Abstract
Objective
To examine whether baseline chronic stress and impulsive risk-taking synergistically predict changes in visceral fat among healthy mothers in an observational, longitudinal, 18-month study.
Methods
A prospective cohort of 113 adult women (age M±SD: 42.83±4.70; BMI M±SD: 24.86±4.32; 74% (n=84 White) completed assessments at baseline and 18-month follow-up. We compared chronically stressed mothers caring for a child with an autism spectrum disorder (ASD; ‘caregivers’; n=72 participants) with lower stress mothers caring for a neurotypical child (‘controls’; n=41). We objectively assessed impulsive risk-taking using the Behavioral Analog Risk Task (BART) at baseline and assessed visceral fat at each baseline and 18-month follow-up using bioelectrical impedance (ViScan).
Results
The interaction of baseline chronic caregiving stress and impulsive risk-taking predicted 18-month change in visceral fat, such that greater impulsive risk-taking was associated with greater 18-month increases in visceral fat among caregivers (β=.423, p=.005), but not among controls (β=−.030, p=.802), both in unadjusted models and after accounting for covariates. Neither chronic stress nor impulsive risk-taking independently predicted 18-month changes in visceral fat.
Conclusions
The combination of high chronic stress and high impulsive risk-taking may increase risk for visceral fat gain over time and therefore may be an important intervention target in obesity prevention.
Keywords: Visceral Fat, Stress, Longitudinal, Weight Gain, Women-s Health
Introduction
The neurobiology of stress overlaps with that of appetite and energy regulation, which may explain why psychological stress is associated with weight change over time1. Stress may lead to weight gain via several pathways, such as increased wanting2 and eating of highly palatable food (HPF)3,4, decreased perceived satiety5, and physiological changes that drive abdominal adiposity6,7. Stress is a greater risk factor for weight gain over time among individuals with obesity8; however, it remains unclear why some people experience stress-related gain weight, whereas others do not.
Impulsive risk-taking, which can involve the tendency to insufficiently think, plan, and control one’s behavior, is associated with weight gain over time9. This association may be due in part to the modern environment’s ever-present opportunities to consume HPF (e.g., processed fast food), to opt for sedentary activities (e.g., television rather than exercise), and to disrupt the natural circadian processes that dictate sleep (e.g., to stay up long after dark using electronic screens). Thus, it may be particularly difficult for individuals higher in impulsive risk-taking to maintain a healthy lifestyle in the modern environment10–13.
How might chronic stress promote impulsive overeating? Chronic stress can impair the prefrontal cortex (PFC), a higher order region of the brain used to self-regulate consumptive behavior14. Under chronic stress, individuals higher in one or more dimensions of impulsivity may have a reduced capacity to regulate consumptive behaviors such as eating, smoking, or other pleasurable activities. One aspect of self-regulation that promotes healthy behaviors is the ability to delay gratification by choosing unprocessed (healthier) foods, rather than highly processed, immediately rewarding foods, such as sweets and desserts. Indeed, individuals higher in both chronic stress and self-reported impulsive risk-taking tend to choose more highly processed snack foods15,16 and report greater levels of alcohol misuse17. Hence, the combination of greater stress and impulsive risk-taking may predispose individuals to engage in poorer dietary habits and to experience difficulties self-regulating consumption.
In these analyses, we used objective measures of chronic stress and impulsive risk-taking to test whether greater levels of stress and impulsive risk-taking together synergistically predict increases in visceral fat over time. Specifically, we hypothesized that chronically stressed women who are higher in trait-like impulsive risk-taking would experience increases in visceral fat over a period of 18 months. In contrast, we hypothesized that impulsive risk-taking would not be associated with changes in visceral fat among women who were not chronically stressed.
Methods
Participants
Data were collected as part of a larger study on maternal caregiver stress and cellular aging18. Participants were 113 mothers living in the San Francisco Bay area recruited through local schools, parenting publications, social media, mailings, child development centers, and (for caregivers) the University of California, San Francisco Sensory Neurodevelopment and Autism Program. Eligible mothers were non-smokers between 20 and 50 years of age, who had at least one child between 2 and 16 years of age. One hundred and 17 potential participants provided data on the primary predictors and outcome (BART and visceral adiposity change), and 4 were excluded from the analyses because they were missing data on covariates, leaving 113 with complete data. Inclusion criteria for mothers in the high-stress group were caring for a child diagnosed with an autism spectrum disorder (ASD; e.g., autism, Asperger syndrome, or pervasive developmental disorder not otherwise specified). High-stress mothers were excluded if their scores on the Perceived Stress Scale (PSS)19,20 were below 13 during the eligibility phone screen. Inclusion criteria for mothers in the low-stress group were caring for a neurotypical child without any chronic disease. Low-stress mothers were excluded if their PSS scores were 19 or higher during the eligibility phone screen. The mean PSS score for women in nationally representative samples is roughly 1620, and an overlap in PSS scores for the two groups was permitted to reduce longitudinal statistical regression to the mean (see18 for details). Additional exclusion criteria included major chronic diseases (e.g., diabetes, cardiovascular, autoimmune, history of stroke, brain injury, cancer, endocrine disorders), regular use of steroid prescription medications, or meeting criteria for current posttraumatic stress, bipolar, or eating disorders. At baseline, chronically stressed mothers were not excluded for antidepressant use, whereas control mothers were excluded for antidepressant use as this would suggest that their non-medicated or true levels of psychological distress could be higher than the allowed cut-off. Two control mothers began taking antidepressants after enrollment, and we elected to statistically control for this factor rather than exclude them from analyses. This research was approved by the Committee for Human Research at the University of California, San Francisco, and all participants provided written consent and were compensated for their time.
