size on electrocardiographic and echocardiographic analyses in children and adults with XLH, regardless of previous treatment with phosphate and calcitriol (42, 43). When LV mass assessed by echocardiography was normalized to patient height, there was a suggestion that children with XLH may be at risk for developing LVH (44). However, these data may reflect the shorter height of patients with XLH or the presence of hyperparathyroidism in 11 of 13 study subjects (44). Alternatively, on the basis of our finding that phosphate induced a hypertrophic signature in C2C12 myocytes, phosphate treatment of the children with XLH we studied could be responsible for the increase in normalized LV mass observed (44).
Recent studies suggest that enhanced FGF23 action in Hyp mice increases distal renal tubule reabsorption of sodium, leading to hypertension and LVH, as quantified by total heart weight normalized to body weight (36, 45). The disparity between these findings and our data may reflect the disproportionately lower weight of Hyp mice relative to their cardiac weight and differences in the sodium and phosphate content of the mouse chow used. Consistent with our findings, studies in DMP1-knockout (KO) mice, characterized by hypophosphatemia in the setting of increased FGF23 level (46–48), revealed a decrease in cardiac weight without evidence of hypertrophy (49), supporting our conclusion that increased FGF23 in the setting of hypophosphatemia does not lead to cardiac hypertrophy.
Activation of FGFRs was associated with development of LVH in mice (24, 50–52). In vitro treatment of cardiomyocytes with FGF23 increased cell size and expression of genes that mediate cardiac hypertrophy, and hearts from mice injected with FGF23 for 2 weeks exhibited increased weight and LV wall thickness (24). FGF23 activated calcineurin/NFAT signaling to mediate these effects on the LV (24, 25). Consistent with FGF23 activation of calcineurin/NFAT signaling in cardiomyocytes, the LVs of Hyp mice had increased expression of NFATc1; however, there were no histological or molecular features of hypertrophy. The lack of LVH in Hyp mice in the presence of high serum levels of FGF23 suggests actions of FGF23 alone are not sufficient to induce LVH. Consistent with this hypothesis, blocking FGF23 action with an anti-FGF23 antibody did not prevent LV hypertrophy in rats with CKD induced by 5/6 nephrectomy (53).
In humans and rats with CKD (24), in mice fed a high-phosphate diet (25, 26), and in mice lacking Klotho, the FGF23 cofactor (24), hyperphosphatemia leads to a compensatory increase in circulating levels of FGF23, which in turn is associated with development of LVH. In contrast, the high circulating levels of FGF23 in mice and humans with XLH, which lead to hypophosphatemia (1, 54), and in DMP1-KO mice (49) do not increase LV size or fibrosis. The different cardiac phenotypes in these human and rodent models suggest that increased FGF23 in the presence of high serum phosphate level underlies the pathogenesis of the LVH observed in CKD. Studies in mice with CKD due to Alport syndrome (Col4a3 KO mice) further support our findings that increased FGF23 does not lead to hypertrophy in the absence of hyperphosphatemia. Although Col4a3 KO mice exhibit increased serum levels of FGF23 by 6 weeks of age, their LV size is not increased at 12 weeks old when they develop hyperphosphatemia (55). In conclusion, our in vivo studies in Hyp mice and in vitro analyses in C2C12 myocytes suggest that cooperative interactions between phosphate and FGF23 promoted cardiac remodeling in the setting of CKD.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health: K08 AR067854 (to E.S.L.), R01 AR061376 and R01 AR 072650 (to M.B.D.), and P30 AR061313.
Author Contributions: Project design: E.S.L., M.B.D., and E.S.B.; mouse colony management: E.P.; molecular biology experiments and histological analyses: E.S.L. and E.P.; cell culture experiments: E.S.L.; tail SBP measurements: B.Y.; echocardiogram analyses: R.T. and M.S.-C.; manuscript preparation: E.S.L., B.Y., E.S.B., M.S.-C., and M.B.D.
Disclosure Summary:
The authors have nothing to disclose.
