Key Points
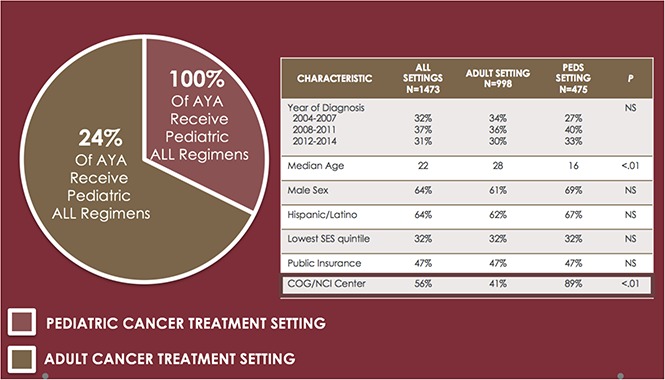
Two-thirds of AYA ALL are treated in the adult cancer setting; one-quarter of AYAs in this setting receive front-line pediatric ALL therapy.
Survival was superior for AYA ALL patients treated in pediatric cancer settings and in NCI-designated cancer centers.
Abstract
Adolescents and young adults (AYAs, 15-39 years) with acute lymphoblastic leukemia (ALL) represent a heterogeneous population who receive care in pediatric or adult cancer settings. Using the California Cancer Registry, we describe AYA ALL patterns of care and outcomes over the past decade. Sociodemographics, treatment location, and front-line therapies administered to AYAs diagnosed with ALL between 2004 and 2014 were obtained. Cox regression models evaluated associations between ALL setting and regimen and overall survival (OS) and leukemia-specific survival (LSS) for the entire cohort, younger AYA (<25 years), and AYAs treated in the adult cancer setting only. Of 1473 cases, 67.7% were treated in an adult setting; of these, 24.8% received a pediatric ALL regimen and 40.7% were treated at a National Cancer Institute (NCI)–designated center. In multivariable analyses, front-line treatment in a pediatric (vs adult) setting (OS HR = 0.53, 95% confidence interval [CI], 0.37-0.76; LSS HR = 0.51, 95% CI, 0.35-0.74) and at an NCI/Children’s Oncology Group (COG) center (OS HR = 0.80, 95% CI, 0.66-0.96; LSS HR = 0.80, 95% CI, 0.65-0.97) were associated with significantly superior survival. Results were similar when analyses were limited to younger AYAs. Outcomes for AYAs treated in an adult setting did not differ following front-line pediatric or adult ALL regimens. Our population-level findings demonstrate that two-thirds of AYAs with newly diagnosed ALL are treated in an adult cancer setting, with the majority receiving care in community settings. Given the potential survival benefits, front-line treatment of AYA ALL at pediatric and/or NCI/COG-designated cancer centers should be considered.
Visual Abstract
Introduction
Since the publication of retrospective cancer cooperative group studies demonstrating a survival benefit for adolescents and young adults (AYAs) treated with pediatric acute lymphoblastic leukemia (ALL) regimens delivered by pediatric oncologists vs adult ALL regimens administered by medical oncologists,1-5 much attention has been given to the type and location of treatment of AYA ALL. Defined by the National Cancer Institute (NCI) as patients diagnosed with cancer between the ages of 15 and 39 years,6 AYAs are a growing proportion of the ALL population such that it is projected that >1300 AYAs will be diagnosed with ALL in 2018 in the United States.7
Prospective clinical trials have confirmed both the feasibility and encouraging outcomes associated with the use of pediatric ALL regimens in the adult medical oncology setting.8-14 In the United States, the Intergroup C10403 prospective clinical trial demonstrated that a complex pediatric ALL chemotherapy regimen could be administered by medical oncologists in the adult medical setting with successful outcomes.9,15 Similar clinical trials conducted by cancer cooperative groups across Europe demonstrated comparable results to C10403, with overall survival (OS) exceeding 60% at 3 to 5 years.10,12,13
A substantial obstacle facing AYAs with ALL remains that they are often caught in the gap between pediatric and adult cancer care, with treatment at an adult or a pediatric cancer resulting from referral patterns, hospital guidelines, or just plain chance. Similarly, if treated at an adult center, an AYA ALL patient may receive a pediatric or adult ALL regimen. Despite a multitude of review articles, society guidelines, and AYA cancer resources championing the pediatric approach,16-18 it is not clear whether AYA ALL patients are increasingly being cared for at pediatric centers or whether pediatric regimens have been universally applied to AYA ALL patients treated in the adult cancer community. To address these gaps in knowledge, we conducted an observational, population-based study of AYAs with newly diagnosed ALL between 2004 and 2014 across the state of California. Using data abstracted from the California Cancer Registry (CCR), we describe front-line ALL regimens received by AYAs in a variety of cancer settings and evaluate associations among treatment setting, ALL regimen, and survival. These data are intended to complement clinical trial findings conducted primarily in research centers and to provide a “real-world” evaluation of AYA ALL care and outcomes in the modern era.
