Abstract
Background
Anorexia and malnutrition are associated with poor outcomes in children with CKD.
Study Design
Observational cohort study.
Setting & Participants
We assessed the changes in body mass index (BMI) as kidney function declines and its association with risk of ESRD among 854 participants followed up inn 2005–2013 in the Chronic Kidney Disease in Children (CKiD) Study.
Predictors
Repeated measures of estimated glomerular filtration rate by serum creatinine (eGFR) in our trajectory analysis using mixed models; change in BMI z score (per year) after eGFR fell below 35 mL/min/1.73 m2 in logistic regression models.
Outcomes
Repeated measures of BMI z score (as a reflection of weight status) in our trajectory analysis; ESRD in logistic regression models.
Results
During mean longitudinal follow-up of 3.4 years, BMI z scores remained stable until eGFR fell below 35 mL/min/1.73 m2. When eGFR fell below 35 mL/min/1.73 m2, a mean decline in BMI z score of 0.13 (95% CI, 0.09–0.17) was noted with each 10-mL/min/1.73 m2 further decline in eGFR. This was statistically significantly different from the weight trajectory when the eGFR was 35 mL/min/1.73m2 or greater (p<0.001). Among children and adolescents with significant weight loss (defined as a decline in BMI z score of >0.2 per year) after eGFR fell below 35 mL/min/1.73 m2, the odds of ESRD was 3.28 (95% CI 1.53–7.05) times greater compared to participants with stable BMI z scores (BMI z-score change per year of 0–0.1).
Limitations
Observational nature of our study, lack of longitudinal assessments of inflammatory markers.
Conclusions
In children and adolescents with CKD, weight loss mostly occurs when eGFR falls below 35 mL/min/1.73 m2, and this weight loss was associated with a higher risk of ESRD. Further studies are needed to define the reasons for the association between weight loss and more rapid progression to ESRD in children and adolescents.
Keywords: body mass index (BMI), BMI trajectory, height, chronic kidney disease (CKD), CKD progression, children, adolescents, pediatric CKD, malnutrition, weight loss, growth, end-stage renal disease (ESRD)
Weight loss is known to occur commonly among adults and children with chronic kidney disease (CKD), including end-stage renal disease (ESRD).1–5 A few prior cross-sectional studies in adults suggested that weight begins to decrease around an estimated glomerular filtration rate (eGFR) of 40 mL/min/1.73 m2.1,2 However, there has been a paucity of data on longitudinal assessments of the timing or degree of weight loss that occurs as CKD progresses in children. Whether weight slowly declines in linear fashion with advancing CKD or remains stable until the late stages of CKD is unclear. Because of the critical importance of nutrition in fostering appropriate growth in children, use of feeding tubes or nutritional supplementation to foster appropriate weight gain during CKD is a common practice.6,7 However, the appropriate timing of such interventions to maintain weight (or foster appropriate weight gain) has not been well examined in children with CKD.
The objective of this study was to determine the trajectory of weight change as kidney function declines in the Chronic Kidney Disease in Children (CKiD) study, a North American observational cohort study of children and adolescents with CKD followed up longitudinally since 2005. We hypothesized that weight trajectory would not be linear with the progression of CKD, and that weight loss would primarily occur after onset of the advanced stages of CKD (stage 4 or beyond). We also aimed to determine the characteristics of CKiD participants who lost significant weight with advancing CKD, and whether such weight changes would be associated with a higher risk of ESRD.
Methods
Study Population
Details of the CKiD study have been previously described.8 Briefly, CKiD is a prospective cohort study of children and adolescents aged 1–16 years with eGFR of 30–90 mL/min/1.73 m2 that began in 2005 and is ongoing.8,9 In the present analysis, we included 854 CKiD participants who had at least one serum creatinine, BMI and height measurement (by stadiometer or measuring table with block and footboard) available for our trajectory analysis. All data were derived from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository in de-identified form, and data were administratively censored as of July 2013. The University of California Institutional Review Board considers this study exempt human subjects research; no additional informed consent was required for this analysis. All data analyses were conducted using Stata 14, and verified by a separate statistician using SAS.
BMI Trajectory During CKD Progression
We examined the association between eGFR and repeated measures of age- and sex-specific BMI z score during longitudinal follow-up of CKiD participants. Our primary predictor of interest was eGFR using the bedside Schwartz equation (eGFRcre),10 given a larger number of missing serum cystatin C levels during follow-up visits. However, in sensitivity analysis, we repeated these analyses using the 2012 cystatin-C based equation (eGFRcys) to estimate kidney function if cystatin C was available.11
Our primary outcome of interest in our trajectory analyses were age- and sex-specific BMI z scores (based on the 2000 Centers for Disease Control [CDC] BMI standards for children aged 2 years or older and the 2006 World Health Organization [WHO] Child Growth Standards for children younger than 2 years of age).12,13 We chose to use BMI z score as our primary outcome, because BMI is a better reflection of weight status since it simultaneously accounts for height accrual that may occur in children. Our secondary outcome of interest was age- and sex-specific weight z score trajectory (also according to 2000 CDC standards).12,13 For visits with BMI z scores that were missing (121 visits), the most recent BMI z score was carried forward.