Measures
Participants completed all measures at baseline and 18-months.
Psychological stress
We included objective (exposure-based) and subjective (perception-based) measures of chronic stress.
Chronic stress (objective)
We operationalized chronic stress (group status) using the objective criteria of providing care for a child with ASD versus caring for a neurotypical child (group status defined as a caregiver versus control, respectively)21.
Perceived stress (subjective)
The Perceived Stress Scale (PSS)19 is a standard 10-item questionnaire that assesses subjective perceptions of average stress levels over the previous month. Items reflect the perception that one’s life is uncontrollable, unpredictable and overwhelm one’s capacity to cope. Response options form a 5-point Likert scale ranging from 0 (never) to 4 (very often).
Impulsive risk-taking
We assessed impulsive risk-taking using the Balloon Analog Risk Task22 (BART). The BART is a computerized, behavioral measure of risk taking and has been modified and used to assess impulsive risk-taking in a variety of contexts22,23. In this study, each participant chose whether to inflate a virtual balloon (linked to increasing hypothetical monetary reward of $0.25) by clicking a computer mouse. With each click, participants were given the opportunity to inflate the virtual balloon (thereby potentially increasing their monetary rewards) or to risk explosion (resulting in no reward). This choice required participants to weigh the risk of losing their accumulated reward versus the possible benefit of accruing even greater rewards. Participants were instructed to imagine that they would receive actual money as depicted in the task, although participants were aware that their true compensation was delivered as a flat fee for participation. Participants viewed a total of 20 balloons, which exploded at a rate of 1/35. Participants could view how many times they had pumped each balloon, their hypothetical earning during each balloon, and how many balloons they had completed. This study used the preferred adjusted score as the outcome22, which is computed as the average number of pumps during the task minus the pumps from the balloons that terminated in explosion.
Visceral fat
We used the commercially available ViScan AB-140 (Tanita Corporation, Tokyo, Japan) bioelectrical impedance analysis (BIA) to assess visceral adipose tissue (VAT)24,25. Participants were fitted with a wireless ‘electrode belt’ on their bare midriff. This belt uses dual frequency bio-impedance (6.25 and 50 kHz) to assess visceral fat resistance and transmits measurements via infrared to the base unit. The ViScan device yields a measure of intra-abdominal adipose tissue, which is expressed as visceral fat (ranging from 1 to 59 in arbitrary units). The intra-abdominal adipose tissue or VAT area measured by computerized axial tomography (CT) in cm2 corresponds to the ViScan visceral fat index multiplied by 10.
Anthropometry
At baseline, participants completed physiological assessments including weight (using a digital scale) and height (using a wall-mounted Stadiometer).
Physical Activity
At baseline, participants answered questions about their daily exercise habits over the course of 7 days and we averaged their responses across the week (see18). We multiplied each activity’s Metabolic Equivalent of Task scores (METsi) by the number of minutes participants reported engaging in that activity that day.
Eating of sweet/dessert foods
We indexed eating of foods that are high-sugar, and often high-fat, using the Food Frequency Questionnaire (FFQ) MESA subscale26, wherein respondents indicated if they ate sweet foods (e.g., cakes, cookies, ice cream, chocolate, candy) on the following scale: 1 (rarely or never), 2 (1x/month), 3 (2–3x/month), 4 (1x/week), 5 (2x/week), 6 (3–4x/week), 7 (5–6x/week), 8 (1x/day), 9 (2x or more/day).
Covariates
We compiled an a priori list of potential covariates (listed in Table 1) to consider for model inclusion. We included covariates in the final adjusted model if they significantly differed by group (Table 1) or were associated with the primary outcome (Table 2). We selected these covariates a priori based on prior associations with weight and/or health-related behavior over time27. Considered covariates at baseline included participant age (years), education (0=No Bachelors; 1=Bachelors or more), child age (years)ii, and race/ethnicity (dichotomized as non-White=0; White=1). Considered covariates at each baseline and 18 months included antidepressant use (0=No; 1=Yes) and physical activity (see Physical Activity, above).
Table 1.
