Key Points
IMGN632 is a conjugate of a novel CD123-targeting antibody with a highly potent DNA alkylating payload.
IMGN632 is active in preclinical models of AML with poor prognosis at concentrations far below levels that impact normal bone marrow cells.
Abstract
The outlook for patients with refractory/relapsed acute myeloid leukemia (AML) remains poor, with conventional chemotherapeutic treatments often associated with unacceptable toxicities, including severe infections due to profound myelosuppression. Thus there exists an urgent need for more effective agents to treat AML that confer high therapeutic indices and favorable tolerability profiles. Because of its high expression on leukemic blast and stem cells compared with normal hematopoietic stem cells and progenitors, CD123 has emerged as a rational candidate for molecularly targeted therapeutic approaches in this disease. Here we describe the development and preclinical characterization of a CD123-targeting antibody-drug conjugate (ADC), IMGN632, that comprises a novel humanized anti-CD123 antibody G4723A linked to a recently reported DNA mono-alkylating payload of the indolinobenzodiazepine pseudodimer (IGN) class of cytotoxic compounds. The activity of IMGN632 was compared with X-ADC, the ADC utilizing the G4723A antibody linked to a DNA crosslinking IGN payload. With low picomolar potency, both ADCs reduced viability in AML cell lines and patient-derived samples in culture, irrespective of their multidrug resistance or disease status. However, X-ADC exposure was >40-fold more cytotoxic to the normal myeloid progenitors than IMGN632. Of particular note, IMGN632 demonstrated potent activity in all AML samples at concentrations well below levels that impacted normal bone marrow progenitors, suggesting the potential for efficacy in AML patients in the absence of or with limited myelosuppression. Furthermore, IMGN632 demonstrated robust antitumor efficacy in multiple AML xenograft models. Overall, these findings identify IMGN632 as a promising candidate for evaluation as a novel therapy in AML.
Visual Abstract
Introduction
Acute myeloid leukemia (AML) is the leading cause of leukemia mortality in the United States.1 Curative treatment involves intensive induction chemotherapy, before proceeding to either consolidation chemotherapy or allogeneic stem cell transplantation based on the patient’s risk for relapse.2 This approach has been employed for >4 decades and, although most individuals achieve complete remissions with front-line therapy,3 the majority of patients ultimately relapse with drug-resistant disease, and overall survival rates remain disappointingly poor.4 The limited ability of many patients to tolerate the intense chemotherapy-based treatments, in particular hematological toxicity, further contributes to the poor outcomes noted in this disease. Thus there exists an urgent, unmet need for new agents that are both effective and not excessively toxic to the patients.
An increased understanding of the biological and genomic complexity of AML has resulted in the investigation of molecularly targeted approaches designed to shift treatment away from broad-based cytotoxic use toward more tailored interventions.2,5 One target of considerable interest is CD123, the α-subunit of the interleukin-3 receptor (IL-3Rα).6 CD123 expression is elevated on AML blasts compared with normal hematopoietic stem and progenitor cells, with high levels reported to be associated with aggressive disease.6-9 Furthermore, CD123 expression levels on leukemic stem cells are similar to those on leukemic blasts,6 suggesting that CD123-directed therapies have the potential to both debulk and eliminate the source of the disease, potentially translating to more durable therapeutic responses. The differential expression pattern of CD123 has been exploited to selectively target AML cells, and a variety of CD123-targeted agents, including a fusion protein of diphtheria toxin with IL-3, antibodies with enhanced antibody-dependent cellular cytotoxicity (ADCC), bispecific molecules, and chimeric antigen receptor (CAR) T cells, have shown promising activity in preclinical models,6,10-17 with a number having advanced into human clinical trials.5,18
The prominent cell surface expression of CD123 makes this antigen well suited for antibody-drug conjugate (ADC)-based therapeutic strategies. An ADC comprises a cytotoxic payload conjugated to a monoclonal antibody directed against a tumor-associated antigen.19 Validation of this approach for leukemia was provided by the regulatory approval of gemtuzumab ozogamicin (GO), a conjugate of a CD33-targeting antibody with a calicheamicin derivative payload, for the treatment of AML.20 The clinical utility of GO, however, can be hampered by limited efficacy in multidrug-resistant disease and significant safety concerns, including hepatotoxicity (veno-occlusive disease) and myelosuppression.21,22
We recently reported on a new chemical class of potent DNA-interacting payloads developed for incorporation into ADCs, indolinobenzodiazepine pseudodimers (IGNs).23 IGNs can be prepared in either a mono- or a diimine form, which act via DNA monoalkylation or DNA crosslinking, respectively. Although both forms are highly active, ADCs with alkylating IGNs demonstrated a better safety profile, and high therapeutic indices in vivo.23,24 Here we describe the development and preclinical characterization of a novel CD123-targeting ADC, IMGN632, consisting of a humanized anti-CD123 antibody conjugated to an alkylating IGN payload via a protease cleavable linker. IMGN632 demonstrated potent cytotoxicity against CD123-positive AML cells and primary patient samples in culture, at concentrations well below those that affected normal bone marrow progenitors. This selective activity against leukemic cells, coupled with favorable tolerability and robust efficacy in xenograft models of AML, provide a rationale for the clinical development of IMGN632 as a treatment of AML with potential therapeutic advantages over existing agents.
Methods
Antibodies and ADCs
The 7G3, 9F5, and 6H6 antibodies were purchased from BD Biosciences and BioLegend. GO was purchased from Mika Pharmaceuticals. The CD123-6 antibody was generated by immunizing BALB/c mice with the mouse pre-B-cell line 300-19 stably expressing human CD123. CD123-6 was humanized by grafting the CDRs into the human IGKV1-16*01 VL and IGHV1-46*03 VH frameworks. Back-mutations were introduced to restore high-affinity binding to CD123, and a cysteine residue was engineered at position 442 (EU numbering) in the CH3 domain of the Gamma1 constant region to allow for site-specific drug attachment. The resulting antibody, G4723A, and a nontargeting control antibody of identical Fc sequences as G4723A, were conjugated with IGN dimers as described24 to generate IMGN632, X-ADC, and control ADCs.