We first used unadjusted linear mixed models to flexibly model the relationship between eGFRcre and BMI (or weight) z score. We modeled eGFRcre as a restricted cubic spline term with knots specified a priori at 15, 30, and 45 mL/min/1.73 m2 (standard CKD stages 5, 4, and 3b, respectively); this spline term served as our primary predictor of repeated measures of BMI (or weight) z score in linear mixed models. Use of splines allows for a large degree of flexibility to accommodate non-linear associations and provides additional flexibility at the knots. These analyses were unadjusted as our primary interest was changes within the same individual over time. Because we noted an inflection point in the BMI z score when the eGFR fell below 35 mL/min/1.73 m2, we tested for differences in the coefficient for the slope terms before versus after eGFR of 35 mL/min/1.73 m2. In secondary analysis, we also adjusted these segmented mixed models for age, sex, race (black, white, versus other), cause of CKD, and proteinuria (as a time-dependent variable, categorized as ≥ or < 2 g/g). We confirmed our results using eGFRcys as the primary predictor of BMI (and weight) z score in unadjusted and adjusted analyses.11 In sensitivity analysis, we also used the clinically relevant cutoff (eGFR of 30 mL/min/1.73 m2 or CKD stage 4) as the inflection point and repeated our trajectory analysis.
We tested for the presence of interaction between repeated measures of eGFR and cause of CKD, given the known faster rate of CKD progression among those with glomerular causes of CKD.14 We performed stratified analysis by cause of CKD (glomerular versus non-glomerular) after we detected the presence of an interaction.
Because BMI z scores are influenced by changes in height, and because height accrual may slow with sexual maturation, we performed a series of additional analyses. First, we used unadjusted mixed models to examine how longitudinal declines in eGFRcre and eGFRcys are associated with height z score measurements over time (based on age- and sex-specific CDC standards).12,13 This analysis provided an assessment of the likelihood that changes in BMI z scores would be secondary to changes in height z score, as opposed to weight changes. In addition, we repeated our models excluding all visits when participants had a sexual maturation rating of Tanner 4 or beyond (for pubic hair, testes, or breasts) in order to isolate the analyses to participants with continued growth potential.
Characteristics of Participants by Weight Change Category
After we noted that the decline in BMI z score occurred after eGFR fell below 35 mL/min/1.73 m2, we classified the children and adolescents by the magnitude of their weight change between an eGFR of 35 mL/min/1.73 m2 and their most recent BMI z score value. This change in BMI z score was annualized, and categorized into quartiles based on the distribution of our data as: 1) stable weight (z score change of 0-<0.1); 2) moderate weight loss (z score change < 0 but >= −0.2); 3) significant weight loss (z score change <−0.2); and 4) weight gain (z score change ≥ 0.1). The −0.2 and 0.1 z score cutoffs were based on the approximate 25th and 75th percentile of BMI z score change in our data, respectively. Prior studies have also suggested that changes in BMI z score of this magnitude are clinically significant.15
Next, we examined the demographic characteristics of participants by weight change category and clinical characteristics including cause of CKD, history of feeding tube use (nasogastric or gastrostomy tubes), proteinuria (categorized as a protein-creatinine ratio ≥ 2 or < 2 g/g), self-reported appetite over the last 7 days prior to CKiD study visit, systolic blood pressure z score, serum albumin, and hemoglobin measured at the first visit when eGFR fell below 35 mL/min/1.73 m2.
Weight Change and Risk of ESRD
ESRD (defined as receiving long-term dialysis or having a kidney transplant) was identified in CKiD through phone contact or in-person during follow-up. We used unadjusted logistic regression models to examine the odds of ESRD by weight change category after eGFRcre fell below 35 mL/min/1.73 m2. We considered these unadjusted models our primary analysis, since we were primarily interested in the association between changes in weight within the same individual as a predictor of risk of adverse outcomes. In sensitivity analysis, we also adjusted these models for age, sex, race, cause of CKD, and proteinuria (≥2 or < 2 g/g) as a categorical variable (Model 1), and then additionally adjusted for hemoglobin, eGFRcre (categorized as < or ≥ 15 mL/min/1.73 m2 on account of non-normality), SBP z score, and serum albumin values (categorized as ≥ or < the median of 4.3 g/dL on account of non-normality) ascertained at the first CkiD visit when eGFR was below 35 mL/min/1.73 m2 (Model 2). We then tested for the presence of interaction between weight change category and cause of CKD, as well as between weight change category and BMI z score at the time when eGFRcre first fell below 35 mL/min/1.73m2. Because of the presence of an interaction by BMI z score when eGFRcre first fell below 35 mL/min/1.73 m2, we further stratified our logistic regression models by BMI (categorized as above or below the mean z-score) when eGFRcre first fell below 35 mL/min/1.73 m2.