Group differences across covariates and adiposity measures over 18 months
| Caregivers (n=41) | Controls (n=72) | |
|---|---|---|
| Maternal Age (M, SD) | 43.93 (.76) | 42.10 (0.53)† |
| Race / Ethnicity (n, %) | 31 (76%) | 53 (74%) |
| Higher Education (n, %) | 13 (32%) | 40 (56%) |
| Child Age (M, SD) | 8.45 (2.94) | 7.12 (3.37)* |
| Food Frequency Questionnaire – Sweets and Desserts | 5.68 (2.10) | 5.70 (2.07) |
| Perceived Stress Scores (Baseline) (M, SD) | 22.01 (4.50) | 15.64 (4.37)** |
| Perceived Stress Scores (18 Months) (M, SD) | 20.38 (5.27) | 15.72 (4.98)** |
| Physical Activity METs (Baseline) (M, SD) | 982.25 (153.46) | 1160.31 (90.04) |
| Physical Activity METs (18 Months) (M, SD) | 894.05 (152.82) | 906.20 (107.28) |
| Antidepressant Use (Baseline) (n, %) | 6 (15%) | 2 (3%)* |
| Antidepressant Use (18 Months) (n, %) | 6 (15%) | 2 (3%)* |
| Visceral Fat (Baseline) (M, SD) | 8.43 (0.65) | 7.16 (0.41)† |
| Visceral Fat (18 Months) (M, SD) | 8.59 (0.69) | 7.19 (0.41)† |
| Body Mass Index (Baseline) (M, SD) | 25.50 (0.76) | 24.50 (0.47) |
| Body Mass Index (18 Months) (M, SD) | 25.58 (0.78) | 24.58 (0.51) |
Note.
p ≤.01,
p ≤.05,
p ≤.10, critical alpha = .05. N=113.
Age=Participant age in years; Race / Ethnicity coded as 0=Non-white, 1=White; Higher education coded as 0=Less than BA, 1=BA or greater; Child Age=Age of child in years; Food Frequency Questionnaire – Sweets and Desserts=Range from 1 (least frequent) to 9 (most frequent), see Method; Physical Activity METs=Physical Activity Metabolic Equivalent of Task Scores. Visceral fat = Intra-abdominal adipose tissue indexed with ViScan, arbitrary values range from 1 to 59. The intra-abdominal adipose tissue or VAT area measured by computerized axial tomography (CT) in cm2 corresponds to the visceral fat in arbitrary units obtained by the ViScan multiplied by 10.
Table 2.
Associations between potential confounds and residualized change in visceral fat over 18 months
| r | |
|---|---|
| Maternal Age | −.051 |
| Child Age | .126 |
| Race / Ethnicity | −.055 |
| Higher Education | −.201* |
| Physical Activity METs (Baseline) | −.194* |
| Physical Activity METs (18 Months) | −.036 |
| Antidepressant Use (Baseline) | −.064 |
| Antidepressant Use (18 Months) | −.130 |
Note.
p ≤.05, critical alpha = .05. N=113.
R-values were computed as Pearson correlations. See Table 1 note for variable descriptions.
Data analysis
Variable preparation
We created a residualized change score representing the change in visceral fat over 18 months by entering visceral fat at baseline as a predictor of visceral fat at 18 months and saving the standardized residuals (there were no statistical outliers: range: −2.15 to 2.42). We mean-centered BART scores (impulsive risk-taking) and coded group status (chronic stress level) as 0 (control) and 1 (caregiver). We computed the interaction term as the product of BART x group.
Covariate and confound testing
We tested whether controls and caregivers differed on covariates using independent samples t-tests and chi-square tests (Criteria 1; Table 1). We tested whether each covariate correlated with residualized change in visceral fat (primary outcome) using Pearson correlations (Criteria 2; Table 2). We only included covariates that met one or both criteria in adjusted analyses. We did not include BMI as a covariate, as this would have yielded high collinearity in the models, rendering them uninterpretable (BMI and visceral fat at baseline, r=.89, p<.001; at 18 months, r=.90, p<.001). Because model coefficients can become biased when including too many covariates (especially highly correlated covariates), we defined antidepressant use covariate as any use at baseline or 18 months, and computed child age as the standardized residual after removing its correlation with maternal age. We divided the METs covariate by 1000, as the unstandardized regression coefficients were otherwise too small to report.
Primary hypothesis testing
We tested the primary hypothesis by examining the ability of the BART x group interaction term to predict residualized change in visceral fat. In this test, a positive regression coefficient indicates that BART has a stronger prospective association with visceral fat increases for caregivers (coded 1) than for controls (coded 0). We computed (1) an unadjusted model including only the main effects and interaction term and (2) a model adjusting for above-described covariates (Table 3). We deconstructed each interaction term to examine the simple effects associations between BART and residualized change in visceral fat within each group separately. All statistical tests used a critical alpha of .05. We used SPSS 24 and Python 2.7.13 for all analyses.
Table 3.
Chronic caregiver stress and impulsive risk-taking synergistically predict increases in visceral fat over 18 months
| B (SE) | t | p | 95% CI (lower, upper) | |
|---|---|---|---|---|
| Unadjusted Model (df=109) | ||||
| Group | 0.200 (0.152) | 1.316 | .191 | (−0.101, 0.178) |
| Impulsive Risk-taking (BART) | 0.005 (0.021) | 0.233 | .816 | (−0.037, 0.047) |
| Group x Impulsive Risk-taking Interaction | 0.088 (0.034) | 2.612 | .010** | (0.021, 0.154) |
|
| ||||
| Adjusted Model (df=101) | ||||
| Group | 0.091 (0.157) | 0.575 | .566 | (−0.222, 0.403) |
| Impulsive Risk-taking (BART) | 0.002 (0.021) | 0.078 | .938 | (−0.039, 0.042) |
| Group x Impulsive Risk-taking Interaction | 0.095 (0.034) | 2.844 | .005** | (0.029, 0.162) |
| Maternal Age | −0.014 (0.015) | −0.888 | .377 | (−0.044, 0.017) |
| Higher Education | −0.276 (0.149) | −1.855 | .066† | (−0.571, 0.019) |
| Physical Activity METs/1000 | −0.168 (0.084) | −2.007 | .047* | (−0.334, −0.002) |
| Child Age | 0.136 (0.073) | 1.869 | .065† | (−0.008, 0.281) |
| Antidepressant Use | −0.005 (0.244) | −0.020 | .984 | (−0.488, 0.478) |
Note.
p ≤.01,
p ≤.05,
p ≤.10, critical alpha = .05.
