Key Points
Human red pulp macrophages are distinct from monocytes and monocyte-derived macrophages in the expression of FcγRs and other surface markers.
Red pulp macrophages phagocytose IgG-opsonized blood cells by activating FcγRs and are sensitive to IV immunoglobulin blocking
Abstract
Tissue-resident macrophages in the spleen play a major role in the clearance of immunoglobulin G (IgG)–opsonized blood cells, as occurs in immune thrombocytopenia (ITP) and autoimmune hemolytic anemia (AIHA). Blood cells are phagocytosed via the Fc-γ receptors (FcγRs), but little is known about the FcγR expression on splenic red pulp macrophages in humans, with only a few previous studies that showed conflicting results. We developed a novel method to specifically isolate red pulp macrophages from 82 human spleens. Surface expression of various receptors and phagocytic capacity was analyzed by flow cytometry and immunofluorescence of tissue sections. Red pulp macrophages were distinct from splenic monocytes and blood monocyte–derived macrophages on various surface markers. Human red pulp macrophages predominantly expressed the low-affinity receptors FcγRIIa and FcγRIIIa. In contrast to blood monocyte–derived macrophages, red pulp macrophages did not express the inhibitory FcγRIIb. Red pulp macrophages expressed very low levels of the high-affinity receptor FcγRI. Messenger RNA transcript analysis confirmed this expression pattern. Unexpectedly and despite these differences in FcγR expression, phagocytosis of IgG-opsonized blood cells by red pulp macrophages was dependent on the same FcγRs as phagocytosis by blood monocyte–derived macrophages, especially in regarding the response to IV immunoglobulin. Concluding, we show the distinct nature of splenic red pulp macrophages in human subjects. Knowledge on the FcγR expression and usage of these cells is important for understanding and improving treatment strategies for autoimmune diseases such as ITP and AIHA.
Visual Abstract
Introduction
Circulating blood cells that are opsonized by immunoglobulin G (IgG) autoantibodies can be rapidly cleared from the circulation. For instance, autoantibodies against platelets result in immune thrombocytopenia (ITP), whereas autoantibodies against erythrocytes result in autoimmune hemolytic anemia. These blood cells are presumably cleared by macrophages that have direct contact with blood cells (ie, macrophages of the reticuloendothelial system), which comprise macrophages in the spleen and the liver.1,2 In vivo studies with radioactively labeled IgG-opsonized platelets and erythrocytes have revealed that, in the majority of patients, the spleen, not the liver, is the predominant site of blood cell destruction,3,4 a notion that is supported by the fact that splenectomy can be an effective last-resort treatment of refractory ITP.5 Macrophages clear these IgG-opsonized blood cells by phagocytosis, which is mediated by the receptors for IgG (Fc-γ receptors [FcγRs]).1,6 However, splenic macrophages have been poorly characterized thus far in humans, especially with regard to FcγR expression. Functional studies of IgG-mediated phagocytosis in humans often use monocyte-derived macrophages instead7,8 because they are readily available. However, because it is not well known whether these macrophages are similar to splenic macrophages, the relevance of such studies can be debated. In fact, recent evidence from studies in rodents has led to a paradigm shift regarding the origin of macrophages. Many tissue-resident macrophage populations, including the red pulp macrophages of the spleen, have been shown to largely consist of self-renewing populations derived from embryonic macrophages, established before birth, instead of differentiating from blood monocytes.9,10 This suggests that splenic macrophages in humans may also be phenotypically very different from monocyte-derived macrophages.
Human splenic tissue can be divided into red pulp, which consists of an open circulation and contains many erythrocytes, and white pulp, which consists of lymphoid tissue including the periarteriolar lymphoid sheaths and follicles.11 The area between the follicles and red pulp has sometimes been designated as a so-called perifollicular zone.11,12 Within these splenic tissues, different subsets of macrophages have been identified in humans. The most abundant type of macrophage is the red pulp macrophage, characterized by expression of CD163, which is thought to be involved in the clearance of aged red blood cells from the circulation and iron metabolism.11,13 The open circulation of the red pulp functions as a filter for blood, resulting in close contact of red pulp macrophages with circulating blood cells, facilitating the uptake of senescent erythrocytes.11 Other types of macrophages have been associated with capillary sheaths in the red pulp and the perifollicular zone.14 These capillary sheath–associated macrophages are CD163−, and the part of this population that is located close to follicles (ie, perifollicular zone) is strongly positive for sialoadhesin (CD169).12,14
Macrophages throughout the red pulp of the spleen are known to express receptors for IgG and bind IgG-opsonized erythrocytes.15 The FcγR are considered important for clearance of IgG complexes and IgG-opsonized cell material. Five of the 6 isoforms of human FcγR, all with differences in affinities for IgG and function, can be found on monocyte-derived macrophages.16 However, there is little conclusive evidence available for the isoforms of FcγR that are expressed on splenic macrophages in humans. Immunohistochemistry studies have shown that FcγRIII is present throughout the red pulp, but was unclear which cell type is responsible for the expression of this IgG receptor isoform.17 Another study using immunohistochemistry suggested that FcγRI, FcγRII, and FcγRIII all are present on macrophages of the spleen.18 These studies could not distinguish between the isoforms of FcγRII and FcγRIII.
Recent studies have investigated FcγR expression and function of splenic phagocytes using isolated splenic cells. This suggested that FcγRI, FcγRIIa, and FcγRIII are all expressed on splenic macrophages, whereas FcγRIIb was expressed on only 30% of cells.19 In phagocytosis assays with ex vivo obtained splenic macrophages, FcγRI was suggested as the critical FcγR, whereas blocking FcγRIII had little or no effect.20,21 However, all of these studies used isolation methods that cannot distinguish between circulating monocytes and red pulp macrophages.
Altogether, the evidence about FcγR expression on human splenic macrophages has remained confusing. In the present study, we developed a protocol to specifically obtain splenic red pulp macrophages, and used a set of FcγR isoform-specific monoclonal antibodies (MoAbs) as well as functional assays to determine the cell type–specific expression and function as compared with monocytes and monocyte-derived macrophages. Our studies show major differences between these different cell types.
Methods
Human subjects
Spleen tissue from organ transplant donors was used in this study, as described previously.22 Written informed consent for organ donation was obtained according to national regulations regarding organ donation. Splenic tissue of the organ donor was obtained during transplantation surgery as part of the standard diagnostic procedure for HLA typing, and was transported in University of Wisconsin Fluid at 4°C. In case of excess splenic tissue for diagnostic procedures, the excess of splenic tissue was used in an anonymous fashion for research in the present study, in accordance with the Dutch law regarding the use of rest material for research purposes. Blood samples used were rest material from blood taken from the same organ donors, drawn at the time of surgery as a standard diagnostic procedure. In some cases, when matched blood samples were not available, control blood from age-matched healthy volunteers was used. Written informed consent was obtained from all healthy volunteers. The study was approved by the Medical Ethics Committee of the Academic Medical Center and Sanquin in Amsterdam, and was performed in accordance with the Declaration of Helsinki.
