Abstract
It was posited that the initial host response to Staphylococcus aureus is a contributing factor in the pathogenesis of acute pneumonia. Having previously observed that T cells play a negative role in the pathogenesis of acute pneumonia to S. aureus the contribution of the CD80/CD86 pathway in pathogenesis was investigated. Mice lacking CD80 and CD86 had significantly improved survival in a mouse model of acute S. aureus pneumonia. This was accompanied by significant reductions in several proinflammatory cytokines, including TNF, MIP-2, IL-1β, IL-17 and IL-6, as well as increased numbers of viable alveolar macrophages. Early during infection reductions in cytokine production were evident and cytokine production in response to S. aureus in bone marrow derived macrophages showed decreases in TNF, KC, IL-1α and GM-CSF. Our data suggest that CD80/CD86 signaling plays a significant role in the initial inflammatory response to S. aureus in the airway and could be a potential acute target to reduce the initial inflammatory insult.
Keywords: Staphylococcus aureus, pneumonia, CD80, CD86, host-pathogen, cytokines
Introduction
S. aureus and in particular methicillin resistant S. aureus (MRSA) is a major problem not only in the hospital setting but in the community, causing significant morbidity, mortality and economic burden [1, 2]. In contrast to hospital-acquired strains, community-acquired strains of S. aureus infect otherwise healthy individuals [3]. The MRSA strain USA300 is the dominant clone and epidemic in the United States [4, 5]. Pneumonias that result from USA300 infection are especially severe and involve significant loss of alveolar architecture, leukocyte infiltration and consolidation of the lung parenchyma [6]. While much has been learned in regards to the cell types important in the response to this pathogen in the airway, many of the signaling pathways that contribute to its pathogenesis remain undefined.
Activation of T cells occurs through interaction of major histocompatibility complex (MHC) presented peptides that interact with the T cell receptor complex, leading to the initiation of various signaling cascades. This signaling complex is also augmented through various co-receptors and their respective ligands. The T cell co-receptor CD28, interacts with CD80 (B7-1) and CD86 (B7-2) that are expressed on activated antigen presenting cells in response to pathogens, which leads to induction of several signaling pathways, such as those controlled by NF-κB, MAPK, PI3K and AKT [7, 8]
The laboratory recently identified that T cells contributed to the pathogenesis of S. aureus in a murine model of pneumonia [9]. Mice deficient in T cells had improved outcomes to S. aureus infection and reductions in proinflammatory cytokine production. This is also consistent with our humanized mouse study whereby NOD scid Il2rγ (NSG) mice also had improved outcomes to S. aureus respiratory infection compared to the standard C57BL/6J mouse model [10]. It has also been shown in models of sepsis and skin infection that T cells are associated with the pathogenesis of S. aureus [11–13].
Here evidence that expression of CD80 and CD86 contributes significantly to the proinflammatory response in the airway in response to S. aureus is provided. Mice deficient in CD80 and CD86 had significant reductions in several proinflammatory cytokines and have significantly improved rates of survival in a murine model of pneumonia. This requirement of CD80 and CD86 in the proinflammatory response to S. aureus was evident early in infection and in vitro using bone marrow derived macrophages. This works lends support to the hypothesis that much of the morbidity and mortality associated with S. aureus pneumonia is the result of excessive cytokine production.
Materials and methods
Animal studies
C57BL/6J and Cd80Cd86−/− mice were purchased from Jackson Laboratories. Mice were infected with Staphylococcus aureus MRSA strain USA300 FPR3757 [5] that was grown in LB broth to mid exponential phase (OD 600nm 1.0) at 37°C prior to suspension in PBS. Mice were anesthetized with ketamine and xylazine before intranasal administration of 4 × 107 cfu for acute or 1 × 108 cfu for mortality studies in 50 μl volumes. Bronchoalveolar lavage fluid (BALF) and homogenized lung tissue was used to enumerate bacteria on selective chromogenic media (Chromagar). Clarified BALF fluid was used to quantitate cytokine levels by multiplex technology (Eve Technologies). Temperatures were measured using a TW2 infrared thermometer (Thermoworks). Animal work in this study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory animals of the National Institutes of Health, the Animal Welfare Act, and U.S. federal law. Protocols were approved by the Animal Care and Use Committee of Columbia University.
