Abstract
Background and Purpose
Peripartum strokes during delivery admissions are rare but have high maternal morbidity. Infections have been proposed as a possible stroke trigger. We hypothesized that women who had infections diagnosed at the time of delivery admission would have higher risk of stroke during their delivery hospitalization.
Methods
We conducted a case-control study using state inpatient administrative databases for California (2007–2011), Florida (2009–2011) and New York (2009–2011). Women whose admission included a vaginal or cesarean delivery, with a new diagnosis of stroke during the admission were considered cases, and were randomly matched to 3 in-state controls by age/admission year and presence and severity of hypertensive disorders of pregnancy. The primary exposure of interest was infection of any type present on admission. Secondary exposures included race/ethnicity, payer status, delivery method, and known vascular risk factors such as chronic hypertension, diabetes, smoking, alcohol abuse, hypercoagulable states, coagulopathies, and renal disease. We used multivariable conditional logistic regression to estimate the odds ratios and 95% confidence intervals (OR, 95%CI) for the association of infections and known vascular risk factors with stroke risk.
Results
A total of 455 cases (mean age 29.8), of whom 195 (42.9%) had hypertensive disorders of pregnancy, were matched with 1365 controls. Infection of any type present on admission increased the odds of stroke diagnosis during the admission (adjusted OR 1.74, 95%CI 1.29–2.35). Risk was higher for genitourinary infections (adjusted OR 2.56, 95%CI 1.25–5.24) and sepsis (adjusted OR 10.4, 95%CI 2.15–20.0). The association between infection and stroke during delivery admission did not differ by presence of hypertensive disorders of pregnancy.
Conclusions
Infections present on admission increased stroke risk during delivery admissions in women with and without hypertensive disorders of pregnancy. The results were driven by genitourinary infections and sepsis. Infections may be an under-recognized precipitant of peripartum stroke.
Keywords: infections, pregnancy, preeclampsia, postpartum, stroke
Introduction
Maternal stroke complicates 30 of every 100,000 pregnancies and is associated with substantial maternal morbidity and mortality.1 Risk factors for peripartum stroke include hypertensive disorders of pregnancy (HDP), including gestational hypertension, preeclampsia and eclampsia; chronic hypertension; migraine headaches; antiphospholipid syndrome and other thrombophilias; sickle cell disease; black race; cesarean delivery; and extremes of age.2,3 Multiple studies have shown an increased risk of stroke in the postpartum period compared to during the pregnancy itself.3–7 However, 30–40% of pregnancy-associated strokes occur during the delivery admission,2,8 and at least one study found the highest risk one day prior and two days after delivery.9 Despite known risk factors, these devastating events remain difficult to predict due to their rarity, hampering efforts to develop preventive strategies.
Infection is a novel risk factor that has emerged as an important and under-recognized trigger for stroke, particularly in young people.10–12 Acute respiratory infections and urinary tract infections were associated with a transient increase in cardiovascular event rates including myocardial infarction and stroke in a large population-based study.13 Several studies have found an association between infections and peripartum or postpartum stroke,3,14,15 with one study showing a 25-fold increase in the odds of postpartum stroke in women with postpartum infections.2 Infections have been noted as a risk factor for peripartum stroke in women with preeclampsia,15 a subgroup that is at 5- to 6-fold higher risk for pregnancy-related stroke compared to women without preeclampsia.8,16 Interestingly, infections have also been implicated in the pathogenesis of preeclampsia; multiple studies have demonstrated a strong association between preexisting infections including Helicobacter pylori,17 Chlamydia pneumoniae, and urinary tract infections18,19 and the development of preeclampsia. However, the relationship between infection and peripartum stroke in the general pregnant population is not well characterized. In particular, infection as a trigger for stroke at the time of delivery has not been described in prior studies, and there are few data regarding timing of infections (present on admission or hospital-acquired). Furthermore, most prior studies of pregnancy-related stroke did not explore infection subtypes as a risk factor.
We hypothesized that women who had infections diagnosed at the time of delivery admission would have higher risk of stroke during their delivery hospitalization.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study design and population
We performed a case-control study using data from the Healthcare Cost and Utilization Project State Inpatient Database for California (2007–2011), Florida (2009–2011) and New York (2009–2011), which includes hospital and emergency department discharge data from all non-federal hospitals in each state. We restricted the sample to female patients aged 15–45 with visits coded by the major diagnostic category of 14 (pregnancy, childbirth and the puerperium). Women with a stroke diagnosis present on admission were excluded from the analysis. Delivery admissions were selected using diagnosis related groups for vaginal and cesarean delivery codes (Supplemental Table I).20,21 Diagnosis related group codes changed in October 2007, so both sets of codes were used to select the cohort from the 2007 datasets.22 Delivery hospitalizations with diagnostic codes for oncology and trauma based on the International Classification of Diseases, 9th edition, Clinical Modification (ICD-9) were excluded from the sample. Stroke and other cerebrovascular outcomes, infections, hypertensive disorders of pregnancy (HDP) and additional stroke risk factors were abstracted using ICD-9 codes.