Methods
Case ascertainment and data collection
The study population consisted of all California residents with newly diagnosed ALL (International Classification of Diseases for Oncology, 3rd ed,19 codes 9826, 9835, 9836, 9811-9818, and 9837 per NCI Surveillance Epidemiology and End Results [SEER] AYA site recode), aged 15 to 39 years at time of diagnosis between 1 January 2004 and 31 December 2014. Data were abstracted from the CCR, California's population-based cancer surveillance system that comprises 4 SEER cancer registries and captures approximately 99% of new cancer cases statewide (www.ccrcal.org). Patient and tumor characteristics were obtained from CCR data routinely collected for the registry via medical records, including age at diagnosis, sex, race/ethnicity, health insurance status at the time of initial diagnosis or treatment, and a previously developed composite measure of neighborhood socioeconomic status (nSES) that incorporates census block group level data on income, education, housing costs, and employment.20,21 Underlying cause of death was determined from death certificates. Patients were assigned to statewide nSES quintiles based on their address at time of diagnosis. Approval for human subjects research was granted by the institutional review board of the Cancer Prevention Institute of California and the Committee for the Protection of Human Subjects for the California Health and Human Services Agency. The study was conducted in accordance with the Declaration of Helsinki.
Facility-level information
We reviewed all available CCR facility-level reports for each patient following methodologies previously described.22 The facility where the initial ALL treatment regimen was administered was designated as either a pediatric or adult hospital (“treatment setting”). Pediatric centers were identified using a list of Children’s Oncology Group (COG) pediatric cancer centers23 and children’s hospitals24 across California. Patients treated at institutions that that do not report cancer cases from their pediatric and adult hospitals separately were identified as treated in a pediatric setting only if the treating physician was a pediatric oncologist; if no information on treating physician was available, treatment setting was considered unknown. Hospitals were classified by their affiliation with an NCI-designated cancer center (CC)25; affiliation as a COG center was taken into account for patients treated in pediatric settings only.
Data text fields were abstracted to identify ALL chemotherapy regimens administered and individual records were reviewed to assign the treatment facility associated with front-line ALL regimen administration. Treatment regimens were categorized as either adult or pediatric/pediatric-inspired ALL regimens (supplemental Table 1). Based on previous work and clinical input, every ALL regimen administered in the pediatric setting was considered a pediatric ALL regimen.22 Missing or unclear data were coded as unknown. A facility initially administering a single agent (such as steroids or hydroxyurea) was not considered the treating facility if a patient was subsequently transferred elsewhere to begin front-line ALL therapy.
Statistical analysis
Descriptive statistics (frequencies, percentages) characterized baseline patient, hospital, and treatment characteristics by treatment setting. Differences in these characteristics between pediatric and adult treatment settings were evaluated using χ2 and Fisher’s exact test. Logistic regression models were used to obtain the odds ratios (ORs) and 95% confidence intervals (CIs) for the factors associated with receipt of a pediatric-inspired ALL regimen in the adult treatment setting, adjusting for clustering by facility. Variables significant at P < .10 in the univariable model were included in the final multivariable model.
The association of treatment setting (pediatric vs adult) with both OS and leukemia-specific survival (LSS) was assessed using Cox proportional hazards regression for AYAs of all ages and for those 15 to 24 years of age because of the superior outcomes and variability in pediatric vs adult treatment setting in these younger AYAs. Cox proportional hazards regression was also used to explore the association of ALL regimen type (pediatric-inspired vs adult) with overall and LSS in the adult ALL treatment setting after 2008 (pediatric ALL regimens were used infrequently in the adult treatment setting before this time). Proportional hazards assumptions were tested using Schoenfeld residuals. In the model of AYAs treated in the adult setting (2009-2014), age violated the proportional hazards assumption and was included as a stratification variable, allowing baseline hazards to vary by age. No evidence of significant multicollinearity was found using the variance inflation factor. Survival time was calculated from the date of diagnosis to the date of death or last known vital status. Vital status data were complete through December 31, 2014; any patient alive at this date was censored. For LSS analyses, patients who died of a cause other than leukemia were censored on the date of death. Patients with incomplete dates of diagnosis or follow-up for whom we could not calculate survival time (N = 33) were excluded from survival analyses. All multivariable models were adjusted for age and sex. Additional covariates considered for inclusion in the multivariable models were race/ethnicity, health insurance, nSES, NCI-designated CC/COG status of treating facility, and year of diagnosis. In the model limited to AYA treated in the adult setting, NCI/COG center status and type of induction protocol were selected for inclusion a priori because the effects of these variables were of primary interest. Using backward selection, any additional variables significant at P < .10 were retained in all the final models. Results are reported in hazard ratios (HR) and 95% CIs. Statistical analyses were performed using SAS, version 9.4 (Cary, NC).