AML cell lines and primary patient samples
Cell lines were obtained from ATCC or DSMZ and grown according to the supplier’s recommendations. Primary samples from AML patients (ConversantBio) and healthy donors (AllCells) were characterized by multiparametric flow cytometry. Cell populations were defined by lineage markers25-27 as presented in supplemental Table 1. The median number of CD123 antibody binding sites (ABCs) was determined by the BD QuantiBRITE assay (BD Biosciences) using G4723A conjugated to phycoerythrin at a 1:1 ratio. MDR1 (P-glycoprotein)-mediated efflux activity was assessed using a flow cytometric method, in which cells were incubated with 0.3 pg/mL of the fluorescent stain Syto16 in either the absence or the presence of 2.4 μg/mL of the MDR1 inhibitor PSC833.28
Epitope mapping of anti-CD123 antibodies
A series of molecules where the extracellular domains of IL-3Rα and GM-CSFRα with a histidine tag had been swapped were expressed in HEK293T cells and added to enzyme-linked immunosorbent assay plates at 1 ng/mL in 50 mM sodium bicarbonate buffer pH 9.6. The plates were incubated at 4°C for 16 hours, washed with Tris-buffered saline containing 0.1% Tween-20, and blocked with Tris-buffered saline containing 1% bovine serum albumin. Serially diluted anti-CD123 antibodies were then added and incubated at room temperature for 1 hour, followed by addition of antihuman immunoglobulin G (IgG)–horseradish peroxidase (Jackson ImmunoResearch) for 1 hour at room temperature and development with 3,3′,5,5′-tetramethylbenzidine substrate (Surmodics).
Internalization, processing, and cytotoxicity studies
Prior to all studies, 200 to 500 nM of a nontargeted huIgG1 was added to culture medium to block Fc receptor–mediated uptake by cells. For internalization studies, AML cells were incubated with 10 nM of Alexa Fluor 488 (AF488; Invitrogen)-labeled G4723A or nonbinding human IgG1 control at 37°C. At selected time points, cell-surface–associated fluorescence was quenched with 10 mg/mL of anti-Alexa Fluor 488 antibody, and internalized fluorescence signals were quantified by flow cytometry. To assess G4723A processing, the antibody was labeled with [3H]-propionate (3H-G4723A) as previously described.29 Cells were exposed to a 30-minute pulse with 2 nM [3H]-G4723A, washed, and cultured for an additional 24 hours at 37°C, prior to harvest and extraction by acetone in order to separate soluble [3H] species from protein-associated species as described.24
Cytotoxicity in cell lines was assessed using the WST-8 viability assay (Dojindo Molecular Technologies) as previously described.30 Samples from AML patients and healthy donors were tested using colony-forming unit (CFU) assays. Cells were treated with serial dilutions of a test compound for 20 hours and then mixed with MethoCult (StemCell Technologies) supplemented with 50 ng/mL of each IL-3, stem cell factor, and FLT-3 ligand (Humanzyme) and seeded into 6-well plates. Plates were incubated for 7 to 14 days until colonies formed. Colonies were counted under a microscope and 90% of maximal inhibitory concentration (IC90) values were estimated by dividing the number of colonies for each treatment by the number of colonies in control wells, plotted against the test-compound concentrations. All untreated control wells had >100 colonies.
Xenograft models
Female athymic nude mice were obtained from Charles River Laboratories (Wilmington, MA). All animal procedures were approved by ImmunoGen’s Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In all studies, mice received an intraperitoneal injection of a nonbinding huIgG1 (400 mg/kg) 24 hours prior to ADC treatment and again on days 5 and 10 post-ADC injection (at 100 mg/kg) to block Fc receptors.
Mice were inoculated subcutaneously with EOL-1 cells (107 cells per mouse), and 6 days later were randomized into study groups by tumor volume (8 mice per group). The next day, the mice received a single IV bolus injection of either vehicle (phosphate-buffered saline [PBS]), IMGN632, the control ADC, G4723A, or FGN849 (the active payload catabolite of IMGN632 and the control ADC24). Other groups began intraperitoneal treatment with either cytarabine or azacitidine. Tumors were measured twice weekly, and tumor volume was calculated as previously described.23
The MOLM-13 disseminated model was established by injecting 107 cells IV into mice. Seven days later, the mice were randomized into study groups by body weight (10 mice per group) and received a single IV bolus injection of vehicle (PBS), IMGN632, or the control ADC. The mice were monitored for body weight loss and clinical signs. The Kasumi-3 disseminated model was established at Molecular Imaging (Ann Arbor, MI) by IV injecting female NSG mice (Jackson Laboratories) with 5 × 106 Kasumi-3-Luc-mCh-Puro cells engineered to express luciferase. Six days later, the mice were injected with luciferin, imaged with IVIS 50 optical imaging (Xenogen), and randomized into the study groups (6 mice per group) based on bioluminescent tumor burden. On days 7 and 41, a single IV bolus injection of vehicle (PBS), IMGN632, or the control ADC was administered. The mice were imaged to quantify tumor burden and monitored for body weight loss and clinical signs.
The patient-derived xenograft model was established at Champions Oncology (Hackensack, NJ) by IV injecting irradiated female NOG mice (Taconic) with 5 × 106 primary leukemia cells from a patient with refractory AML. Eight weeks postinjection, the engraftment was confirmed by flow cytometric staining of peripheral blood cells for human CD45+/CD33+ cells, followed by randomization into study groups and treatment with a single bolus IV injection of IMGN632 or the control ADC. Fifteen days later, all mice were euthanized, and tissues were collected for immunophenotyping.
Results
Characterization of a novel anti-CD123 antibody G4723A
Initial screening of monoclonal antibodies generated against human CD123 (IL-3Rα) identified CD123-6 as the lead. The CD123-6 antibody exhibited greater antigen binding affinity than 3 well-characterized commercially available anti-CD123 antibodies 7G3, 9F5, and 6H6 (supplemental Table 2), and, similar to 7G3, inhibited IL-3–dependent cell proliferation (supplemental Figure 1). CD123-6 was subsequently humanized to create G4723A, an IgG1 that retained the functional properties of its parental antibody.