Finally, we used linear regression models to explore whether the rate of eGFR decline would be associated with annualized weight changes that occurred after eGFR fell below 35 mL/min/1.73 m2 in unadjusted and adjusted models. We categorized rate of decline in eGFR as rapid (defined as eGFR ≥3 mL/min/1.73 m2 per year) versus slow (< 3 mL/min/1.73 m2 per year) based on prior definitions used in the literature.16
Results
The baseline characteristics of CkiD participants included for analysis are shown in Table 1. Mean age was 10.8 years, and 63% were boys. Of the 854 participants included for our trajectory analysis, BMI and serum creatinine data from an average of 3.8 visits per participant were included.
Table 1.
Baseline characteristics of Chronic Kidney Disease in Children Cohort
| Characteristic | Value |
|---|---|
|
| |
| Age category | |
| < 5 y | 93 (10.9) |
| 5-<12 y | 353 (41.3) |
| ≥12 y | 408 (47.8) |
|
| |
| Race | |
| White | 561 (65.7) |
| Black | 152 (17.8) |
| Other | 141 (16.5) |
|
| |
| Male sex | 535 (62.7) |
|
| |
| Cause of CKD | |
| Glomerular | 244 (28.6) |
| CAKUT | 553 (64.8) |
| Other | 57 (6.7) |
|
| |
| Maternal education | |
| High school | 331 (38.8) |
| Some college | 233 (27.3) |
| College graduate | 269 (31.5) |
| Unknown or missing | 21 (2.5) |
|
| |
| Income | |
| <$24,000/y | 223 (26.1) |
| $24,000–$75,000/y | 116 (13.6) |
| >$75,000/y | 494 (57.9) |
| Unknown or missing | 21 (2.5) |
|
| |
| z Score | |
|
| |
| Age- and sex-adjusted BMI* | 0.47 ± 1.2 |
|
| |
| Age- and sex-adjusted weight | 0.05 ± 1.4 |
|
| |
| Age- and sex-adjusted height* | −0.56 ± 1.2 |
|
| |
| Serum albumin (g/dL)* | 4.3 ± 0.5 |
|
| |
| Serum bicarbonate (mmol/L) | 21.2 ± 3.2 |
|
| |
| eGFRcr (mL/min/1.73m2)* | 51.7 [38.2–67.2] |
|
| |
| Protein-creatinine ratio (g/g)* | 0.37 [0.12–1.1] |
|
| |
| Corticosteroid use at baseline* | 78 (9.1) |
|
| |
| Growth hormone use at baseline* | 77 (9.0) |
Note: N=854. Values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation or median [interquartile range].
Data for BMI z score missing in n=21; height z score missing in n=16; serum albumin and bicarbonate missing in n=8; eGFR missing in n=2; steroid use and growth hormone use missing in n=1; protein-creatinine ratio missing in n=32.
BMI, body mass index; CAKUT, congenital anomalies of the kidney and urinary tract; CKD, chronic kidney disease; eGFRcr, serum-creatinine based estimated glomerular filtration rate (calculated using the Schwartz equation)
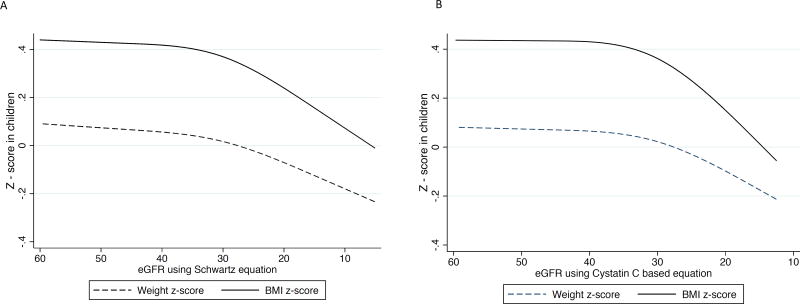
Prior to an eGFRcre of 35 mL/min/1.73 m2, every 10 mL/min/1.73 m2 decline in eGFRcre was associated with a mean decline in BMI z score of 0.008 (95% CI, −0.01 to 0.02). After eGFRcre fell below 35 mL/min/1.73 m2, every 10 mL/min/1.73 m2 further decline in eGFRcre was associated with a mean decrease in BMI z score of 0.13 (95% CI, 0.09 to 0.17) (Figure 1A). Adjustment for demographic characteristics, cause of CKD, and proteinuria did not significantly alter results: a decline in BMI z score of 0.14 (95% CI, 0.10 to 0.18) was noted with every 10 mL/min/1.73m2 decrease in eGFRcre after eGFRcre fell below 35 mL/min/1.73 m2. The difference in the association between repeated measures of BMI z score and kidney function before and after an eGFRcre threshold of 35 mL/min/1.73 m2 was statistically significant (p<0.001). The findings were similar using repeated measures of BMI z scores from the 840 participants in whom eGFRcys was available (Figure 1B). Results were also similar after exclusion of visits where participants were noted to have a Tanner IV or V sexual maturity rating (Figure S1, available as online supplementary material).