Preparation of single-cell suspension of splenocytes
Splenocytes were isolated by injecting a piece of spleen at several sites with collagenase buffer containing 100 U/mL collagenase (CLSPA; Worthington Biochemical Corporation), 2 Kunitz units/mL DNase (deoxyribonuclease I, bovine recombinant) (Sigma-Aldrich), 0.5 μg/mL Aggrastat (Merck, Sharp & Dohme), 1 mg/mL glucose (Sigma-Aldrich), and 1 mM calcium chloride (Merck) in HEPES. Connective tissue was removed and subsequently incubated in the collagenase buffer for 30 minutes at 37°C. Tissue was then filtered using a 100-μm filter. Subsequently, erythrocytes were lysed with an isotonic ammonium chloride buffer for 5 minutes at 4°C, after which the lysis buffer was washed away.
Preparation of blood leukocytes and monocyte-derived macrophages
Whole blood leukocytes were isolated from heparin or EDTA blood (spleen donors) or from heparin blood (healthy controls) by lysis of red blood cells with an isotonic ammonium chloride buffer. Monocyte-derived macrophages were cultured for 9 days with either macrophage colony-stimulating factor (M-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) from monocytes isolated from heparin blood of healthy controls, as previously described.8
Elutriation
To enrich for large cells from the single cell suspension of splenocytes before sorting, the splenocytes were purified by counterflow centrifugal elutriation (JE-6B Beckman-Coulter centrifuge; Beckman Instruments Inc, Palo Alto, CA). During this purification, the fractions were monitored on a Forward scatter, Side scatter plot on a FACS Canto II machine (BD).
Cell sorting
The elutriated fraction of splenocytes was stained with CD163-PE (Trillium Diagnostics) and CD14-PE-Cy7 (BD Pharmingen) for 30 minutes in the dark, shaken, at 4°C, and washed twice. Cells were sorted on a FACS ARIA II machine (BD), and were collected at 4°C. Purity of the sorted fractions was checked by flow cytometry on a FACS Canto II machine (BD). Red pulp macrophages were subjected to a second round of sorting, consistently yielding a purity >95%. Cytospins were made after sorting and stained with May-Grünwald Giemsa for morphological analysis.
Flow cytometry
For determination of expression levels on different cell populations from the spleen, the single-cell suspension of splenocytes was stained with directly labeled specific MoAb. Samples were measured on a FACS CANTO II (BD). For a list of MoAb, see supplemental Table 1.
Genetic analysis
Genomic DNA was isolated from splenocytes with the QIAamp Blood Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Copy number variations and single nucleotide polymorphisms in the low-affinity FCGR genes FCGR2A, FCGR2B, FCGR2C, FCGR3A, and FCGR3B were routinely determined with an FCGR-specific multiplex ligation-dependent probe amplification assay (MRC-Holland, Amsterdam, The Netherlands) as described previously.23
Quantitative mRNA analysis
Messenger RNA (mRNA) was isolated from the different cell populations with the QIAamp RNA blood mini kit (Qiagen, Hilden, Germany). Complementary DNA (cDNA) synthesis was performed as described previously.24 Real-time quantitative polymerase chain reaction (qPCR) on the cDNA was performed on a LightCycler machine (Roche Applied Science), and expression was measured as described previously24 for FCGR1A1 (encoding FcγRI), FCGR2A (encoding FcγRIIa), FCGR2B2 (encoding FcγRIIb on myeloid cells16), FCGR3A (encoding FcγRIIIa), FCGR3B (encoding FcγRIIIb), and housekeeping genes glyceraldehyde-3-phosphate dehydrogenase and β-glucuronidase. A list of primers used is shown in supplemental Table 2. cDNA from pooled whole blood leukocytes of 4 healthy blood donors was used as a standard curve, with serial 10-fold dilutions of this cDNA quantified with the method described in Technical Note No. LC 13/2001 (Roche Applied Science). Expression levels in the different cell populations were always compared with this standard curve of pooled whole blood leukocytes, as calculated by Lightcycler software. Subsequently, the values obtained were corrected for cDNA input by dividing the values obtained for each transcript of interest by the value of the geometric mean of housekeeping genes glyceraldehyde-3-phosphate dehydrogenase and β-glucuronidase of that sample, following the Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines.25 This resulted in the relative expression reported in the figures.
Immunofluorescence
Small pieces of human spleen were embedded in Tissue Tek (Sakura, Alphen aan de Rijn, The Netherlands), frozen in liquid nitrogen vapor, and stored at −80°C. Cryostat sections of 7-µm thickness were cut, fixed in acetone for 10 minutes, and rehydrated in phosphate-buffered saline (PBS). The sections were blocked with 5% human serum in PBS for 15 minutes at room temperature. The sections were subsequently stained with directly labeled specific MoAb (supplemental Table 1) identifying macrophages and FcγR for 30 minutes at room temperature. After washing with PBS, the sections were embedded in vinol containing 4′6-diamidino-2-phenylindole to stain the nuclei. Isotype-matched antibodies were used as negative controls. For each wavelength, the detection limit of the microscope was calibrated to the isotype control (ie, the detection limit was increased until the level was reached at which the isotype control did not show any fluorescent signal anymore, thereby correcting for aspecific binding as well as autofluorescence). Stainings were analyzed using a DM6000 Leica immunofluorescence microscope.
Phagocytosis assays
Red blood cells of healthy volunteers positive for the rhesus D (RhD) blood group were isolated and stained with carboxyfluorescein diacetate succinimidyl ester (CFSE; Life Technologies) for 30 minutes. The stained erythrocytes were subsequently opsonized with human polyclonal anti-RhD (RheDQuin, Sanquin) for 30 minutes or left unopsonized, after which the cells were washed twice.
Spleen macrophages and monocytes were purified as described previously, after which the cells were resuspended in Iscove modified Dulbecco medium (Gibco), containing 10% fetal calf serum (Bodinco), glutamine, and antibiotics. Cells were incubated with erythrocytes in a ratio of 1:10. After a 2-hour incubation at 37°C, phagocytosis was stopped by transferring the cells to ice and lysing the nonphagocytosed erythrocytes by an isotone ammonium chloride lysis buffer for 5 minutes at 4°C. After washing in PBS, the ingestion of CFSE-positive erythrocytes by macrophages was quantified by flow cytometry on a FACS CANTO II (BD Biosciences). IgG-mediated phagocytosis was calculated as percentage positive macrophages minus the percentage positive macrophages of the unopsonized control, as described previously.8 FcγRs were blocked by adding monoclonal antibodies at 10 µg/mL 5 minutes before the addition of erythrocytes: intact anti-CD64 clone 10.1 (BioLegend), F(ab′)2 fragment anti-CD64 clone 10.1 (Ancell), F(ab′)2 fragment anti-CD32 clone 7.3 (Ancell), F(ab′)2 fragment anti-CD16 clone 3G8 (Ancell), and F(ab′)2 fragment of mouse IgG1 isotype control (Ancell).