Women who met our inclusion criteria and had a stroke diagnosis during the delivery admission, defined as a diagnostic ICD-9 code for stroke not present on admission, were considered cases, and were randomly matched to three controls by age, admission year and HDP status. We categorized HDP status as “absent”, “mild” (including gestational hypertension [642.3x, 642.9x] and mild preeclampsia [642.4x, 642.7x]), or “severe” (including severe preeclampsia [642.5x] and eclampsia [642.6x]). We chose to match on HDP status due to the concern that preeclampsia and other HDP were potentially related to both the exposure of interest (infection) and the outcome (stroke). We also conducted pre-planned subgroup analyses to test whether the hypothesized relationship between infection and peripartum stroke changed based on presence of HDP.
Exposures and outcome of interest
The primary exposure of interest was presence of any infection at the time of admission, indicated by a “present on admission” diagnostic code modifier. “Infections” included diagnostic codes for the following conditions as delineated by the Healthcare Cost and Utilization Project:23 sepsis, genitourinary infections, gastrointestinal infections, respiratory infections, sexually transmitted infections, and other infections. Additional pre-specified exposures of interest included race/ethnicity, payer status, delivery method, and known vascular risk factors such as chronic hypertension, diabetes, smoking, alcohol abuse, hypercoagulable states, coagulopathies, and renal disease. The “stroke diagnosis” outcome variable was comprised of the following diagnostic codes: ischemic stroke (433.x [where “x” is any fourth or fifth digit], 434.x, 436.0), intracerebral hemorrhage (431.0), subarachnoid hemorrhage (430.0), cerebral venous thrombosis (325.0, 671.5), and puerperium-related strokes (674.0x, where x is a fifth-digit modifier indicating timing of stroke before or after delivery). A complete listing of diagnostic codes employed can be found in Supplemental Tables II and III in the online supplement.
Informed consent and approvals
Approval was obtained for the study from the Institutional Review Board of Columbia University Medical Center, and the requirement for written informed consent was waived due to the public and de-identified nature of the data.
Statistical analysis
We compared demographics and risk factors between cases and controls using proportions for categorical variables and mean/standard deviation for continuous variables. Crude and adjusted conditional logistic regression models were used to estimate the odds ratios (OR) and 95% confidence intervals (95%CI) for the association between infections present on admission and stroke during delivery admission. We included in the adjusted models only those variables which showed a significant association both with the exposure of interest (infection) and the outcome (stroke). We performed subgroup analyses of the same variables in women without HDP, with mild HDP, and with severe HDP. All analyses were conducted using SAS statistical software (SAS version 9.4, Cary, NC). An alpha of 0.05 was considered significant.
Results
Of the 2,799,020 delivery admissions during the study period, 455 received a new diagnostic code for stroke during the admission and were considered cases, suggesting a cumulative incidence of 16.3 new stroke diagnoses per 100,000 delivery admissions. Mean age among cases was 29.8 years, and HDP were diagnosed in 195 of the 455 women (42.9%). Demographics, vascular risk factors, and pregnancy complications are detailed in Table 1. Compared with the control group (n=1365), cases had higher proportions of vascular risk factors such as chronic hypertension, chronic kidney disease, alcohol abuse, and coagulopathies/hypercoagulable states. More cases than controls delivered via cesarean section (359 of 455 cases [78.9%] vs. 763 of 1365 controls [55.9%]).
Table 1.