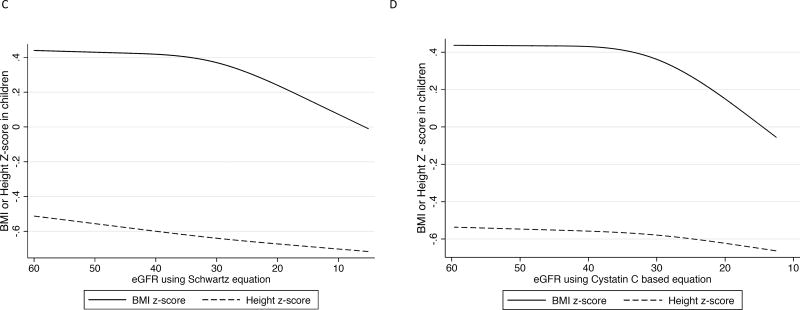
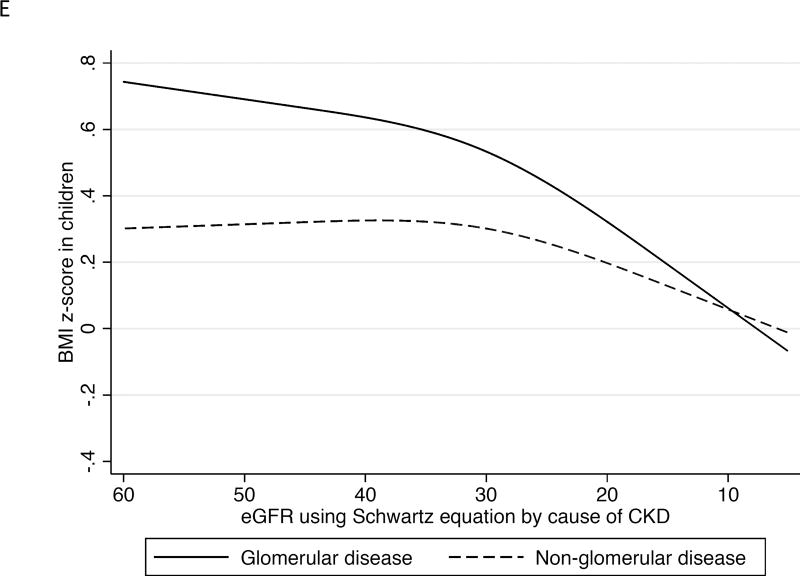
Figure 1.
Association between longitudinal repeated measures of body mass index, weight, and height z score with repeated measures of kidney function over time in the Chronic Kidney Disease in Children study in the overall cohort, and by cause of CKD (glomerular versus non-glomerular disease).
1A. Repeated measures of BMI and weight z scores with repeated measures of eGFR by serum creatinine.
1B. Repeated measures of BMI and weight z scores with repeated measures of eGFR by cystatin C.
1C. Repeated measures of BMI and height z scores with repeated measures of eGFR by serum creatinine.
1D. Repeated measures of BMI and height z scores with repeated measures of eGFR by cystatin C.
1E. Repeated measures of BMI with repeated measures of eGFR by serum creatinine by glomerular versus non-glomerular causes of CKD.
In sensitivity analysis when eGFR of 30 mL/min/1.73 m2 was used as the inflection point, we found similar results: for every 10 mL/min/1.73 m2 further decline in eGFRcre after eGFRcre fell below 30 mL/min/1.73 m2, a mean decrease in BMI z score of 0.16 (95% CI, 0.10– 0.21) was noted. However, in eGFRcre ranges above 30 mL/min/1.73 m2, every 10 mL/min/1.73m2 decline in eGFR was associated with a non-statistically significant decrease in BMI z score of 0.014 (95% CI, −0.005 to 0.03).
When we examined the change in weight z score with advancing CKD, the results were similar to results from our BMI analysis (Figures 1A-1B). We also found no statistically significant associations between repeated measures of declines in eGFRcre or eGFRcys and height z scores (Figure 1D-1E).
When we stratified our BMI z score trajectories by glomerular versus non-glomerular causes of CKD, we found more profound weight loss with progression of CKD among children and adolescents with a glomerular cause of CKD (Figure 1E, p<0.001 for interaction). Every 10 mL/min/1.73m2 decline in eGFRcre was associated with a decline in BMI z score of 0.22 (95% CI, 0.15–0.29) after eGFRcre fell below 35 mL/min/1.73 m2 among participants with a glomerular cause of CKD (n=244) versus a decline in BMI z score of just 0.10 (95% CI, 0.06–0.15) among those with non-glomerular causes of CKD (n=610).
Among the 268 children and adolescents who had at least two BMI and serum creatinine measurements after eGFRcre fell below 35 mL/min/1.73 m2, 130 cases of ESRD and two deaths occurred. Approximately 25% of participants had significant weight loss (Table 2) during a median follow-up of 2.7 years. Participants who lost significant weight were more likely to have a glomerular cause of CKD (Table 2), whereas those who gained weight were most likely to have lower BMI z scores at the visit when eGFR first fell below 35 mL/min.1.73 m2. There was no evidence of an association between the prevalence of poor appetite by self-report and weight change categories (Table 2).
Table 2.