Results
AYA patient and treatment setting characteristics
A total of 1795 AYA patients with newly diagnosed ALL were diagnosed and reported to the CCR between 1 January 2004 and 31 December 2014. Five cases were excluded because of multiple leukemia diagnoses and 30 cases were excluded because they were found to have been miscoded as ALL. Cases were also excluded if missing essential data for characterization of treatment or treatment setting (N = 270) or if death occurred before administration of any therapy (N = 17), resulting in a total of 1473 AYAs with ALL included in the analyses.
Patient and treatment setting characteristics are detailed in Table 1. The median age at diagnosis was 22 years; 32.3% were 15 to 18 and 56.8% were <25 years. Two-thirds of the study population was male and 63.7% were of Hispanic race/ethnicity. The majority of patients had public insurance (47.2%; including Medicaid/Medicare) or private insurance (43.1%). Most (67.7%) patients were treated in an adult cancer setting. The proportion of AYAs treated in a pediatric setting increased significantly over the study period, from 27.3% from 2004 through 2007 to 34.9% from 2012 through 2014 (P = .002). Treatment setting differed significantly by age, with 86.9% and 16.0% of AYA aged 15 through 18 and 19 through 24 years, respectively, treated in a pediatric setting, and >98% of AYA aged 25 through 39 years treated in an adult setting (P < .001). The proportion of public and private insurance holders was similar between AYA treated in a pediatric vs adult setting (P = .384).
Table 1.
Baseline patient and treating facility characteristics according to adult or pediatric treatment setting among AYA with ALL, ages 15-39 y, 2004-2014, California
| All settings (N = 1473) | Adult setting (N = 998) | Pediatric setting (N = 475) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Age at diagnosis, y | ||||||
| 15-18 | 476 | 32.3 | 62 | 6.2 | 414 | 87.1 |
| 19-24 | 362 | 24.5 | 304 | 30.4 | 58 | 12.2 |
| 25-29 | 212 | 14.3 | 210 | 21.0 | <5 | 0.4 |
| 30-34 | 208 | 14.1 | 208 | 20.8 | 0 | 0 |
| 35-39 | 215 | 14.5 | 214 | 21.4 | <5 | 0.2 |
| Year of diagnosis | ||||||
| 2004-2007 | 469 | 31.8 | 341 | 34.1 | 128 | 26.9 |
| 2008-2011 | 548 | 37.2 | 360 | 36.0 | 188 | 39.5 |
| 2012-2014 | 456 | 30.9 | 297 | 29.7 | 159 | 33.4 |
| Sex | ||||||
| Male | 943 | 64.0 | 613 | 61.4 | 330 | 69.4 |
| Female | 530 | 35.9 | 385 | 38.5 | 145 | 30.5 |
| Race/ethnicity | ||||||
| NH white | 340 | 23.0 | 250 | 25.0 | 90 | 18.9 |
| NH African American | 45 | 3.0 | 33 | 3.3 | 12 | 2.5 |
| Hispanic | 939 | 63.7 | 622 | 62.3 | 317 | 66.7 |
| Asian/PI | 141 | 9.5 | 86 | 8.6 | 55 | 11.5 |
| Other/unknown | 8 | 0.5 | 7 | 0.7 | <5 | 0.2 |
| Neighborhood SES quintile | ||||||
| 1 Lowest | 472 | 32.0 | 324 | 32.4 | 148 | 31.1 |
| 2 | 334 | 22.6 | 225 | 22.5 | 109 | 22.9 |
| 3 | 262 | 17.7 | 175 | 17.5 | 87 | 18.3 |
| 4 | 215 | 14.5 | 147 | 14.7 | 68 | 14.3 |
| 5 Highest | 190 | 12.8 | 127 | 12.7 | 63 | 13.2 |
| Health insurance | ||||||
| Uninsured/self-pay | 38 | 2.5 | 32 | 3.2 | 6 | 1.2 |
| Insured NOS | 78 | 5.2 | 53 | 5.3 | 25 | 5.2 |
| Private/military | 636 | 43.1 | 417 | 41.7 | 219 | 46.1 |
| Public/Medicaid/Medicare | 696 | 47.2 | 472 | 47.2 | 224 | 47.1 |
| Unknown | 25 | 1.6 | 24 | 2.4 | <5 | 0.2 |
| Induction facility is COG center or NCI CC | ||||||
| No | 645 | 43.7 | 592 | 59.3 | 53 | 11.1 |
| Yes | 828 | 56.2 | 406 | 40.6 | 422 | 88.8 |
NH, non-Hispanic; NOS, not otherwise specified; PI, Pacific Islander; SES, socioeconomic status.
Across the total cohort, 56.2% received their initial ALL regimen at either an NCI CC or COG center. Among the 43.7% of AYAs who did not receive care at either an NCI-CC or COG center, a significantly higher proportion of AYAs were treated in an adult vs pediatric setting (59.3% vs 11.1%, P < .001) (Table 1).
Treatment regimens applied to AYA ALL across adult treatment settings
Of those treated in an adult setting, 24.8% of AYAs received a pediatric ALL regimen (Table 2). The administration of pediatric ALL regimens to AYAs treated in adult settings increased over time from 10.3% between 2004 and 2007 to 34.7% between 2012 and 2014 (P < .001). Factors associated with receipt of a pediatric ALL regimen in an adult setting include younger age (P = .007), treatment at an NCI CC (P < .001), residence in low SES neighborhoods (P = .015), and diagnosis in a more recent year (P < .001) (supplemental Table 2).