Preparations of IV immunoglobulin (Nanogam, Sanquin) were used in 10, 1, and 0.1 µg/mL and also added 5 minutes before the addition of red blood cells.
Results
FcγR-expression on splenic macrophages as detected by immunofluorescence
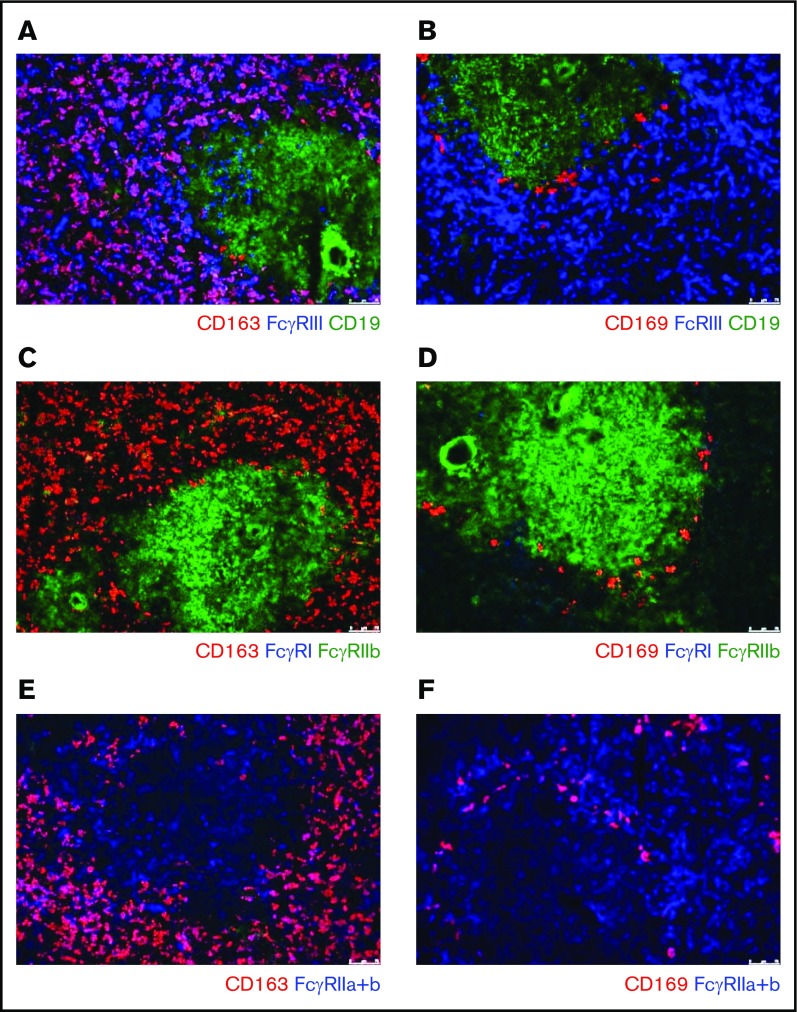
To investigate the FcγR expression pattern of splenic macrophages, we performed immunofluorescent stainings on sections of human spleen tissue. FcγRIIa and FcγRIII were expressed on CD163pos macrophages in the red pulp, whereas FcγRI and FcγRIIb were not detectable on these cells (Figure 1A,C,E). In the tissue sections, we also detected the CD169+ macrophages in the perifollicular zone. These perifollicular zone macrophages only demonstrated a clear expression of FcγRIIa, possibly a low expression of FcγRIIb, but no detectable expression of FcγRI and FcγRIII (Figure 1B,D-E).
Figure 1.
Immunofluorescence of spleen tissue shows FcγR expression pattern of splenic macrophages. Immunofluorescent costainings of (A) CD163, FcγRIII, and CD19; (B) CD169, FcγRIII, and CD19; (C) CD163, FcγRI, and FcγRIIb; (D) CD169, FcγRI, and FcγRIIb; (E) CD163 and FcγRIIa+b; and (F) CD169 and FcγRIIa+b. Original magnification ×10. CD163 marks the red pulp macrophages, CD169 marks the perifollicular zone macrophages, CD19 marks B cells in the follicles. B cells in the follicle are also positive for FcγRIIb. Figures are representative of n = 3 spleens from different donors.
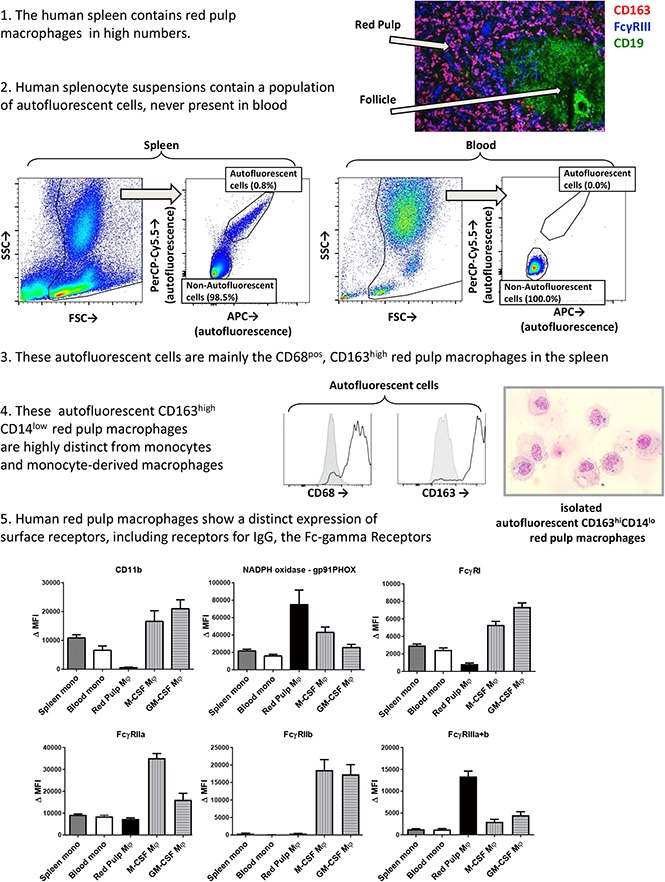
The spleen contains an autofluorescent cell population of the monocyte/macrophage lineage
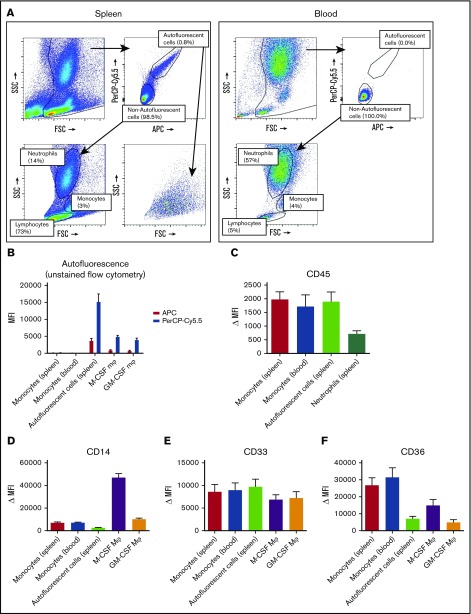
To be able to more specifically investigate splenic macrophages for FcγR surface expression and functional experiments, we set out to isolate resident splenic macrophages from splenic tissue by gentle collagenase digestion. We analyzed the single-cell suspensions of splenocytes by flow cytometry, which revealed a consistent population of autofluorescent cells in unstained samples, which did not occur in suspensions of whole blood leukocytes (Figure 2A). Apart from the autofluorescent cells, the splenocyte suspension contained, based on canonical forward sideward scatter patterns, populations of neutrophils, monocytes, and a large population of lymphocytes (Figure 2A). Autofluorescence is a well-known characteristic of macrophages,26 and these cells showed a similar pattern in autofluorescence as monocyte-derived macrophages, albeit somewhat more pronounced (Figure 2B). To confirm that the autofluorescent splenic cells represented macrophages, we stained for canonical lineage markers of leukocytes, corrected for appropriate isotype controls. First, these cells were positive for the leukocyte common antigen CD45 (Figure 2C), confirming hematopoietic origin. Second, these cells were positive for the common monocyte/macrophage markers CD14, CD33, and CD36 (Figure 2D-F), while being negative for the lymphocyte markers CD3 (T cells), CD19 (B cells), and CD56 (NK cells) (supplemental Figure 1). Thus, we can conclude that the autofluorescent cells are of the monocyte/macrophage lineage.
Figure 2.
