Abstract
Osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BM-MSCs) is a central event in bone formation. However, oxidative stress has a deleterious impact on BM-MSC osteogenesis. In this study, we hypothesized that oxidative stress influenced BM-MSC osteogenesis differently in the early or late stages, in which silent information regulator type 1 (SIRT1) played a critical role. A continuous exposure to sublethal concentrations of hydrogen peroxide (H2O2), ranging from 25 μM to 100 μM for 21 days, resulted in the complete inhibition of BM-MSC osteogenesis. We found that a 7-day treatment with H2O2 inhibited the lineage commitment of BM-MSCs toward osteoblasts, as evidenced by a significant reduction of alkaline phosphatase activity (a typical marker for early osteogenesis). However, moderate oxidative stress did not affect late-differentiated BM-MSCs, as there were comparable levels of matrix mineralization (a typical marker for late osteogenesis). In addition, we observed a spontaneous up-regulation of SIRT1 and intracellular antioxidant enzymes such as superoxide dismutase 2, catalase, and glutathione peroxidase 1, which accounted for the enhanced resistance to oxidative stress upon osteogenic differentiation. Activation of SIRT1 by resveratrol rescued the effect of H2O2 on early-differentiated BM-MSCs and inhibition of SIRT1 by nicotinamide intensified the effect of H2O2 on late-differentiated BM-MSCs, indicating that the SIRT1-mediated pathway was actively involved in MSC osteogenesis and antioxidant mechanisms. Our findings uncovered the relationship between SIRT1 and resistance to H2O2-induced oxidative stress during BM-MSC osteogenesis, which could provide a new strategy for protecting MSCs from extracellular oxidative stress.
Keywords: MESENCHYMAL STEM CELLS, OSTEOGENIC DIFFERENTIATION, OXIDATIVE STRESS, HYDROGEN PEROXIDE, SIRT1, ANTIOXIDANT ENZYMES
INTRODUCTION
Bone marrow-derived mesenchymal stem cells (BM-MSCs) are crucial in maintaining the balance of bone metabolism as they differentiate into osteoblasts and synthesize calcified bone matrices. Osteogenic differentiation of BM-MSCs is a central event of bone formation and can be divided into four stages: BM-MSCs are induced toward an osteogenic lineage commitment, followed by rapid proliferation of osteoprogenitors, synthesis of the extracellular matrix (ECM), and deposition of minerals in the ECM [Franceschi and Iyer, 1992]. Two typical biomarkers have been used to evaluate the stages of osteogenic differentiation, alkaline phosphatase (ALP) activity for the early stage and matrix mineralization for the late stage.
Growing evidence has demonstrated that osteogenic differentiation can be influenced by various factors such as growth factors [Chen et al., 2012] and oxygen tension [Nicolaije et al., 2012] Intracellular basal levels of reactive oxygen species (ROS) including superoxide anion, hydrogen peroxide (H2O2), hydroxyl radical, and nitric oxide [Atashi et al., 2015], are indispensable to multiple cellular processes in mesenchymal stem cells (MSCs), such as cell survival, proliferation, migration, and differentiation. However, complete elimination of ROS resulted in a cell cycle arrest by inhibiting MSCs from entering the S phase [Lyublinskaya et al., 2015]. In the meantime, to protect MSCs from the over-accumulation of ROS, intracellular antioxidant enzymes are responsible for eliminating deleterious ROS. For example, superoxide dismutase (SOD) converts superoxide anions to H2O2, which is subsequently decomposed by catalase (CAT) or glutathione peroxidases (GPXs) [Mates and Sanchez-Jimenez, 1999]. However, oxidative stress, representing an imbalance between ROS generation and neutralization, induces cell damage by the oxidation of DNA, lipids, and proteins. Exposure of MSCs to sublethal concentrations of H2O2 causes premature senescence-associated phenotypes, such as a G0/G1 cell cycle arrest, positive senescence-associated β-galactosidase activity, and an up-regulation of cell-cycle inhibitors [Zhou et al., 2015]
Oxidative stress has been shown to have deleterious effects on osteogenic differentiation and bone formation. Exposing bone osteoblastic cells (MC3T3-E1) [Mody et al., 2001] or MSCs [Ho et al., 2013; Liu et al., 2016] to exogenous H2O2 resulted in increases in intracellular oxidative stress and inhibition of osteogenic differentiation. However, over-accumulation of ROS induces the calcification of vascular smooth muscle cells (VSMCs) in atherosclerosis [Byon et al., 2016]. Exposure to exogenous H2O2 directly induced the phenotypic switch of VSMCs from a contractile to an osteogenic phenotype and promoted arterial calcification [Byon et al., 2008]. Therefore, the effects of varying doses of oxidative stress on either the early or late stage of osteogenic differentiation have not yet been determined, nor have potential mechanisms been identified.
Recently, silent information regulator type 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, has been shown to be involved in several cellular processes of MSCs, such as energy metabolism, proliferation, cellular senescence, and lineage-specific differentiation [Chen et al., 2014]. Yoon et al. demonstrated that SIRT1 played an important role in maintaining the self-renewal capability and multipotency of BM-MSCs by directly regulating SRY (sex determining region Y)-box 2 (SOX2) [Yoon et al., 2014]. A previous study showed that activation of SIRT1 by resveratrol (ResV) promoted osteogenic differentiation of MSCs while inhibiting adipocyte formation [Backesjo et al., 2006]. In addition, SIRT1 has been implicated in metabolic and ROS control by regulating several antioxidant genes, such as SOD2, CAT, peroxiredoxins, thioredoxin 2, and uncoupling protein 2 [Olmos et al., 2013]. Studies from our laboratory suggested that activation of SIRT1 by melatonin or extracellular matrix was effective to prevent premature senescence and rescue the proliferative capacity of MSCs by inhibiting the expression of the senescence-associated proteins such as p16INK4α [Zhou et al., 2015; Zhou et al., 2017]. However, little is known about the changes undergone by SIRT1 during MSC osteogenesis and whether osteogenic induction of SIRT1 is associated with its antioxidant mechanisms. Understanding the response of SIRT1 to oxidative stress in osteogenesis will lead to further advances in stem cell-based bone tissue engineering and optimize current clinical strategies.
In this study, oxidative stress was induced by adding H2O2 at sublethal concentrations in the medium for osteogenic induction of BM-MSCs followed by the evaluation of oxidative stress and antioxidant enzymes, such as catalase (CAT) and glutathione peroxidases (GPXs), in the early and late stages of osteogenic differentiation. To elucidate the role of SIRT1 in different stages of osteogenic differentiation, ResV (a SIRT1 agonist) or nicotinamide (NAM, a SIRT1 inhibitor) was supplemented in the presence of H2O2 and the effects of SIRT1 activation or inhibition on osteogenic differentiation of BM-MSCs were evaluated.
MATERIALS AND METHODS
REAGENTS AND ANTIBODIES
Human adult BM-MSCs were purchased from Cyagen Biosciences Inc. (HUXMA-01001, Guangzhou, China). Standard cell culture plates and flasks were purchased from Costar (Tewksbury, MA, USA). Fetal bovine serum (FBS), alpha minimum essential medium (α-MEM), Dulbecco’s modified Eagle medium (DMEM), penicillin, streptomycin, TRIzol® reagent, protease inhibitor tablets, horseradish peroxidase-conjugated secondary antibodies, and SuperSignal West Pico Substrate were purchased from Thermo Fisher Scientific (Waltham, MA, USA). ResV, NAM, phosphate buffered saline (PBS), H2O2, dexamethasone, L-ascorbic acid, β-glycerol phosphate, paraformaldehyde, fluorescein diacetate (FDA), and 2′,7′-dichlorofluorescein diacetate (DCF-DA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies against SIRT1, SOD1, SOD2, CAT, GPX1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Abcam (Cambridge, MA, USA). Cell lysis buffer, polyacrylamide gels, nitrocellulose membranes, and X-OMAT BT Film were purchased from Beyotime Institute of Biotechnology (Haimen, China).
CELL CULTURE AND OSTEOGENIC DIFFERENTIATION OF BM-MSCS
BM-MSCs at the first passage were cultured in growth medium containing α-MEM, 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C with 5% CO2. BM-MSCs were passaged two more passages to obtain a sufficient number of cells and then cryopreserved for the subsequent experiments. To induce toward an osteogenic lineage commitment, BM-MSCs at the fourth passage were incubated in osteogenic differentiation medium containing DMEM, 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL L-ascorbic acid, 100 nM dexamethasone, and 10 mM β-glycerol phosphate at 37°C with 5% CO2. The differentiation medium was changed every 3 days. At the day 7, 14, and 21 time points, differentiated cells were subjected to subsequent tests as specified in the individual experiments.
TREATMENT WITH H2O2, RESVERATROL, OR NICOTINAMIDE
To evaluate the effects of H2O2 on cell viability of BM-MSCs, cells were exposed to different dosages of H2O2 ranging from 25 to 400 μM for 72 h. At the specific time points, BM-MSCs were analyzed by both qualitative and quantitative cell viability assays. To evaluate the influence of H2O2 on the stages of osteogenic differentiation, BM-MSCs were treated with 25 μM, 50 μM, and 100 μM H2O2 for 21 days. Untreated cells served as a control. To investigate the role of SIRT1 in the early stage of osteogenesis, BM-MSCs were treated with 5 μM ResV in the presence of 100 μM H2O2 for 7 days and subjected to subsequent experiments. To investigate the role of SIRT1 in the late stage of osteogenesis, BM-MSCs were treated with 10 mM NAM in the presence of 50 μM H2O2 for the last 7 days and subjected to subsequent experiments.