Flow cytometry
Cells in BALF were stained with the following fluorescently labeled antibodies: MARCO-FITC (MCA1849F; Biorad), CD11c-BV605 (N418), CD86-BV421 (GL-1), CD103-BV510 (M290; BD Biosciences), CD11b-AF594 (M1/70), Ly6G-PerCP Cy5.5 (1A8), CD206-BV650 (C086C2), Siglec-F-AF647 (E50-2440; BD Biosciences), CD45-AF700 (30-F11), MHCII-APC-Cy7 (M5/114.15.2), BUV395 (Life Technologies), CD200R-PE (123908), Ly6C-PE-Texas Red (AL-2; BD Biosciences) and NK1.1-BV650 (PK136). Viability was assessed using BUV395 live/dead dye (Life Technologies). Antibodies were purchased from Biolegend unless otherwise stated. Cells in the airway were classified as follows [14]: Alveolar macrophages-CD45+Ly6C−SiglecF+CD11b−, eosinophils-CD45+Ly6C−SiglecF+CD11b+, neutrophils-CD45+Ly6C+CD11b+MHCII−Ly6G+, Ly6C+ monocytes-CD45+Ly6C+CD11b+MHCII−Ly6G−, Ly6C− monocytes-CD45+Ly6C−SiglecF−CD11b+CD11c+MHCII−, interstitial macrophages-CD45+Ly6C−SiglecF−CD11b+CD11c+MHCII+, CD11b dendritic cells (DC)-CD45+Ly6C+CD11b+MHCII+CD11c+, CD103 DC-CD45+Ly6C−SiglecF−CD11b−MHCII+CD103+ and plasmacytoid DC (pDC)-CD45+Ly6C+CD11b−CD11c+MHCII+.
Cell culture
Macrophages were generated by extracting bone marrow from mouse tibias and femurs, and differentiated using 20ng/ml of M-CSF (Peprotech) in RPMI 1640 medium with 10% heat inactivated fetal bovine serum and penicillin and streptomycin for seven days. Media was changed to no antibiotics 2 h prior to infection with S. aureus at an MOI of 10 for 24 h before supernatants were collected for multiplex analysis (Eve Technologies).
Statistics
Animal data were assessed using a nonparametric Mann-Whitney test. Mortality data was assessed using a Fishers exact test. Statistics were performed with Prism software (GraphPad, La Jolla LA USA).
Results
CD80/CD86 contributes to pathogenesis of S. aureus pneumonia
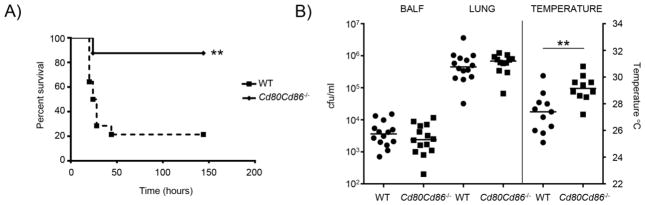
Having previously identified T cells as being a major contributor to the pathogenesis of S. aureus pneumonia [9] the role of CD80/CD86 signaling in infection was investigated. In a model of S. aureus pneumonia mice missing CD80/CD86 had significantly improved survival. On day 7 post-infection 87.5% of Cd80Cd86−/− mice had survived compared to 21.4% of WT mice (Fig. 1A; P<0.01). When the mice were given a lower inoculum to examine the role of CD80/CD86 in the context of non-lethal acute pneumonia no differences in bacterial burden were observed (Fig. 1B). Bacterial counts in both the bronchoalveolar lavage fluid (BALF) and lung homogenate were not different between the WT and Cd80Cd86−/− mice (Fig. 1B). Consistent with the improved outcome in the lethal pneumonia model the temperatures of Cd80Cd86−/− infected mice were improved compared to similarly infected WT mice (Fig. 1B). Temperatures in Cd80Cd86−/− infected mice were on average two degrees higher (P<0.01) than WT infected mice. These data show a role for CD80/CD86 in the pathogenesis of S. aureus pneumonia independent of bacterial clearance.
Figure 1.