Splenocytes contain an autofluorescent cell population of the monocyte/macrophage lineage. (A) Representative flow cytometry plots of unstained single-cell suspensions of splenocytes (left) and blood leukocytes (right) showing an autofluorescent cell population in splenocytes but not in blood. Another consistent feature of splenocytes is the large proportion of lymphocytes, which are in part larger than blood lymphocytes. Percentages are indicated, figure is representative of n = 82 spleens. (B) MFIs for allophycocyanin (APC) and peridinin-chlorophyll protein-Cy5.5 (PerCP-Cy5.5) in unstained samples, comparing the autofluorescent cells in the spleen and spleen monocytes gated as in panel A, with blood monocytes, M-CSF Mφ, and GM-CSF Mφ cultured monocyte-derived macrophages. (C-F) Stainings of CD45, CD14, CD33, and CD36 on various cell types in spleen and blood. Mean ± standard error of the mean (SEM) of n ≥ 8 are shown for each group. FSC, forward scatter; MFI, median fluorescence intensity; Δ MFI, MFI corrected for staining with an isotype control; M-CSF Mφ , monocyte-derived macrophages cultured for 9 days with M-CSF; GM-CSF Mφ, monocyte-derived macrophages cultured for 9 days with GM-CSF; SSC, side scatter.
Splenic red pulp macrophages can be identified as autofluorescent CD163highCD14low cells
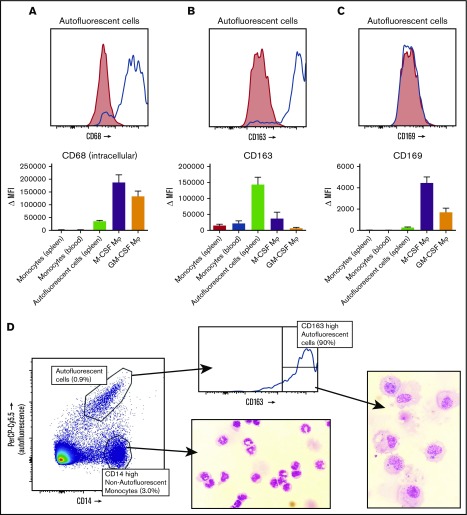
Intracellular staining with the macrophage marker CD68 identified the autofluorescent cells to be macrophages, not monocytes (Figure 3A). We then set out to stain these macrophages with the known markers for splenic macrophages, CD163 and CD169. The autofluorescent cells were highly positive for the red pulp macrophage marker CD163 (Figure 3B), although a small percentage (∼10%) of the cells was usually negative, especially in spleens that had been removed >24 hours before handling. These CD163− autofluorescent events consisted merely of cellular debris and dying cells upon morphological analysis (data not shown). CD169 appeared to be present in very low levels, just above background staining, but we never detected a CD169high population, suggesting that we did not recover the small population of perifollicular zone macrophages in our spleen suspensions (Figure 3C). The monocytes in the spleen also expressed low levels of CD163 (Figure 3B) and no CD169 (Figure 3C). To further confirm that the autofluorescent CD163+ cells in the spleen are red pulp macrophages, we set out to obtain pure fractions of these cells for morphological analysis. We first enriched for large cells by elutriation of the splenocytes, which increased the percentage of autofluorescent CD163highCD14low cells by approximately 4 times. Cells were subsequently sorted for autofluorescent CD163highCD14low cells and nonautofluorescent CD163intCD14high monocytes (Figure 3D). Morphological analysis of autofluorescent CD163highCD14low cells revealed a typical macrophage phenotype, with a round nucleus, multiple granular bodies, and, in some cases, whole ingested erythrocytes (Figure 3D). The monocytes were similar to normal blood monocytes (Figure 3D).
Figure 3.
Spleen autofluorescent cells are macrophages and represent the red pulp macrophages of the spleen. Flow cytometry stainings on monocytes in the spleen, blood monocytes, spleen autofluorescent cells, M-CSF, and GM-CSF cultured monocyte-derived macrophages. (A-C, top) Representative histogram on spleen autofluorescent cells (blue line, specific staining; red shading, isotype control). (A-C, bottom) Summary of Δ MFIs of multiple stainings. (A) Intracellular staining for CD68. (B) CD163 staining. (C) CD169 staining. (D) Sorting strategy for splenocytes sorted into autofluorescent CD163highCD14low red pulp macrophages and nonautofluorescent CD163intCD14high spleen monocytes, and May-Grünwald-Giemsa stainings (original magnification ×40) of the sorted cell populations, representative of n = 8 experiments. Mean ± SEM of n ≥ 7 are shown for each group.