β = standardized regression coefficient. Df = degrees of freedom. Group = (0=controls, 1=caregivers). See Table 1 note for variable descriptions. All models were conducted as linear regression analyses, including main effects, the interaction term, and (for the adjusted model) the covariates. Covariates were selected based on their association with the outcome or differing by group (see data analysis). Change in visceral fat was computed as a residualized change score representing the change in visceral fat over 18 months by entering visceral fat at baseline as a predictor of visceral fat at 18 months and saving the standardized residuals. We transformed METs by dividing by 1000 to place the units on a scale where the regression coefficients would be larger and more interpretable.
Results
Covariate and confound testing
As shown in Table 1, caregivers had significantly lower likelihood of obtaining a bachelor’s degree (p=.015). By study design, caregivers had significantly higher likelihood of having a higher PSS score (p<.001) and being on antidepressants (as described in Methods; p=.018). Hence, we included education and antidepressant use as covariates in the adjusted models. Caregivers in this sample tended to be slightly older than controls (p=.060), and their target child was also slightly older at baseline (p=.039); hence, we included both maternal and child age as covariates. Other covariates (race/ethnicity, METs) did not statistically significantly differ across groups (Table 1). Caregivers evidenced a trend toward higher levels of visceral fat than control mothers at baseline (p=.086) and 18 months (p=.065).
Associations between covariates at baseline and 18 months with the residualized change in visceral fat appear in Table 2. Participants who obtained a bachelor’s degree or greater (p=.033) or reported more physical activity (p=.039) had significantly smaller 18-month increases in visceral fat. Participant age, child age, race/ethnicity and antidepressant use were not significantly correlated with change in visceral fat.
Primary hypothesis testing
As hypothesized, the synergistic combination of being in the high-stress group (caregivers) and BART scores (greater impulsive risk-taking) predicted 18-month residualized change in visceral fat (Table 3). Simple effects analyses showed that higher BART scores predicted visceral fat increases among caregivers (B(SE)=0.093(0.029), t(39)=3.202, p=.003), but did not predict such increases among controls (B(SE)=0.005(0.020), t(70)=0.250, p=.803), unadjusted for covariates (see Figure 1)iii. Furthermore, the BART x group interaction term remained statistically significant in analyses adjusting for covariates. Higher BART scores continued to predict increases in visceral fat, but only among caregivers (B(SE)=0.086(0.029), t(34)=2.989, p=.005). Among controls, BART scores did not predict change in visceral fat (B(SE)=−0.005(0.019), t(62)= −0.252, p=.802)iv. Of note, higher education (B(SE)=−0.276(0.149), t(101)= −1.855, p=.066) and greater exercise at baseline (B(SE)=−.0.168(0.084), t(101)= −2.007, p=.047) were associated with lesser increases in visceral fat, which suggests that these might be protective factors. In contrast, higher age of target child was marginally associated with greater increases in visceral fat (Table 3), which may suggest a cumulative impact of parenting. In the unadjusted and adjusted models, neither group (control versus caregiver) nor impulsive risk-taking individually predicted 18-month changes in visceral fat. To determine whether these effects were specific to visceral fat, we also examined the interactive effect of group and BART on the 18-month change in BMI, which was not significant (B(SE)=−0.010(0.044), t(109)=−0.218, p=.828).
Figure 1. Two-panel scatterplot depicting 18-month changes in visceral fat by impulsive risk-taking as indexed by the Behavioral Analogue Risk Task (BART) among mothers of children with an autism spectrum disorder (caregivers, high stress) and mothers of neurotypical children (controls, low stress).