CELL VIABILITY ASSAY
BM-MSCs were seeded into a 96-well plate at the initial density of 10,000 cells/well and, after 12 h, BM-MSCs were washed with fresh growth medium to remove unattached cells and then subjected to H2O2 at concentrations of 25 μM, 50 μM, 100 μM, 200 μM, and 400 μM (diluted in growth medium). The culture medium was changed every 24 h. After 72 h, cell viability was qualitatively observed by an FDA staining assay. BM-MSCs were washed with PBS and then incubated in 5 μg/mL of FDA solution at 37°C for 10 min. The fluorescent images of live cells were captured using an Olympus IX51 microscope (Olympus Corporation, Tokyo, Japan). To quantitatively determine the cell viability of BM-MSCs, a cell counting kit-8 (CCK-8; Beyotime) was used following the manufacturer’s protocol. BM-MSCs were washed with PBS and 10 μL of the CCK-8 solution (diluted in growth medium) was added to each well. The cells were incubated at 37°C for 1 h. Absorbance was measured at 450 nm using a microplate spectrophotometer (BioTek, Winooski, VT, USA).
MEASUREMENT OF INTRACELLULAR ROS
The effects of H2O2 treatments on intracellular ROS in BM-MSCs were determined through a fluorescent method; 2 × 105 cells were incubated in 10 μM of DCF-DA solution at 37°C for 10 min. The fluorescence intensity was measured using a Cytomics FC500 Flow Cytometer (Beckman-Coulter, Brea, CA, USA) and 10,000 events from each cell sample were analyzed using the WinMDI (Windows Multiple Document Interface for Flow Cytometry) 2.9 software (The Scripps Research Institute, La Jolla, CA, USA).
MEASUREMENT OF SIRT1 ACTIVITY
To quantitatively measure SIRT1 activity, BM-MSCs were induced toward osteogenesis and treated with H2O2, ResV, or NAM. At the day 7 or day 21 time points, monolayer cells were first collected and the nuclear protein was extracted using a commercial Nuclear Extraction Kit (Abcam). The protein concentrations were determined using a BCA Protein Assay Kit (Beyotime). SIRT1 activity was quantitatively evaluated using a fluorometric SIRT1 Activity Assay Kit (Abcam) according to the manufacturer's instructions. The fluorescence intensity of samples was measured using a SynergyMx multi-mode microplate reader (BioTek) with excitation at 350 nm and emission at 460 nm alongside a standard curve.
ALP STAINING AND ACTIVITY MEASUREMENT
After a 7-day osteogenic induction, intracellular ALP activity was measured using commercially available kits. To qualitatively observe ALP-positive cells, BM-MSCs were washed three times with PBS, fixed with 4% paraformaldehyde, and stained with a BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime) for 15 min according to the manufacturer's instructions. Digital images were captured using an Olympus IX51 microscope.
To quantitatively measure the intracellular ALP activity, BM-MSCs were first dissolved in ice-cold cell lysis buffer and, after centrifuging, the supernatant was divided into two parts. One part was evaluated colorimetrically using an Alkaline Phosphatase Assay Kit (Beyotime) for 30 min at 37°C according to the manufacturer's instructions. The other part was used to determine the concentration of the extracted protein using a BCA protein assay kit (Beyotime). The ALP activity was normalized to the total protein content.
EVALUATION OF MATRIX MINERALIZATION USING ALIZARIN RED S STAINING
After a 21-day osteogenic induction, MSC-differentiated osteoblasts were fixed in 4% paraformaldehyde and incubated in 1% Alizarin Red S solution (pH = 4.3; Sigma-Aldrich) at room temperature for 15 min. After washing with PBS three times, images of calcium deposition were captured using an Olympus IX51 microscope. To quantify the level of matrix mineralization, 200 μL of 1% hydrochloric acid (Sigma-Aldrich) was added to each well to dissolve the stain from the calcified layers and the absorbance was measured at 420 nm using a microplate spectrophotometer.
SIRT1 RNA INTERFERENCE
Small interfering RNAs (siRNAs) targeting human SIRT1 were designed and synthesized by GenePharma Biological Company (Shanghai, China). BM-MSCs in 12-well plates were induced toward osteoblasts and, after a 14-day osteogenic induction, cells were transfected with 40 pmol siRNA and 2 μL LipofectamineTM 2000 (Thermo Fisher Scientific) for 6 h according to the manufacturer’s instructions. Non-silencing siRNA that does not recognize any known genes was used as a negative control (NC). The sequences of siRNA for SIRT1 were 5′-GCGGGAAUCCAAAGGAUAATT-3′ and 5′-UUAUCCUUUGGAUUCCCGCTT -3′. The sequences of NC were 5′- UUCUCCGAACGUGUCACGUTT -3′ and 5′- ACGUGACACGUUCGGAGAATT -3′.
TOTAL RNA EXTRACTION AND REAL-TIME REVERSE TRANSCRIPTION POLYMERASE CHAIN REACTION (RT-PCR)
Total RNA was extracted using TRIzol® reagent and 1 μg of total RNA was reverse-transcribed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). To quantify mRNA expression, an amount of cDNA equivalent to 50 ng of total RNA was amplified by real-time PCR using the iTaq™ Universal SYBR® Green Supermix kit (Bio-Rad, Hercules, CA, USA). Transcript levels of osteogenic marker genes, including ALP, COL1A1 (type I collagen α1), RUNX2 (runt-related transcription factor 2), and BGLAP (bone gamma carboxyglutamate protein or osteocalcin) were evaluated. Transcript levels of SIRT1 and antioxidant enzymes, including CAT, GPX1, SOD1, and SOD2 were also evaluated. GAPDH served as an internal standard. The primer sequences are listed in Table 1. Real-time PCR was performed on a CFX96™ Real-Time RT-PCR System (Bio-Rad) following the manufacturer’s protocol. Relative transcript levels were calculated as χ = 2-ΔΔCt, in which ΔΔCt = ΔE - ΔC, ΔE = Ctexp - CtGAPDH, and ΔC = Ctct1 - CtGAPDH.
Table 1.
Primers used for real-time RT-PCR
| Gene | Forward Primer sequence(5′-3′) | Reverse Primer sequence(5′-3′) |
|---|---|---|
| GAPDH | AGAAAAACCTGCCAAATATGATGAC | TGGGTGTCGCTGTTGAAGTC |
| COL1A1 | CAGCCGCTTCACCTACAGC | TTTTGTATTCAATCACTGTCTTGCC |
| RUNX2 | AGAAGGCACAGACAGAAGCTTGA | AGGAATGCGCCCTAAATCACT |
| BGLAP | GAGCCCCAGTCCCCTACC | GACACCCTAGACCGGGCCGT |
| ALP | AGCACTCCCACTTCATCTGGAA | GAGACCCAATAGGTAGTCCACATTG |
| SIRT1 | GCGGGAATCCAAAGGATAAT | CTGTTGCAAAGGAACCATGA |
| CAT | TGGGATCTCGTTGGAAATAACAC | TCAGGACGTAGGCTCCAGAAG |
| GPX1 | TATCGAGAATGTGGCGTCCC | TCTTGGCGTTCTCCTGATGC |
| SOD1 | GGTGGGCCAAAGGATGAAGAG | CCACAAGCCAAACGACTTCC |
| SOD2 | GGGGATTGATGTGTGGGAGCACG | AGACAGGACGTTATCTTGCTGGGA |
WESTERN BLOT ASSAY
BM-MSCs were lysed in ice-cold cell lysis buffer containing protease inhibitors and the protein concentration in cell extracts was quantified using a BCA protein assay kit (Beyotime). Equal amounts of protein from each extract were denatured and separated in a 10% polyacrylamide gel, and then transferred by electrophoresis onto a nitrocellulose membrane. The membrane was incubated in properly diluted primary antibodies at 4°C overnight, and then incubated in secondary antibodies at room temperature for 1 h. The membranes were developed using SuperSignal West Pico Substrate and X-OMAT BT Film. The intensity of bands was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
STATISTICAL ANALYSIS
All data were expressed as means ± standard error (S.E.). Statistical analysis was conducted using SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA). Multiple comparisons of data were analyzed by one-way Analysis of Variance (ANOVA), followed by the Tukey’s post hoc test. The two-tailed Student's t-test was performed for single comparisons. Significance was indicated by a p-value < 0.05 (*) or < 0.01 (**).