Red pulp macrophages are distinct from monocytes and monocyte-derived macrophages
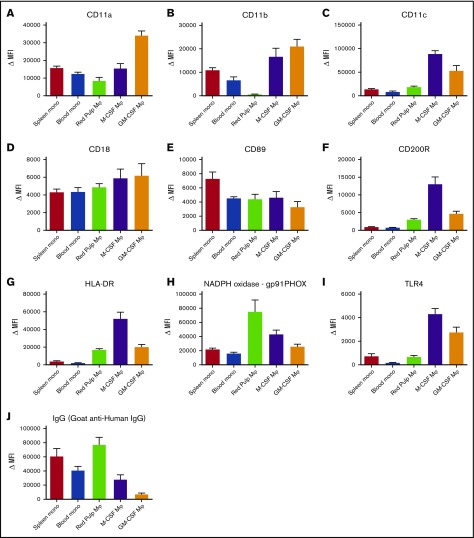
Next, we compared these autofluorescent CD163highCD14low red pulp macrophages with monocytes and monocyte-derived macrophages for several canonical phagocyte surface receptors. This revealed several marked differences, such as the absence of the integrin CD11b on red pulp macrophages (Figure 4D), whereas the other integrins that we tested for were present (Figure 4A,C-D). Various other surface markers (Figure 4E-I) showed marked differences from both monocytes and monocyte-derived macrophages, most notably the very high surface expression of gp91phox, one of the essential proteins of the reduced NADPH oxidase complex required for the production of reactive oxygen species (Figure 4H). Altogether, our results indicate that red pulp macrophages are highly distinct from both monocytes and monocyte-derived macrophages. On the other hand, monocytes of spleen and blood were similar to each other in their expression of all tested surface markers.
Figure 4.
Red pulp macrophages exhibit an expression pattern distinct from monocytes and monocyte-derived macrophages. Flow cytometry stainings on nonautofluorescent CD163intCD14high spleen monocytes, CD14high blood monocytes gated on FSC/SSC patterns, autofluorescent CD163highCD14low red pulp macrophages from spleen, and M-CSF and GM-CSF cultured monocyte-derived macrophages, (A) CD11a, (B) CD11b, (C) CD11c, (D) CD18, (E) CD89, (F) CD200R, (G) HLA-DR, (H) gp91PHOX (reduced NAD phosphate [NADPH] oxidase complex), (I) TLR4, and (J) IgG (polyclonal goat anti-human IgG). Mean ± SEM of n ≥ 8 are shown for each group.
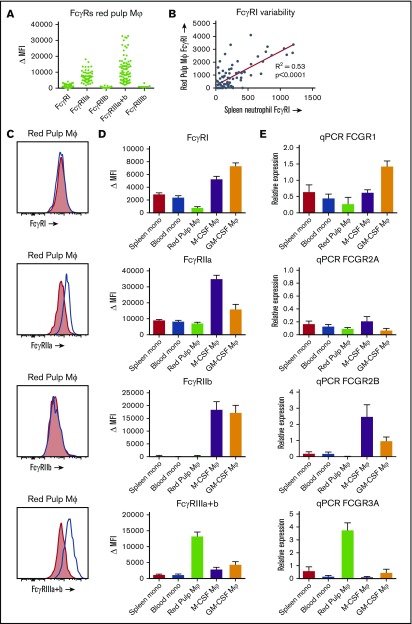
Red pulp macrophages have a distinct FcγR expression pattern
FcγR isoform expression on spleens from 82 individuals revealed a distinct expression pattern (Figure 5A,C). When compared with monocytes and monocyte-derived macrophages, levels of the high-affinity FcγRI (CD64) were low (Figure 5D). In fact, red pulp macrophages from some individuals showed no detectable expression at all, whereas others showed clear expression of this receptor. Because FcγRI is known to be induced in inflammatory states in neutrophils,27 we investigated whether this could be the case in our spleen donors. Indeed, there was a strong correlation of FcγRI expression on red pulp macrophages with FcγRI levels on neutrophils in the same spleen (Figure 5B). FcγRIIa (CD32a) was expressed in both monocytes and red pulp macrophages at similar levels, although lower than the levels detected in monocyte-derived macrophages (Figure 5C-D). The inhibitory FcγRIIb (CD32b) appeared not to be present on red pulp macrophages at all (Figure 5C-D). On monocytes, only a small subset expressed FcγRIIb,16 whereas FcγRIIb levels on monocyte-derived macrophages were high (Figure 5D). FcγRIIc (CD32c), an activating Fc receptor that has an extracellular domain identical to FcγRIIb, was not present (supplemental Figure 2). Finally, FcγRIII (CD16) was always expressed by the whole population of red pulp macrophages (Figure 5C-D), which is strikingly different from both monocytes and monocyte-derived macrophages. Blood and spleen monocytes and monocyte-derived macrophages only show a small subset of CD16+ cells, with CD16 being low or absent on the majority of cells (data not shown).8,16 We then investigated which isoform of FcγRIII was present on the red pulp macrophages by staining with MoAb 5D7 (also known as CLBgran11), which specifically recognizes the neutrophil-specific, phospatidyl inositol–anchored FcγRIIIb. The negative staining with this MoAb revealed that red pulp macrophages exclusively express the transmembrane form of FcγRIII, FcγRIIIa (Figure 5A; supplemental Figure 3A). Finally, we performed a qPCR analysis of mRNA expression of the FcγR isoforms, confirming the expression differences between the different cell types (Figure 5E; supplemental Figure 3B).
Figure 5.
FcγR expression on red pulp macrophages is distinct from the expression on monocytes and monocyte-derived macrophages. (A) Overview of expression of the FcγR isoforms on autofluorescent CD163highCD14low red pulp macrophages from spleen, showing individual values. FcγRI (MoAb 10.1), n = 82; FcγRIIa (MoAb IV.3), n = 57; FcγRIIb (MoAb 2B6), n = 43; FcγRIIIa+b (MoAb 3G8), n = 82; FcγRIIIb (MoAb 5D7), n = 7. (B) Correlation of FcγRI expression on red pulp Mφ with FcγRI levels on neutrophils in the same spleen (n = 82). (C) Representative histograms on autofluorescent CD163highCD14low red pulp macrophages from spleen for FcγRI, FcγRIIa, FcγRIIb, and FcγRIIIa+b (blue line: specific staining, red shading: isotype control). (D) Comparison of flow cytometry stainings on nonautofluorescent CD163intCD14high spleen monocytes, CD14high blood monocytes gated on FSC/SSC pattern, autofluorescent CD163highCD14low red pulp macrophages from spleen, and M-CSF and GM-CSF cultured monocyte-derived macrophages. FcγRI: n ≥ 24 for each group. FcγRIIa: n ≥ 10 for each group. FcγRIIb: n ≥ 15 for each group. To ensure specificity for FcγRIIb, only samples from individuals that cannot express FcγRIIc and with wild-type FCGR2B promoters are presented. FcγRIIIa+b: n ≥ 23 for each group. Mean ± SEM are shown for each group. (E) Quantitative mRNA analysis of various FCGR transcripts encoding FcγRs. The relative expression compared with the expression in pooled whole blood leukocytes, corrected for housekeeping genes, is shown for each transcript. Mean ± SEM of n = 3 are shown for each group.