Table 2.
Chemotherapy regimens administered to AYA with ALL, ages 15-39 y, 2004-2014, California
| Setting | All years | 2004-2007 | 2008-2011 | 2012-2014 | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Adult treatment | ||||||||
| Adult ALL regimen | 543 | 75.6 | 181 | 89.6 | 213 | 73.4 | 149 | 65.9 |
| Hyper-CVAD | 306 | 42.6 | 74 | 36.6 | 123 | 42.4 | 109 | 48.2 |
| Adult cooperative group regimens* | 125 | 17.4 | 61 | 30.1 | 40 | 13.7 | 24 | 10.6 |
| Linker regimen | 84 | 11.6 | 28 | 13.8 | 43 | 14.8 | 13 | 5.7 |
| Other | 28 | 3.8 | 18 | 8.9 | 7 | 2.4 | <5 | 1.3 |
| Pediatric ALL regimen | 175 | 24.3 | 21 | 10.3 | 77 | 26.5 | 77 | 34.0 |
| C10403 | 67 | 9.3 | 0 | 0 | 36 | 12.4 | 31 | 13.7 |
| BFM | 45 | 6.2 | 12 | 5.9 | 20 | 6.8 | 13 | 5.7 |
| Pediatric cooperative group regimens | 38 | 5.2 | 5 | 2.4 | 16 | 5.5 | 17 | 7.5 |
| Other pediatric regimen | 25 | 3.4 | <5 | 1.9 | 5 | 1.7 | 16 | 7.0 |
| Pediatric treatment | ||||||||
| Pediatric ALL regimen | 472 | 100.0 | 126 | 100.0 | 187 | 100.0 | 159 | 100.0 |
| Pediatric cooperative group regimens^ | 328 | 69.4 | 79 | 62.6 | 123 | 65.7 | 126 | 79.2 |
| Other pediatric regimen | 144 | 30.5 | 47 | 37.3 | 64 | 34.2 | 33 | 20.7 |
BFM, Berlin-Frankfurt-Münster; CVAD, cyclophosphamide, vincristine sulfate, Adriamycin, and dexamethasone.
Adult cooperative group regimens with the exception of C10403.
OS and LSS
For the total cohort of AYAs, the median follow-up for alive patients was 3.5 years (range, 0-10.9 years) and the 3-year OS and LSS were 61.8% (95% CI, 58.9-64.5) and 66.4% (95% CI, 63.5-69.1), respectively. In multivariable adjusted models, treatment of AYA ALL patients in a pediatric (vs adult) setting was associated with significantly higher overall (HR = 0.53; 95% CI, 0.37-0.76) and leukemia-specific (HR = 0.51; 95% CI, 0.35-0.74) survival (Table 3; supplemental Figure 1). Similarly, treatment of AYA ALL at an NCI CC/COG center was associated with significantly higher overall (HR = 0.80; 95% CI, 0.66-0.96) and leukemia-specific (HR = 0.80; 95% CI, 0.65-0.97) survival. AYA ALL patients of Hispanic (OS: HR = 1.64; 95% CI, 1.30-2.06; LSS: HR = 1.76, 95% CI, 1.39-2.24) or African American race/ethnicity (overall survival: HR = 2.07; 95% CI, 1.28-3.35, LSS: HR = 2.01; 95% CI, 1.19-3.39) experienced lower survival than non-Hispanic white AYAs (Table 3). Results were similar when analyses were limited to younger AYAs, 15 to 24 years of age (Table 4; supplemental Figure 1).
Table 3.
Multivariable associations of patient and treatment characteristics with OS and LSS among AYA with ALL, ages 15-39 y, 2004-2014, California
| OS | LSS | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex | .27 | .64 | ||
| Female | 0.91 (0.76-1.08) | 0.96 (0.79-1.16) | ||
| Male | Ref. | Ref. | ||
| Age at diagnosis, y | .07 | .38 | ||
| 15-18 | 0.75 (0.51-1.10) | 0.86 (0.58-1.29) | ||
| 19-24 | 0.83 (0.63-1.08) | 0.85 (0.64-1.14) | ||
| 25-29 | 1.13 (0.84-1.51) | 1.08 (0.79-1.49) | ||
| 30-34 | 1.08 (0.81-1.44) | 1.09 (0.80-1.48) | ||
| 35-39 | Ref. | Ref. | ||
| Race/ethnicity | <.001 | <.001 | ||
| Asian/PI | 1.1 (0.79-1.08) | 1.14 (0.76-1.71) | ||
| Hispanic | 1.64 (1.30-2.06) | 1.76 (1.39-2.24) | ||
| NH African American | 2.07 (1.28-3.35) | 2.01 (1.19-3.39) | ||
| Other/ unknown | 1.97 (0.72-5.40) | 2.41 (0.88-6.61) | ||
| NH white | Ref. | Ref. | ||
| Treatment setting | .001 | <.001 | ||
| Pediatric | 0.53 (0.37-0.76) | 0.51 (0.35-0.74) | ||
| Adult | Ref. | Ref. | ||
| Induction facility is COG center or NCI CC | .019 | .026 | ||
| Yes | 0.80 (0.66-0.96) | 0.80 (0.65-0.97) | ||
| No | Ref. | Ref. | ||
Final model includes age, sex, and all variables significant at the P < .10 level.