Glossary
Abbreviations:
- CKD
chronic kidney disease
- ERK
extracellular signal–regulated kinase
- FGF23
fibroblast growth factor 23
- FGFR
fibroblast growth factor receptor
- Hyp mice
mice with X-linked hypophosphatemia
- KO
knockout
- LV
left ventricle
- LVH
left ventricular hypertrophy
- MAPK
mitogen-activated protein kinase
- NFAT
nuclear factor of activated T cell
- PCR
polymerase chain reaction
- RV
right ventricle
- SBP
systolic blood pressure
- WT
wild-type
- XLH
X-linked hypophosphatemia
References
- 1. Liu ES, Martins JS, Raimann A, Chae BT, Brooks DJ, Jorgetti V, Bouxsein ML, Demay MB. 1,25-Dihydroxyvitamin D alone improves skeletal growth, microarchitecture and strength in a murine model of XLH, despite enhanced FGF23 expression. J Bone Miner Res. 2016;31(5):929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holm IA, Huang X, Kunkel LM. Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am J Hum Genet. 1997;60(4):790–797. [PMC free article] [PubMed] [Google Scholar]
- 3. Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. [DOI] [PubMed] [Google Scholar]
- 4. Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291(1):E38–E49. [DOI] [PubMed] [Google Scholar]
- 5. Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98(11):6500–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]
- 7. Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297(2):F282–F291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gattineni J, Alphonse P, Zhang Q, Mathews N, Bates CM, Baum M. Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4. Am J Physiol Renal Physiol. 2014;306(3):F351–F358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chanakul A, Zhang MY, Louw A, Armbrecht HJ, Miller WL, Portale AA, Perwad F. FGF-23 regulates CYP27B1 transcription in the kidney and in extra-renal tissues. PLoS One. 2013;8(9):e72816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohata Y, Yamazaki M, Kawai M, Tsugawa N, Tachikawa K, Koinuma T, Miyagawa K, Kimoto A, Nakayama M, Namba N, Yamamoto H, Okano T, Ozono K, Michigami T. Elevated fibroblast growth factor 23 exerts its effects on placenta and regulates vitamin D metabolism in pregnancy of Hyp mice. J Bone Miner Res. 2014;29(7):1627–1638. [DOI] [PubMed] [Google Scholar]
- 11. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. [DOI] [PubMed] [Google Scholar]
- 12. Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32(5):1454–1459. [DOI] [PubMed] [Google Scholar]
- 13. McLenachan JM, Henderson E, Morris KI, Dargie HJ. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med. 1987;317(13):787–792. [DOI] [PubMed] [Google Scholar]
- 14. Otterstad JE, Davies M, Ball SG, Erikssen J, Birkin E, Virk S, Rynning SE, Rodevand O, Hansson L, Bergbrandt A, Findlay I, Khokhar AA, Areskog NH, Nylander E, Smith S, Marlow HF Left ventricular hypertrophy and myocardial ischaemia in hypertension: the THAMES Study. Eur Heart J. 1993;14(12):1622–1628. [DOI] [PubMed] [Google Scholar]
- 15. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 16. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134(8):629–636. [DOI] [PubMed] [Google Scholar]
- 17. Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med. 2015;5(4):254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. 2005;46(2):320–327. [DOI] [PubMed] [Google Scholar]
- 19. McMahon LP, Roger SD, Levin A; Slimheart Investigators Group . Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J Am Soc Nephrol. 2004;15(6):1640–1647. [DOI] [PubMed] [Google Scholar]
- 20. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. [DOI] [PubMed] [Google Scholar]
- 21. Martin KJ, González EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18(3):875–885. [DOI] [PubMed] [Google Scholar]
- 22. Navab KD, Hama SY, Safarpour S, Hough GP, Vakili L, Reddy ST, Navab M, Vaziri ND. Chronic inflammatory disorders and accelerated atherosclerosis: chronic kidney disease. Curr Pharm Des. 2011;17(1):17–20. [DOI] [PubMed] [Google Scholar]
- 23. Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22(6):1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro-O M, Hill JA, Moe OW. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26(6):1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marie PJ, Travers R, Glorieux FH. Healing of bone lesions with 1,25-dihydroxyvitamin D3 in the young X-linked hypophosphatemic male mouse. Endocrinology. 1982;111(3):904–911. [DOI] [PubMed] [Google Scholar]
- 28. Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117(15):1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A, Chen C, Chao W, Panagia M, Sosnovik DE, Puppala D, Armoundas AA, Hindle A, Bloch KD, Buys ES, Scherrer-Crosbie M. Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol. 2015;84:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scherrer-Crosbie M, Steudel W, Ullrich R, Hunziker PR, Liel-Cohen N, Newell J, Zaroff J, Zapol WM, Picard MH. Echocardiographic determination of risk area size in a murine model of myocardial ischemia. Am J Physiol. 1999;277(3 Pt 2):H986–H992. [DOI] [PubMed] [Google Scholar]