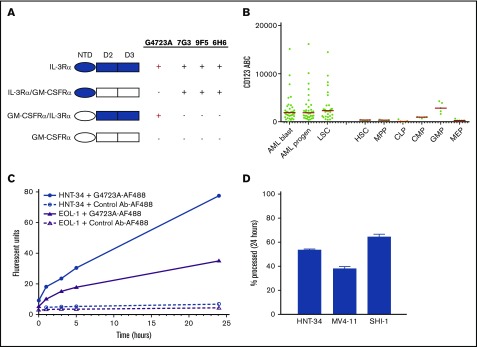
Using chimeric proteins that comprised extracellular domains of the IL-3Rα and GM-CSFRα (Figure 1A), the G4723A epitope was localized to an area within the 2 discrete folding domains (D2 and D3) of CD123, which differs from the reported binding regions for 7G3, 9F5, and 6H6 in the N-terminal domain.31 Next, G4723A binding was examined on a panel of primary samples from 5 healthy donors and 36 AML patients (Figure 1B). Among the normal cells, the highest ABC (antibodies bound per cell) values were found on granulocyte macrophage progenitors and common myeloid progenitors (median of 2829 and 943 ABC, respectively). The other subtypes, including HSCs, multipotent progenitors, megakaryocyte-erythroid progenitors, and common lymphoid progenitors, each had <500 ABCs (Figure 1B). Higher ABC values were observed in the AML blasts, progenitors, and leukemic stem cells cell populations (median of 1883, 1850, and 2300 ABCs, respectively).
Figure 1.
Characterization of CD123-6 and G4723A antibodies. (A) Binding of the CD123 antibodies to IL-3Rα, GM-CSFRα, and their swapped domain molecules as assessed by enzyme-linked immunosorbent assay. (B) The number of G4723A binding sites (CD123 ABC) on bone marrow cells from AML and healthy donors. Cell populations were defined by lineage markers as presented in supplemental Table 1. (C) Internalization of G4723A antibody by EOL-1 or HNT-34 cells. Cells were incubated with AF488-labeled G4723A or nonbinding huIgG1 (Ab, antibody); then the surface-bound fluorescence was quenched, and internalized AF488 signal was quantified by flow cytometry at indicated time points. (D) Processing of [3H]-propionate-G4723A antibody by HNT-34, MV4-11, and SHI-1 cells. Cells were exposed to G4723A antibody labeled with [3H]-propionate for 30 minutes followed by wash and 24 hours of incubation. The proteolytic catabolism of G4723A was quantified by comparing the protein and non-protein-associated radioactivity present in the cell lysate and culture medium. AML progen, AML progenitor; CLP, common lymphoid progenitors; CMP, common myeloid progenitors; GMP, granulocyte macrophage progenitors; HSC, hematopoietic stem cells; LSC, leukemic stem cells; MEP, megakaryocyte-erythroid progenitors; MPP, multipotent progenitors; NTD, N-terminal domain.
Antigen-directed internalization and subsequent intracellular processing of the antibody moiety are both prerequisites for ADC activity. Therefore, G4723A internalization was first assessed in 2 CD123-positive AML cell lines, EOL-1 and HNT-34 (Figure 1C). In each line, rapid internalization and intracellular accumulation of fluorescently labeled G4723A, but not of a nontargeting control antibody, was observed. Next, antibody proteolytic processing studies were performed using G4723A labeled with [3H]-propionate (Figure 1D). Following a pulse exposure to 3H-G4723A, the mean percentage of antibody degraded in HNT-34, MV4-11, and SHI-1 cells in 24 hours ranged between 40% and 66%. Taken together, these data are consistent with selective target-mediated uptake and processing of G4723A in CD123-positive AML cells.
Generation of anti-CD123 ADCs
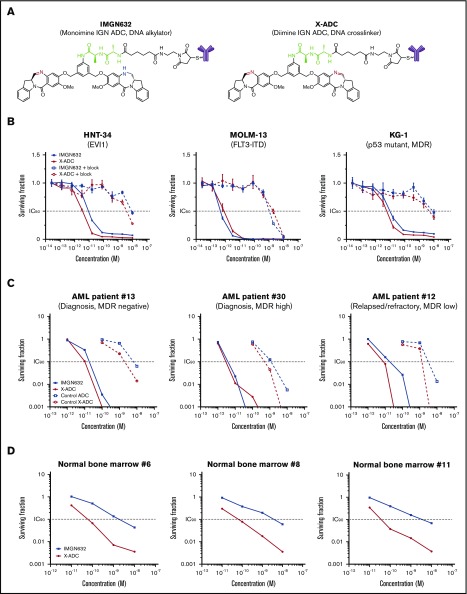
To select an optimal cytotoxic payload, monoimine (alkylating) or diimine (crosslinking) containing IGN molecules were conjugated to engineered cysteines of the G4723A antibody via a peptide linker, yielding IMGN632 or X-ADC, respectively (Figure 2A). Mass spectrometric analysis revealed that IMGN632 and X-ADC were each uniformly loaded with 2 payload molecules per antibody (supplemental Figure 2). The binding affinities of G4723A, IMGN632, and X-ADC to HNT-34 cells were similar (supplemental Tables 3 and 4), indicating that conjugation had only a minimal impact on antigen-specific binding.
Figure 2.
Evaluation of G4723A ADCs with monoimine or diimine IGN payloads. (A) Chemical structures of IMGN632 and X-ADC. Imine is highlighted in red; amine is highlighted in blue, and peptide linker is highlighted in green. (B) In vitro potency of IMGN632 and X-ADC toward CD123-positive HNT-34, MOLM-13, and KG-1 cells without and with blocking antibody. (C) Potency of IMGN632, X-ADC, and their control nonbinding ADCs toward leukemic progenitors from AML patients with various treatment and MDR status in CFU assays. (D) Potency of IMGN632 and X-ADC toward normal myeloid progenitors from healthy donors in CFU assays.
In vitro cytotoxicity against AML cells
The cytotoxicities of IMGN632 and X-ADC were first assessed in a panel of AML cell lines, which expressed CD123 at levels similar to AML blast cells (ABC values, 1100-13 000) and encompassed a variety of poor prognostic markers (Table 1). Both ADCs were very potent against all AML lines examined, with half maximal inhibitory concentration (IC50) values in the low picomolar range. This activity was antigen-dependent, because CD123 blocking experiments with an unconjugated G4723A antibody reduced the potency by >1000-fold (Figure 2B), and each ADC showed only a minimal activity against CD123-negative Namalwa (B-cell lymphoma) cells (Table 1). The unconjugated G4723A antibody alone had no antiproliferative effects on these cell lines at concentrations as high as 1 μM (supplemental Figure 3).