Ref, reference.
Table 4.
Multivariable associations of patient and treatment characteristics with OS and LSS among AYA with ALL, ages 15-24 y, 2004-2014, California
| OS | LSS | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex | .26 | .53 | ||
| Female | 0.86 (0.66-1.12) | 0.92 (0.70-1.21) | ||
| Male | Ref. | Ref. | ||
| Age at diagnosis, y | .51 | .94 | ||
| 15-18 | 0.89 (0.64-1.25) | 1.01 (0.71-1.44) | ||
| 19-24 | Ref. | Ref. | ||
| Race/ethnicity | <.001 | <.001 | ||
| Asian/PI | 1.30 (0.72-2.37) | 1.45 (0.78-2.76) | ||
| Hispanic | 2.30 (1.59-3.31) | 2.48 (1.66-3.94) | ||
| NH African American | 3.10 (1.63-5.88) | 3.13 (1.56-6.32) | ||
| Other/ unknown | 0.86 (0.12-6.28) | 1.09 (0.15-7.99) | ||
| NH white | Ref. | Ref. | ||
| Treatment setting | .001 | .001 | ||
| Pediatric | 0.51 (0.35-0.74) | 0.51 (0.34-0.76) | ||
| Adult | Ref. | Ref. | ||
| Induction facility is COG center or NCI CC | .07 | .029 | ||
| Yes | 0.77 (0.58-1.02) | 0.72 (0.53-0.97) | ||
| No | Ref. | Ref. | ||
Final model includes age, sex, and all variables significant at the P < .10 level.
Among AYAs treated in the adult treatment setting only between 2009 and 2014, receipt of a pediatric (vs adult) ALL regimen was not associated with OS or LSS (Table 5; supplemental Figure 1). Similarly, there was no significant difference in OS (HR, 0.83; 95% CI, 0.58-1.20) or LSS (HR, 0.99; 95% CI, 0.66-1.47) among AYA receiving “hyper cyclophosphamide, vincristine sulfate, Adriamycin, and dexamethasone” as opposed to pediatric ALL regimens. Consistent with prior analyses, Hispanics experienced worse survival (OS: HR = 1.82, 95% CI 1.22-2.74; LSS: HR = 2.10; 95% CI, 1.31-3.35) than non-Hispanic white AYA with ALL, and treatment at an NCI CC was nonsignificantly associated with better LSS (HR = 0.70; 95% CI, 0.46-1.07). Results were similar when analyses were limited to younger AYAs, 15 to 24 years of age, treated in the adult setting (data not shown).
Table 5.
Multivariable associations of patient and treatment characteristics with OS and LSS among AYA with ALL treated in an adult setting, ages 15-39 y, 2009-2014, California
| OS | LSS | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex | .27 | .59 | ||
| Female | 0.82 (0.58-1.16) | 0.90 (0.62-1.31) | ||
| Male | Ref. | Ref. | ||
| Race/ethnicity | .60 | .04 | ||
| Asian/PI | 1.39 (0.73-2.62) | 1.73 (0.85-3.51) | ||
| Hispanic | 1.82 (1.22-2.74) | 2.10 (1.31-3.35) | ||
| NH African American | 2.04 (0.78-5.36) | 2.19 (0.74-6.51) | ||
| Other/ unknown | 2.02 (0.48-8.56) | 2.80 (0.64-12.17) | ||
| NH white | Ref. | Ref. | ||
| Front-line ALL regimen | .83 | .21 | ||
| Pediatric | 1.05 (0.70-1.57) | 1.33 (0.85-2.08) | ||
| Adult | Ref. | Ref. | ||
| Induction facility is COG center or NCI CC | 0.28 | 0.10 | ||
| Yes | 0.81 (0.56-1.19) | 0.70 (0.46-1.07) | ||
| No | Ref. | Ref. | ||
Final model is stratified on age and includes sex, type of induction, NCI/COG CC status, and all variables significant at the P < .10 level.
Discussion
In the current study of 1473 newly diagnosed AYA ALL patients in California from 2004 through 2014, we found a significant survival advantage for AYAs with ALL treated in a pediatric, as opposed to an adult cancer setting, as well as for AYA ALL patients cared for at NCI CC/COG centers. This study was based on population data enhanced with a previously piloted method of abstracting ALL treatment setting and chemotherapy regimen data from cancer registry free-text fields.22 We show that two-thirds of AYAs with newly diagnosed ALL are treated in an adult cancer setting, of which approximately 60% are community-based centers, and that as of 2014, only 33% of AYAs treated in an adult cancer setting received a pediatric-inspired ALL regimen. Our population-based findings complement those of prospective clinical trials by providing a “real-world” analysis of AYA ALL and identifying concerning gaps in patient management and outcomes that may be improved upon through educational, practice, and/or policy interventions.