Characteristics of patients when eGFR first fell below 35 mL/min/1.73 m2 by change in BMI z score category
| Characteristic | Significant Weight Loss (n=66) |
Moderate Weight Loss (n=93) |
Maintained Weight (n=51) |
Gained Weight (n=58) |
P value |
|---|---|---|---|---|---|
|
| |||||
| Age category | 0.2 | ||||
| < 5 y | 3 (5%) | 3 (3) | 3 (6) | 6 (10) | |
| 5-<12 y | 17 (26) | 39 (42) | 16 (31) | 19 (33) | |
| ≥12 y | 46 (70) | 51 (55) | 32 (63) | 33 (57) | |
|
| |||||
| Race | 0.1 | ||||
| White | 46 (70) | 71 (76) | 27 (53) | 35 (60) | |
| Black | 11 (17) | 13 (14) | 13 (26) | 11 (19) | |
| Other | 9 (14) | 9 (10) | 11 (22) | 12 (21) | |
|
| |||||
| Male sex | 50 (76) | 61 (66) | 32 (63) | 34 (59) | 0.2 |
|
| |||||
| Cause of CKD | 0.04 | ||||
| Glomerular | 21 (32) | 12 (13) | 8 (16) | 9 (16) | |
| CAKUT | 43 (65) | 71 (76) | 40 (78) | 46 (79) | |
| Other | 2 (3) | 10 (11) | 3 (6) | 3 (5) | |
|
| |||||
| Maternal education* | 0.8 | ||||
| High school | 32 (50) | 39 (43) | 21 (43) | 22 (39) | |
| Some college | 13 (20) | 25 (28) | 12 (25) | 19 (34) | |
| College graduate | 19 (30) | 27 (30) | 16 (33) | 15 (27) | |
|
| |||||
| Income* | 0.5 | ||||
| <$24,000/y | 19 (30) | 17 (19) | 8 (16) | 16 (28) | |
| $24,000–$75,000/y | 10 (16) | 16 (18) | 9 (18) | 12 (21) | |
| >$75,000/y | 35 (55) | 55 (63) | 32 (65) | 29 (51) | |
|
| |||||
| Ever had an NG or G tube* | 1 (2) | 2 (2) | 4 (8) | 4 (7) | 0.2 |
|
| |||||
| Fair/poor appetite in last 7 d when eGFR first < 35 mL/min/1.73 m2* # | 7 (21) | 6 (7) | 2 (4) | 5 (9) | 0.4 |
|
| |||||
| BMI z score when eGFR first< 35 mL/min/1.73 m2 | 0.32 [−0.3 to 1.1] | 0.45 [−0.5 to 1.2] | 0.56 [0 to 1.4] | −0.3 [−0.8 to 0.2] | <0.001 |
|
| |||||
| Most recent BMI z score | −0.44 [−1.1 to 0.2] | 0.05 [−0.9 to 0.8] | 0.76 [0.2 to 1.5] | 0.37 [−0.2 to 0.9] | <0.001 |
|
| |||||
| Systolic BP* z score when eGFR first <35 mL/min/1.73 m2 | 0.35 ± 1.3 | 0.33 ± 1.3 | 0.28 ± 1.1 | 0.22 ± 1.3 | 0.9 |
|
| |||||
| Serum albumin when eGFR first <35 mL/min/1.73 m 2*, g/dL | 4.1 ± 0.6 | 4.3 ± 0.3 | 4.2 ± 0.4 | 4.3 ± 0.4 | 0.1 |
|
| |||||
| Most recent serum albumin (g/dL)* | 4.2 ± 0.5 | 4.4 ± 0.4 | 4.3 ± 0.4 | 4.2 ± 0.5 | 0.1 |
|
| |||||
| Proteinuria (PCR ≥ 2g/g)* | 22 (34.9) | 18 (19.8) | 11 (22.9) | 12 (21.8) | 0.2 |
|
| |||||
| Hb* when eGFR first <35 mL/min/1.73m2 | 12.1 ± 1.7 | 12.0 ± 1.4 | 12.2 ± 1.6 | 12.1 ± 1.8 | 0.9 |
Note: n=268. Values for categorical variables are given as number (column percentage); for continuous variables, as mean ± standard deviation or median [interquartile range].
Data for maternal education missing in n=8; income missing or unknown in n=10; NG tube missing in n= 15; albumin missing in n=7; SBP missing in n=6; appetite missing in n=162; serum albumin at baseline missing in n=3 and most recent missing in n=7; proteinuria missing in n=11; hemoglobin missing in n=4
BMI, body mass index; CAKUT, congenital anomalies of the kidney and urinary tract; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; G, gastrostomy; NG, nasogastric; Hb, hemoglobin; PCR, protein-creatinine ratio
By self- or parent-report
Among the subset of children and adolescents with two or more BMI measurements after eGFRcre first fell below 35 mL/min/1.73 m2, we noted a graded association between the degree of weight loss and odds of ESRD in unadjusted and adjusted analyses (Table 3). Weight gain was also associated with a higher odds of ESRD, although these findings did not achieve statistical significance (Table 3).
Table 3.