Table 1.
Cytotoxicity of IMGN632 and X-ADC in AML cell lines
| Cell line | CD123 ABC | IMGN632 IC50, pM | X-ADC IC50, pM | Prognostic marker |
|---|---|---|---|---|
| EOL-1 | 2600 | 2 | 1 | — |
| HNT-34 | 13 000 | 9 | 4 | EVI1 ↑ |
| KASUMI-3 | 9 000 | 3 | 0.9 | EVI1 ↑, MDR1 |
| KG-1 | 3 500 | 10 | 6 | p53 mut, MDR1 |
| KO52 | 1 200 | 6.6 | 2 | — |
| MOLM-13 | 5 300 | 0.5 | 0.8 | FLT3-ITD |
| MV4-11 | 8 800 | 1 | 0.4 | FLT3-ITD |
| SHI-1 | 4 300 | 5 | 2 | p53 mut |
| SKM-1 | 1 100 | 40 | 40 | p53 mut |
| UCSD-AML1 | 3 200 | 0.6 | 0.2 | EVI1 ↑ |
| Namalwa (B-cell lymphoma) | 0 | 10 000 | 8000 | — |
—, No known prognostic markers were present.
Next, IMGN632 and X-ADC were evaluated in primary samples from 16 AML patients with newly diagnosed or relapsed/refractory AML (Table 2) using CFU assays designed to induce proliferation of myeloid progenitors. IMGN632 and X-ADC were both highly potent (IC90 ranges, 3-56 and 2-31 pM, respectively), irrespective of multidrug resistance or disease status (Table 2; Figure 2C). Corresponding control (nonbinding) ADCs were at least 100-fold less active than IMGN632 and X-ADC, confirming the antigen dependence of the effects (Figure 2C). The G4723A antibody was inactive at the highest dose tested (10 000 pM), indicating that the IGN payload is needed for ADC activity.
Table 2.
Cytotoxicity of IMGN632, X-ADC, and GO in primary AML samples
| Sample no. | Patient status | BM blasts, % | MDR1 status | IMGN632 IC90, pM | X-ADC IC90, pM | GO IC90, pM |
|---|---|---|---|---|---|---|
| 39 | Diagnosis | 88 | − | 3.4 | 2.8 | 30 |
| 30 | Diagnosis | 28 | + | 3.6 | 2.8 | 300 |
| 35 | Refractory | 18 | + | 7 | NT | 1 000 |
| 9 | Diagnosis | 87 | + | 7.6 | 2.4 | 44 |
| 41 | Diagnosis | 90 | + | 9 | NT | 5 000 |
| 16 | Relapsed | 93 | − | 13 | 11 | 13 |
| 32 | Diagnosis | 83 | +/− | 13 | 2.3 | 2 500 |
| 31 | Diagnosis | 18 | +/− | 15 | 4.8 | >10 000 |
| 12 | Relapsed/refractory | 91 | +/− | 18 | 7.9 | 1 200 |
| 13 | Diagnosis | NT | − | 18 | 10 | NT |
| 21 | Diagnosis | 80 | NT | 18 | NT | 2 000 |
| 37 | Diagnosis | 83 | − | 20 | NT | 2 000 |
| 45 | Relapsed/refractory | 90 | + | 26 | 14 | 30 |
| 36 | Diagnosis | NT | + | 40 | NT | 800 |
| 38 | Diagnosis | 95 | +/− | 40 | 19 | 30 |
| 28 | Diagnosis | 30 | + | 56 | 31 | 10 000 |
+, Greater than twofold shift in Syto16 in the presence of MDR1 inhibitor; +/−, up to twofold shift in Syto16 in the presence of MDR1 inhibitor; −, no shift; NT, not tested.
Sensitivity of normal myeloid progenitors to IMGN632 and X-ADC
IMGN632 and X-ADC toxicity to normal myeloid progenitors was then assessed in a panel of bone marrow cell samples from healthy human donors in the CFU assay (Table 3; Figure 2D). Unexpectedly, X-ADC treatment was >40-fold more cytotoxic to the normal myeloid progenitors than IMGN632 (median IC90 values of 45 pM vs 1900 pM, respectively, in matched samples). To confirm the finding, normal bone marrow cells were exposed to either IMGN632 or X-ADC for 72 hours at a concentration highly active against AML cells (100 pM), and the degree of caspase 3 activation, a marker of apoptosis, in different cell populations was assessed by flow cytometry (supplemental Figure 4). Staurosporine treatment, a known inducer of apoptosis, increased caspase 3 signal in the populations of mature cells (CD34−CD45high) and early progenitors/HSC (CD34+CD38−CD45med), but these same populations were unaffected by either ADC in this assay. However, exposure to X-ADC, but not IMGN632, resulted in robust caspase 3 activation in the late myeloid progenitors (CD34+CD38+CD45med). Taken together, these data suggest that the ADC with monoimine containing IGN payload (IMGN632) is less myelotoxic than the ADC with the diimine payload (X-ADC). Based on these results, IMGN632 was selected for further evaluation.
Table 3.