RESULTS
THE EFFECTS OF H2O2-INDUCED OXIDATIVE STRESS ON CELL VIABILITY AND INTRACELLULAR ROS OF BM-MSCS
To investigate the influence of H2O2 treatments on osteogenic differentiation of BM-MSCs, it was necessary to exclude the possibility that cytotoxic levels of H2O2 reduced cell survival. We first determined cell viability by treating BM-MSCs with varying doses of H2O2 ranging from 25 to 400 μM. After 72 h, BM-MSCs in the untreated, 25 μM, 50 μM, and 100 μM groups showed similar and confluent cell density. The cell number in the 200 μM group was much lower than the other groups and BM-MSCs barely survived after treatment with 400 μM H2O2 (Fig. 1A). Quantitative results of the CCK-8 assay showed that treatments with H2O2 lower than 100 μM did not significantly decrease cell viability of BM-MSCs, but treatment with 200 μM H2O2 induced a significant reduction of cell viability by 19.9% after 24 h, by 43.5% after 48 h, and by 46.0% after 72 h compared to the untreated cells. The cell viability in the 400 μM group was 13.1% after 24 h, 21.0% after 48 h, and 16.1% after 72 h compared to the control group (Fig. 1B). We further analyzed the cell viability of BM-MSCs during osteogenic differentiation and the CCK-8 assay data confirmed that treatment with 100 μM H2O2 did not significantly affect cell viability of BM-MSCs (Supplementary Fig. 1). To determine the effects of H2O2 treatments on intracellular ROS, BM-MSCs were stained with DCF-DA solution and the levels of ROS were measured by flow cytometry (Fig. 1C). The results of the DCF fluorescence density indicated that, after treating with H2O2 at concentrations of 25 μM, 50 μM, 100 μM, 200 μM, and 400 μM, the levels of intracellular ROS were increased by 17.3%, 42.3%, 85.0%, 1.8-fold, and 4.2-fold, compared to untreated cells, respectively (Fig. 1D). Though high dosages of H2O2 increased intracellular ROS in BM-MSCs, they also led to a significant decrease in cell viability. Therefore, we decided to use H2O2 at 25 μM, 50 μM, and 100 μM concentrations in the following experiments.
Fig. 1.
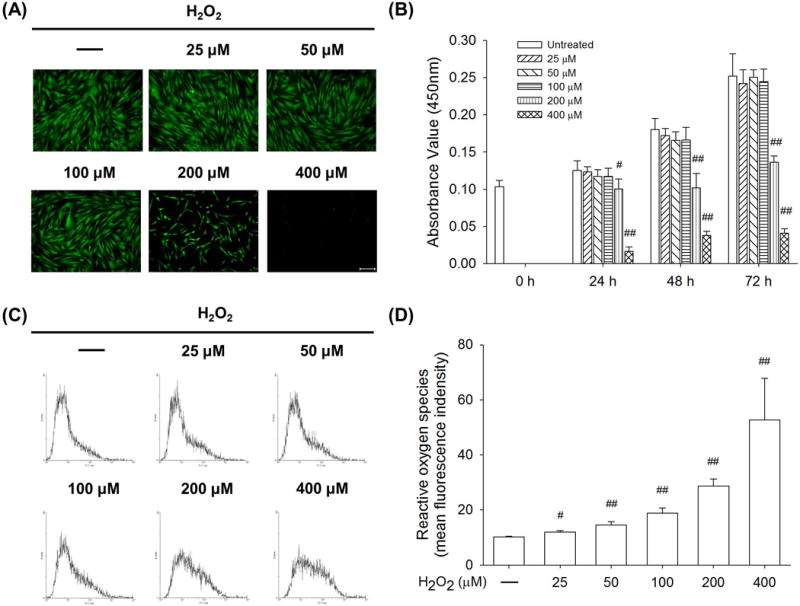
The effects of H2O2-induced oxidative stress on cell viability and ROS accumulation in BM-MSCs. The cells were exposed to H2O2 at concentrations ranging from 25 μM to 400 μM for 72 h. (A) Cell density of live BM-MSCs was observed by labeling with fluorescein diacetate (FDA). Scale bar = 200 μm. (B) The quantitative results of the CCK-8 assay revealed that treatments with H2O2 less than 100 μM did not significantly affect the cell viability of BM-MSCs. (C) After exposure to varying concentrations of H2O2, intracellular ROS of BM-MSCs was labeled by 2′,7′-dichlorofluorescein diacetate (DCF-DA) and measured with flow cytometry. (D) Quantitative results showed that H2O2 treatments increased intracellular ROS in BM-MSCs in a dose-dependent manner. Values are the mean ± S.E. of six independent experiments (n = 6) in CCK-8 assays and four independent experiments (n = 4) in ROS assays. Statistically significant differences are indicated by # p < 0.05 or ## p < 0.01 vs. the untreated group.
CONTINUOUS EXPOSURE TO H2O2 SUPPRESSED OSTEOGENESIS IN A DOSE-DEPENDENT MANNER
We then evaluated the long-term effect of H2O2 treatments on osteogenic differentiation of BM-MSCs. As shown in Fig. 2A, BM-MSCs were incubated in osteogenic differentiation medium in the presence of 25 μM, 50 μM, or 100 μM H2O2 for 21 days. The results of matrix mineralization, as a typical marker for the late stage of osteogenesis, demonstrated that H2O2-induced oxidative stress suppressed calcium deposition during osteogenic differentiation (Fig. 2B). The quantitative measurement of mineralization confirmed that H2O2 treatments inhibited matrix calcification in a dose-dependent manner, notably by 5.1% at 25 μM, by 32.0% at 50 μM, and by 83.7% at 100 μM, as compared to the control group (Fig. 2C). The transcript levels of osteoblast-specific genes were evaluated, including COL1A1, RUNX2, and BGLAP. Exposure to 100 μM H2O2 resulted in a reduction of 21.5% in mRNA expression of COL1A1 compared to the untreated cells (Fig. 2D). The transcript level of RUNX2 decreased in the presence of H2O2 by 7.2% at 25 μM, 21.5% at 50 μM, and 26.7% at 100 μM, compared to the control group (Fig. 2E). Treatment with H2O2 reduced the mRNA expression of BGLAP by 15.8% at 50 μM and 28.9% at 100 μM, in contrast to the untreated cells (Fig. 2F).
Fig. 2.
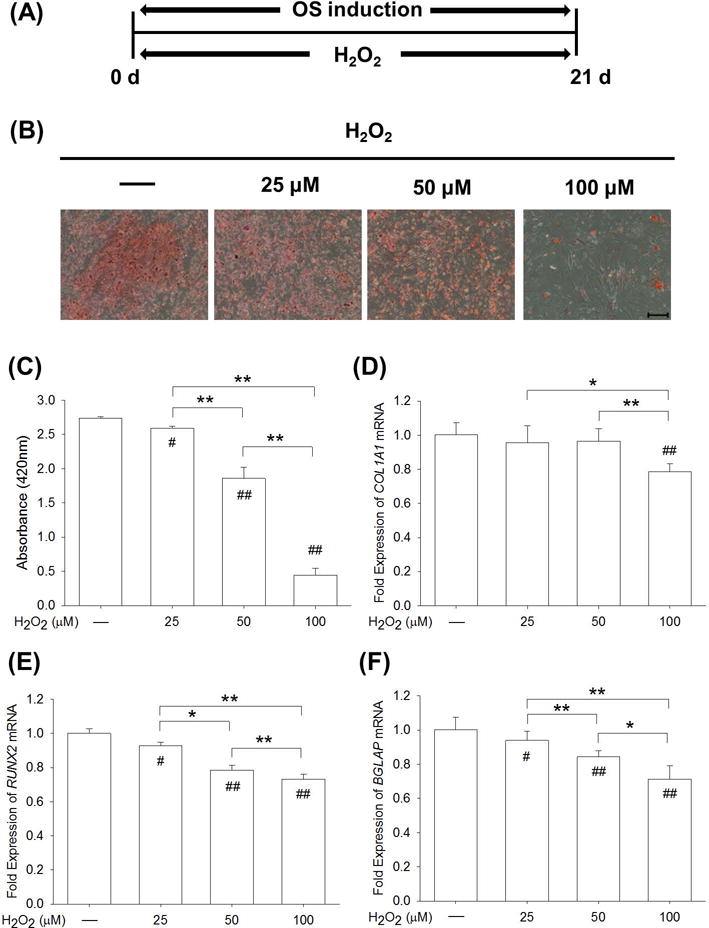
A continuous exposure to H2O2-induced oxidative stress inhibited osteogenic differentiation of BM-MSCs. (A) From the beginning of osteogenic induction, BM-MSCs were treated with H2O2 at concentrations of 25 μM, 50 μM, and 100 μM for 21 days. (B) Representative images of mineralized extracellular matrix stained by Alizarin Red S. Scale bar = 200 μm. (C) Quantification of the stained mineral layers demonstrated that H2O2 treatments suppressed osteogenic differentiation of BM-MSCs in a dose-dependent manner. (D-F) The mRNA levels of osteoblast-specific marker genes, including COL1A1 (D), RUNX2 (E), and BGLAP (F) were quantified with real-time RT-PCR using GAPDH for normalization. Values are the mean ± S.E. of four independent experiments (n = 4) in Alizarin Red S staining assays and four independent experiments (n = 4) in real-time RT-PCR assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups. Statistically significant differences are indicated by # p < 0.05 or ## p < 0.05 vs. the CTRL group.
H2O2-INDUCED OXIDATIVE STRESS INHIBITED THE EARLY STAGE OF OSTEOGENIC DIFFERENTIATION
The early stage of osteogenic differentiation mostly involved the lineage commitment of BM-MSCs toward osteoblasts. As shown in Fig. 3A, BM-MSCs were induced to osteogenesis with exposure to 25 μM, 50 μM, or 100 μM H2O2 for 7 days. The ALP staining assay showed fewer positively-stained cells in the 50 μM and 100 μM groups than in the control group (Fig. 3B). The quantitative analysis revealed that ALP activity of H2O2-treated BM-MSCs was reduced in a dose-dependent manner by 28.3% at 25 μM, by 41.7% at 50 μM, and by 69.3% at 100 μM, compared with the untreated cells (Fig. 3C). In agreement, the real-time RT-PCR data showed that ALP mRNA levels were significantly decreased by 46.2% at 50 μM and by 70.4% at 100 μM, as compared to the control group (Fig. 3D). Similarly, the transcript levels of COL1A1 (Fig. 3E) and RUNX2 (Fig. 3F) were significantly lower in the 50 μM and 100 μM groups.
