Abstract
Purpose
Prior studies have shown nuclear reactivity for BAP1 yields prognostic information for paraffin embedded uveal melanomas. Lacking are immunocytochemical studies of BAP1 on fine needle aspiration biopsies of uveal melanoma that correlate with prognosis or other markers of prognosis. Our purpose was to fill this gap.
Design
Experimental laboratory study
Methods
Fine needle aspiration biopsies were performed prospectively on 113 patients with uveal melanomas garnering limited subsets of cases for comparison. Agreement between immunocytochemistry for BAP1 nuclear staining versus chromosome 3 ploidy analysis and gene expression profiling was assessed by 2 × 2 contingency table analysis.
Results
The presence or absence of suppression of nuclear expression of BAP1 was strongly associated (73%, P=.000002) with monosomy and disomy chromosome 3, respectively. BAP1 nuclear expression was also correlated with gene expression profiling. Chromosome 3 ploidy analysis correlated with gene expression profiles.
Conclusion
When adequate material is obtained, immunocytology using BAP1 is a potentially informative tool for prognostication of uveal melanoma.
Recent work has significantly advanced the development of prognostic markers for uveal melanoma.1–4 Cytogenetic analysis for monosomy 3, either by fluorescence in situ hybridization and or multiplex ligation dependent probe amplification correlate with a worse prognosis. Gene expression profiling has also been shown to provide excellent prognostic information.2,5 These tests are in common use for fine needle aspirates of uveal melanoma. The correlation between these prognostic markers has been somewhat variable in both histologic studies and recent cytologic studies.6,7 Recently, empiric evidence from several retrospective studies show immunohistochemistry of BAP1 in paraffin tissue sections of uveal melanoma effectively predicts prognosis in terms of both metastasis and death.3,8,9 In these studies the lack of nuclear expression of BAP1 also connotes a worse prognosis in uveal melanoma. A poor prognosis is presumably related to the lack of functional BAP1 protein molecules in the nucleus to suppress tumor proliferation. One intact allele of the BAP1 gene on chromosome 3 is requisite for the expression and translocation to the nucleus where BAP1 can inhibit tumor proliferation.2 Suppression of the nuclear localization of BAP1 (biallelic suppression) occurs in monosomy 3 because it is combined with assorted mutations on the remaining allele that may truncate the BAP1 protein, or affect nuclear localizer regions.2 BAP1 has definite additional prognostic value compared to the aforementioned tests and unlike the other prognostic tests permits microscopic confirmation of tumor. Immunohistochemistry for BAP1 has been well studied in tissue sections, but immunocytochemistry for BAP1 has neither been studied nor is in general clinical use in patients undergoing fine needle aspiration. In many institutions tissue samples are collected by fine needle aspiration for diagnosis and smears are readily available. Immunocytochemical studies for BAP1 protein nuclear localization are lacking and correlation with gene expression profiling and monosomy 3 is needed in fine needle aspirates of uveal melanoma. This study was undertaken to fill this gap.
Materials and Methods
This study and data accumulation were carried out with approval from the UCLA Institutional Review Board (IRB). Informed consent for the research was obtained from the patients in accordance with HIPAA regulations. Intraocular fine needle aspiration biopsies (27–30 gauge needles) were performed on 113 cases over a 2 year period for the clinical evaluation of uveal melanoma. The aspirates were allocated as follows: one alcohol fixed slide for hematoxylin and eosin (H&E) stains for microscopic analysis, one alcohol fixed slide for immunocytochemistry for BAP1, one air dried smear for fluorescence in situ hybridization using probes for chromosome 3, cell samples sent for gene expression profiling, as well as multiplex ligation dependent probe amplification as previously described.10,11 For categorization purposes the ploidy analysis used the results of both fluorescence in situ hybridization and multiplex ligation dependent probe amplification.