Inactivation of CD80/CD86 improves survival of mice in a model of S. aureus pneumonia. A) WT and Cd80Cd86−/− mice were infected with 1 × 108 cfu of S. aureus USA300 and survival was followed for seven days. Data are from three independent experiments. n=WT-14 and Cd80Cd86−/−-8. B) C57BL/6J WT and Cd80Cd86−/− mice were infected with 4 × 107 cfu of S. aureus intranasally for 24 h. Bacterial counts were assessed in BALF and lung homogenate, and external body temperatures were measured before euthanasia. Data are from four independent experiments. Each point represents a mouse. Lines display median. **P<0.01.
Alveolar macrophages are increased in the absence of CD80/CD86
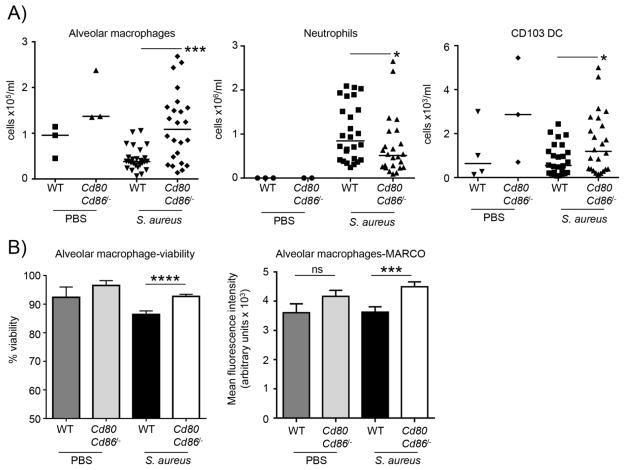
To better understand the improved outcome of mice lacking CD80/CD86 multicolor flow cytometry was performed on immune cell populations in the BALF. A preservation of alveolar macrophage cell numbers was observed in addition to CD103 positive dendritic cells in Cd80Cd86−/− mice compared to WT mice (Fig. 2A). Alveolar macrophages have been shown to be important in protection against S. aureus [15] so the 2.6-fold increase (P<0.001) has important consequences for pathogenesis. Neutrophils are also important in the clearance of S. aureus but excessive numbers can contribute to inflammation. It was observed that Cd80Cd86−/− mice to have 30% less (P<0.05) neutrophils in BALF compared to WT infected mice (Fig. 2A). In addition to the difference in alveolar macrophage numbers an overall improvement in their viability was also observed. Alveolar macrophages from Cd80Cd86−/− infected mice had a mean viability of 93% compared to 86% (P<0.0001) in WT infected mice (Fig. 2B). The scavenger receptor MARCO [16, 17], was expressed at a level 24% (P<0.001) greater on alveolar macrophages from Cd80Cd86−/− infected compared to WT infected mice (Fig. 2B). Given this improvement in macrophage numbers it would be expected that this may influence bacterial clearance. As a clearance phenotype was not observed, but a protection against mortality phenotype was observed, the host immune response to S. aureus in WT and Cd80Cd86−/− mice was further investigated.
Figure 2.
Improved immune cell responses in the airway of Cd80Cd86−/− mice. C57BL/6J WT and Cd80Cd86−/− mice were infected with 4 × 107 cfu of S. aureus intranasally for 24 h. A) Immune cell populations in BALF were characterized by flow cytometry. B) Viability and surface expression analysis of alveolar macrophages from the BALF. Alveolar macrophage viability; n=3 for PBS/uninfected mice, 25 for WT and 22 for Cd80Cd86/−/− infected mice. For MARCO expression; n=4 for uninfected WT mice and 3 for uninfected Cd80Cd86−/− mice, 26 for WT infected and 22 for infected Cd80Cd86−/− mice. Each point represents a mouse. Lines display median. Graphs display mean with standard error. ****P<0.0001, ***P<0.001 and *P<0.05.