Fig. 3.
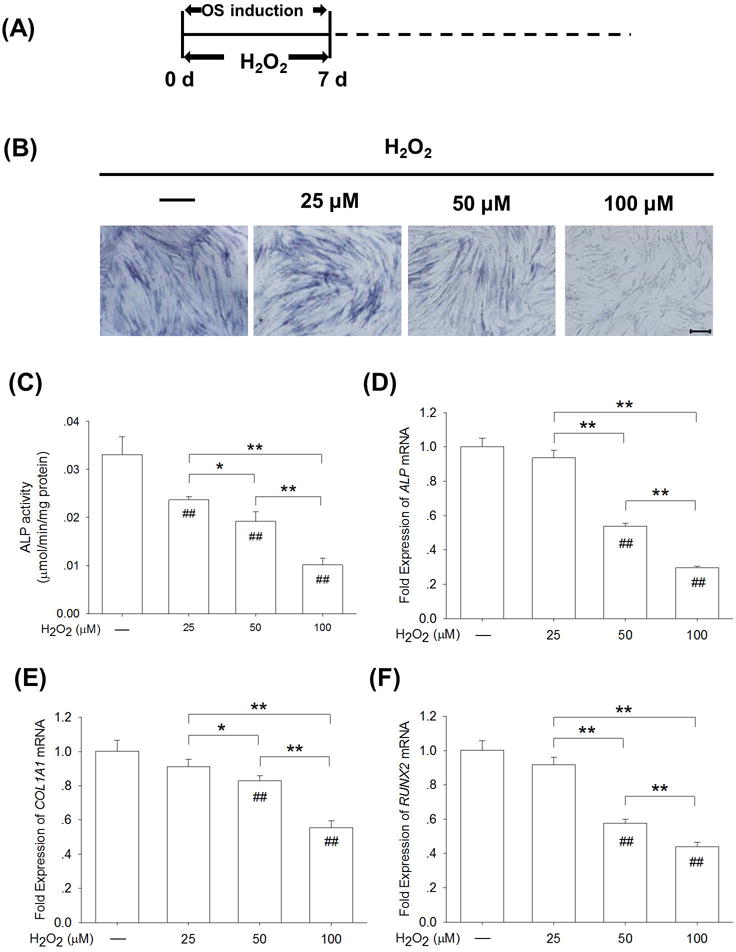
H2O2-induced oxidative stress suppressed lineage commitment toward osteoblasts in the early stage of osteogenic differentiation of BM-MSCs. (A) From the beginning of osteogenic induction, BM-MSCs were treated with H2O2 at concentrations of 25 μM, 50 μM, and 100 μM for 7 days. (B) Staining of intracellular ALP, a typical marker of the early stage of osteogenesis, was observed in H2O2-treated and untreated BM-MSCs. (C) Quantitative results demonstrated that H2O2 treatments decreased ALP activity in early-differentiated BM-MSCs in a dose-dependent manner. (D-F) The mRNA levels of early osteogenic markers, including ALP (D), COL1A1 (E), and RUNX2 (F) were quantified with real-time RT-PCR using GAPDH for normalization. Values are the mean ± S.E. of four independent experiments (n = 4) in ALP activity assays and four independent experiments (n = 4) in real-time RT-PCR assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups. Statistically significant differences are indicated by # p < 0.05 or ## p < 0.05 vs. the CTRL group.
H2O2-INDUCED OXIDATIVE STRESS DID NOT AFFECT MATRIX MINERALIZATION IN THE LATE STAGE OF OSTEOGENIC DIFFERENTIATION
To investigate the effect of oxidative stress on the late stage of osteogenic differentiation, BM-MSCs were first incubated in osteogenic induction medium and, after 14 days, exogenous H2O2 at 25 μM, 50 μM, and 100 μM was supplemented into the differentiation medium (Fig. 4A). The Alizarin Red S staining assay showed that calcified matrix could be observed in both the H2O2-treated and untreated groups (Fig. 4B). The results of quantitative experiments confirmed that H2O2 treatments had no negative effects on matrix mineralization in the late stage of osteogenic differentiation and notably, the level of calcium deposition was 4.5% higher in the 50 μM group than the control group (Fig. 4C). The treatments with H2O2 (lower than 50 μM) did not alter the mRNA expression of COL1A1, but the treatment with 100 μM H2O2 resulted in a significant decrease (Fig. 4D). The transcript levels of BGLAP were maintained at similar levels in H2O2-treated and untreated cells (Fig. 4E). With regard to the gene expression of RUNX2, H2O2-treated cells showed a decrease in a dose-dependent manner by 5.5% at 25 μM (p = 0.15), by 18.6% at 50 μM, and by 32.6% at 100 μM, compared with the untreated cells, respectively (Fig. 4F).
Fig. 4.
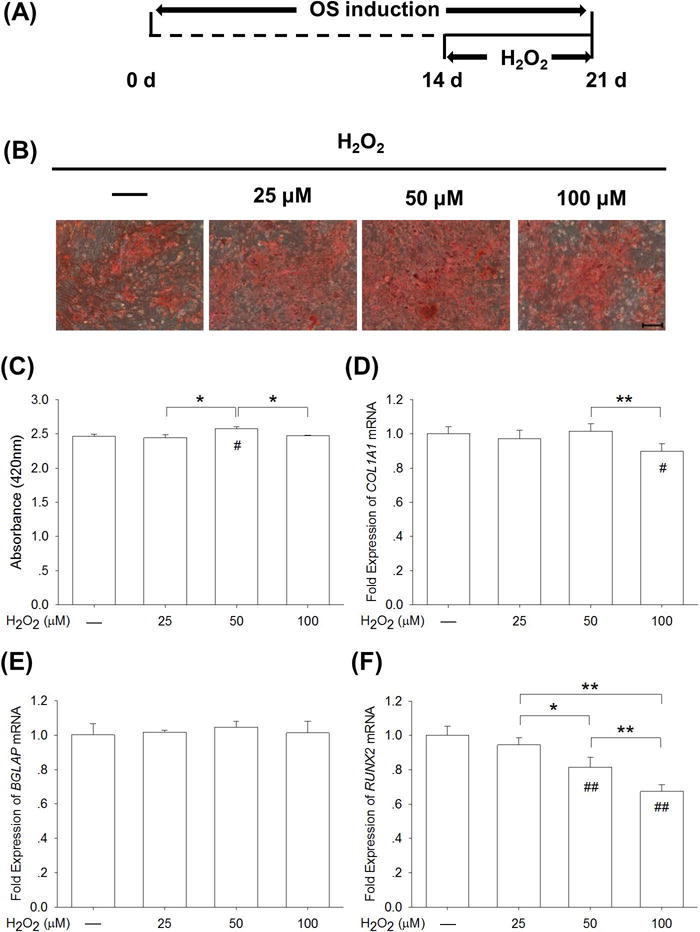
H2O2-induced oxidative stress did not affect matrix mineralization in the late stage of osteogenic differentiation of BM-MSCs. (A) After a 14-day osteogenic induction, late-differentiated BM-MSCs were treated with H2O2 at concentrations of 25 μM, 50 μM, and 100 μM for 7 days. (B) Representative images of mineralized extracellular matrix stained by Alizarin Red S. Scale bar = 200 μm. (C) Quantification of the stained mineral layers demonstrated that moderate H2O2-induced oxidative stress did not decrease calcium deposition in late-differentiated BM-MSCs. (D-F) The mRNA levels of late-differentiated osteoblast marker genes, including COL1A1 (D), RUNX2 (E), and BGLAP (F) were quantified with real-time RT-PCR using GAPDH for normalization. Values are the mean ± S.E. of four independent experiments (n = 4) in Alizarin Red S staining assays and four independent experiments (n = 4) in real-time RT-PCR assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups. Statistically significant differences are indicated by # p < 0.05 or ## p < 0.05 vs. the CTRL group.