Cytotoxicity of IMGN632, X-ADC, and GO in normal bone marrow samples
| Sample no. | IMGN632 IC90, pM | X-ADC IC90, pM | GO IC90, pM |
|---|---|---|---|
| 1 | 3500 | NT | 770 |
| 2 | 5700 | NT | 10 000 |
| 3 | 910 | NT | >10 000 |
| 4 | 210 | NT | 3 600 |
| 5 | 1000 | 45 | >10 000 |
| 6 | 1900 | 60 | >10 000 |
| 7 | 290 | 26 | >10 000 |
| 8 | 3700 | 66 | 760 |
| 9 | 180 | 14 | 2 700 |
| 10 | 3500 | 77 | 10 000 |
| 11 | 3500 | 37 | >10 000 |
IMGN632, but not GO, exhibits pronounced and selective cytotoxicity against AML progenitors
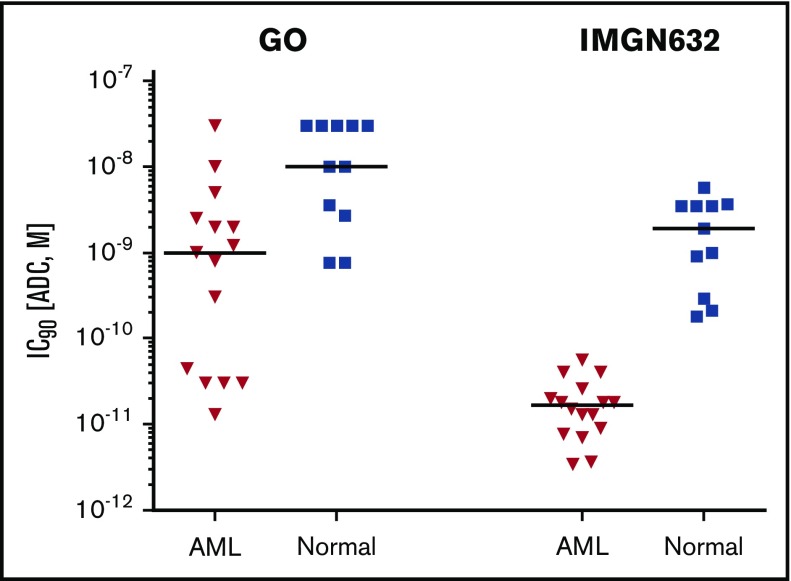
The potency of IMGN632 was then compared with GO activity in an extended panel of commercially available bone marrow cell samples from randomly selected AML patients and healthy human donors using the CFU assay (Tables 2 and 3). IMGN632 demonstrated similar potency to GO in 3/15 AML samples; for the others it was up to >600-fold more active (Table 2). On average, in comparison with GO, IMGN632 was 70-fold more cytotoxic to AML progenitors and only fivefold more cytotoxic to normal myeloid progenitors. Moreover, only 6 out of 15 AML samples were sensitive to GO at concentrations below those affecting normal myeloid progenitors from healthy donors (Figure 3). In contrast, AML progenitors from all patients were acutely sensitive to IMGN632 and, most importantly, at concentrations 120-fold lower than those that impact the normal samples (Figure 3), suggesting a favorable balance between antileukemic activity and myelotoxicity.
Figure 3.
IMGN632 displays preferential killing of leukemic over normal progenitors. Bone marrow mononuclear cells from 15 AML patients and 11 healthy donors were treated with serial dilutions of IMGN632 and GO for 20 hours, followed by CFU assay. The ADC concentrations that kill 90% of the cells are presented. Horizontal bars indicate median value for the sample set.
Antitumor activity of IMGN632 in AML xenograft models
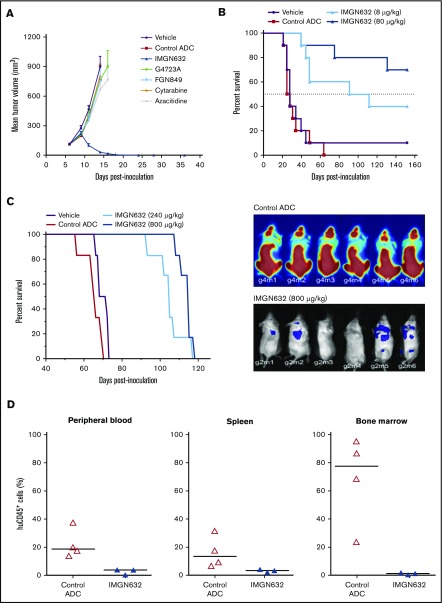
Having established a favorable therapeutic profile in vitro, the in vivo efficacy of IMGN632 was examined in human AML xenograft models. It has previously been established that the conjugate is well tolerated in mice at doses up to at least 8000 μg/kg.24 In mice bearing subcutaneous EOL-1 tumors that were resistant to high doses of cytarabine and azacitidine, a single administration of IMGN632 (240 μg/kg) induced complete and durable regressions in 8/8 animals (Figure 4A). In contrast, unconjugated G4723A antibody, active payload catabolite (FGN849), or the control (nonbinding) ADCs were all inactive at IMGN632-matched doses, indicating that both payload-to-antibody conjugation and CD123-targeted delivery were required for activity. Of note, IMGN632 was also efficacious at single doses of 40 and 80 μg/kg in this model (supplemental Figure 5).
Figure 4.
IMGN632 exhibits potent antitumor efficacy in multiple AML xenograft models. (A) Nude mice bearing EOL-1 subcutaneous xenografts were treated with vehicle or a single dose of either IMGN632 (240 μg/kg), control nonbinding ADC (240 μg/kg), unconjugated antihuman CD123 antibody (G4723A, 240 μg/kg), active payload catabolite (FGN849, 3 μg/kg), or a regimen of cytarabine (75 mg/kg, daily × 5) or azacitidine (3.75 mg/kg, every 3 days × 5). Complete regressions (8/8) in the IMGN632 group were monitored to the end of the study (day 116). (B) Kaplan-Meier analysis of overall survival in a disseminated Molm-13 xenograft model where tumor-bearing nude mice were treated with either vehicle (purple) or a single dose of a control nonbinding ADC (80 μg/kg) or IMGN632 (8 or 80 μg/kg), as indicated. Survival was monitored to day 152. (C) Kaplan-Meier analysis of overall survival (left) and bioluminescent images of mice (right; day 27) in a disseminated Kasumi-3-Luc-mCh-Puro xenograft model where tumor-bearing NSG mice were treated with either vehicle or 2 doses (on days 7 and 41) of a control nonbinding ADC (800 μg/kg) or IMGN632 (240 or 800 μg/kg) as indicated. Survival was monitored to day 118. (D) Mice bearing a primary patient-derived AML model were treated with a single dose of either a control nonbinding ADC (800 μg/kg) or IMGN632 (800 μg/kg). All animals were euthanized 15 days posttreatment, and cells from peripheral blood, bone marrow, and spleen were harvested and stained for murine CD45, human CD45, and human CD33 and analyzed by flow cytometry. Percentage of human CD45+/human CD33+/murine CD45− of all viable cells is presented on the graphs.