Signaling from CD80/CD86 contributes significantly to the proinflammatory response to S. aureus in the airway
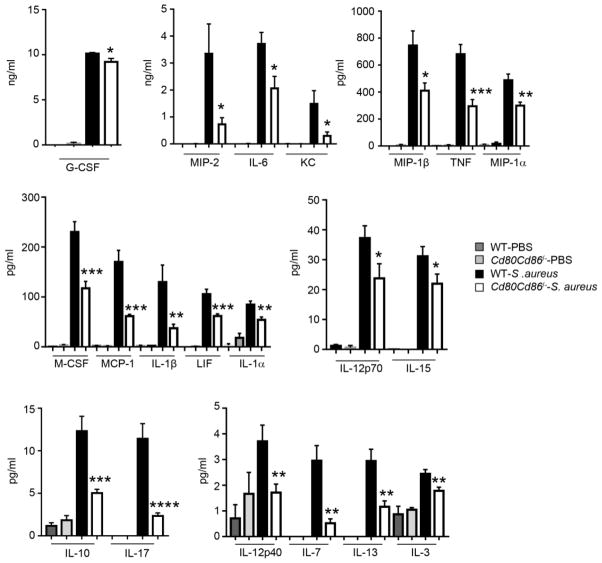
To gain a better understanding of the improved phenotype observed in Cd80Cd86−/− mice cytokine levels were quantified in the BALF from control and infected, WT and Cd80Cd86−/− mice (Fig. 3). A significant reduction in several proinflammatory cytokines was noted, even in the absence of changes to bacterial counts. This included the major cytokines TNF (56% reduction; P<0.001) and IL-6 (44% reduction; P<0.05), as well as the neutrophil chemokine KC (78% reduction; P<0.05) (Fig. 3), consistent with our reduced neutrophil numbers (Fig. 2). IL-17 signaling has been shown to be important in the clearance of extracellular pathogens including S. aureus [18]. In the absence of any changes in bacterial numbers levels of IL-17 were reduced by 79 % (P<0.0001) in S. aureus infected Cd80Cd86−/− mice compared to WT infected mice (Fig. 3). The proinflammatory cytokine IL-1β is a readout for the inflammasome, however, in the airway overproduction of IL-1β can contribute to immunopathology [19]. Levels of IL-1β were reduced by 63% (P<0.01) in Cd80Cd86−/− infected mice compared to WT infected mice (Fig. 3). Reductions in several other cytokines were also observed: G-CSF, MIP-2, MIP-1β, MIP-1α, M-CSF, MCP-1, LIF, IL-1α, IL-12p40, IL-12p70, IL-15, IL-10, IL-7, IL-13 and IL-3 (Fig. 3). Changes in cytokine levels were not evident with: eoxtaxin, GM-CSF, IL-2, IL-5, IL-9, CXCL9, CXCL10, RANTES and VEGF (data not shown). These data suggest that CD80/CD86 signaling contributes significantly to the production of proinflammatory cytokines in response to S. aureus in the airway.
Figure 3.
Cd80Cd86−/− mice exhibit a decreased inflammatory response to S. aureus in a model of acute pneumonia. C57BL/6J WT and Cd80Cd86−/− mice were infected with 4 × 107 cfu of S. aureus intranasally for 24 h. Cytokines were quantified from BALF. Data is from four independent experiments. Graphs display means with standard error. n=3-PBS/uninfected and 14 for infected animals. ****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05 compared to WT infected.
Reduced cytokine production in the absence of CD80/CD86 is evident early during infection
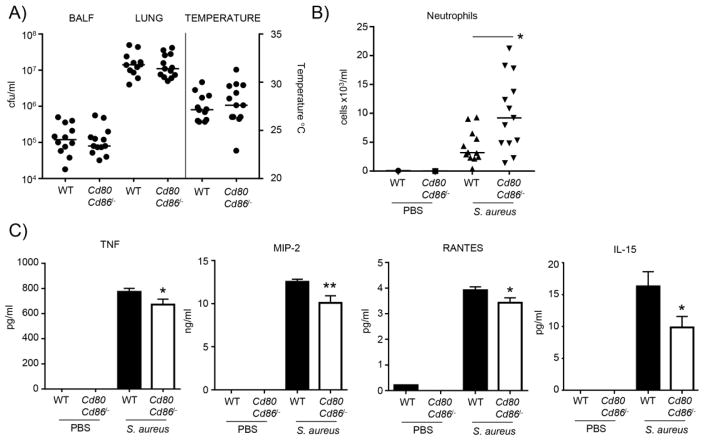
To determine if the influence of CD80/CD86 signaling was evident early during infection, WT and Cd80Cd86−/− mice were infected with S. aureus USA300 for four hours. Consistent with the data from animals infected for 24 h, significant differences in the bacterial counts from the BALF or lung homogenate were not observed (Fig. 4A). In contrast to the 24 h data (Fig. 1B), a difference in the body temperature of WT and Cd80Cd86−/− mice infected with S. aureus was noted (Fig. 4A). This early time point may be too soon to detect a difference in body temperature. At this early time point a 2.3-fold increase was observed (P<0.05) in neutrophil numbers of Cd80Cd86−/− mice infected with S. aureus compared to similarly infected WT animals (Fig. 4B). Analysis of the cytokine response in BALF did indicate some early changes (Fig. 4C). TNF levels were 13% (P<0.05) less in Cd80Cd86−/− infected mice, MIP-2 was reduced by 20% (P<0.01), RANTES by 12% (P<0.05) and IL-15 by 40% (P<0.05). These early changes in cytokine responses indicate that resident cells such as macrophages have a reduced cytokine response.