Odds of ESRD by weight change category between eGFR 35 mL/min/1.73 m2 and last available BMI z score
| Weight change categorya | Unadjusted OR (95% CI) |
Adjusted OR (95% CI) | |
|---|---|---|---|
| Model 1* | Model 2** | ||
| Significant weight loss (n=66) | 3.28 (1.53–7.05) | 2.86 (1.20–6.81) | 3.01 (1.20–7.54) |
| Moderate weight loss (n=93) | 1.80 (0.88–3.65) | 1.93 (0.88–4.24) | 1.97 (0.87–4.48) |
| Maintained weight (n=51) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Weight gain (n=58) | 1.87 (0.86–4.06) | 2.08 (0.88–4.91) | 2.18 (0.90–5.32) |
Note: n=268.
BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; OR, odds ratio
Adjusted for age, sex, race, cause of chronic kidney disease, and proteinuria category (analysis includes n=257 due to covariates)
Adjusted for model 1 covariates + hemoglobin, serum albumin (categorical variable as ≥ 4.3 versus < 4.3 g/dL), systolic blood pressure z score, and eGFR ascertained when eGFR first fell below eGFR 35 mL/min/1.73 m2 as a categorical variable (analysis includes n=246 due to missing covariates)
Stable weight defined as annualized BMI z score change 0–<0.1; moderate weight loss defined if annualized BMI z score change was <0 but ≥−0.2; significant weight loss defined as annualized BMI z score change < −0.2; weight gain defined as annualized z score change ≥ 0.1.
There was an interaction between significant weight loss and BMI z score starting at the time when eGFRcre first fell below 35 mL/min/1.73 m2 (p=0.04). Among children and adolescents with a BMI z score above 0.3 (n=122) when the eGFRcre first fell below 35 mL/min/1.73 m2, the adjusted odds of ESRD was 5.8 (95% CI, 1.61–21.0) times higher (Model 2) among patients who had significant weight loss compared to the reference group. In contrast, among participants with a BMI z score ≤0.3 (n=125) when eGFRcre first fell below 35 mL/min/1.73 m2, the odds of ESRD in those with significant weight loss was not statistically significantly different from that in those who maintained weight (odds ratio, 0.86; 95% CI, 0.18–4.00).
We tested for but did not find presence of interaction between weight change category and cause of CKD for the risk of ESRD (all p>0.05). Finally, we did not find any association between rates of eGFR loss per year after eGFR fell below 35 mL/min/1.73m2 and change in BMI z score per year (p=0.2).
Discussion
In our study, we found that intensified weight loss (reflected by BMI changes) begins earlier than previously reported in the literature,17 specifically after eGFR falls below 35 mL/min/1.73 m2. This weight loss was, in particular, more pronounced among children and adolescents who had glomerular causes of CKD, and was associated with a higher risk of ESRD. We believe our study provides clinically useful data on the patterns of weight change in children and adolescents with CKD as kidney function declines over time. We also believe our study highlights the need for greater attention to weight status and nutrition during the course of CKD progression, given that weight changes may serve to identify the subset of participants at higher risk for developing ESRD.
The reasons for our finding of a strong association between weight loss during the advanced stages of CKD and risk of ESRD are not entirely clear. Because anorexia and weight loss are commonly considered symptoms of uremia,2,20 children and adolescents who lose significant weight may be started on renal replacement therapy earlier to improve their weight status and treat uremia, which could explain our observations. This is consistent with observations within a large randomized controlled trial in adults of early versus late dialysis initiation, where anorexia was cited as one of the reasons that providers started RRT early.21 In a recent survey of pediatric nephrologists, weight loss was also cited as a factor that prompted earlier initiation of dialysis.22 It is also possible that the weight loss observed in our study serves as marker of illness severity or greater inflammation, which may predispose to more rapid progression to ESRD.23 Finally, non- adherence to nutritional interventions (such as nutritional supplementation)24 or late presentation for nephrology care25,26 may explain the association between greater weight loss and a higher risk of ESRD. We believe it is less likely that nutritional supplementation would slow the progression of CKD, although our study is observational in nature and was not designed to answer this question.
Interestingly, weight loss appeared to be more strongly associated with ESRD if this weight loss occurred among children and adolescents who had a BMI z score above the mean at the visit when eGFR first fell below 35 mL/min/1.73 m2. The reasons for the effect modification by BMI z score when eGFR first dropped below 35 mL/min/1.73 m2 are unclear. One possibility is that those who already had a below-average BMI by CKD stage 3b (eGFR <45 ml/min/1.73 m2) had lower protein intake and thus reduced muscle mass and lower serum urea levels, leading to a consistent underestimation of their degree of kidney failure and uremic toxicity and therefore lower likelihood of initiating RRT.27 Another possibility is that participants with above average BMI z score developed this weight loss rapidly during periods of severe illness, which therefore prompted earlier initiation of dialysis or transplantation. 21,22
Our data suggest that more frequent monitoring of protein-energy wasting may be especially important among children and adolescents with glomerular causes of CKD, in whom weight loss was observed to be more profound compared to those with nonglomerular causes of CKD. We speculate that genetic and congenital causes of CKD may assume a more indolent course, and that greater attention to nutrition (by [naso]gastric feeding or caloric supplementation) due to the chronicity of kidney disease and the younger age of onset of CKD may occur in this subset of patients. In contrast, due to the older age of most children and adolescents who acquire CKD due to glomerulonephritis, it is possible that less attention is paid to nutrition among this subgroup, leading to more profound weight loss with advancing CKD. It is also possible that children and adolescents with glomerulonephritis have more generalized inflammation, and therefore increased protein-energy wasting and weight loss.19
Given the observational nature of our data, it is unclear whether nutritional supplementation to maintain weight can reduce the risk of ESRD.7,28 Of note, there was no difference in the prevalence of poor appetite by self-report across different weight categories, which is consistent with the results of a prior study.29 We speculate that this finding could be related to heightened awareness of symptoms such as loss of appetite by participants and their families regardless of whether objective weight changes occurred.29 However, there are also known limitations to the use of self-report when determining appetite,30 especially among children and adolescents with CKD where reduced appetite may become the norm and go unnoticed in the setting of a chronic illness. While our study was not designed to determine whether nutritional supplementation would improve clinical outcomes and we do not have access to repeated measurements of caloric intake, such a study would be warranted, given the worse outcomes of children and adolescents who lost weight during their course of CKD.