Immunocytochemistry for BAP1 was performed with a mouse monoclonal antibody, IgG1 (kappa light chain) C-4 (Santa Cruz antibodies, CA) following the manufacturer’s suggested protocol. If the unstained alcohol fixed slide had insufficient material, available cells were transferred from the H&E stained slides using the Diatex method to coated slides for anti-BAP1 staining.12 To avoid obfuscation of immunoreactivity with melanin pigment a red chromogen (bond polymer refine red detection, Leica Biosystems Inc., IL) was used. Positive controls included histologic sections of skin nevi or smears of retinal cells internal positive control) if present. Deletion of the primary antibody served as a negative control. The results were interpreted in a binary fashion, either positive or negative nuclear reactivity to anti-BAP1 in nuclei of tumor cells. In some cases heterogeneity of staining was noted. In such instances the test was repeated but if a similar result was obtained these cases were considered non-diagnostic for the purposes of the study.
Correlative analysis was performed with 2×2 contingency tables using the published formulas for evaluation of significance using Fisher’s two tailed exact test.13 A positive correlation was deemed significant if p<.05. In addition, the McNemar test was also applied using the null hypothesis that for the tests compared were not different or that the marginal probabilities (p) for each test are the same Ho: pb=pc where b and c refer to discordant cells in the contingency table. Finally, the kappa statistic was also applied as a measure of agreement.14
Results
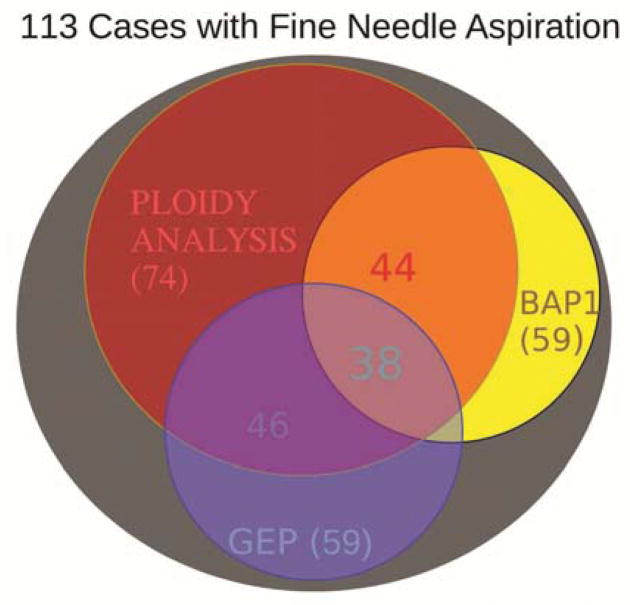
Of the 113 cases in which fine needle aspiration biopsy was performed, only 79 had sufficient cells for morphologic assessment on H&E staining. The distribution of tests successfully obtained for prognostication is shown in the Venn diagram, Figure 1. Because ploidy analysis could be done by either fluorescence in situ hybridization or multiplex ligation dependent probe amplification, the yield was highest for chromosomal analysis (74 cases). In cases in which both tests were performed the results were identical. However, many cases had sufficient material for only one or two tests. Correlative analysis between tests could only be done in the overlap areas of the Venn diagram (Figure 1). Enough cases with sufficient material were gathered for correlation with 2 × 2 contingency tables for paired analysis of combinations that include BAP1, ploidy analysis (monosomy 3), and/or the gene expression profile. The contingency Tables 1–3 show the correlations. The strongest correlation (73%) emerged for nuclear reactivity for anti-BAP1 compared to chromosomal 3 ploidy analysis. Six cases appeared discordant. Significant correlation was also found between BAP1 and gene expression profile (Table 2) as well as chromosomal analysis versus gene expression profile (Table 3). The odds ratios and confidence intervals are given in the tables. Analysis with the McNemar test showed no differences between any of the paired test results.
Figure 1.
Venn diagram of overlap of prognostic characteristics to predict prognosis from fine needle aspirates of uveal melanomas. The number of cases available in each prognostic category are shown by the numbers in parentheses. The numbers without parentheses are those in the overlapped categories available for correlative analysis. These numbers are color coded to show the areas involved, e.g. (purple=red+blue).
Table 1.