Figure 4.
During early infection CD80/CD86 signaling contributes to cytokine production. C57BL/6J WT and Cd80Cd86−/− mice were intranasally infected with 4 × 107 cfu of S. aureus for 4 h. A) Bacterial counts from BALF and lung homogenate. External body temperatures prior to euthanasia. B) Neutrophil numbers in BALF. C) Cytokine levels in BALF. n=2 for uninfected animals, WT infected n=11 and Cd80Cd86−/− infected n=12. Each point represents a mouse. Lines display medians. Graphs display means with standard error. **P<0.01 and *P<0.05.
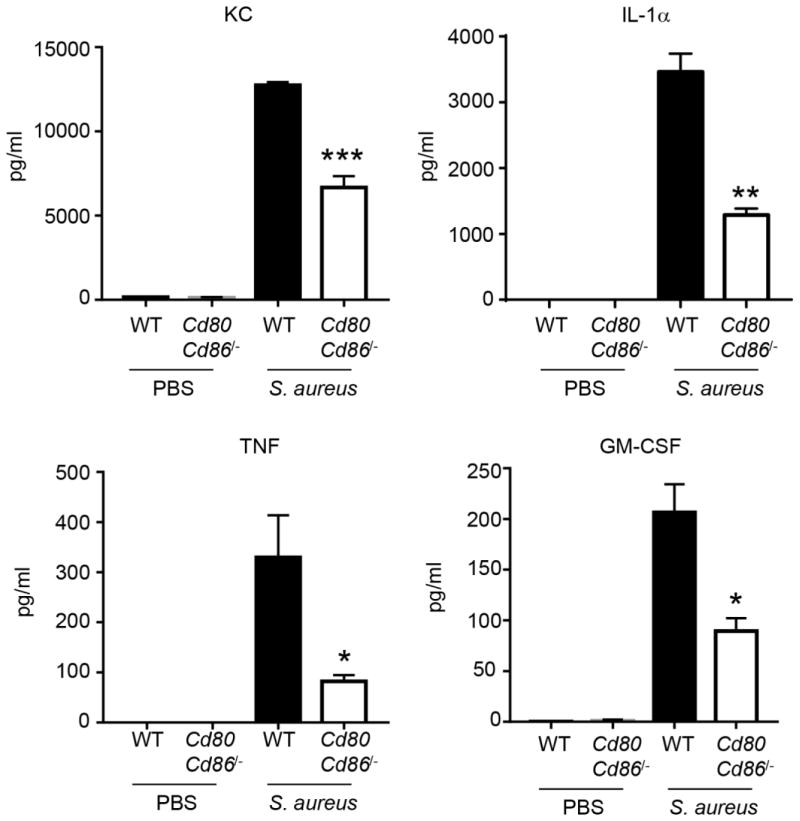
Macrophages from CD80/CD86 deficient mice have reduced cytokine production
To examine any potential macrophage signaling alternations caused by a lack of CD80/CD86 in response to S. aureus, bone marrow from WT and Cd80Cd86−/− mice was isolated and differentiated into macrophages. The bone marrow macrophages were then stimulated with S. aureus USA300 for 24 h prior to collection of supernatants for cytokine analysis. Multiple cytokines were observed to be reduced in the absence of CD80/CD86 (Fig. 5). The neutrophil chemokine KC/CXCL1 was reduced by 48% (P<0.001), IL-α was decreased 63% (P<0.01), TNF by 75% (P<0.05) and GM-CSF by 57% (P<0.05). These data indicate that in the absence of CD80/CD86 the proinflammatory capacity of macrophages is reduced and likely contributes to our cytokine phenotype in vivo.