Next, IMGN632 antitumor activity was evaluated in 2 models of disseminated disease. In the first, mice were inoculated IV with MOLM-13 cells, and initiation of therapy was delayed for 7 days to allow engraftment and outgrowth of AML cells. Mice received a single administration of either vehicle, nonbinding control ADC (80 μg/kg) or IMGN632 (8 μg/kg or 80 μg/kg), and survival was monitored out to 152 days (Figure 4B). The majority (7/10) of mice treated with 80 μg/kg IMGN632 survived until the end of study, whereas all control ADC-treated animals succumbed to disease by day 64. Even at the lower, suboptimal 8-μg/kg dose level, IMGN632 increased the lifespan of the animals by 263% compared with vehicle treatment (median survival, 102 vs 28 days, respectively). Robust antileukemic activity was also observed in the multidrug-resistant Kasumi-3 model, with 2 doses (240 μg/kg or 800 μg/kg) of IMGN632 leading to increase in lifespan by 50% and 64% over control animals, respectively (Figure 4C). Bioluminescent imaging confirmed the substantial reduction in tumor burden in all IMGN632-treated animals compared with those treated with the control ADC.
Finally, tissue-specific effects of IMGN632 treatment were examined using a patient-derived xenograft model established with cells derived from an individual with refractory AML. Following engraftment, mice received a single bolus dose of 800 μg/kg IMGN632 or control ADC. Two weeks posttreatment all mice were euthanized, and immunophenotyping for human CD45+ cells revealed that only IMGN632 reduced tumor burden in the peripheral blood, spleen, and most notably, bone marrow (Figure 4D).
Discussion
Here we report the development and preclinical characterization of a novel CD123-targeting ADC, IMGN632, which utilizes a highly potent DNA-alkylating payload, resulting in an ADC with robust preclinical activity in multiple models of AML. In contrast to previously reported CD123-targeted agents, such as CSL360/362, MGD006, and SGN-CD123A,12,13,32,33 which employ variable domains of the 7G3 antibody and bind to the N-terminal domain of CD123,31 IMGN632 binds to the D2-D3 region in the extracellular domain via its unique G4723A antibody moiety. This may be therapeutically advantageous, given that 2 CD123 isoforms have been reported: the full-length protein and an alternative splicing variant that lacks an N-terminal domain, but still has the D2-D3 region.34 In addition, G4723A was similarly effective as 7G3 in inhibiting IL-3-mediated growth of an AML cell line. IL-3 initially binds to the D2-D3 region of CD123, followed by a secondary interaction with the N-terminal domain of the receptor.35 It is reasonable to speculate that binding of G4723A to the D2-D3 region is sufficient to prevent primary IL-3 interaction, whereas the 7G3 antibody interferes with the IL-3 secondary binding to CD123.31,35 In preclinical studies, 7G3 reduced AML engraftment and burden in murine models,36 and the humanized version of this antibody, CSL360, as well as a derivative with enhanced antibody-dependent cellular cytotoxicity activity, CSL362, has been evaluated in human trials. Both CSL360 and CSL36212 demonstrated favorable safety profile in humans.32 However, only minimal activity was observed in a phase 1 study for CSL360, leaving the authors to conclude that CD123 blockade and abolition of IL-3 signaling alone was an ineffective therapeutic strategy for AML.32
As an ADC, IMGN632 couples the targeting and pharmacokinetic features of the antibody with the additional cancer-killing impact of its payload.19 A majority of ADCs in development use highly potent tubulin inhibitors as their effector molecules.37,38 However, the clinical experience with these types of agents suggests that they are more suitable as payloads for targeting antigens that are expressed at levels higher that of CD123.39 Cytotoxic agents that target DNA are broadly used as AML therapy, making them a logical option for use in ADCs in this disease. However, ADCs that use derivatives of calicheamicin, which promotes double-stranded DNA breaks, such as GO and inotuzumab ozogamicin,40,41 have limited activity in cells with high multidrug resistance protein activity, a phenotype found in a significant fraction of AML patients.42 Another ADC, SGN-CD123A, comprising an engineered form of the CD123-targeting 7G3 antibody conjugated to a synthetic pyrrolobenzodiazepine dimer, which acts via DNA crosslinking, has entered clinical trials after showing robust activity in preclinical models of AML, including those with multidrug resistance.33
During the development of the IGN payloads, it was found that changing the IGN mechanism of action from a DNA crosslinker to a DNA alkylator improved the tolerability of the resultant ADC.23,24 Mechanistically, IGNs confer cytotoxic activity via DNA damage, which in turn leads to cell-cycle arrest and apoptosis.43 As part of the payload selection process, we directly compared the activity of IMGN632 (monoimine-containing, alkylating IGN) with another CD123-targeting conjugate, X-ADC (diimine-containing, crosslinking IGN). In vitro, IMGN632 and X-ADC each demonstrated highly selective and potent activity in AML cell lines that encompassed a variety of genetic alterations linked to poor prognosis in AML, such as the internal tandem duplication in FLT3 (FLT3-ITD), ecotropic viral integration site 1 (EVI1) protein overexpression, and p53 mutations. Importantly, however, IMGN632 was >40-fold less toxic to normal myeloid progenitors than X-ADC. In addition, IMGN632 was highly active and well tolerated in both subcutaneous and disseminated AML xenografts, including drug-resistant and MDR1-positive models. Of note, activity was observed at doses that represented 0.1% to 10% of maximally tolerated levels for the ADC in mice. In all cases, the antitumor activity was target specific, because a nonbinding control ADC exhibited no activity at comparable dosing.