Correlation of ploidy analysis for chromosome 3 versus nuclear reactivity for anti-BAP1
| Ploidy Analysis | BAP1 Positive | BAP1 Negative |
|---|---|---|
| Disomy 3 | 18 | 3 |
| Monosomy 3 | 3 | 20 |
Fisher’s exact test two tailed P=.000002
Odds ratio mean= 40, (95% confidence interval 7.1–223)
Kappa statistic=.73 (substantial agreement between the tests))
Table 3.
Correlation of Gene Expression Profiling verus ploidy analysis for chromosome 3
| Gene Expression Profiling | Disomy 3 | Monosomy 3 |
|---|---|---|
| Class 1 | 24 | 7 |
| Class 2 | 3 | 11 |
Fisher’s exact test two tailed P=.0001
Odds ratio =12.6 (95% confidence interval 2.7–58.1)
Kappa statistic =.52 (moderate agreement)
Table 2.
Correlation of Gene Expression Profiling versus nuclear reactivity for anti-BAP1
| Gene Expression Profiling | BAP1 Positive | BAP1 negative |
|---|---|---|
| Class 1 | 17 | 7 |
| Class 2 | 1 | 13 |
Fisher’s exact test two tailed P=.0001
Odds ratio mean =.035 (95% confidence interval .004–.32)
Kappa statistic= .59 (moderate agreement
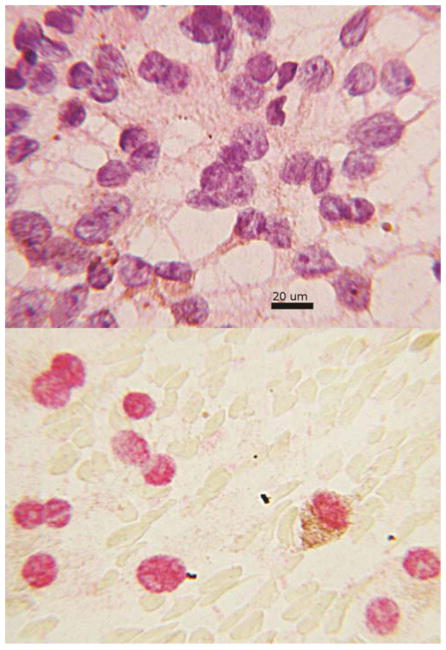
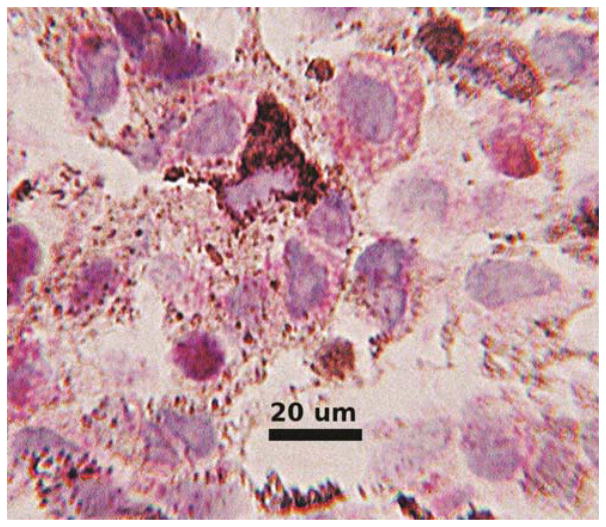
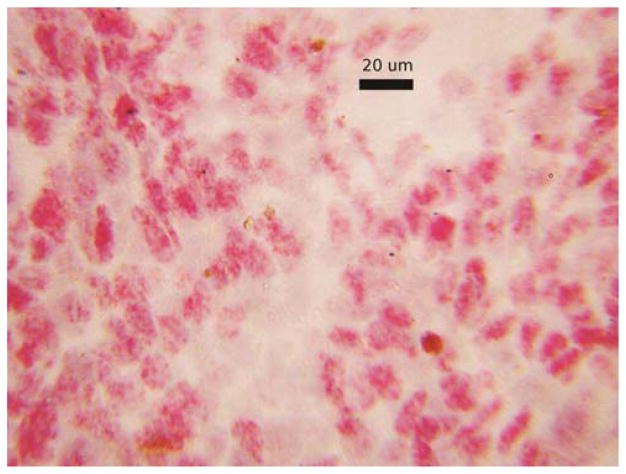
For the cases that were being compared in contingency tables there were no ambiguous interpretations. In all cases with sufficient cells on H&E stained slides there were typical cytologic findings of uveal melanoma (Figure 2A). In aspirates with anti-BAP1 reactivity nearly all tumor cell nuclei showed nuclear translocation (Figure 2B). Anti-BAP1 staining with a red chromogen permitted interpretation in the presence of melanin pigment (Figure 2B). In the negative cases nuclear reactivity was absent in almost all tumor cell nuclei. However, in a few cases scattered reactivity over the nucleus appeared to be of the same intensity as cytoplasmic staining and obfuscated the nucleus (Figure 3). Other prognostic tests were not available for these cases so correlation was not possible and the analysis was not affected. In cases with the transfer of cells for immunocytochemistry some cell nuclei appeared smudged despite the positive reactivity (Figure 4). Interpretation was still rendered because the original H&E stained slide showed a homogeneous population of tumor cells, diagnostic for melanoma.
Figure 2.
Figure 2A., above cytology smear from a fine needle aspirate diagnosed as melanoma. Enlarged nuclei, prominent nucleoli and brown cytoplasmic pigment are predominant features. The cytoplasm tapers in a dendritiform pattern, hematoxylin and eosin. B., below, same case reacted with anti-BAP1 by immunocytochemistry. The red staining areas indicate the presence of protein is localized to the nucleus. The melanin pigment appears brown and features the same dendritiform configuration seen in 2A.
Figure 3.
Immunocytochemical reactivity for anti-BAP1 from a fine needle aspirate of uveal melanoma shows red cytoplasmic granules. In some cells the red color appears to overlie the nucleus and obfuscating the interpretation. Nuclei did not appear to contain red chromogen in cells without accompanying cytoplasmic reactivity suggesting interpretation as a negative reaction.
Figure 4.