SIRT1-MEDIATED SPONTANEOUS ENHANCEMENT OF ANTIOXIDANT DEFENSE DURING OSTEOGENIC DIFFERENTIATION
Since H2O2 treatments showed different effects on the early and late stages of osteogenic differentiation, we hypothesized that there was an increase in spontaneous resistance to oxidative stress during the osteogenic process. Since SIRT1 played an important role in protecting stem cells from oxidative stress, we compared the levels of SIRT1 and related antioxidant enzymes in undifferentiated and osteogenic differentiated BM-MSCs. We found that the transcript levels of SIRT1 were 90.9%, 109.2%, and 83.7% higher following induction of osteogenesis than those in undifferentiated BM-MSC cultures on day 7, 14, and 21, respectively (Fig. 5A). Western blot analysis confirmed that the protein levels of SIRT1 in BM-MSCs increased during osteogenesis in a time-dependent manner (Fig. 5B), which was 67.1% and 130.6% higher in differentiated cells compared with undifferentiated cells on day 14 and day 21, respectively (Supplementary Fig. 2A). Similarly, SIRT1 activity was increased by 42.7 % and 86.5% in the differentiated group compared with the control group on day 14 and 21, respectively (Fig. 5C). Two important H2O2-eliminating enzymes, CAT and GPX1, are responsible for converting intracellular H2O2 into water and oxygen. After inducing BM-MSCs toward osteoblasts, the mRNA levels of CAT were significantly higher than those in undifferentiated cells (by 76.3% on day 7, 62.5% on day 14, and 35.7% on day 21) (Fig. 5D). The gene expression of GPX1 in differentiated BM-MSCs showed a greater increase that was 2.4-fold on day 7, 2.6-fold on day 14, and 2.4-fold on day 21 in relation to the undifferentiated cells (Fig. 5E). Western blot analysis confirmed that the protein levels of CAT and GPX1 were significantly up-regulated in differentiated BM-MSCs (Fig. 5F, Supplementary Fig. 2B–C). We also evaluated the expression of antioxidant enzymes that scavenge intracellular superoxide radicals. The mRNA levels of SOD1 (Fig. 5G) and SOD2 (Fig. 5H) in differentiated BM-MSCs were significantly higher than those in undifferentiated cells. However, the gene expression of SOD2 showed the largest difference, which was 6.7-fold on higher day 7, 8.5-fold on higher day 14, and 16.1-fold higher on day 21 than those in undifferentiated cells, respectively (Fig. 5H). The protein levels of SOD1 and SOD2 showed an upward tendency similar to their mRNA expressions (Fig. 5I, Supplementary Fig. 2D–E).
Fig. 5.
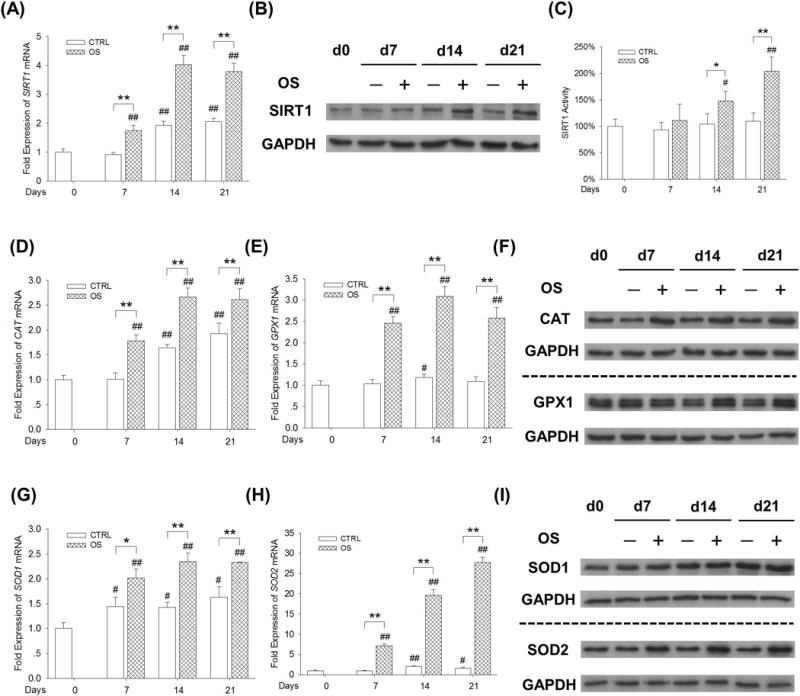
Up-regulation of SIRT1 correlated with spontaneous enhancement of antioxidant enzymes during osteogenic differentiation of BM-MSCs. BM-MSCs were incubated in osteogenic induction medium (OS) for 21 days; undifferentiated BM-MSCs cultured in growth medium served as a control (CTRL). (A) The mRNA and protein (B) levels of SIRT1 showed a time-dependent increase upon osteogenesis of BM-MSCs. (C) The SIRT1 activity was up-regulated in differentiated BM-MSCs after 14-day and 21-day incubation in osteogenic medium. SIRT1 activity results were normalized to the level of day 0 and values are the mean ± S.E. of three independent experiments (n = 3). (D-E) The mRNA levels of CAT (D) and GPX1 (E) were up-regulated during osteogenic differentiation. (F) The increase in protein levels of CAT and GPX1 in differentiated BM-MSCs was confirmed by Western blot assays. (G-H) The mRNA levels of SOD1 (F) and SOD2 (G) were increased during osteogenic differentiation. (I) The protein levels of SOD1 and SOD2 in differentiated BM-MSCs were confirmed by Western blot assays. Values are the mean ± S.E. of four independent experiments (n = 4) in real-time RT-PCR and Western blot assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups. Statistically significant differences are indicated by # p < 0.05 or ## p < 0.05 vs. Day 0.
ACTIVATION OF SIRT1 RESCUED H2O2-INHIBITED EARLY STAGE OF OSTEOGENESIS
We first evaluated the effects of ResV and NAM on osteogenesis of BM-MSCs. From the beginning of osteogenic induction, BM-MSCs were treated with 5 μM ResV or 10 mM NAM for 7 days. The early osteogenic differentiation was enhanced by ResV treatment but inhibited by NAM treatment (Supplementary Fig. 3). By treating BM-MSCs for 21 days, ResV treatment enhanced matrix mineralization and expression of osteoblast genes, while NAM treatment inhibited the late osteogenic markers (Supplementary Fig. 4). In order to test whether the poor resistance to H2O2-induced oxidative stress in the early stage of osteogenesis correlated with low levels of SIRT1, ResV was supplemented to activate SIRT1 in BM-MSCs in the presence of 100 μM H2O2 (Fig. 6A). The ALP staining assay demonstrated that the ALP-positive BM-MSCs were barely observed when exposed to H2O2; however, some positive cells became observable after the addition of ResV (Fig. 6B). Quantitative analysis revealed that ResV treatment barely affected the ALP activity in early-differentiated BM-MSCs, but it significantly improved the ALP activity by 51.3% in H2O2-treated cells (Fig. 6C). The mRNA expression of ALP was up-regulated by 16.4% and by 54.4% following treatment with ResV in untreated and H2O2-treated cells, respectively (Fig. 6D). Similarly, compared with H2O2-treated BM-MSCs, the addition of ResV significantly increased the transcript levels of COL1A1 by 58.3% (Fig. 6E) and RUNX2 by 50.7% (Fig. 6F). Since the levels of GPX1 and SOD2 showed the largest differences when BM-MSCs were induced toward osteoblasts, we further examined the protein expressions of GPX1, SOD2, and SIRT1 in H2O2- and ResV-treated BM-MSCs. Western blot assays showed that, after a 7-day osteogenic induction, H2O2 treatments resulted in a dramatic decrease in the protein levels of GPX1 and SOD2 but supplementation with ResV recovered the expression of these two antioxidant enzymes (Fig. 6G, Supplementary Fig. 5A–C). As well, the 7-day treatment with ResV up-regulated the protein expression of SIRT1 by 53.2% (Supplementary Fig. 5C) compared with H2O2-treated BM-MSCs. Similarly, H2O2 treatment significantly decreased SIRT1 activity by 52.0% compared to untreated cells, but ResV treatment significantly increased SIRT1 activity by 104.0% in contrast to H2O2-treated cells (Fig. 6H).
Fig. 6.
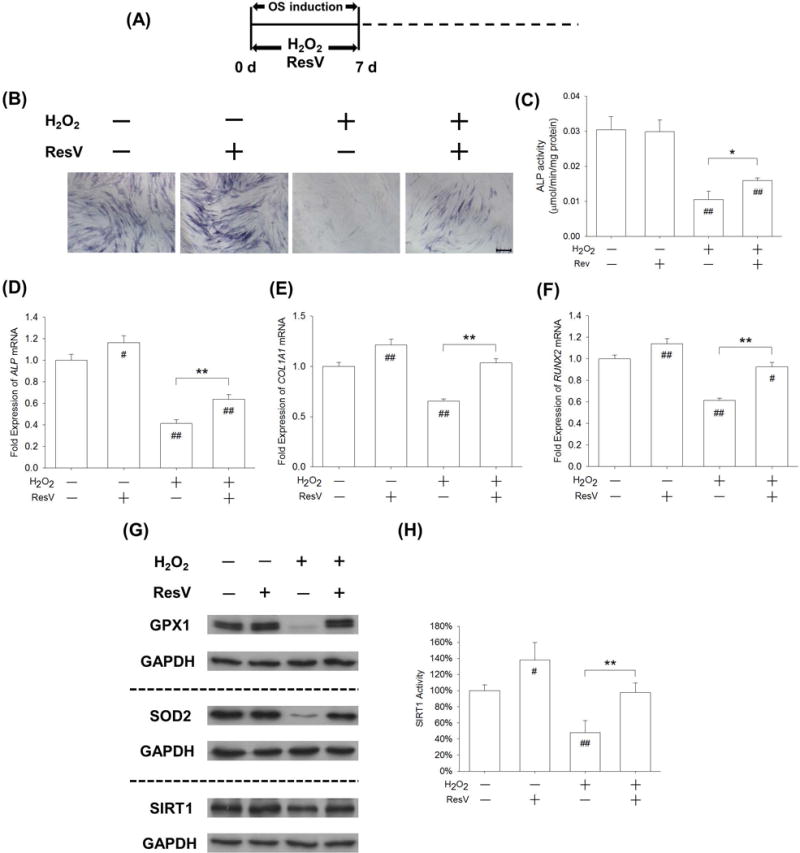
Activation of SIRT1 by ResV rescued H2O2-inhibited early osteogenic differentiation of BM-MSCs. (A) BM-MSCs were incubated in osteogenic induction medium supplemented with 100 μM H2O2 and 5 μM ResV for 7 days. (B) Staining of intracellular ALP as a typical marker for the early stage of osteogenesis was observed in BM-MSCs. (C) Quantitative results demonstrated that ResV treatment significantly increased ALP activity compared with H2O2-treated BM-MSCs. (D-F) The mRNA levels of early osteogenic markers, including ALP (D), COL1A1 (E), and RUNX2 (F) were quantified with real-time RT-PCR using GAPDH for normalization. (G) The protein levels of GPX1, SOD2, and SIRT1 were measured by Western blot assays. (H) The SIRT1 activity was up-regulated by ResV treatment compared with H2O2-treated BM-MSCs. SIRT1 activity results were normalized to the level of untreated cells and values are the mean ± S.E. of three independent experiments (n = 3). Values are the mean ± S.E. of four independent experiments (n = 4) in ALP activity assays, real-time RT-PCR assays, and Western blot assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups. Statistically significant differences are indicated by # p < 0.05 or ## p < 0.05 vs. the CTRL group.