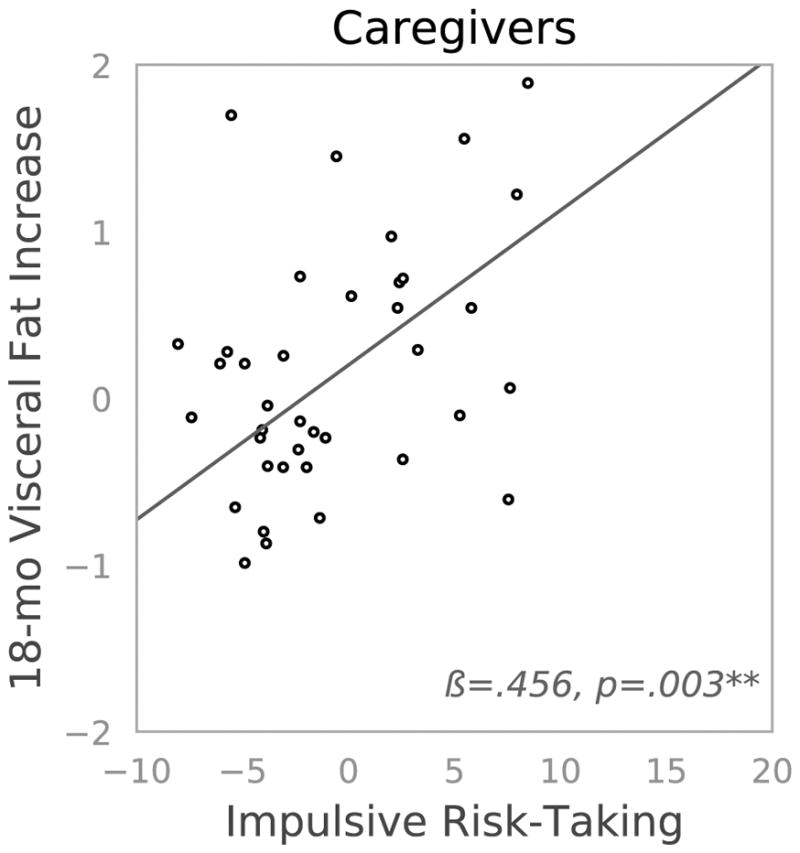

**p ≤.01. Mothers of a child with an autism spectrum disorder (ASD; caregivers) are depicted on the left and mothers of neurotypical children (controls) are depicted on the right. We indexed impulsive risk-taking using the Balloon Analog Risk Task (BART), and mean-centered scores. We quantified 18-month increases in visceral fat as the standardized residualized change score (see data analyses).
Potential Explanatory Factors
To ascertain whether impulsive risk-taking was related to eating of highly palatable foods, specifically, sweets and desserts, we conducted regression tests. Higher BART scores were significantly associated with more frequent eating of sweets (r=.203, p=.032), and this association remained significant after adjusting for caregiver group and covariates (B(SE)=0.102(0.045), t(102)=2.236, p=.028)v (Figure 2). To investigate whether chronic stress increases risk-taking or eating of sweets, we conducted t-tests to evaluate whether caregivers, relative to controls, have greater (1) impulsive risk-taking, or (2) frequency of sweets consumption (e.g., cakes, cookies, candy). First, caregivers (M±SE: 9.340±0.725) did not have greater BART scores than controls (M±SE: 10.597±0.507; t(111)=0.149, p=.149). Second, caregivers (M±SE: 5.675±0.333) did not report eating more sweets than controls (M±SE: 5.700±0.247; t(108)=0.061, p=.952). We also tested the association between self-reported stress (PSS) and eating of sweets, and found that they were not significantly correlated (r=.056, p=.546).
Figure 2. Scatterplot depicting the association between impulsive risk-taking as indexed by the Behavioral Analogue Risk Task (BART) and frequency of eating of sweet/dessert foods as indexed by the Food Frequency Questionnaire (FFQ) MESA subscale.
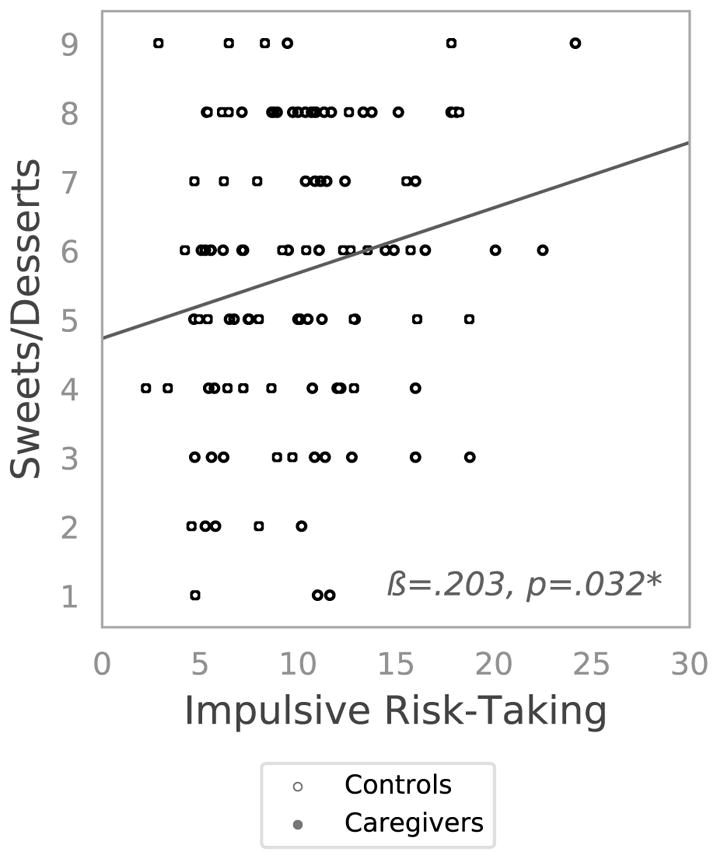
*p ≤.05. Mothers of children with an autism spectrum disorder (caregivers, high stress) are depicted as filled circles, whereas mothers of neurotypical children (controls, low stress) are depicted with open circles. The association did not significantly differ by group.