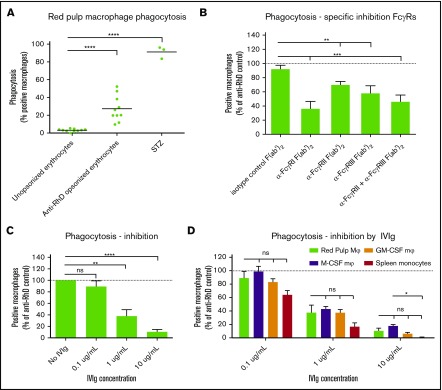
Isolated red pulp macrophages phagocytose IgG-opsonized red blood cells, using all FcγRs, most notably FcγRI
To determine whether red pulp macrophages do indeed phagocytose IgG-opsonized erythrocytes, we isolated pure fractions of red pulp macrophages as shown in Figure 3D. Incubating these cells with CFSE-labeled, RhD+ human erythrocytes, opsonized with human anti-RhD antibodies, we observed that these cells phagocytosed IgG-opsonized erythrocytes, whereas unopsonized erythrocytes were essentially not phagocytosed at all (Figure 6A). Phagocytic capacity of the vast majority of red pulp macrophages was shown by their capacity to phagocytose serum-treated zymosan particles. Because the FcγR expression pattern of the red pulp macrophages is notably different from the expression on monocyte-derived macrophages (Figure 5), we determined the relative contribution of the isoforms of FcγR on the phagocytosis by blocking with FcγR-specific F(ab′)2 fragments. Despite low-to-absent surface expression, FcγRI was involved in phagocytosis, because blocking this receptor greatly reduced phagocytosis of IgG-opsonized erythrocytes (Figure 6B). This was the case using F(ab′)2 fragments as well as intact antibodies of MoAb clone 10.1, which showed similar blocking potential (supplemental Figure 4A). Blocking of FcγRIIa and FcγRIIIa with well-characterized inhibiting F(ab′)2 fragments also significantly reduced phagocytosis, but to a lesser extent than the FcγRI MoAb 10.1 (Figure 6B). Comparing the relative contribution of the different FcγRs to phagocytosis between red pulp macrophages, monocyte-derived macrophages and spleen monocytes showed a similar pattern (supplemental Figure 4B-G). M-CSF macrophages were more dependent on FcγRIIa (supplemental Figure 4C) and GM-CSF macrophages were more dependent on FcγRI when blocked with intact MoAb 10.1 (supplemental Figure 4F). Finally, we showed that IgG-mediated phagocytosis by red pulp macrophages can be inhibited by preparations of IV immunoglobulins (IVIg) in a dose-dependent manner (Figure 6C). This inhibition was similar to IVIg-mediated inhibition in monocyte-derived macrophages and spleen monocytes (Figure 6D).
Figure 6.
Red pulp macrophages phagocytose IgG-opsonized erythrocytes. (A) Sorted red pulp macrophages phagocytose erythrocytes opsonized with human anti-RhD antibodies, but do not phagocytose unopsonized erythrocytes. Individual values are shown for n = 10 experiments. Serum treated zymosan (STZ) serves as a positive control indicating phagocytic capacity of these cells (n = 3). (B) Phagocytosis of anti-RhD opsonized erythrocytes by red pulp macrophages can be inhibited by F(ab′)2 fragment against specific FcγRs. Isotype control, n = 9; α-FcγRI; n = 3; α-FcγRII, n = 10; α-FcγRIII, n = 10; α-FcγRII+III, n = 9. Data are normalized against phagocytosis by unblocked macrophages. (C) Phagocytosis of anti-RhD opsonized erythrocytes by red pulp macrophages can be inhibited by IVIg in a concentration-dependent manner. n = 4 for each concentration. (D) Comparison of inhibition by IVIg in various cell types. Red pulp macrophages, n = 4; spleen monocytes, n = 3, M-CSF Mφ, n = ≥22; M-CSF, n = 26. Data in panels B-D are normalized against phagocytosis by unblocked macrophages. Mean ± SEM are shown for each group throughout this figure.
Discussion
We have characterized the major resident macrophages in the human spleen, the red pulp macrophages, as a unique population with major differences in expression for surface receptors such as the FcγRs, as opposed to monocytes and monocyte-derived macrophages. Our newly developed isolation method takes advantage of the autofluorescence of these cells and allows for specific isolation of red pulp macrophages from the spleen through a combination of autofluorescence, CD163, and CD14. We did not find any single surface marker that could absolutely distinguish red pulp macrophages from monocytes on its own. We show that human red pulp macrophages express the low-affinity FcγRIIa and FcγRIIIa, but not the inhibitory FcγRIIb, and appear to have an inducible expression of the high-affinity FcγRI, because we detected FcγRI in these cells only under inflammatory conditions. This is in stark contrast with monocytes (either from spleen or blood) and monocyte-derived macrophages, which constitutively express FcγRI and FcγRIIIa only on a small subset of the population. The difference in surface expression of various receptors between red pulp macrophages and monocyte-derived macrophages may reflect a different origin of these cells, similar to the situation in rodents, where red pulp macrophages consist of a self-renewing and highly specialized population established before birth.9 However, because there are many differences between rodent and human spleen, caution must be taken in the translation of these findings to the human situation.
Although it is not exactly known which phagocyte population in the spleen is responsible for the uptake of IgG-opsonized blood cells from the circulation, red pulp macrophages, which we have now shown to phagocytose IgG-opsonized erythrocytes via FcγRs ex vivo, are likely candidates. First, they are the most abundant macrophages in the spleen. Second, they are in direct contact with the cells in the circulation, as opposed to the minor fraction of CD163− macrophages in the perifollicular zone, which surround endothelial cells in capillary sheaths.12 Possibly, neutrophils may also play a role in the uptake of IgG-opsonized blood cells because neutrophils from the human spleen were shown to be highly potent in the phagocytosis of erythrocytes opsonized with high concentrations of murine IgG antibodies.28 However, neutrophils were not capable of phagocytozing anti-RhD opsonized erythrocytes.28
Because we have only tested phagocytosis by red pulp macrophages with anti–RhD-opsonized erythrocytes, we cannot be sure that our findings can be extrapolated to other opsonized antigens or blood groups. However, we have previously shown that different antibodies and antigens showed similar results in erythrophagocytosis by monocyte-derived macrophages.29
Our finding of FcγRIIIa being constitutively expressed on all red pulp macrophages is in line with several older publications showing FcγRIII in the red pulp of the spleen, but is in direct contrast with an immunohistochemistry study by Wu et al that proposed that FcγRIII is only expressed on splenic macrophages in a minority of individuals.30 However, we consistently observed FcγRIIIa on red pulp macrophages of 82 individuals, as tested by immunofluorescence of spleen sections and flow cytometry of freshly isolated single-cell suspensions. Supporting evidence for an important role of the low-affinity FcγRIIa and FcγRIIIa in the uptake of IgG-opsonized blood cells comes from the finding that genetic variation in these receptors is associated with ITP.31 Similarly, the removal of transfused IgG-opsonized erythrocytes in human volunteers correlated with SNPs in FcγRIIa and FcγRIIIa.32 However, such studies do not indicate which immune cell may be predominantly involved.