The strengths of our study include the national representativeness of the cohort, the repeated and well-characterized anthropometric measurements in CKiD, and the availability of repeated measures of kidney function over time. However, there are several limitations to our study. First, our study is limited by the likely underestimation of the degree of non-edematous weight loss that occurred, given the potential for the presence of fluid weight to accumulate with advancing CKD. We also have a limited number of longitudinal measurements of inflammatory markers such as C-reactive protein and cholesterol to include for analysis. Although a number of studies of children with CKD have expressed BMI z scores for height-age (the age at which a children’s height would be at the 50th percentile) rather than for chronological age, we have chosen to use chronological age because height z score did not change substantially during longitudinal follow-up, and because expression of BMI z score by height-age is less practical in a busy clinical setting. We also note that there may be limitations in the ability for BMI measurements to distinguish between adiposity versus fluid weight, although BMI z scores remain the most commonly used marker of body size in children with CKD, and we are limited by the lack of availability of other measures of body composition, such as bioelectrical impedance, in the CKiD study.34,35 We also acknowledge that the threshold of decline in eGFR that was selected for our primary analysis (eGFR of 35 mL/min/1.73 m2) was determined post hoc, and should be confirmed in additional studies. Finally, given the observational nature of our study, we cannot rule out the possibility of residual confounding.
In conclusion, significant weight loss appears to occur primarily after eGFR falls below 35 mL/min/1.73 m2 in children and adolescents with CKD during longitudinal follow-up. The development of significant weight loss in children and adolescents with CKD was associated with a higher risk of ESRD. Careful attention to nutritional parameters starting in CKD stage 3 may be warranted, with earlier and more frequent assessments than currently recommended. Further research is needed to determine reasons behind the association between weight loss and risk of ESRD in children and adolescents.
Supplementary Material
Acknowledgments
We thank Feng Lin, MS, for statistical support and analysis verification.
Support: This work was supported by the National Institutes of Health to Drs Ku (HL131023) and Mitsnefes (DK090070). This work was also supported by a National Kidney Foundation Satellite Dialysis Clinical Investigator Grant (Dr Ku). The CKiD Cohort Study was conducted by the CKiD Investigators and supported by the NIDDK, with additional funding from the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). The data and samples from the CKiD Study reported herein were supplied by the NIDDK Central Repositories. This study does not necessarily reflect the opinions or views of the CKiD study, the NIDDK Central Repositories, or the NIDDK. The funders of this study did not have any role in the study design, collection, analysis, or interpretation of data, or drafting of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions: Research idea and study design: EK, JK, MM, CEM; data acquisition: EK, JK SLF, BAW, RM, MM; data analysis/interpretation: EK, CEM, JK, MM, SLF, BAW, RM, BAG; statistical analysis EK, CEM, BAG, MM. Each author contributed important intellectual content during manuscript drafting or revision, and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
References
- 1.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney international. 2000;57(4):1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 2.Ikizler TA, Greene JH, Wingard RL, Parker RA, Hakim RM. Spontaneous dietary protein intake during progression of chronic renal failure. Journal of the American Society of Nephrology: JASN. 1995;6(5):1386–1391. doi: 10.1681/ASN.V651386. [DOI] [PubMed] [Google Scholar]
- 3.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney international. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 4.Chazot C. Why are chronic kidney disease patients anorexic and what can be done about it? Seminars in nephrology. 2009;29(1):15–23. doi: 10.1016/j.semnephrol.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Mak RH, Cheung WW, Zhan JY, Shen Q, Foster BJ. Cachexia and protein-energy wasting in children with chronic kidney disease. Pediatric nephrology (Berlin, Germany) 2012;27(2):173–181. doi: 10.1007/s00467-011-1765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sienna JL, Saqan R, Teh JC, et al. Body size in children with chronic kidney disease after gastrostomy tube feeding. Pediatric nephrology. 2010;25(10):2115–2121. doi: 10.1007/s00467-010-1586-y. [DOI] [PubMed] [Google Scholar]
- 7.Hobbs DJ, Bunchman TE, Weismantel DP, et al. Megestrol acetate improves weight gain in pediatric patients with chronic kidney disease. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2010;20(6):408–413. doi: 10.1053/j.jrn.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clinical journal of the American Society of Nephrology: CJASN. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL. CKiD (CKD in children) prospective cohort study: a review of current findings. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;60(6):1002–1011. doi: 10.1053/j.ajkd.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology: JASN. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney international. 2012;82(4):445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data; Hyattsville MUSDoH: [Google Scholar]