Demographics and characteristics of women aged 15–45 admitted for delivery in California (2007–2011), Florida (2009–2011) and New York (2009–2011), with (cases) and without new stroke diagnostic codes (controls) during delivery hospitalization
| Cases of stroke during delivery hospitalization n=455 |
Controls n=1365 |
p-value | |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Matching characteristics | |||
| Age, Mean | 29.8 | 28.8 | p=0.009 |
| HDP status | p=1.0 | ||
| No HDP | 260 (57.1) | 780 (57.1) | |
| Mild HDP | 80 (17.6) | 240 (17.6) | |
| Severe HDP | 115 (25.3) | 345 (25.3) | |
| Other characteristics | |||
| Race/ethnicity* | p<0.0001 | ||
| White | 169 (38.0) | 489 (36.8) | |
| Black | 71 (16.0) | 367 (27.6) | |
| Hispanic | 145 (32.6) | 258 (19.4) | |
| Asian or Pacific Islander | 41 (9.2) | 135 (10.2) | |
| Native American | 0 | 1 (0.1) | |
| Other | 19 (4.3) | 79 (5.9) | |
| Payer Status | p=0.30 | ||
| Medicare | 9 (2.0) | 13 (1.0) | |
| Medicaid | 212 (46.6) | 688 (50.4) | |
| Private insurance | 214 (47.0) | 614 (45.0) | |
| Self-pay | 9 (2.0) | 25 (1.8) | |
| Other | 11 (2.4) | 25 (1.8) | |
| Comorbidities/complications | |||
| Chronic hypertension | 57 (12.5) | 61 (4.5) | p<0.0001 |
| Coagulopathy | 71 (15.6) | 56 (4.1) | p<0.0001 |
| Hypercoagulable state | 47 (10.33) | 42 (3.1) | p<0.0001 |
| Sickle cell disease | 8 (1.76) | 10 (0.7) | p=0.06 |
| Deep vein thrombosis/pulmonary embolism | 25 (5.49) | 11 (0.8) | p<0.0001 |
| Diabetes | 12 (2.6) | 29 (2.1) | p=0.52 |
| Renal disease | 6 (1.3) | 5 (0.4) | p=0.02 |
| Smoking | 21 (4.6) | 71 (5.2) | p=0.62 |
| Alcohol abuse | 17 (3.7) | 16 (1.2) | p=0.0004 |
| Cesarean delivery | 359 (78.9) | 763 (55.9) | p<0.0001 |
Women with a new stroke diagnosis code during the delivery admission were considered cases and were matched with stroke-free controls on age, state, year of admission, and HDP status. Women with gestational hypertension or mild preeclampsia were considered to have “mild” HDP. Women with severe preeclampsia or eclampsia were considered to have “severe” HDP. HDP: hypertensive disorder of pregnancy.
Data on race/ethnicity were not available for all subjects.
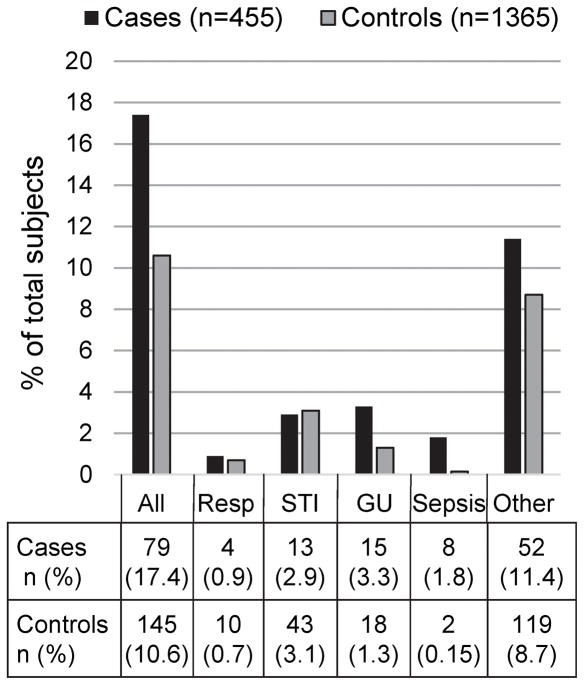
The primary exposure of interest, infection of any type present on admission, was significantly associated with stroke diagnosis during hospitalization (Table 2). Women who had an infection had 1.76 (95%CI 1.31–2.37) times the odds of a stroke diagnosis during their delivery admission compared to controls, and when adjusted for race and insurance payer status the relationship remained (OR 1.74, 95%CI 1.29–2.35). Infection subtypes are shown in Figure 1. Sepsis and genitourinary infections were more highly associated with stroke diagnoses than other types of infection: women with sepsis had 10.4 (95%CI 2.15–50.0) times the odds of stroke diagnosis during the delivery hospitalization, and women with genitourinary infections had 2.56 (95%CI 1.25–5.24) times the odds of stroke diagnosis after adjustment. There was no significant association between sexually transmitted infections (OR 1.06, 95%CI 0.56–2.02) or respiratory infections (OR 0.88, 95%CI 00.247–3.21) and stroke diagnosis in adjusted models. Subgroup analyses stratified by presence/severity of HDP showed an effect of infection in all three groups, although the effects were not statistically significant in the individual groups.
Table 2.