Figure 5.
Macrophages derived from Cd80Cd86−/− mice have reduced cytokine production. Bone marrow derived macrophages from WT and Cd80Cd86−/− mice were stimulated with S. aureus USA300 for 24 h and cytokines quantified. Data is representative of two independent experiments. Graphs display means with standard error. n=3. ***P<0.001, **P<0.01 and *P<0.05.
Discussion
It is shown here that the immune response can be a contributing factor to the pathogenesis of infection. T cells are known to contribute to the pathogenesis of S. aureus infection in the lung [9] and it is shown here that mice missing CD80/CD86 also demonstrate improved outcomes. Mice lacking CD80/86 were significantly protected against mortality with S. aureus and exhibited significant decreases in proinflammatory cytokine production.
In many cases excessive inflammation or an increased immune response can be detrimental to the host, our data with S. aureus being no exception. Conditions whereby individuals have enhanced activation of the interferon signaling pathway, interferonopathies, suffer from several inflammatory based symptoms [20]. Sufferers of cystic fibrosis are also afflicted with chronic inflammation, in part due to dysregulation of their immune response and an incapacity to resolve infections with Pseudomonas aeruginosa [21]. S. aureus possess many virulence factors and surface proteins to induce a robust immune response, including various superantigen and superantigen-like proteins [22, 23] in addition to cell wall components.
Typically, the immune response observed to S. aureus in the airway is commensurate with the bacterial load present. In this study significant alternations, reductions, in cytokine production were observed in the absence of changes in bacterial counts in the airway or lung tissue. These data indicate that the reductions in cytokine production were a direct result of the absence of CD80/CD86. Our mortality phenotype is likely due to excessive cytokine production or cytokine storm in the absence of alterations in bacterial burden. Consistent with an absence of CD80/CD86 signaling causing reduced cytokine production, bone marrow macrophages from CD80/CD86 deficient mice were also observed to induce lower cytokine levels in response to S. aureus compared to WT stimulated macrophages.
Inactivation of CD80/CD86 has been observed to improve the outcomes in other models of microbial pathogenesis. In a model of cecal ligation and puncture, mice lacking CD80/CD86 also had reduced mortality and decreased proinflammatory cytokine production [24, 25]. Models of Leishmania and acute parasitemia also saw improvements in outcome [26, 27]. Inhibition of T cells in the context of pulmonary Streptococcus pneumoniae infection also led to improvements in outcome [28]. This is in contrast to Trypanosoma cruzi, whereby in the absence of CD80/CD86, cytotoxic T cells were impaired that led to exacerbated infection [29]. One difference about the T. cruzi study compared to the current study is that it was a chronic infection model. Our data is supportive that removal or reduction in the early inflammatory signaling of S. aureus in an acute model leads to a reduction in morbidity and mortality.
Through these studies further evidence is provided that the initial acute response to S. aureus in the airway leads to an excessive and detrimental level of cytokine production. This proinflammatory response is directed by many resident cells in the airway and here evidence that expression of CD80/CD86 is a significant contributor to this response is provided. Given that the initial host response to S. aureus in the airway can contribute to pathogenesis, acute therapies to limit this inflammation may prove useful in reducing morbidity and mortality to this important pathogen.
Highlights.