A key observation of this study was the marked, selective cytotoxicity of IMGN632 against leukemic vs normal myeloid progenitors. Myelosuppression is a well-established side effect of current AML therapies, with prolonged events leading to an increased risk of infections and death.44 Consequently, therapies that preferentially kill leukemic cells over normal bone marrow cells are urgently required, and the overexpression of CD123 on AML leukemic stem cells and blasts compared with normal hematopoietic cells contributes to its attractiveness as a therapeutic target in this disease.6,45 However, severe myelosuppression and myeloablation have emerged for some investigational CD123-targeted treatment modalities. For example, CD123-targeted CAR T cells markedly impaired human myeloid hematopoiesis in xenograft mice models.11 In a separate study, CAR T-based treatment resulted in specific cytolysis of nonmalignant bone marrow progenitors and HSC in vitro.15 To identify populations of normal cells that might be affected by CD123-targeted therapies, antigen expression was assessed on bone marrow progenitors from healthy donors, with the most notable levels being observed on common myeloid progenitors and granulocyte macrophage progenitors. In GM-CFU assays, an established method to assess toxicity of cytotoxic agents to these progenitors,46-48 IMGN632 was cytotoxic to leukemic progenitors from AML patients at concentrations 120-fold lower than those that impacted viability of normal bone marrow cells. This remarkable difference in sensitivity between AML and normal progenitors to IMGN632 likely reflects both higher endogenous CD123 levels on AML progenitors and a lower sensitivity of normal progenitors to the monoimine IGN payload. The selective cytotoxicity of IMGN632 to AML progenitors suggests an opportunity for a significant therapeutic window; nevertheless, the potential for myelosuppression cannot be excluded given the activity against normal progenitors observed at much higher concentrations. In addition to bone marrow cells, significant CD123 protein levels have also been reported on basophils, plasmacytoid dendritic cells, and monocytes.6,13 Although IMGN632 exposure did not induce an apoptotic signal in monocytes derived from the bone marrow, these populations could present potential pharmacodynamic biomarkers and should be monitored during clinical trials of CD123-targeted agents.
In conclusion, we have developed and characterized a CD123-targeting ADC that combines a unique antibody and a DNA-alkylating payload. This ADC demonstrated potent antitumor activity in preclinical models of AML, including those with poor prognostic markers and multidrug resistance. Importantly, IMGN632 was effective at concentrations well below those that affected normal bone marrow cells, suggesting the potential for efficacy in AML patients with reduced likelihood of myelosuppressive side effects and favorable safety profile. These findings identify IMGN632 as a promising new therapeutic candidate for the treatment of this disease, and a first-in-human study in patients with relapsed/refractory CD123-positive AML has recently been initiated (NCT03386513).
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank their colleagues Michael Miller, Katie O’Callaghan, and Neeraj Kohli for their contributions to the present study and also John Lambert and Patrick Zweidler-McKay for their support and thoughtful review of this work. The authors are also grateful to Richard Bates for drafts and editorial assistance during production of this manuscript.
Footnotes
Presented in part in poster form at the 21st European Hematology Association Congress, Copenhagen, Denmark, 11 June 2016, and as an oral presentation at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 5 December 2016.
Authorship
Contribution: Y.K., S.A., L.R., Y.S., N.C.Y., and T.C. designed the experiments and interpreted the data; G.E.J., L.H., C.A.A., A.W., C.B., L.R., R.L., F.L., and O.A. performed experiments and analyzed data; guidance was provided by V.S.G., R.V.J.C., J.P., and T.C.; and Y.K. wrote the manuscript and led the project; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: All authors are current or former employees of ImmunoGen, Inc.
Correspondence: Yelena Kovtun, Science, Technology, and Translation, ImmunoGen Inc, 830 Winter St, Waltham, MA 02451; e-mail: yelena.kovtun@immunogen.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Kavanagh S, Murphy T, Law A, et al. Emerging therapies for acute myeloid leukemia: translating biology into the clinic. JCI Insight. 2017;2(18):e95679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackl H, Astanina K, Wieser R. Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. J Hematol Oncol. 2017;10(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saygin C, Carraway HE. Emerging therapies for acute myeloid leukemia. J Hematol Oncol. 2017;10(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gbolahan OB, Zeidan AM, Stahl M, et al. Immunotherapeutic concepts to target acute myeloid leukemia: focusing on the role of monoclonal antibodies, hypomethylating agents and the leukemic microenvironment. Int J Mol Sci. 2017;18(8):E1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Testa U, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res. 2014;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graf M, Hecht K, Reif S, Pelka-Fleischer R, Pfister K, Schmetzer H. Expression and prognostic value of hemopoietic cytokine receptors in acute myeloid leukemia (AML): implications for future therapeutical strategies. Eur J Haematol. 2004;72(2):89-106. [DOI] [PubMed] [Google Scholar]
- 8.Ehninger A, Kramer M, Röllig C, et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4(6):e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini DF, Ottone T, Guerrera G, et al. A leukemia-associated CD34/CD123/CD25/CD99+ immunophenotype identifies FLT3-mutated clones in acute myeloid leukemia. Clin Cancer Res. 2015;21(17):3977-3985. [DOI] [PubMed] [Google Scholar]
- 10.Mardiros A, Dos Santos C, McDonald T, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122(18):3138-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill S, Tasian SK, Ruella M, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busfield SJ, Biondo M, Wong M, et al. Targeting of acute myeloid leukemia in vitro and in vivo with an anti-CD123 mAb engineered for optimal ADCC. Leukemia. 2014;28(11):2213-2221. [DOI] [PubMed] [Google Scholar]