To understand whether the effects of caregiver stress could be explained by subjective reports of stress over the last month, we regressed the interaction between PSS scores and BART scores on 18-month increase in visceral fat. This interaction was not statistically significant (p=.906). BART remained a significant predictor of 18-month change in visceral fat among caregivers (p=.020), whereas PSS did not predict visceral fat change in either group (all p’s>.210). Additionally, 18-month change in PSS was not significantly associated with 18-month change in visceral fat (r=−.043, p=.654), and 18-month change in PSS did not interact with BART to predict 18-month change in visceral fat, B(SE)=.002(.005), p=.738.
Discussion
To our knowledge, this is the first prospective observational study to show that, among premenopausal women, greater trait-like impulsive risk-taking and chronic psychological stress synergistically predict increases in visceral fat over 18 months. Specifically, greater impulsive risk-taking predicted increases in visceral fat, but only among chronically stressed women (mothers caregiving for a child with ASD). Indeed, these mothers report more stress28 than mothers of neurotypical children, possibly in part because children with ASD may engage in unresponsive, oppositional, or aggressive behaviors more often than neurotypical children29. In these analyses, neither impulsive risk-taking nor chronic stress alone predicted increases in mothers’ visceral fat over this 18-month period; however, together they significantly predicted larger increases in visceral fat.
Impulsive risk-taking can increase how often individuals seek out rewarding experiences like eating or drinking30,31, which can contribute to weight gain. Accordingly, we found that women with greater impulsive risk-taking reported eating more sweets, which, over time, can adversely affect metabolic health. Whereas caregivers reported significantly higher stress levels than controls, they did not have greater impulsive risk-taking or consume more sweets. Hence, we cannot conclude that being a highly stressed mother of a child with ASD alone causes women to eat more sweets or make riskier choices. Furthermore, impulsive risk-taking (BART) in isolation did not predict increases in visceral fat. However, caregivers (relative to controls) who also had higher levels of impulsive risk-taking gained significantly more visceral fat over the following 18 months, and these effects remained after adjusting for covariates including physical activity.
Even if caregiving stress does not directly increase impulsive risk-taking or eating behavior, it may amplify any negative effects of dietary choices through neural or peripheral biological pathways. Although caregivers and controls may not differ in terms of basal impulsive risk-taking, chronic stress can impair aspects of top-down, prefrontal cortex (PFC) regulatory capacities needed to restrain impulsive behavior14. Dysregulations in stress-related hormones such as cortisol, together with impaired insulin sensitivity, can further enhance the drive to eat comfort foods4,6. If true, we might have expected caregivers to eat differently (e.g., consume more sweet foods), which we did not observe. However, self-report measures of food intake, such as those used herein, are subject to a myriad biases32. It is also possible that the sweets subscale does not capture relevant differences in eating behavior. Future research might employ a laboratory eating paradigm to test stress-related eating in this population.
Another possibility is that the stress of caregiving may work through biological rather than behavioral pathways. In animals, chronic stress enhances the secretion of peripheral neuropeptide Y (NPY), which helps fat tissue expand more effectively, even with the same HPF consumption. Moreover, these effects are strongest in highly vascularized visceral fat33, which may explain why we observed these effects in visceral fat and not overall BMI. In humans, we have found that the chronic stress of caregiving in combination with HPF consumption to be associated with greater metabolic risk7, and this increased risk may have been mediated via increases in peripheral NPY. In sum, animal and human data suggest that the physiologic burden of chronic stress may increase risk for visceral fat expansion, with no changes in impulsive risk-taking or consumption of sweet foods.
In contrast, neither women’s one-time self-reports of stress levels over the last month nor changes in these self-reported stress levels worked synergistically with impulsive risk-taking to predict changes in visceral fat. This raises the question of why subjective perceived stress was not as powerful a predictor as the objective stress of caring for a child with ASD. Unlike caregiving statuse.g., 34, self-reported perceived stress levels are more susceptible to self-report bias and random recent events. Caregiving captures stress that persists over many years, whereas these subjective reports of perceived stress only reflected the past month. Furthermore, perceived stress levels do not consistently track with measures of biological stress responsese.g., 35. Taken together, these data may reflect that stress may need to be chronic over years to impact visceral fat, the Perceived Stress Scale may be too global to capture the nature of caregiving stress, and/or that caregiving as an “umbrella” variable may reflect a multifaceted burden that goes beyond the subjective perception of stress.
Whereas many studies examining longitudinal change in adiposity have focused on weight or BMIe.g., 36, this study focused on visceral (intra-abdominal) fat. Visceral fat, often indexed by the less accurate proxy waist circumference, is more tightly associated with metabolic health than subcutaneous (beneath the skin) fat37. Changes in visceral fat are likely more important than changes in weight or BMI in the identification of obesity-related disease risk38,39. Whereas many weight loss and diabetes prevention programs target changes in diet and physical activity40, this study identifies two alternative psychological targets (impulsive risk-taking and chronic stress).
These results fit with a growing literature suggesting that facets of impulsivity, such as impulsive responding, work synergistically with other psychological processes involved in reward-related behavior to predict health risks. The combination of high impulsive responding (indexed using delayed discounting) and high sensitivity to food reward (which has been termed ‘reinforcement pathology’) has been linked to overeating in several studies41. For example, Appelhans and colleagues42 found that among individuals with higher levels of impulsive responding, greater food-related sensitivity to reward was associated with greater consumption of highly palatable snacks. That is, sensitivity to food reward impacted eating behavior only in the context of high impulsive responding. Similarly, prior work has shown that greater impulsive purchasing tendencies are associated with greater eating of sweets, but only among individuals who report difficulty controlling their eating43. In other words, having heightened food-related sensitivity, in and of itself, does not constitute an independent risk factor, so long as individuals with such sensitivity can exercise self-control when confronted with food-related decisions.