Perhaps unsurprisingly, we found that the majority (87%) of AYAs aged 15 to 18 years were treated in a pediatric cancer setting, whereas nearly 100% of AYAs age ≥25 years were treated in an adult cancer setting. Nineteen- to 24-year-old AYAs were more commonly treated in an adult setting throughout the decade, but over time a shift occurred such that ALL patients in this age group were increasingly treated in a pediatric setting, up to 27.8% between 2012 and 2014. Regardless, in multivariable analyses taking into account age, we found that in AYAs ≤25 years, treatment in a pediatric cancer setting was associated with superior OS and LSS. As best as we could discern, our observations do not appear to be due to the pediatric regimen alone because pediatric vs adult regimen in the adult setting was not a significant predictor of outcome in AYA of all ages in our study. Instead, our observations may have resulted from unmeasured factors that have been shown to favor the pediatric setting such as minimal residual disease-driven ALL treatment algorithms,26 greater clinical trial enrollment,27,28 and improved therapeutic adherence.29 Our data highlight that referral of younger AYAs, up to at least age 25 years, to pediatric centers for ALL management should be strongly considered.
The association between management of AYA and adult cancer patients in NCI CC/COG centers and superior outcomes has been previously shown in population studies using data from local/regional cancer registries.30-32 Our data confirm a striking difference in whether AYAs are treated at an NCI CC/COG center based upon adult or pediatric cancer setting of care, with 88.9% and 40.6% of AYA treated in a pediatric vs adult setting receiving care at an NCI CC/COG center, respectively. NCI-designated CC status is granted based upon recognition of scientific leadership, resources, and the depth and breadth of research in basic, clinical, and/or population science.25 Similarly, in the pediatric cancer setting, COG center member institutions are expected to adhere to COG’s conduct of clinical research responsibilities and performance requirements.33 Given that ALL represents just 0.4% of all newly diagnosed cancers,34 and a tiny fraction of all cancers treated in the adult cancer setting, it is perhaps anticipated that outcomes of this rare malignancy are likely superior at centers with greater expertise and infrastructure to incorporate research and comprehensive leukemia care. The results of our study and others demonstrating the importance of NCI CC/COG centers to improving AYA ALL outcomes warrant further attention in the development of systematic quality metrics and improving access to specialty care in this vulnerable population.
Our findings demonstrate that over the past decade, only 25% of AYAs with ALL treated in an adult cancer setting received a pediatric ALL regimen as front-line therapy. Receiving a diagnosis of ALL in more recent years and treatment at an NCI CC were associated with receipt of a pediatric ALL regimen in the adult setting, yet even as recently as 2014, only one-third of AYAs were receiving pediatric-inspired therapy. This is striking, given that concurrent outcome comparisons of pediatric and adult treatment regimens performed in 10 countries as well as a large meta-analysis have demonstrated superiority of the pediatric regimen for young adults with ALL.7,35
In the current analyses, our ability to evaluate the long-term survival benefit of pediatric ALL regimens delivered in an adult cancer setting was limited by short median follow-up time (median follow-up of 2.3 years for surviving patients), in addition to our inability to reliably track sequential therapies (such as hematopoietic cell transplantation) and their influence on outcomes. This is an important limitation given that the large Medical Research Council/Eastern Cooperative Oncology Group ALL study was reported during this time and demonstrated improved outcomes with consolidative hematopoietic cell transplantation for young adults with standard-risk ALL who achieved first remission with front-line adult ALL therapy.36 The pediatric approach administered in the adult ALL setting has not been compared with traditional adult ALL regimens in a randomized fashion, and, because of the complexity and ethical constraints of such a study design, this is unlikely to be feasible. Although the pediatric approach appears superior in nearly all retrospective cooperative group studies and in single-arm phase 2 prospective studies, a large US single-center report showed equivalent survival between the pediatric approach and an optimized adult ALL regimen in young adults with ALL.14 Estimates of the long-term impact of pediatric ALL regimens administered by medical oncologists will require mature clinical trial results and future assessments of population-level data.
AYAs of Hispanic and African American race/ethnicity experienced worse OS and LSS relative to non-Hispanic white AYAs. Several studies in childhood ALL have reported inferior outcomes in Hispanic populations, likely, in part, due to a higher reported frequency of adverse biological risk variants, such as alterations in CRLF2, found in Hispanic and African American ALL populations that confer adverse leukemic risk.37-39 Additional factors, such as clinical trial enrollment, adherence to therapy, and access to specialized care have also been shown to contribute to disparities in ALL outcomes among racial/ethnic groups.29,31,40-42 Interestingly, we did find that there were significant differences in ALL regimens received based upon race/ethnicity; for example, C10403 was not administered to any non-Hispanic African American AYA in our sample. These findings warrant attention and focused interventions in order to narrow health outcome disparities as the US population continues to grow and diversify.