- 13.World Health Organization. WHO child growth standards: length/height-for- age w-f-a, weight-for-height and body mass index-for-age: methods and development. World Health Organization; Geneva: 2006. Available at: http://www.who.int/childgrowth/publications/technical_report_pub/en/index.htm. [Google Scholar]
- 14.Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;65(6):878–888. doi: 10.1053/j.ajkd.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinh A, Campbell M, Ukoumunne OC, Gerner B, Wake M. Physical activity and 3-year BMI change in overweight and obese children. Pediatrics. 2013;131(2):e470–477. doi: 10.1542/peds.2012-1092. [DOI] [PubMed] [Google Scholar]
- 16.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Archives of internal medicine. 2008;168(20):2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman LJ, Coleman JE, Macdonald IA, Tomsett AM, Watson AR. Nutrition and growth in relation to severity of renal disease in children. Pediatric nephrology (Berlin, Germany) 2000;15(3–4):259–265. doi: 10.1007/s004670000465. [DOI] [PubMed] [Google Scholar]
- 18.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. Journal of hypertension. 2014;32(1):3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Norris KC. Is the malnutrition-inflammation complex the secret behind greater survival of African-American dialysis patients? Journal of the American Society of Nephrology: JASN. 2011;22(12):2150–2152. doi: 10.1681/ASN.2011101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer TW, Hostetter TH. Uremia. The New England journal of medicine. 2007;357(13):1316–1325. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 21.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. The New England journal of medicine. 2010;363(7):609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 22.Saban JA, Zappitelli M, Samuel SM, et al. Perceptions of pediatric nephrologists regarding timing of dialysis initiation in children in Canada. Canadian journal of kidney health and disease. 2016;3:31. doi: 10.1186/s40697-016-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amdur RL, Feldman HI, Gupta J, et al. Inflammation and Progression of CKD: The CRIC Study. Clinical journal of the American Society of Nephrology: CJASN. 2016;11(9):1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akchurin OM, Schneider MF, Mulqueen L, et al. Medication adherence and growth in children with CKD. Clinical journal of the American Society of Nephrology: CJASN. 2014;9(9):1519–1525. doi: 10.2215/CJN.01150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney international. 1999;55(5):1945–1951. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 26.Pupim LB, Caglar K, Hakim RM, Shyr Y, Ikizler TA. Uremic malnutrition is a predictor of death independent of inflammatory status. Kidney international. 2004;66(5):2054–2060. doi: 10.1111/j.1523-1755.2004.00978.x. [DOI] [PubMed] [Google Scholar]
- 27.von Scholten BJ, Persson F, Svane MS, Hansen TW, Madsbad S, Rossing P. Effect of large weight reductions on measured and estimated kidney function. BMC nephrology. 2017;18(1):52. doi: 10.1186/s12882-017-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aparicio M, Chauveau P, De Precigout V, Bouchet JL, Lasseur C, Combe C. Nutrition and outcome on renal replacement therapy of patients with chronic renal failure treated by a supplemented very low protein diet. Journal of the American Society of Nephrology: JASN. 2000;11(4):708–716. doi: 10.1681/ASN.V114708. [DOI] [PubMed] [Google Scholar]
- 29.Ayestaran FW, Schneider MF, Kaskel FJ, et al. Perceived appetite and clinical outcomes in children with chronic kidney disease. Pediatric nephrology (Berlin, Germany) 2016;31(7):1121–1127. doi: 10.1007/s00467-016-3321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subar AF, Freedman LS, Tooze JA, et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. The Journal of nutrition. 2015;145(12):2639–2645. doi: 10.3945/jn.115.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA. Adverse clinical outcomes associated with short stature at dialysis initiation: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics. 2002;109(5):909–913. doi: 10.1542/peds.109.5.909. [DOI] [PubMed] [Google Scholar]
- 32.Ku E, Fine RN, Hsu CY, et al. Height at First RRT and Mortality in Children. Clinical journal of the American Society of Nephrology: CJASN. 2016;11(5):832–839. doi: 10.2215/CJN.08250815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham AG, Mak RH, Mitsnefes M, et al. Protein energy wasting in children with chronic kidney disease. Pediatric nephrology (Berlin, Germany) 2014;29(7):1231–1238. doi: 10.1007/s00467-014-2768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao T, Leonard MB, Zemel B, Kalkwarf HJ, Foster BJ. Interpretation of body mass index in children with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(4):558–564. doi: 10.2215/CJN.09710911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ku E, Glidden DV, Hsu CY, Portale AA, Grimes B, Johansen KL. Association of Body Mass Index with Patient-Centered Outcomes in Children with ESRD. Journal of the American Society of Nephrology: JASN. 2016;27(2):551–558. doi: 10.1681/ASN.2015010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.