INHIBITION OF SIRT1 INTENSIFIED THE NEGATIVE EFFECT OF H2O2-INDUCED OXIDATIVE STRESS ON THE LATE STAGE OF OSTEOGENESIS
Finally, we tested whether the inactivation of SIRT1 would affect matrix mineralization and late stage osteogenesis in H2O2-treated BM-MSCs. BM-MSCs were first incubated in osteogenic induction medium and, after a 14-day induction, NAM was supplemented in osteogenic differentiation medium in the presence of 50 μM H2O2 (Fig. 7A). An Alizarin Red S staining assay showed that matrix mineralization was not affected by either NAM or H2O2 treatment but was dramatically suppressed by the treatment with NAM + H2O2 (Fig. 7B). The quantitative experiment confirmed that, when exposed to NAM and H2O2 together, BM-MSCs showed the lowest levels of calcium deposition, only 62.1% and 57.5% of the CTRL and H2O2 group, respectively (Fig. 7C). The real-time RT-PCR data demonstrated that the transcript levels of COL1A1 were significantly down-regulated by treatment with NAM + H2O2; the levels were 50.3% and 45.3% lower than in the CTRL and H2O2 groups, respectively (Fig. 7D). Similarly, treatment with NAM + H2O2 reduced the level of RUNX2 mRNA by 38.9% (Fig. 7E) and BGLAP mRNA by 49.8% (Fig. 7F) compared with the CTRL group. Western blot assays showed that, after treating BM-MSCs with NAM and H2O2 together, the protein levels of GPX1, SOD2, and SIRT1 were down-regulated compared with the CTRL or H2O2 group (Fig. 7G, Supplementary Fig. 6A–C). In the presence of 50 μM H2O2, the protein expression of SIRT1 was decreased by 40.8% (Supplementary Fig. 6C) and the activity was down-regulated by 51.5% with NAM treatment (Fig. 7H).
Fig. 7.
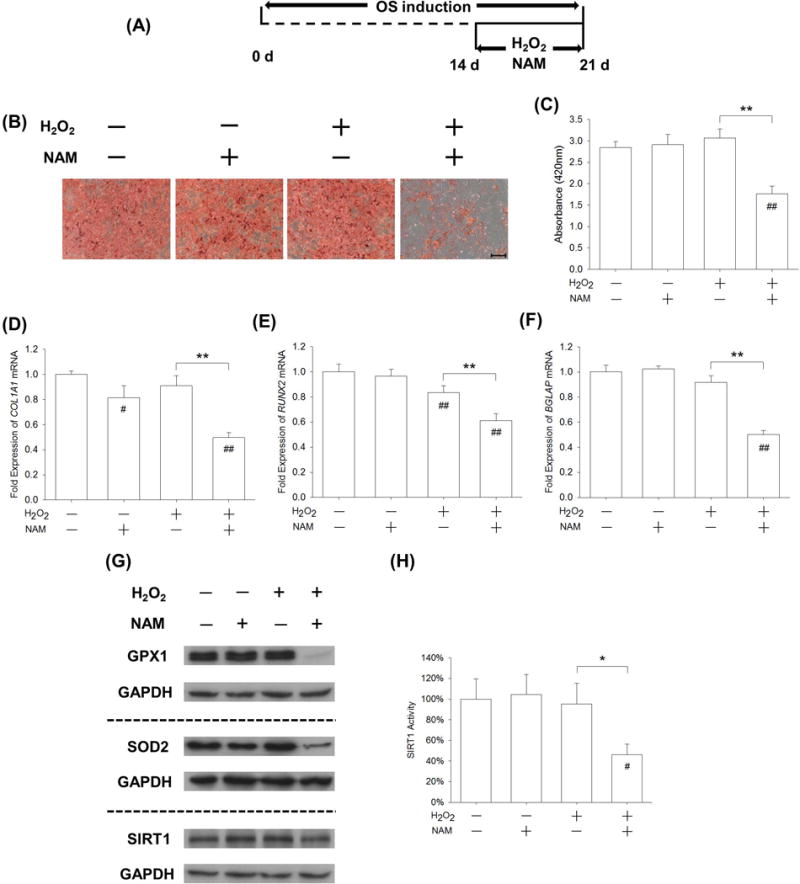
Inhibition of SIRT1 by NAM intensified the effects of H2O2-induced oxidative stress on the late stage of osteogenic differentiation of BM-MSCs. (A) After a 14-day osteogenic induction, late-differentiated BM-MSCs were exposed to 50 μM H2O2 in the presence of 10 mM NAM for 7 days. (B) Mineral depositions in ECM were stained by Alizarin Red S. Scale bar = 200 μm. (C) Quantification of the stained mineral layers demonstrated that calcium deposition was significantly inhibited by treatments with H2O2 and NAM in the late-differentiated BM-MSCs. (D-F) The mRNA levels of late-differentiated osteoblast marker genes, including COL1A1 (D), RUNX2 (E), and BGLAP (F) were quantified with real-time RT-PCR using GAPDH for normalization. (G) The protein levels of GPX1, SOD2, and SIRT1 were measured by Western blot assays. (H) SIRT1 activity was down-regulated by NAM treatment compared with untreated and H2O2-treated BM-MSCs. SIRT1 activity results were normalized to the level of untreated cells and values are the mean ± S.E. of three independent experiments (n = 3). Values are the mean ± S.E. of four independent experiments (n = 4) in Alizarin Red S staining assays, real-time RT-PCR assays, and Western blot assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups. Statistically significant differences are indicated by # p < 0.05 or ## p < 0.05 vs. the CTRL group.
We further used SIRT1 siRNA to confirm its effect on the resistance to H2O2–induced oxidative stress in late-differentiated BM-MSCs. BM-MSCs were incubated in osteogenic induction medium for 14 days and, after cells were transfected with SIRT1 siRNA, BM-MSCs were subjected to 50 μM H2O2 for 7 days. SIRT1 targeted siRNA transfection in BM-MSCs significantly decreased the expression of SIRT1 by 69.7% compared with the CTRL group (Fig. 8A&B). An Alizarin Red S staining assay showed that matrix mineralization was suppressed by SIRT1 targeted siRNA transfection (Fig. 8C). The level of matrix mineralization in the siRNA group was 22.4% lower than the CTRL group, and in the presence of H2O2, treatment with SIRT1 siRNA decreased matrix mineralization by 36.1% compared with the CTRL group (Fig. 8D). The real-time RT-PCR data showed that the transcript levels of osteoblast genes were significantly down-regulated by SIRT1 targeted siRNA transfection. In the presence of H2O2, treatment with SIRT1 siRNA reduced the level of COL1A1 mRNA by 48.5% (Fig. 8E), RUNX2 mRNA by 60.3% (Fig. 8F), and BGLAP mRNA by 51.9% (Fig. 8G) compared with the CTRL group.
Fig. 8.