Population-based cancer registries, such as the CCR, provide invaluable data on cancer epidemiology and outcomes across the population that may not be gleaned from clinical trials or single-center reports and lack the associated selection biases.43 In addition to basic demographic, cancer type, and survival information, we were able to enrich our report on this AYA ALL cohort through detailed examination, first applying a text mining approach and then an individual record review, of facility-level reporting, yielding granular data on treatment setting and front-line ALL chemotherapy regimen administered. However, the CCR and other SEER registries lack important disease-specific information (such as ALL cytogenetic and molecular risk), detailed treatment data beyond front-line therapy, and critical treatment response metrics such as minimal residual disease. Further, we were unable to characterize differences in treatment toxicities between regimens, as well as therapeutic adherence, an area of heightened importance in the AYA population. Future population-science research incorporating detailed clinical, treatment, and patient-reported information, perhaps through comprehensive database development or database linkages, will be essential to improving access to optimal care and improving outcomes across the entire population of AYA ALL.
In summary, our data demonstrate a significant survival advantage for AYA ALL associated with treatment in a pediatric, as opposed to an adult cancer setting, suggesting that referral of younger AYAs, up to at least age 25 years, to pediatric centers for ALL management should be strongly considered. The results of our study and others demonstrate the importance of NCI CC/COG centers to improving AYA ALL outcomes and warrant further attention as only a minority of AYA ALL treated in the adult setting is cared for at these centers. Given the relatively recent adoption of pediatric ALL regimens by medical oncologists, mature population-level data are needed to better quantify the evolving impact of pediatric ALL regimens administered by medical oncologists in adult cancer settings.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer-reporting program mandated by California Health and Safety Code Section 103885; the National Institutes of Health, National Cancer Institute’s Surveillance, Epidemiology and End Results Program (contract HHSN261201000140C awarded to the Cancer Prevention Institute of California [principal investigator, S.L.G.], contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute); and the Centers for Disease Control and Prevention’s National Program of Cancer Registries (agreement U58DP003862-01 awarded to the California Department of Public Health). The study was supported by investigator initiated research funding from Shire Pharmaceuticals (principal investigator, L.M.).
The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Authorship
Contribution: L.M. designed research, analyzed data, and wrote the paper; E.A. designed research and wrote the paper; D.L. designed research, analyzed data, and wrote the paper; R.A. designed research and wrote the paper; S.L.G. designed research, analyzed data, and wrote the paper; and T.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lori Muffly, Department of Medicine, Division of Blood and Marrow Transplantation, Stanford University, 300 Pasteur Dr, H0144, Stanford, CA 94305; e-mail: lmuffly@stanford.edu.
References
- 1.Stock W, La M, Sanford B, et al. ; Cancer and Leukemia Group B studies. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boissel N, Auclerc MF, Lhéritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21(5):774-780. [DOI] [PubMed] [Google Scholar]
- 3.de Bont JM, van der Holt B, Dekker AW, van der Does-van den Berg A, Sonneveld P, Pieters R. Adolescents with acute lymphatic leukaemia achieve significantly better results when treated following Dutch paediatric oncology protocols than with adult protocols [in Dutch]. Ned Tijdschr Geneeskd. 2005;149(8):400-406. [PubMed] [Google Scholar]
- 4.Hallböök H, Gustafsson G, Smedmyr B, Söderhäll S, Heyman M;. Swedish Adult Acute Lymphocytic Leukemia Group; Swedish Childhood Leukemia Group. Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer. 2006;107(7):1551-1561. [DOI] [PubMed] [Google Scholar]
- 5.Ramanujachar R, Richards S, Hann I, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48(3):254-261. [DOI] [PubMed] [Google Scholar]
- 6.Adolescent and Young Adult Oncology Progress Review Group. Closing the gap: research and care imperatives for adolescents and young adults with cancer. United States Department of Health and Human Services, National Cancer Institute, and the LiveStrong Young Adult Alliance. Available at: https://deainfo.nci.nih.gov/advisory/ncab/archive/139_0906/presentations/AYAO.pdf. Accessed 26 February 2018. [Google Scholar]
- 7.Siegel SE, Stock W, Johnson RH, et al. Pediatric-inspired treatment regimens for adolescents and young adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: a review of advantages and challenges [published online ahead of print 15 February 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29(3):526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stock W, Luger SM, Advani AS, et al. Favorable outcomes for older adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL): early results of U.S. Intergroup Trial C10403 [abstract]. Blood. 2014;124(21). Abstract 796. [Google Scholar]
- 10.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study [published correction appears in J Clin Oncol. 2009;27(15):2574]. J Clin Oncol. 2009;27(6):911-918. [DOI] [PubMed] [Google Scholar]
- 11.Haïat S, Marjanovic Z, Lapusan S, et al. Outcome of 40 adults aged from 18 to 55 years with acute lymphoblastic leukemia treated with double-delayed intensification pediatric protocol. Leuk Res. 2011;35(1):66-72. [DOI] [PubMed] [Google Scholar]
- 12.Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia [published online ahead of print 18 August 2017]. Leukemia. doi:10.1038/leu.2017.265. [DOI] [PubMed] [Google Scholar]
- 13.Ribera JM, Oriol A, Sanz MA, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26(11):1843-1849. [DOI] [PubMed] [Google Scholar]
- 14.Rytting ME, Jabbour EJ, Jorgensen JL, et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Münster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91(8):819-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Advani AS, Sanford B, Luger S, et al. Frontline-treatment of acute lymphoblastic leukemia (ALL) in older adolescents and young adults (AYA) using a pediatric regimen is feasible: toxicity results of the Prospective US Intergroup Trial C10403. Blood. 2013;122(21):3903. [Google Scholar]
- 16.NCCN Guidelines, version 1.2017. Acute lymphoblastic leukemia. https://www.nccn.org/patients/guidelines/all/index.html#11. Accessed 26 February 2018.