Immunocytochemical stain for anti-BAP on cells transferred from an alcohol fixed hematoxylin and eosin stained aspirate smeared on a glass slide. Although the nuclei are smudged, positive reactivity (red color) is evident in the nuclei.
Discussion
The key findings of this study are that anti-BAP1 reactivity in fine needle aspiration cytologic smears correlates with chromosome ploidy analysis, and gene expression profile. Chromosome 3 ploidy analysis correlated well with gene expression profile. These findings have important clinical implications for the algorithm of sample utilization in cases of uveal melanoma.
In many centers including our own fine needle aspiration biopsy has emerged as the predominant method to obtain tissue for melanoma. However, fine needle aspiration is almost always performed for prognostication not usually for diagnosis.15 The diagnosis of uveal melanoma is generally predicated on the characteristic clinical findings including ultrasonographic findings of a dome shaped mass with low to medium internal reflectivity. The high degree of accuracy of clinical diagnosis (99.7%) has obviated the diagnostic need for biopsies except in ambiguous cases.13,16
With the realization that enucleation does not affect the prognosis of uveal melanoma, eye-sparing therapies are generally implemented to eradicate uveal tumor growth. In most of our cases radioactive brachytherapy plaques are placed concurrently with fine needle aspiration biopsy but before cytologic results are finalized. Therefore, enucleations are generally unnecessary and fine needle aspiration prior to treatment may be the only opportunity at tissue procurement for diagnosis and prognosis. To minimize risk of tumor seeding and damage to the eye small diameter needles are used at our center.15,17,18 The aspiration samples are often sparse demanding maximum utilization of the sample for diagnosis and prognostication. Since adequate sampling may vary among the tests, the importance of correlation of immunocytochemistry of BAP1 to the other tests is apparent. BAP1 is the only prognostic test that allows simultaneous morphologic confirmation and prognostication from needle aspiration biopsy. Fluorescence in situ hybridization shows only nuclear features in counterstaining as the cytoplasmic pigment and cytoplasmic size and shapes are lost with the requisite digestion. If retinal cells, inflammatory cells or a benign tumor are obtained the fluorescence in situ hybridization may be reported as disomy.19 Inflammatory cells were easily discerned from tumor cells in the aspirates of this study. However, if the morphologic character has been altered, smudging of the cells could result in focal false positive cells. Similarly, multiplex ligation dependent probe amplification and the gene expression profiling start by extracting RNA/DNA and no morphologic features can be obtained. The uncertainty and heterogeneity of the sample have lead to erroneous prognostication.10,20 As demonstrated here the ability to transfer the cells from slides may permit secondary use in other tests although only immunocytochemistry was tested here. Therefore, the correlation of BAP1 with the other prognostic markers is an important step. In this study a positive correlation with other prognostic markers is established.