Previous publications have reported the expression of FcγRI19-21 and FcγRIIb19,33 on splenic macrophages. Our data are in contrast with these findings. We clearly observed that red pulp macrophages do not constitutively express the high levels of FcγRI that are expressed by monocytes or monocyte-derived macrophages. Nevertheless, its expression can be induced under inflammatory conditions, and even these very low levels of FcγRI expression on red pulp macrophages appeared to be functional.
Also, the inhibitory FcγRIIb is absent or at most very lowly expressed on red pulp macrophages, both in normal state and inflammatory circumstances. Given that FcγRIIb is often attributed an important role in balancing the immune response and preventing uncontrolled immune activation, its near absence is remarkable.
The most likely explanation for the differences between our findings and previous studies with regard to FcγR expression on isolated splenic macrophages is the difference in isolation protocols. The previously used isolation methods (ie, selecting for adherent cells21 or CD14 bead isolation),19,20 assumingly result in cell preparations with mainly monocytes, which are numerous in the spleen,34 or cell preparations with monocyte-derived macrophages cultured from spleen monocytes. We now show that a specific isolation of red pulp macrophages as we described is crucial for correct interpretation.
Although FcγR expression differed greatly between red pulp macrophages and monocyte-derived macrophages, FcγR usage in phagocytosis of IgG-opsonized erythrocytes was unexpectedly similar for red pulp macrophages and monocyte-derived macrophages. The relative contribution of FcγRII was higher in M-CSF macrophages, reflecting the very high expression of this receptor on this type of macrophage. Other differences could not be found. Most remarkable was the effect of FcγRI in phagocytosis by red pulp macrophages, because this receptor has a low expression on red pulp macrophages. Blocking experiments against FcγRI with MoAb 10.1 should be interpreted with caution, because the blocking effect of F(ab′)2 fragments of this MoAb is only partial.8 We assume that the actual contribution of FcγRI in our assay is somewhere in between the effect seen with F(ab′)2 fragments and the intact form of MoAb 10.1. F(ab′)2 fragments of MoAb 10.1 specifically block FcγRI but are weak in the competition with human IgG for this receptor.8 On the other hand, the intact form of MoAb 10.1 may not be specific for FcγRI because the Fc region of the MoAb may interact with the other FcγR on the cell surface, especially when MoAb 10.1 is cell-bound by molecules of FcγRI, a phenomenon known as the Kurlander effect.35 In any case, our data showed that the low levels of FcγRI still cause a major contribution to the phagocytosis by these macrophages, in cooperation with FcγRIIa and FcγRIIIa. Possibly, a rapid upregulation of surface expression of FcγRI after initial stimulation of FcγRIIa and FcγRIIIa by inside-out signaling takes place, as has been suggested before.36 Our qPCR analysis indeed showed that mRNA transcripts of FCGR1 were present in red pulp macrophages, albeit lower than in (splenic) monocytes and monocyte-derived macrophages (Figure 5E). These mRNA transcripts were also present in red pulp macrophages of 2 individuals that did not show any detectable surface expression of FcγRI on red pulp macrophages by flow cytometry (data not shown).
Corresponding with the similarity between red pulp macrophages and monocyte-derived macrophages in FcγR usage in IgG-opsonized blood cell phagocytosis, the different types of macrophages also responded similarly to inhibition by soluble human IgG in the form of IVIg. The dose-response to IVIg was identical across different types of macrophages. Our data suggest that, despite differences in FcγR expression levels, monocyte-derived macrophages are an equally suitable model as isolated red pulp macrophages when studying the in vitro effects of IVIg on macrophage phagocytosis.8,29
Concluding, we describe the splenic architecture and FcγR expression on splenic macrophages both in situ and with a novel method for the specific isolation of splenic red pulp macrophages. These red pulp macrophages have an expression profile that is highly distinct from monocytes and monocyte-derived macrophages. Our data demonstrate that all activating FcγR, but not the inhibitory FcγRIIb, are involved in the process of clearance of IgG-opsonized blood cells from the human circulation by red pulp macrophages. This knowledge is important for the design of targeted therapies for ITP and autoimmune hemolytic anemia.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Swi Fan Cheung (Sanquin, Amsterdam, The Netherlands) for help in providing tissue samples, Ellen Borg (VU University Medical Center, Amsterdam, The Netherlands), Judy Geissler (Sanquin, Amsterdam, The Netherlands) for assistance with the experiments, and Georg Kraal (VU University Medical Center, Amsterdam, The Netherlands) for intellectual input.
This work was supported by a grant from the Landsteiner Foundation for Bloodtransfusion Research (LSBR #0916).
Authorship
Contribution: S.Q.N. designed the study, performed experiments, analyzed data, and wrote the manuscript; C.W.B. performed experiments and analyzed data; E.P.J.M. performed and designed experiments; J.M.M.d.H. and T.K.v.d.B. discussed data; R.v.B. designed experiments and discussed data; and T.W.K. discussed data, designed the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Sietse Q. Nagelkerke, Plesmanlaan 125, Sanquin Research, 1066CX Amsterdam, The Netherlands; e-mail: s.nagelkerke@sanquin.nl.