- 31. Rodrigues AC, Hataishi R, Ichinose F, Bloch KD, Derumeaux G, Picard MH, Scherrer-Crosbie M. Relationship of systolic dysfunction to area at risk and infarction size after ischemia-reperfusion in mice. J Am Soc Echocardiogr. 2004;17(9):948–953. [DOI] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 33. Miedlich SU, Zalutskaya A, Zhu ED, Demay MB. Phosphate-induced apoptosis of hypertrophic chondrocytes is associated with a decrease in mitochondrial membrane potential and is dependent upon Erk1/2 phosphorylation. J Biol Chem. 2010;285(24):18270–18275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu ES, Zalutskaya A, Chae BT, Zhu ED, Gori F, Demay MB. Phosphate interacts with PTHrP to regulate endochondral bone formation. Endocrinology. 2014;155(10):3750–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science. 1985;230(4727):758–766. [DOI] [PubMed] [Google Scholar]
- 36. Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6(6):744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA. 2005;102(27):9637–9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dyar KA, Ciciliot S, Tagliazucchi GM, Pallafacchina G, Tothova J, Argentini C, Agatea L, Abraham R, Ahdesmäki M, Forcato M, Bicciato S, Schiaffino S, Blaauw B. The calcineurin-NFAT pathway controls activity-dependent circadian gene expression in slow skeletal muscle. Mol Metab. 2015;4(11):823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schulz RA, Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004;266(1):1–16. [DOI] [PubMed] [Google Scholar]
- 40. Komuro I, Yazaki Y. Control of cardiac gene expression by mechanical stress. Annu Rev Physiol. 1993;55(1):55–75. [DOI] [PubMed] [Google Scholar]
- 41. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vered I, Vered Z, Perez JE, Jaffe AS, Whyte MP. Normal left ventricular performance in children with X-linked hypophosphatemic rickets: a Doppler echocardiography study. J Bone Miner Res. 1990;5(5):469–474. [DOI] [PubMed] [Google Scholar]
- 43. Takashi Y, Kinoshita Y, Hori M, Ito N, Taguchi M, Fukumoto S. Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr Res. 2017;42(2):132–137. [DOI] [PubMed] [Google Scholar]
- 44. Nehgme R, Fahey JT, Smith C, Carpenter TO. Cardiovascular abnormalities in patients with X-linked hypophosphatemia. J Clin Endocrinol Metab. 1997;82(8):2450–2454. [DOI] [PubMed] [Google Scholar]
- 45. Han X, Ross J, Kolumam G, Pi M, Sonoda J, King G, Quarles LD. Cardiovascular effects of renal distal tubule deletion of the FGF receptor 1 gene. J Am Soc Nephrol. 2018;29(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38(11):1248–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295(2):E254–E261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wacker MJ, Touchberry CD, Silswal N, Brotto L, Elmore CJ, Bonewald LF, Andresen J, Brotto M. Skeletal muscle, but not cardiovascular function, is altered in a mouse model of autosomal recessive hypophosphatemic rickets. Front Physiol. 2016;7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheinowitz M, Kotlyar A, Zimand S, Ohad D, Leibovitz I, Bloom N, Goldberg I, Nass D, Engelberg S, Savion N, Eldar M. Basic fibroblast growth factor induces myocardial hypertrophy following acute infarction in rats. Exp Physiol. 1998;83(5):585–593. [DOI] [PubMed] [Google Scholar]
- 51. Corda S, Mebazaa A, Gandolfini MP, Fitting C, Marotte F, Peynet J, Charlemagne D, Cavaillon JM, Payen D, Rappaport L, Samuel JL. Trophic effect of human pericardial fluid on adult cardiac myocytes: differential role of fibroblast growth factor-2 and factors related to ventricular hypertrophy. Circ Res. 1997;81(5):679–687. [DOI] [PubMed] [Google Scholar]
- 52. Cilvik SN, Wang JI, Lavine KJ, Uchida K, Castro A, Gierasch CM, Weinheimer CJ, House SL, Kovacs A, Nichols CG, Ornitz DM. Fibroblast growth factor receptor 1 signaling in adult cardiomyocytes increases contractility and results in a hypertrophic cardiomyopathy. PLoS One. 2013;8(12):e82979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122(7):2543–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chesney RW, Mazess RB, Rose P, Hamstra AJ, DeLuca HF, Breed AL. Long-term influence of calcitriol (1,25-dihydroxyvitamin D) and supplemental phosphate in X-linked hypophosphatemic rickets. Pediatrics. 1983;71(4):559–567. [PubMed] [Google Scholar]
- 55. Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B, Bonewald LF, Stubbs JR, Wacker MJ. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304(8):E863–E873. [DOI] [PMC free article] [PubMed] [Google Scholar]