Although BAP1 immunocytochemistry appears to be an adequate surrogate for the other tests in most cases there are notable discrepancies. The ultimate meaning of these discrepancies will be evident in the analysis of future patient metastatic outcome. To date only one patient of this series has documented metastases and BAP1 and gene expression profile were concordant. In this case chromosomal analysis by fluorescence in situ hybridization or multiplex ligation dependent probe amplification were obviated because of inadequate material. The basis of these discrepancies can be multifold. A partial list includes the unrecognized source material (false negative in gene expression profile and chromosomal analysis), the presence of point mutations that affect function but not nuclear translocation (false negative BAP1), the presence of isodisomy (false negative for chromosomal analysis), and the presence of germ line mutations (false negative chromosomal analysis). The potential exists to improve the prognostication by using all 3 tests in concert. Probably the best approach would be to pool the material from all aspirates and then determine adequacy with morphology and BAP1 first. This would save time and expense. Further, discordant results between BAP1 nuclear reactivity and other tests could be examined in a stepwise manner. Bialleic suppression can also result from mutations that affect both alleles such as in isodisomy of chromosome 3p. Multiplex ligation-dependent probe amplification will be enlightening if probes are chosen to discover isodisomies. Gene expression profiling in concert with BAP1 show the end result of a final common pathway, which is tumor suppression. Point mutations may negate the function of the BAP1 as a tumor suppressor gene without hampering its nuclear localization. Target gene sequencing for specific germline mutations may be also help resolve discrepancies.
A distinct advantage of BAP 1 immunocytochemistry of the other tests at our institution is the reduced cost. Immunocytochemistry is automated and is a fraction of the cost of gene expression profiling or multiplex ligation-dependent probe amplification. Further, immunocytochemistry is readily available to most pathology laboratories. Because the test is not generally sent to another laboratory the turn around time for the test is about 1–2 days compared to weeks for the other tests. Finally, immunocytochemistry for BAP1 can be performed on H&E stained slides, conserving material.
The use of fine needle aspiration for obtaining prognostic material has notable challenges. In our study multiple aspirates may be performed without adequate cellular material for any test, especially in smaller tumors. Advances are needed in the approach to obtain a consistently high yield and discrepant results among tests need clarification.