- 17.Curran E, Stock W. How I treat acute lymphoblastic leukemia in older adolescents and young adults [published correction appears in Blood. 2015;126(15):1868]. Blood. 2015;125(24):3702-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boissel N. How should we treat the AYA patient with newly diagnosed ALL? Best Pract Res Clin Haematol. 2017;30(3):175-183. [DOI] [PubMed] [Google Scholar]
- 19.Fritz A, Percy C, Jack A, et al., eds. World Health Organization. International Classification of Diseases for Oncology. 3rd ed. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 20.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703-711. [DOI] [PubMed] [Google Scholar]
- 21.Shariff-Marco S, Yang J, John EM, et al. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2014;23(5):793-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muffly L, Lichtensztajn D, Shiraz P, et al. Adoption of pediatric-inspired acute lymphoblastic leukemia regimens by adult oncologists treating adolescents and young adults: a population-based study. Cancer. 2017;123(1):122-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. https://childrensoncologygroup.org/index.php/survivorshipguidelines. Accessed 1 March 2017.
- 24.Directory of Children’s Hospitals. https://www.childrenshospitals.org/Directories/Hospital-Directory?state=CA. Accessed 10 November 2017.
- 25.NCI Designated Cancer Centers. 2016. http://www.cancer.gov.laneproxy.stanford.edu/researchandfunding/extramural/cancercenters/abou. Accessed 1 June 2017.
- 26.Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017;129(14):1913-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potosky AL, Harlan LC, Albritton K, et al. ; AYA HOPE Study Collaborative Group. Use of appropriate initial treatment among adolescents and young adults with cancer. J Natl Cancer Inst. 2014;106(11):dju300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol. 2012;30(17):2094-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho G, Jonas BA, Li Q, Brunson A, Wun T, Keegan THM. Early mortality and complications in hospitalized adult Californians with acute myeloid leukaemia. Br J Haematol. 2017;177(5):791-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfson J, Sun CL, Wyatt L, Stock W, Bhatia S. Adolescents and young adults with acute lymphoblastic leukemia and acute myeloid leukemia: impact of care at specialized cancer centers on survival outcome. Cancer Epidemiol Biomarkers Prev. 2017;26(3):312-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfson JA, Sun CL, Wyatt LP, Hurria A, Bhatia S. Impact of care at comprehensive cancer centers on outcome: results from a population-based study. Cancer. 2015;121(21):3885-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Children’s Oncology Group. https://childrensoncologygroup.org/downloads/MI_ConductofClinicalResearchFINAL.pdf. Accessed 1 June 2017.
- 34.Surveillance, Epidemiology, and End Results Program. SEER*Stat software: incidence - SEER 18. http://www.seer.cancer.gov. Accessed 1 October 2017.
- 35.Ram R, Wolach O, Vidal L, Gafter-Gvili A, Shpilberg O, Raanani P. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am J Hematol. 2012;87(5):472-478. [DOI] [PubMed] [Google Scholar]
- 36.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833. [DOI] [PubMed] [Google Scholar]
- 37.Karol SE, Larsen E, Cheng C, et al. Genetics of ancestry-specific risk for relapse in acute lymphoblastic leukemia. Leukemia. 2017;31(6):1325-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahn JM, Keegan TH, Tao L, Abrahão R, Bleyer A, Viny AD. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122(17):2723-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez E, Keegan T, Johnston EE, et al. Adolescent and young adult oncology patients: Disparities in access to specialized cancer centers. Cancer. 2017;123(13):2516-2523. [DOI] [PubMed] [Google Scholar]
- 41.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirtane K, Lee SJ. Racial and ethnic disparities in hematologic malignancies. Blood. 2017;130(15):1699-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visvanathan K, Levit LA, Raghavan D, et al. Untapped potential of observational research to inform clinical decision making: American Society of Clinical Oncology Research Statement. J Clin Oncol. 2017;35(16):1845-1854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