CD80/CD86 signaling contributes to mortality due to S. aureus pneumonia
Absence of CD80/Cd86 leads to improved levels of alveolar macrophages
CD80/CD86 signaling significantly contributes to inflammatory cytokine production
Acknowledgments
This work was supported by funding through the American Lung Association (RG-310706) and NIH (R56HL125653 and R01HL134870). The author declares no conflicts of interest. Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under award S10RR027050
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, MI Active Bacterial Core surveillance, Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Lee BY, Singh A, David MZ, Bartsch SM, Slayton RB, Huang SS, Zimmer SM, Potter MA, Macal CM, Lauderdale DS, Miller LG, Daum RS. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) Clin Microbiol Infect. 2013;19(6):528–36. doi: 10.1111/j.1469-0691.2012.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40(1):100–7. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 4.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41(11):5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367(9512):731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 6.Garnier F, Tristan A, Francois B, Etienne J, Delage-Corre M, Martin C, Liassine N, Wannet W, Denis F, Ploy MC. Pneumonia and new methicillin-resistant Staphylococcus aureus clone. Emerg Infect Dis. 2006;12(3):498–500. doi: 10.3201/eid1203.051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12(2):130–46. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker D, Ryan CL, Alonzo F, 3rd, Torres VJ, Planet PJ, Prince AS. CD4+ T cells promote the pathogenesis of Staphylococcus aureus pneumonia. J Infect Dis. 2015;211(5):835–45. doi: 10.1093/infdis/jiu525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prince A, Wang H, Kitur K, Parker D. Humanized Mice Exhibit Increased Susceptibility to Staphylococcus aureus Pneumonia. J Infect Dis. 2017;215(9):1386–1395. doi: 10.1093/infdis/jiw425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzianabos AO, Chandraker A, Kalka-Moll W, Stingele F, Dong VM, Finberg RW, Peach R, Sayegh MH. Bacterial pathogens induce abscess formation by CD4(+) T-cell activation via the CD28-B7-2 costimulatory pathway. Infect Immun. 2000;68(12):6650–5. doi: 10.1128/iai.68.12.6650-6655.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmaler M, Jann NJ, Ferracin F, Landmann R. T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88-dependent T cell activation. J Immunol. 2011;186(1):443–52. doi: 10.4049/jimmunol.1001407. [DOI] [PubMed] [Google Scholar]
- 13.McLoughlin RM, Solinga RM, Rich J, Zaleski KJ, Cocchiaro JL, Risley A, Tzianabos AO, Lee JC. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc Natl Acad Sci U S A. 2006;103(27):10408–13. doi: 10.1073/pnas.0508961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49(4):503–10. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin FJ, Parker D, Harfenist BS, Soong G, Prince A. Participation of CD11c(+) leukocytes in methicillin-resistant Staphylococcus aureus clearance from the lung. Infect Immun. 2011;79(5):1898–904. doi: 10.1128/IAI.01299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLoid GM, Sulahian TH, Imrich A, Kobzik L. Heterogeneity in macrophage phagocytosis of Staphylococcus aureus strains: high-throughput scanning cytometry-based analysis. PLoS One. 2009;4(7):e6209. doi: 10.1371/journal.pone.0006209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palecanda A, Paulauskis J, Al-Mutairi E, Imrich A, Qin G, Suzuki H, Kodama T, Tryggvason K, Koziel H, Kobzik L. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp Med. 1999;189(9):1497–506. doi: 10.1084/jem.189.9.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186(3):1666–74. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32(4):311–8. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 20.Rodero MP, Crow YJ. Type I interferon-mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J Exp Med. 2016;213(12):2527–2538. doi: 10.1084/jem.20161596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012;18(4):509–19. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev. 2013;26(3):422–47. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huvenne W, Hellings PW, Bachert C. Role of staphylococcal superantigens in airway disease. Int Arch Allergy Immunol. 2013;161(4):304–14. doi: 10.1159/000350329. [DOI] [PubMed] [Google Scholar]
- 24.Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, Gold JA. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med. 2008;177(3):301–8. doi: 10.1164/rccm.200703-515OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, Karulf MR, Rom WN, Weiden MD, Gold JA. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS One. 2009;4(8):e6600. doi: 10.1371/journal.pone.0006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JA, Titus RG, Nabavi N, Glimcher LH. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J Infect Dis. 1996;174(6):1303–8. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- 27.Taylor-Robinson AW, Smith EC. Modulation of experimental blood stage malaria through blockade of the B7/CD28 T-cell costimulatory pathway. Immunology. 1999;96(3):498–504. doi: 10.1046/j.1365-2567.1999.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeMessurier K, Hacker H, Tuomanen E, Redecke V. Inhibition of T cells provides protection against early invasive pneumococcal disease. Infect Immun. 2010;78(12):5287–94. doi: 10.1128/IAI.00431-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyahira Y, Katae M, Kobayashi S, Takeuchi T, Fukuchi Y, Abe R, Okumura K, Yagita H, Aoki T. Critical contribution of CD28-CD80/CD86 costimulatory pathway to protection from Trypanosoma cruzi infection. Infect Immun. 2003;71(6):3131–7. doi: 10.1128/IAI.71.6.3131-3137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]