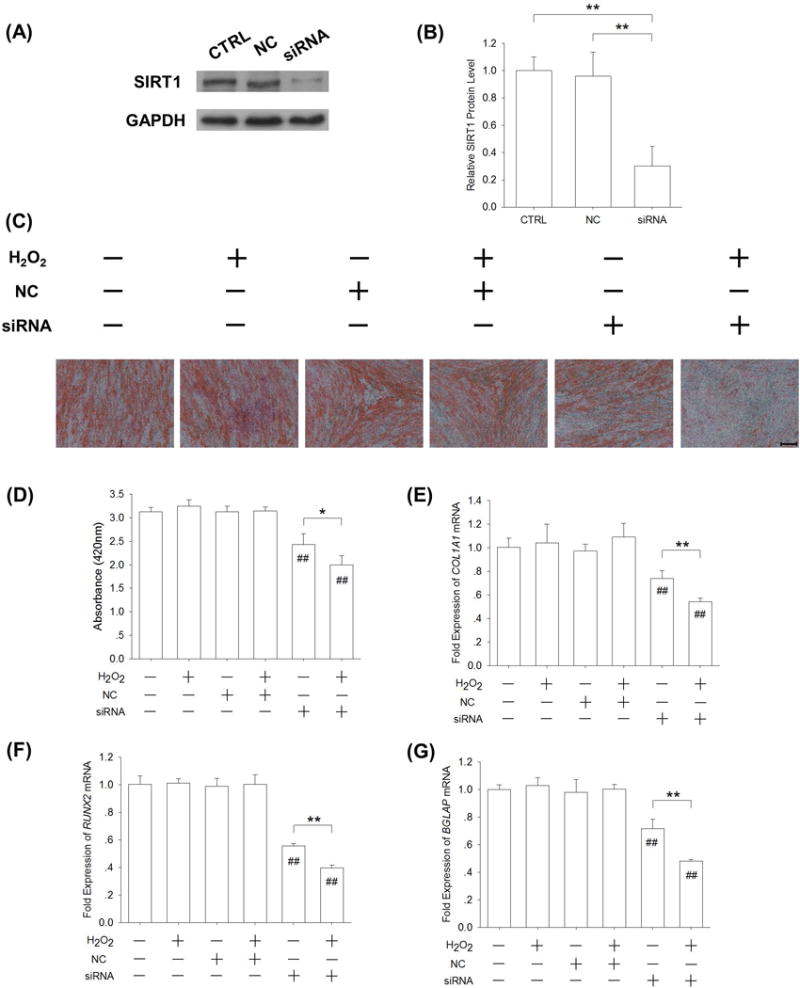
SIRT1 targeted siRNA transfection in late-differentiated BM-MSCs inhibited matrix mineralization in the presence of H2O2-induced oxidative stress. (A-B) After a 14-day osteogenic induction, BM-MSCs were transfected with SIRT1 siRNA and then subjected to 50 μM H2O2 for 7 days. SIRT1 targeted siRNA transfection in BM-MSCs significantly decreased the expression of SIRT1. (C) Mineral depositions in ECM were stained with Alizarin Red S. Scale bar = 200 μm. (D) Quantification of the stained mineral layers demonstrated that matrix mineralization was suppressed by SIRT1 targeted siRNA transfection. (E-G) The mRNA levels of late-differentiated osteoblast marker genes, including COL1A1 (E), RUNX2 (F), and BGLAP (G) were quantified with real-time RT-PCR using GAPDH for normalization. Values are the mean ± S.E. of four independent experiments (n = 4) in Western blot assays, Alizarin Red S staining assays, and real-time RT-PCR assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups. Statistically significant differences are indicated by # p < 0.05 or ## p < 0.05 vs. the CTRL group.
DISCUSSION
In the present study, we found that low doses of H2O2 barely affected MSC viability; however, high doses of H2O2, such as 200 μM or 400 μM, significantly suppressed MSC proliferation and even caused cell death. These findings are in agreement with previous reports in which oxidative stress was shown to activate MSCs’ apoptotic signaling pathways [Urao and Ushio-Fukai, 2013; Wei et al., 2010; Zhou et al., 2015]. In the following osteogenic induction experiments, only moderate doses of H2O2, ranging from 25 μM to 100 μM, were chosen in order to minimize the influence of high doses on MSC survival. We found that H2O2-induced oxidative stress did not suppress matrix calcification and SIRT1 and antioxidant enzymes were up-regulated in osteogenic induction of BM-MSCs. These results indicate that the resistance to oxidative stress was spontaneously enhanced in BM-MSCs upon osteogenic induction and the SIRT1-mediated signaling pathway was involved in the intracellular antioxidant defense system.
In agreement with previous studies [Kim et al., 2010; Mody et al., 2001], we observed that a long-term exposure to oxidative stress showed deleterious effects on osteogenic differentiation of BM-MSCs, which might be associated with the extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway [Bai et al., 2004] and/or the Wnt/β-catenin canonical pathway [Almeida et al., 2007]. Several studies have shown that pretreatment with antioxidants, such as N-acetyl cysteine and melatonin, effectively protect osteoblastic cells and MSCs from H2O2-induced [Lee et al., 2015] or proinflammatory cytokine-induced oxidative stress [Liu et al., 2013]. However, the effects of antioxidant treatments, especially on osteogenic differentiation and matrix calcification, are still controversial. Yamada et al. [Yamada et al., 2012] demonstrated that application of an antioxidant tempol attenuated the accumulation of oxidative stress and suppressed osteogenic transdifferentiation of smooth muscle cells. Overexpression of antioxidant enzymes such as SOD and CAT has been shown to reverse ROS-mediated calcium deposition in aortic valve sclerosis-derived cells [Branchetti et al., 2013]
Interestingly, we found that exogenous H2O2-induced oxidative stress barely affected the process of matrix calcification in the late stage of osteogenesis. Our data indicated that undifferentiated BM-MSCs had a weak tolerance to moderate oxidative stress, as opposed to embryonic stem cells (ESCs). The latter has shown superior resistance to oxidative stress in the undifferentiated stage compared to their spontaneously differentiated fibroblastic progenies [George et al., 2009]; this strong tolerance to ROS might correlate to the high activity of glycolytic enzymes and elevated glycolytic flux in undifferentiated ESCs [Kondoh et al., 2007]. Previous studies indicated that there were alterations in energy metabolism and cellular redox status during the spontaneous differentiation of ESCs. Therefore, we speculated that osteoblasts differentiated from BM-MSCs have a different resistance compared to the same level of H2O2-induced oxidative stress from undifferentiated BM-MSCs.
Recent studies have indicated that osteogenic differentiation of MSCs is also influenced by their antioxidant capacity. In this study, we found a time-dependent up-regulation of CAT, GPX1, and SOD2 upon osteogenic induction. These results demonstrated that, during osteogenic differentiation, the intracellular antioxidant defense system was spontaneously enhanced, which might contribute to the improved resistance to exogenous H2O2-induced oxidative stress. Our findings are consistent with a previous report by Chen and co-workers [Chen et al., 2008] which demonstrated that, upon osteogenic induction, mitochondrial biogenesis in MSCs changed from glycolysis into an aerobic respiratory form for energy supply and a significant reduction in intracellular ROS was observed, including the accumulation of intracellular H2O2 and superoxide anions. However, another important antioxidant enzyme, SOD1 in differentiated BM-MSCs, was slightly up-regulated in mRNA expression and showed no significant differences in the protein levels compared to undifferentiated cells. A recent study indicated that the levels of SOD3, the only extracellular antioxidant enzyme that scavenged toxic free radicals, were not affected during osteogenic differentiation in BM-MSCs, but they were increased upon adipogenic differentiation [Nightingale et al., 2012]. Therefore, additional experiments are necessary to determine the relationship between individual antioxidant enzymes and lineage-specific differentiation (e.g., osteogenesis, adipogenesis, and chondrogenesis) of MSCs.
For the first time, in this study, we found that there was a spontaneous increase in SIRT1, with a peak level after 2-week osteogenic induction, suggesting that there may be a link between up-regulation of SIRT1 and osteogenic lineage commitment of BM-MSCs. Previous studies have shown that the activation of SIRT1 by ResV treatment promoted the lineage commitment of MSCs toward osteogenesis by enhancing FOXO3A-dependent transcriptional activity to stimulate RUNX2 gene transcription [Tseng et al., 2011]. However, the underlying signaling pathways regulating SIRT1 expression and activity during osteogenic differentiation are still unclear. Activation of AMPK (adenosine monophosphate-activated protein kinase) may play an important role in promoting SIRT1 upon osteogenesis, because phosphorylation of AMPK contributed to MSC differentiation to osteoblasts [Pantovic et al., 2013]. It has also been reported that the activation of AMPK induced an increase in SIRT1 activity, as evidenced by the up-regulated levels of deacetylation and downstream SIRT1 targeted genes, such as PPARγ (peroxisome proliferators-activated receptor-gamma) and FOXO3A [Canto et al., 2009].
Furthermore, we tested the hypothesis of a link between an increase in spontaneous resistance to H2O2-oxidative stress and an up-regulation of SIRT1 in differentiated BM-MSCs. In the early stage of osteogenic induction, treatment with ResV reversed the effect of exogenous H2O2 on early-differentiated MSCs. An explanation is that SIRT1 could induce expression of antioxidant enzymes such as SOD2, CAT, peroxiredoxins, and uncoupling protein 2 (UCP-2) by directly deacetylating FOXO3A and the transcriptional coactivator peroxisome proliferator activated receptor γ-coactivator 1α (PGC-1α) [Olmos et al., 2013]. Another underlying molecular mechanism by which SIRT1 activated the intracellular antioxidant system might be through the Nrf2 (nuclear factor E2-related factor 2)/ARE (antioxidant response element) signaling pathway. SIRT1 promoted the transcriptional activities of Nrf2 by its deacetylation effect and subsequently increased the expression of Nrf2 downstream antioxidant genes, such as SOD1 [Huang et al., 2013]. Finally, we found that the inhibiting or silencing SIRT1 intensified the effect of H2O2 treatment on late-differentiated BM-MSCs. In agreement with our study, silencing SIRT1 was proven to exacerbate mitochondrial oxidative stress induced by ischemia reperfusion injury in cardiomyocytes [Yang et al., 2013]. Therefore, our results showed that the up-regulation of SIRT1 contributed to spontaneously enhanced antioxidant enzyme expression in BM-MSCs and improved resistance to oxidative stress during osteogenic differentiation.