Reference List
- 1.Crow AR, Lazarus AH. Role of Fcgamma receptors in the pathogenesis and treatment of idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2003;25(suppl 1):S14-S18. [DOI] [PubMed] [Google Scholar]
- 2.Sandler SG. The spleen and splenectomy in immune (idiopathic) thrombocytopenic purpura. Semin Hematol. 2000;37(1 suppl 1):10-12. [DOI] [PubMed] [Google Scholar]
- 3.Najean Y, Rain JD, Billotey C. The site of destruction of autologous 111In-labelled platelets and the efficiency of splenectomy in children and adults with idiopathic thrombocytopenic purpura: a study of 578 patients with 268 splenectomies. Br J Haematol. 1997;97(3):547-550. [DOI] [PubMed] [Google Scholar]
- 4.Dambuyant C, Thivolet J, Viala JJ, Ville D, Boyer J. Clearance mediated by splenic macrophage membrane receptors for immune complexes in cutaneous vasculitis. J Invest Dermatol. 1982;78(3):194-199. [DOI] [PubMed] [Google Scholar]
- 5.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168-186. [DOI] [PubMed] [Google Scholar]
- 6.Clarkson SB, Bussel JB, Kimberly RP, Valinsky JE, Nachman RL, Unkeless JC. Treatment of refractory immune thrombocytopenic purpura with an anti-Fc gamma-receptor antibody. N Engl J Med. 1986;314(19):1236-1239. [DOI] [PubMed] [Google Scholar]
- 7.Leidi M, Gotti E, Bologna L, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182(7):4415-4422. [DOI] [PubMed] [Google Scholar]
- 8.Nagelkerke SQ, Dekkers G, Kustiawan I, et al. Inhibition of FcγR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcγRIIb in human macrophages. Blood. 2014;124(25):3709-3718. [DOI] [PubMed] [Google Scholar]
- 9.Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis [published correction appears in Immunity. 2013;38(5)1073-1079]. Immunity. 2013;38(1):79-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15(12):731-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606-616. [DOI] [PubMed] [Google Scholar]
- 12.Steiniger B, Barth P, Herbst B, Hartnell A, Crocker PR. The species-specific structure of microanatomical compartments in the human spleen: strongly sialoadhesin-positive macrophages occur in the perifollicular zone, but not in the marginal zone. Immunology. 1997;92(2):307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4(5-6):446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiniger BS, Seiler A, Lampp K, Wilhelmi V, Stachniss V. B lymphocyte compartments in the human splenic red pulp: capillary sheaths and periarteriolar regions. Histochem Cell Biol. 2014;141(5):507-518. [DOI] [PubMed] [Google Scholar]
- 15.Schrieber L, Penny R. Tissue distribution of IgG Fc receptors. Clin Exp Immunol. 1982;47(3):535-540. [PMC free article] [PubMed] [Google Scholar]
- 16.Nagelkerke SQ, Kuijpers TW. Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol. 2015;5:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleit HB, Kobasiuk CD, Peress NS, Fleit SA. A common epitope is recognized by monoclonal antibodies prepared against purified human neutrophil Fc gamma RIII (CD16). Clin Immunol Immunopathol. 1992;62(1 Pt 1):16-24. [DOI] [PubMed] [Google Scholar]
- 18.Tuijnman WB, Van Wichen DF, Schuurman HJ. Tissue distribution of human IgG Fc receptors CD16, CD32 and CD64: an immunohistochemical study. APMIS. 1993;101(4):319-329. [DOI] [PubMed] [Google Scholar]
- 19.Audia S, Santegoets K, Laarhoven AG, et al. Fcγ receptor expression on splenic macrophages in adult immune thrombocytopenia. Clin Exp Immunol. 2017;188(2):275-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwana M, Okazaki Y, Ikeda Y. Splenic macrophages maintain the anti-platelet autoimmune response via uptake of opsonized platelets in patients with immune thrombocytopenic purpura. J Thromb Haemost. 2009;7(2):322-329. [DOI] [PubMed] [Google Scholar]
- 21.Wiener E, Mawas F, Dellow RA, Singh I, Rodeck CH. A major role of class I Fc gamma receptors in immunoglobulin G anti-D-mediated red blood cell destruction by fetal mononuclear phagocytes. Obstet Gynecol. 1995;86(2):157-162. [DOI] [PubMed] [Google Scholar]
- 22.Nagelkerke SQ, aan de Kerk DJ, Jansen MH, van den Berg TK, Kuijpers TW. Failure to detect functional neutrophil B helper cells in the human spleen. PLoS One. 2014;9(2):e88377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang-A-Sjoe MWP, Nagelkerke SQ, Bultink IE, et al. Fc-gamma receptor polymorphisms differentially influence susceptibility to systemic lupus erythematosus and lupus nephritis. Rheumatology (Oxford). 2016;55(5):939-948. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijden J, Breunis WB, Geissler J, de Boer M, van den Berg TK, Kuijpers TW. Phenotypic variation in IgG receptors by nonclassical FCGR2C alleles. J Immunol. 2012;188(3):1318-1324. [DOI] [PubMed] [Google Scholar]
- 25.Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611-622. [DOI] [PubMed] [Google Scholar]
- 26.Njoroge JM, Mitchell LB, Centola M, Kastner D, Raffeld M, Miller JL. Characterization of viable autofluorescent macrophages among cultured peripheral blood mononuclear cells. Cytometry. 2001;44(1):38-44. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Huang X, Chen Z, et al. Neutrophil CD64 expression as a biomarker in the early diagnosis of bacterial infection: a meta-analysis. Int J Infect Dis. 2013;17(1):e12-e23. [DOI] [PubMed] [Google Scholar]
- 28.Meinderts SM, Oldenborg PA, Beuger BM, et al. Human and murine splenic neutrophils are potent phagocytes of IgG-opsonized red blood cells. Blood Adv. 2017;1(14):875-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruggeman CW, Dekkers G, Bentlage AEH, et al. Enhanced effector functions due to antibody defucosylation depend on the effector cell Fcγ receptor profile. J Immunol. 2017;199(1):204-211. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Zhou J, Prsoon P, Wei X, Liu X, Peng B. Low expression of FCGRIIB in macrophages of immune thrombocytopenia-affected individuals. Int J Hematol. 2012;96(5):588-593. [DOI] [PubMed] [Google Scholar]
- 31.Breunis WB, van Mirre E, Bruin M, et al. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood. 2008;111(3):1029-1038. [DOI] [PubMed] [Google Scholar]
- 32.Miescher S, Spycher MO, Amstutz H, et al. A single recombinant anti-RhD IgG prevents RhD immunization: association of RhD-positive red blood cell clearance rate with polymorphisms in the FcgammaRIIA and FcgammaIIIA genes. Blood. 2004;103(11):4028-4035. [DOI] [PubMed] [Google Scholar]
- 33.Lux A, Seeling M, Baerenwaldt A, et al. A humanized mouse identifies the bone marrow as a niche with low therapeutic IgG activity. Cell Reports. 2014;7(1):236-248. [DOI] [PubMed] [Google Scholar]
- 34.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurlander RJ. Blockade of Fc receptor-mediated binding to U-937 cells by murine monoclonal antibodies directed against a variety of surface antigens. J Immunol. 1983;131(1):140-147. [PubMed] [Google Scholar]
- 36.van der Poel CE, Karssemeijer RA, Boross P, et al. Cytokine-induced immune complex binding to the high-affinity IgG receptor, FcγRI, in the presence of monomeric IgG. Blood. 2010;116(24):5327-5333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.