Interventions that merely target impulsive eating may miss the importance of a stressful context. For example, many parents may have had the experience of trying to cook dinner and maintain a resolution to eat less and lose weight, all the while coping with a child’s tantrum. These challenging behaviors are more frequent and intense among children with ASD29. Interventions targeting dimensions of impulsivity (e.g., cognitive retraining44) may not capture the extent to which coping with daily stressors exhausts the capacity for effective self-regulation of emotions and eating. Similarly, interventions targeting general stress-reduction (e.g., mindfulness-based stress reduction) may not provide specific skills that allow individuals to recognize, in the moment, how their circumstances affect food-related decision-making. Behavioral interventions that target the intersections among dimensions of impulsivity, stress, and reward-driven processes hold promise for reducing problematic overeating. For example, prior work has shown that training in eating mindfully - which teaches individuals to recognize links between stress, reward, and eating - can lead to reductions in reward-related eating, and that such reductions do predict weight loss45.
Limitations
We cannot confirm whether stress and impulsive risk-taking specifically altered caloric intake as we did not collect complete dietary recall data. As is typical22, the Balloon Analog Risk Task featured hypothetical, rather than actual, monetary rewards, which could have been less motivating and attenuated the effects of impulsive risk-taking. This study operationalized caregiving as an objective index of chronic stress; however, other unknown factors that differ across caregivers for children with versus without ASD may be responsible for the observed results. Although this study focused on a stress-impulsive risk-taking interaction, these results are not mutually exclusive with stress-eating pathways or with peripheral stress-diet pathways; rather, they likely work together. Finally, it is unknown whether these results are generalizable to men or post-menopausal women.
Conclusions and Future Directions
These results identify chronic stress (using a maternal caregiving model) and greater impulsive risk-taking as synergistic risk factors for increases in visceral fat over time. Further, we posit that interventions focusing on one target at a time may not address this synergistic effect. Future work should evaluate the extent to which mechanisms driving this synergistic effect are behavioral (eating more and possibly differently) versus biological (gaining more fat with the same intake).
What is already known about this subject?
Associations between stress and weight gain over time are mixed and may depend on the type or chronicity of the stressor
Laboratory assessments of impulsive risk-taking best predict weight gain over time when examined in tandem with other risk factors
Greater levels of visceral fat increase risk for metabolic disease states
What does this study add?
These data show that chronic caregiver stress interacts with laboratory-assessed impulsive risk-taking to predict 18-month increases in visceral fat
When considered with laboratory-assessed impulsive risk-taking, objective stress (i.e., caregiver status), but not subjective stress (i.e., perceived stress), may be a more potent predictor of 18-month increases in visceral fat
The combination of high impulsive risk-taking and chronic stress may be a potent risk factor for poorer metabolic health
Acknowledgments
Funding: The research was supported by awards from the National Institutes of Health (NIH) to Ashley E. Mason (NHLBI; K23HL133442), Kirstin Aschbacher (NHLBI; K23HL112955), and Elissa S. Epel (NIA; R01AG030424-01A2). Additional support was provided to Kirstin Aschbacher from The Institute for Integrative Health (TIIH) and the UCSF NORC (P30DK098722). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Available at: https://community.plu.edu/~chasega/met.html
Data on child age were missing for three participants.
The interaction term remained significant with bootstrapping; hence, relatively low or high points do not drive the finding.
We also confirmed that the pattern of results for unadjusted and adjusted analyses remained the same when employed a delta change score, after accounting for baseline.
Three participants were missing data on eating of sweet/dessert foods. The association between impulsive risk-taking and eating of sweets did not differ by group status.
Disclosure: The authors declare no conflict of interest.