There are several limitations we would like to point out regarding this study. First, we focused on the effect of oxidative stress on osteogenic differentiation of BM-MSCs. Previous studies suggested that over-accumulation of ROS induced an osteogenic phenotype and the calcification of VSMCs. Our future study will compare differences in SIRT1 expression and resistance to oxidative stress between BM-MSCs and VSMCs. Second, we found that the antioxidant defense system was spontaneously enhanced in normal adult human BM-MSCs during osteogenic differentiation. However, nothing is known about the levels of SIRT1 and antioxidant enzymes in BM-MSCs from patients with bone diseases, such as osteoporosis and osteoarthritis. In future studies, we will investigate the response to oxidative stress and the changes of antioxidant enzymes in abnormal BM-MSCs during osteogenic differentiation. Third, animal studies are necessary to confirm the effect of SIRT1 on protecting BM-MSCs from oxidative stress and our future work will focus on in vivo bone formation, especially in an oxidative environment.
CONCLUSIONS
In summary, we demonstrated that exogenous H2O2-induced oxidative stress inhibited the osteogenic lineage commitment of BM-MSCs, but late-differentiated BM-MSCs showed an enhanced resistance to oxidative stress. Our findings uncovered a critical role of SIRT1 in regulating the spontaneous enhancement of the antioxidant defense system and resistance to oxidative stress in BM-MSCs upon osteogenic induction. Understanding the relationship between SIRT1 and antioxidant enzymes during osteogenic differentiation will provide a new strategy that could potentially protect MSCs from extracellular oxidative stress and facilitate the clinical application of MSCs in bone tissue engineering. Moreover, our results highlight a potential therapeutic role of SIRT1 in bone repair or regeneration based on MSCs, especially when faced with an oxidative stress laden environment.
Supplementary Material
Acknowledgments
The authors are grateful to Suzanne Danley (West Virginia University, USA) and Paula Sahyoun (University of Waterloo, Canada) for carefully reviewing and editing the manuscript. This work was supported by the National Natural Science Foundation of China [31570978, 31771063, 81702146, 81401768]; the Natural Science Foundation of Jiangsu Province [BK20140323, BK20140289]; the National Institutes of Health (NIH) [AR062763-01A1, AR067747-01A1] and an Established Investigator Grant from Musculoskeletal Transplant Foundation (MTF) to M.P.; and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Grant Information:
Grant sponsor: National Natural Science Foundation of China; Grant number: 31570978, 31771063, 81702146, 81401768.
Grant sponsor: Natural Science Foundation of Jiangsu Province; Grant number: BK20140323, BK20140289.
Grant sponsor: National Institutes of Health (NIH); Grant number: AR062763-01A1, AR067747-01A1.
Grant sponsor: Musculoskeletal Transplant Foundation (MTF).
Grant sponsor: Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Abbreviations
- AMPK
adenosine monophosphate-activated protein kinase
- ALP
alkaline phosphatase
- BGLAP
bone gamma carboxyglutamate protein
- BM-MSCs
bone marrow-derived mesenchymal stem cells
- CAT
catalase
- CCK-8
cell counting kit-8
- COL1A1
type I collagen α1
- CTRL
control cells
- ECM
extracellular matrix
- ERK1/2
extracellular signal-regulated kinase 1/2
- ESCs
embryonic stem cells
- FOXO
forkhead box O
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GPX1
glutathione peroxidases 1
- H2O2
hydrogen peroxide
- MSCs
mesenchymal stem cells
- NAM
nicotinamide
- PPARγ
peroxisome proliferators-activated receptor-gamma
- ResV
resveratrol
- ROS
reactive oxygen species
- RUNX2
runt-related transcription factor 2
- SIRT1
silent information regulator type 1
- SOD
superoxide dismutase
- VSMCs
vascular smooth muscle cells
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;24:1150–1163. doi: 10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backesjo CM, Li Y, Lindgren U, Haldosen LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res. 2006;21:993–1002. doi: 10.1359/jbmr.060415. [DOI] [PubMed] [Google Scholar]
- Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, Ferrari G. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 2013;33:e66–74. doi: 10.1161/ATVBAHA.112.300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–253. doi: 10.1016/j.redox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Liu X, Chen H, Cao J, Zhang L, Hu X, Wang J. Role of SIRT1 and AMPK in mesenchymal stem cells differentiation. Ageing Res Rev. 2014;13:55–64. doi: 10.1016/j.arr.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- George S, Heng BC, Vinoth KJ, Kishen A, Cao T. Comparison of the response of human embryonic stem cells and their differentiated progenies to oxidative stress. Photomed Laser Surg. 2009;27:669–74. doi: 10.1089/pho.2008.2354. [DOI] [PubMed] [Google Scholar]
- Ho PJ, Yen ML, Tang BC, Chen CT, Yen BL. H2O2 accumulation mediates differentiation capacity alteration, but not proliferative decline, in senescent human fetal mesenchymal stem cells. Antioxid Redox Signal. 2013;18:1895–1905. doi: 10.1089/ars.2012.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, Liu P, Huang H. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2013;65:528–540. doi: 10.1016/j.freeradbiomed.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Kim WK, Meliton V, Bourquard N, Hahn TJ, Parhami F. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J Cell Biochem. 2010;111:1199–1209. doi: 10.1002/jcb.22846. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, Gil J, Beach D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- Lee D, Kook SH, Ji H, Lee SA, Choi KC, Lee KY, Lee JC. N-acetyl cysteine inhibits H2O2-mediated reduction in the mineralization of MC3T3-E1 cells by down-regulating Nrf2/HO-1 pathway. BMB Rep. 2015;48:636–641. doi: 10.5483/BMBRep.2015.48.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gong Y, Xiong K, Ye Y, Xiong Y, Zhuang Z, Luo Y, Jiang Q, He F. Melatonin mediates protective effects on inflammatory response induced by interleukin-1 beta in human mesenchymal stem cells. J Pineal Res. 2013;55:14–25. doi: 10.1111/jpi.12045. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou L, Chen X, Liu T, Pan G, Cui W, Li M, Luo Z, Pei M, Yang H, He F. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;61:437–448. doi: 10.1016/j.msec.2015.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyublinskaya OG, Borisov YG, Pugovkina NA, Smirnova IS, Obidina JV, Ivanova JS, Zenin VV, Shatrova AN, Borodkina AV, Aksenov ND, Zemelko VI, Burova EB, Puzanov MV, Nikolsky NN. Reactive Oxygen Species Are Required for Human Mesenchymal Stem Cells to Initiate Proliferation after the Quiescence Exit. Oxid Med Cell Longev. 2015;2015:502105. doi: 10.1155/2015/502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates JM, Sanchez-Jimenez F. Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci. 1999;4:D339–D345. doi: 10.2741/mates. [DOI] [PubMed] [Google Scholar]
- Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Nicolaije C, Koedam M, van Leeuwen JP. Decreased oxygen tension lowers reactive oxygen species and apoptosis and inhibits osteoblast matrix mineralization through changes in early osteoblast differentiation. J Cell Physiol. 2012;227:1309–1318. doi: 10.1002/jcp.22841. [DOI] [PubMed] [Google Scholar]
- Nightingale H, Kemp K, Gray E, Hares K, Mallam E, Scolding N, Wilkins A. Changes in expression of the antioxidant enzyme SOD3 occur upon differentiation of human bone marrow-derived mesenchymal stem cells in vitro. Stem Cells Dev. 2012;21:2026–2035. doi: 10.1089/scd.2011.0516. [DOI] [PubMed] [Google Scholar]
- Olmos Y, Sanchez-Gomez FJ, Wild B, Garcia-Quintans N, Cabezudo S, Lamas S, Monsalve M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex. Antioxid Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, Trajkovic V. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52:524–531. doi: 10.1016/j.bone.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML, Yen ML. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res. 2011;26:2552–2563. doi: 10.1002/jbmr.460. [DOI] [PubMed] [Google Scholar]
- Urao N, Ushio-Fukai M. Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic Biol Med. 2013;54:26–39. doi: 10.1016/j.freeradbiomed.2012.10.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Li Z, Hu S, Chen X, Cong X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem. 2010;111:967–978. doi: 10.1002/jcb.22785. [DOI] [PubMed] [Google Scholar]
- Yamada S, Taniguchi M, Tokumoto M, Toyonaga J, Fujisaki K, Suehiro T, Noguchi H, Iida M, Tsuruya K, Kitazono T. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res. 2012;27:474–485. doi: 10.1002/jbmr.539. [DOI] [PubMed] [Google Scholar]
- Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, Wang N, Deng C, Zhang S, Li Y, Chen W, Yu S, Yi D, Jin Z. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013;65:667–679. doi: 10.1016/j.freeradbiomed.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Yoon DS, Choi Y, Jang Y, Lee M, Choi WJ, Kim SH, Lee JW. SIRT1 directly regulates SOX2 to maintain self-renewal and multipotency in bone marrow-derived mesenchymal stem cells. Stem Cells. 2014;32:3219–3231. doi: 10.1002/stem.1811. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan G, Cui W, Luo ZP, Pei M, Yang H, He F. Melatonin reverses H2O2-induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res. 2015;59:190–205. doi: 10.1111/jpi.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chen X, Liu T, Zhu C, Si M, Jargstorf J, Li M, Pan G, Gong Y, Luo ZP, Yang H, Pei M, He F. SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells. J Tissue Eng Regen Med. 2017 doi: 10.1002/term.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