- 13.Chichili GR, Huang L, Li H, et al. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci Transl Med. 2015;7(289):289ra82. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hussaini M, Rettig MP, Ritchey JK, et al. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood. 2016;127(1):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thokala R, Olivares S, Mi T, et al. Redirecting specificity of T cells using the sleeping beauty system to express chimeric antigen receptors by mix-and-matching of VL and VH domains targeting CD123+ tumors. PLoS One. 2016;11(8):e0159477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yalcintepe L, Frankel AE, Hogge DE. Expression of interleukin-3 receptor subunits on defined subpopulations of acute myeloid leukemia blasts predicts the cytotoxicity of diphtheria toxin interleukin-3 fusion protein against malignant progenitors that engraft in immunodeficient mice. Blood. 2006;108(10):3530-3537. [DOI] [PubMed] [Google Scholar]
- 17.Frankel AE, McCubrey JA, Miller MS, et al. Diphtheria toxin fused to human interleukin-3 is toxic to blasts from patients with myeloid leukemias. Leukemia. 2000;14(4):576-585. [DOI] [PubMed] [Google Scholar]
- 18.Assi R, Kantarjian H, Ravandi F, Daver N. Immune therapies in acute myeloid leukemia: a focus on monoclonal antibodies and immune checkpoint inhibitors. Curr Opin Hematol. 2018;25(2):136-145. [DOI] [PubMed] [Google Scholar]
- 19.Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed Engl. 2014;53(15):3796-3827. [DOI] [PubMed] [Google Scholar]
- 20.Bross PF, Beitz J, Chen G, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7(6):1490-1496. [PubMed] [Google Scholar]
- 21.Godwin CD, Gale RP, Walter RB. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. 2017;31(9):1855-1868. [DOI] [PubMed] [Google Scholar]
- 22.Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130(22):2373-2376. [DOI] [PubMed] [Google Scholar]
- 23.Miller ML, Fishkin NE, Li W, et al. A new class of antibody-drug conjugates with potent DNA alkylating activity. Mol Cancer Ther. 2016;15(8):1870-1878. [DOI] [PubMed] [Google Scholar]
- 24.Miller ML, Shizuka M, Wilhelm A, et al. A DNA-interacting payload designed to eliminate cross-linking imporves the therapeutic index of antibody-drug conjugates (ADCs) [published online ahead of print 13 April 2018]. Mol Cancer Ther. doi:10.1158/1535-7163.MCT-17-0940. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94(10):5320-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacombe F, Durrieu F, Briais A, et al. Flow cytometry CD45 gating for immunophenotyping of acute myeloid leukemia. Leukemia. 1997;11(11):1878-1886. [DOI] [PubMed] [Google Scholar]
- 27.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broxterman HJ, Schuurhuis GJ, Lankelma J, et al. Highly sensitive and specific detection of P-glycoprotein function for haematological and solid tumour cells using a novel nucleic acid stain. Br J Cancer. 1997;76(8):1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai KC, Deckert J, Setiady YY, et al. Evaluation of targets for maytansinoid ADC therapy using a novel radiochemical assay. Pharm Res. 2015;32(11):3593-3603. [DOI] [PubMed] [Google Scholar]
- 30.Kovtun YV, Audette CA, Mayo MF, et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70(6):2528-2537. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q, Woodcock JM, Rapoport A, et al. Monoclonal antibody 7G3 recognizes the N-terminal domain of the human interleukin-3 (IL-3) receptor alpha-chain and functions as a specific IL-3 receptor antagonist. Blood. 1996;87(1):83-92. [PubMed] [Google Scholar]
- 32.He SZ, Busfield S, Ritchie DS, et al. A Phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leuk Lymphoma. 2015;56(5):1406-1415. [DOI] [PubMed] [Google Scholar]
- 33.Li F, Sutherland MK, Yu C, et al. Characterization of SGN-CD123A, a potent CD123-directed antibody-drug conjugate for acute myeloid leukemia. Mol Cancer Ther. 2018;17(2):554-564. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Olsen J, Ford S, et al. A new isoform of interleukin-3 receptor alpha with novel differentiation activity and high affinity binding mode. J Biol Chem. 2009;284(9):5763-5773. [DOI] [PubMed] [Google Scholar]
- 35.Broughton SE, Hercus TR, Hardy MP, et al. Dual mechanism of interleukin-3 receptor blockade by an anti-cancer antibody. Cell Reports. 2014;8(2):410-419. [DOI] [PubMed] [Google Scholar]
- 36.Jin L, Lee EM, Ramshaw HS, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5(1):31-42. [DOI] [PubMed] [Google Scholar]
- 37.Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337. [DOI] [PubMed] [Google Scholar]
- 38.Lambert JM, Berkenblit A. Antibody-drug conjugates for cancer treatment. Annu Rev Med. 2018;69(1):191-207. [DOI] [PubMed] [Google Scholar]
- 39.Lapusan S, Vidriales MB, Thomas X, et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs. 2012;30(3):1121-1131. [DOI] [PubMed] [Google Scholar]
- 40.Linenberger ML, Hong T, Flowers D, et al. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood. 2001;98(4):988-994. [DOI] [PubMed] [Google Scholar]
- 41.Takeshita A, Shinjo K, Yamakage N, et al. CMC-544 (inotuzumab ozogamicin) shows less effect on multidrug resistant cells: analyses in cell lines and cells from patients with B-cell chronic lymphocytic leukaemia and lymphoma. Br J Haematol. 2009;146(1):34-43. [DOI] [PubMed] [Google Scholar]
- 42.Legrand O, Perrot JY, Baudard M, et al. The immunophenotype of 177 adults with acute myeloid leukemia: proposal of a prognostic score. Blood. 2000;96(3):870-877. [PubMed] [Google Scholar]
- 43.Kovtun Y, Noordhuis P, Whiteman KR, et al. IMGN779, a novel CD33-targeting antibody-drug conjugate with DNA alkylating activity, exhibits potent antitumor activity in models of AML [published online ahead of print 27 March 2018]. Mol Cancer Ther. doi:10.1158/1535-7163.MCT-17-1077. [DOI] [PubMed] [Google Scholar]
- 44.Gurion R, Belnik-Plitman Y, Gafter-Gvili A, et al. Colony-stimulating factors for prevention and treatment of infectious complications in patients with acute myelogenous leukemia. Cochrane Database Syst Rev. 2012; (6):CD008238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14(10):1777-1784. [DOI] [PubMed] [Google Scholar]
- 46.Parchment RE, Gordon M, Grieshaber CK, Sessa C, Volpe D, Ghielmini M. Predicting hematological toxicity (myelosuppression) of cytotoxic drug therapy from in vitro tests. Ann Oncol. 1998;9(4):357-364. [DOI] [PubMed] [Google Scholar]
- 47.Pessina A, Albella B, Bayo M, et al. Application of the CFU-GM assay to predict acute drug-induced neutropenia: an international blind trial to validate a prediction model for the maximum tolerated dose (MTD) of myelosuppressive xenobiotics. Toxicol Sci. 2003;75(2):355-367. [DOI] [PubMed] [Google Scholar]
- 48.Volpe DA, Warren MK. Myeloid clonogenic assays for comparison of the in vitro toxicity of alkylating agents. Toxicol In Vitro. 2003;17(3):271-277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.