References
- 1.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013;73:827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemmens SG, Rutters F, Born JM, Westerterp-Plantenga MS. Stress augments food ‘wanting’ and energy intake in visceral overweight subjects in the absence of hunger. Physiol Behav. 2011;103:157–163. doi: 10.1016/j.physbeh.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 4.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Drapeau V, et al. Behavioural and metabolic characterisation of the low satiety phenotype. Appetite. 2013;70:67–72. doi: 10.1016/j.appet.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aschbacher K, et al. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14–22. doi: 10.1016/j.psyneuen.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. Am J Epidemiol. 2009;170:181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. J Pers Soc Psychol. 2011;101:579–592. doi: 10.1037/a0024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Akker K, Jansen A, Frentz F, Havermans RC. Impulsivity makes more susceptible to overeating after contextual appetitive conditioning. Appetite. 2013;70:73–80. doi: 10.1016/j.appet.2013.06.092. [DOI] [PubMed] [Google Scholar]
- 11.Gruber R, Cassoff J. The interplay between sleep and emotion regulation: Conceptual framework empirical evidence and future directions. Curr Psychiatry Rep. 2014;16:500. doi: 10.1007/s11920-014-0500-x. [DOI] [PubMed] [Google Scholar]
- 12.Cheval B, Sarrazin P, Pelletier L. Impulsive approach tendencies towards physical activity and sedentary behaviors, but not reflective intentions, prospectively predict non-exercise activity thermogenesis. PLOS ONE. 2014;9:e115238. doi: 10.1371/journal.pone.0115238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pentz MA, Spruijt-Metz D, Chou CP, Riggs NR. High calorie, low nutrient food/beverage intake and video gaming in children as potential signals for addictive behavior. Int J Environ Res Public Health. 2011;8:4406–4424. doi: 10.3390/ijerph8124406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Blyderveen S, et al. Personality differences in the susceptibility to stress-eating: The influence of emotional control and impulsivity. Eat Behav. 2016;23:76–81. doi: 10.1016/j.eatbeh.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Georgii C, Goldhofer P, Meule A, Richard A, Blechert J. Food craving, food choice and consumption: The role of impulsivity and sham-controlled tDCS stimulation of the right dlPFC. Physiol Behav. doi: 10.1016/j.physbeh.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Helen CF, Keri LB, Peihua G, Rajita S. Interactive Effects of Cumulative Stress and Impulsivity on Alcohol Consumption. Alcohol Clin Exp Res. 2010;34:1376–1385. doi: 10.1111/j.1530-0277.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aschbacher K, et al. Chronic stress is associated with reduced circulating hematopoietic progenitor cell number: A maternal caregiving model. Brain Behav Immun. 2017;59:245–252. doi: 10.1016/j.bbi.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 20.Cohen S, Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J Appl Soc Psychol. 2012;42:1320–1334. [Google Scholar]
- 21.Hayes SA, Watson SL. The impact of parenting stress: A meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord. 2013;43:629–642. doi: 10.1007/s10803-012-1604-y. [DOI] [PubMed] [Google Scholar]
- 22.Lejuez CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 23.Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: An fMRI Study of the Balloon Analog Risk Task (BART) NeuroImage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Ambrosi J, et al. Clinical usefulness of abdominal bioimpedance (ViScan) in the determination of visceral fat and its application in the diagnosis and management of obesity and its comorbidities. Clin Nutr Edinb Scotl. 2017 doi: 10.1016/j.clnu.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Thomas EL, et al. Estimation of abdominal fat compartments by bioelectrical impedance: The validity of the ViScan measurement system in comparison with MRI. Eur J Clin Nutr. 2010;64:525–533. doi: 10.1038/ejcn.2010.18. [DOI] [PubMed] [Google Scholar]
- 26.Nettleton JA, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Beydoun MA. The Obesity Epidemic in the United States—Gender, Age, Socioeconomic, Racial/Ethnic, and Geographic Characteristics: A Systematic Review and Meta-Regression Analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 28.Montes G, Halterman JS. Psychological functioning and coping among mothers of children with autism: A population-based study. Pediatrics. 2007;119:e1040–e1046. doi: 10.1542/peds.2006-2819. [DOI] [PubMed] [Google Scholar]
- 29.Jang J, Dixon DR, Tarbox J, Granpeesheh D. Symptom severity and challenging behavior in children with ASD. Res Autism Spectr Disord. 2011;5:1028–1032. [Google Scholar]
- 30.Hamilton KR, Ansell EB, Reynolds B, Potenza MN, Sinha R. Self-reported impulsivity, but not behavioral choice or response impulsivity, partially mediates the effect of stress on drinking behavior. Stress Amst Neth. 2013;16:3–15. doi: 10.3109/10253890.2012.671397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pool E, Brosch T, Delplanque S, Sander D. Stress increases cue-triggered ‘wanting’ for sweet reward in humans. J Exp Psychol Anim Learn Cogn. 2015;41:128–136. doi: 10.1037/xan0000052. [DOI] [PubMed] [Google Scholar]
- 32.Dhurandhar NV, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2015;39:1109–1113. doi: 10.1038/ijo.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo LE, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 34.Allen AP, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: Focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev. 2017;73:123–164. doi: 10.1016/j.neubiorev.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 35.van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Hunt LP, Ford A, Sabin MA, Crowne EC, Shield JPH. Clinical measures of adiposity and percentage fat loss: which measure most accurately reflects fat loss and what should we aim for? Arch Dis Child. 2007;92:399–403. doi: 10.1136/adc.2006.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox CS, et al. Abdominal visceral and subcutaneous adipose tissue compartments. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 38.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: The paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 39.Poirier P, Despres JP. Waist circumference, visceral obesity, and cardiovascular risk. J Cardpulm Rehabil. 2003;23:161. doi: 10.1097/00008483-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP) Description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiol Behav. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appelhans BM, et al. Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity. 2011;19:2175–2182. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honkanen P, Olsen SO, Verplanken B, Tuu HH. Reflective and impulsive influences on unhealthy snacking. The moderating effects of food related self-control. Appetite. 2012;58:616–622. doi: 10.1016/j.appet.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Yokum S, Stice E. Cognitive regulation of food craving: Effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes. 2013;37:1565–1570. doi: 10.1038/ijo.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason A, et al. Reduced reward-driven eating accounts for the impact of a mindfulness-based diet and exercise intervention on weight loss: Data from the SHINE randomized controlled trial. Appetite. 2016;100:86–93. doi: 10.1016/j.appet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