Acknowledgments
This study was supported by the Edith and Lew Wasserman Endowed Professorship (BG), National Eye Institute Award R01-EY11224 (BG), the George E. and Ruth Moss Trust (TAM) and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Disclosure/Duality of Interest. The authors have no duality of interest to disclose. TAM is an uncompensated consultant for Impact Genetics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol. 2014;25(3):234–239. doi: 10.1097/ICU.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harbour JW, Onken MD, Roberson EDO, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Nes JAP, Nelles J, Kreis S, et al. Comparing the prognostic value of BAP1 mutation pattern, chromosome 3 status, and BAP1 immunohistochemistry in uveal melanoma. Am J Surg Pathol. 2016;40(6):796–805. doi: 10.1097/PAS.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 4.Vaarwater J, van den Bosch T, Mensink HW, et al. Multiplex ligation-dependent probe amplification equals fluorescence in-situ hybridization for the identification of patients at risk for metastatic disease in uveal melanoma. Melanoma Res. 2012;22(1):30–37. doi: 10.1097/CMR.0b013e32834e6a67. [DOI] [PubMed] [Google Scholar]
- 5.Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010;12(4):461–468. doi: 10.2353/jmoldx.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrêa ZM, Augsburger JJ. Independent Prognostic Significance of Gene Expression Profile Class and Largest Basal Diameter of Posterior Uveal Melanomas. Am J Ophthalmol. 2016;162:20–27. doi: 10.1016/j.ajo.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Klufas MA, Richter E, Itty S, Moreno C, McCannel CA, McCannel TA. Comparison of gene expression profiling and chromosome 3 analysis by fluorescent in situ hybridization and multiplex ligation probe amplification in fine-needle aspiration biopsy specimens of uveal melanoma. Ocul Oncol Pathol. 2018;4(1):16–20. doi: 10.1159/000468941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalirai H, Dodson A, Faqir S, Damato BE, Coupland SE. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer. 2014;111(7):1373–1380. doi: 10.1038/bjc.2014.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yavuzyigitoglu S, Mensink HW, Smit KN, et al. Metastatic Disease in Polyploid Uveal Melanoma Patients Is Associated With BAP1 Mutations. Invest Ophthalmol Vis Sci. 2016;57(4):2232–2239. doi: 10.1167/iovs.15-18608. [DOI] [PubMed] [Google Scholar]
- 10.Klufas MA, Itty S, McCannel CA, Glasgow BJ, Moreno C, McCannel TA. Variable Results for Uveal Melanoma-Specific Gene Expression Profile Prognostic Test in Choroidal Metastasis. JAMA Ophthalmol. 2015;133(9):1073–1076. doi: 10.1001/jamaophthalmol.2015.1790. [DOI] [PubMed] [Google Scholar]
- 11.Young TA, Rao NP, Glasgow BJ, Moral JN, Straatsma BR. Fluorescent in situ hybridization for monosomy 3 via 30-gauge fine-needle aspiration biopsy of choroidal melanoma in vivo. Ophthalmology. 2007;114(1):142–146. doi: 10.1016/j.ophtha.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Joseph D, Gangi MD. Application of Diatex compound in cytology: use in preparing multiple slides from a single routine smear. Acta Cytol. 1986;30(4):446–447. [PubMed] [Google Scholar]
- 13.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85(1):87–94. [Google Scholar]
- 14.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37–46. [Google Scholar]
- 15.McCannel TA. Fine-needle aspiration biopsy in the management of choroidal melanoma. Curr Opin Ophthalmol. 2013;24(3):262–266. doi: 10.1097/ICU.0b013e32835ff001. [DOI] [PubMed] [Google Scholar]
- 16.Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol. 1998;125(6):745–766. doi: 10.1016/s0002-9394(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 17.Sellam A, Desjardins L, Barnhill R, et al. Fine needle aspiration biopsy in uveal melanoma: technique, complications, and outcomes. Am J Ophthalmol. 2016;162:28–34. doi: 10.1016/j.ajo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Glasgow BJ, Brown HH, Zargoza AM, Foos RY. Quantitation of tumor seeding from fine needle aspiration of ocular melanomas. Am J Ophthalmol. 1988;105(5):538–546. doi: 10.1016/0002-9394(88)90248-6. [DOI] [PubMed] [Google Scholar]
- 19.Chang MY, Rao NP, Burgess BL, Johnson L, McCannel TA. Heterogeneity of monosomy 3 in fine needle aspiration biopsy of choroidal melanoma. Mol Vis. 2013;19:1892–1900. [PMC free article] [PubMed] [Google Scholar]
- 20.Miller AK, Benage MJ, Wilson DJ, Skalet AH. Uveal Melanoma with Histopathologic Intratumoral Heterogeneity Associated with Gene Expression Profile Discordance. Ocul Oncol Pathol. 2017;3(2):156–160. doi: 10.1159/000453616. [DOI] [PMC free article] [PubMed] [Google Scholar]