Unadjusted and adjusted odds ratios for the association between infections present on admission and new stroke diagnosis code during a delivery hospitalization
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| All infections | 1.76 | 1.31–2.37 | 1.74 | 1.29–2.35 |
| Sepsis | 12.0 | 2.55–56.5 | 10.4 | 2.15–50.0 |
| Genitourinary infections | 2.50 | 1.23–5.12 | 2.56 | 1.25–5.24 |
| Sexually transmitted infections | 0.90 | 0.48–1.79 | 1.06 | 0.56–2.02 |
| Respiratory infections | 1.20 | 0.37–3.82 | 0.88 | 0.24–3.21 |
| Other infections | 1.35 | 0.95–1.90 | 1.31 | 0.92–1.86 |
| Subgroup analysis by HDP status | ||||
| No HDP | 1.79 | 1.21–2.67 | 1.74 | 1.16–2.60 |
| Mild HDP | 2.22 | 1.11–4.42 | 2.26 | 1.13–4.53 |
| Severe HDP | 1.41 | 0.77–2.55 | 1.35 | 0.74–2.45 |
Women with gestational hypertension or mild preeclampsia were considered to have “mild” HDP. Women with severe preeclampsia or eclampsia were considered to have “severe” HDP. OR: odds ratio. CI: confidence interval. HDP: hypertensive disorder of pregnancy.
Adjusted models included only those variables found to be associated both with the primary exposure (infection) and the outcome (stroke diagnosis code); these were race/ethnicity and payer status.
Figure 1. Number and subtypes of infections on admission in women with (cases) and without new stroke diagnostic codes (controls) during delivery hospitalization.
Bar graphs and table show total infections and infection subtypes present on admission in women aged 15–45 admitted for delivery in California (2007–2011), Florida (2009–2011) and New York (2009–2011). Some women had multiple infection subtypes.
Among cases, there were 90 diagnoses of ischemic stroke (19.8%), 60 of subarachnoid hemorrhage (13.2%), 58 of intracerebral hemorrhage (12.8%), 3 of venous sinus thrombosis (0.66%) and 244 diagnoses of unspecified pregnancy-related stroke (53.6%). Among cases that had a pregnancy-specific stroke diagnostic code (n=388) which included a fifth-digit modifier for timing, 275 (70.9%) of strokes occurred prior to delivery, and 113 (29.1%) occurred after delivery. Discharge disposition data were incomplete; however, among the available records, a lower proportion of cases than controls were discharged home following delivery (258 of 455 cases [76.8%] vs. 936 of 1365 [93.7%], p<0.0001). In-hospital mortality was 5.7% among cases (26 of 455) and 0% among controls.
Discussion
Infection has emerged as an important risk factor and precipitant for stroke,24 particularly in younger patients, but has not been extensively studied as a stroke risk factor within a pregnant population. In our analysis of three large statewide administrative datasets, we found that infections present on admission, particularly genitourinary infections and sepsis, increased the risk of stroke diagnosis during delivery admission, even when matched on presence and severity of HDP. We previously showed that infections, especially genitourinary, raised the risk of pregnancy-related stroke in women with preeclampsia.15 However, this association has not been previously described in the general pregnant population, and the timing of infections in relation to stroke diagnosis in this population has not been explored.
Infection has been described as a stroke “trigger,”10,25 with proposed mechanisms including increased platelet aggregation due to systemic inflammation, endothelial dysfunction, infection-induced cardiac arrhythmias, and dehydration-related thrombosis.26 Infections can also directly cause strokes, such as in the case of infectious arteriopathies or septic emboli from infectious endocarditis.27 Pregnancy is known to alter immune system function and increase the severity of certain infections, such as influenza;28 whether pregnant women are more vulnerable to contracting infections is debated.29 However, pregnant women do seem to have an enhanced inflammatory response to infections, particularly towards the end of pregnancy.30 It is plausible that this enhanced inflammatory response could play a role in increasing the risk of peripartum stroke. This is supported by our finding of a markedly higher risk of peripartum stroke in women with sepsis, which could be due to a dose-response to high levels of systemic inflammation. Another possibility is that treatment of infection, rather than infections themselves, was associated with stroke; however, we were unable to determine medication effects, as medications are not tracked in billing data.
An association between infections and peripartum stroke has important implications for clinical management. Guidelines for preventing infection in pregnancy and the puerperium recommend against routine antibiotic prophylaxis in the second and third trimester.31 Current evidence does not support routine prophylaxis of infection after vaginal delivery.32 However, various antibiotic strategies have been employed for prevention of post-operative infections in women undergoing cesarean delivery,33–35 which was highly prevalent among the cases in our study (likely due to the high proportion of HDP). Universal adoption of evidence-based practices aimed at infection prevention may have a side benefit of reducing the risk of stroke. Of note, genitourinary infections showed a higher risk than infections overall in our study; these infections are common during pregnancy and can be easily screened for and treated.36 Whether routine antibiotic prophylaxis could reduce the risk of peripartum stroke in selected high-risk individuals is not known, and is difficult to test in a clinical trial due to the rarity of the outcome. However, our results suggest that women identified as having genitourinary infections on admission for delivery might warrant closer monitoring for neurological complications in the peripartum period, regardless of HDP status. Women with sepsis are likely to be admitted to an intensive care unit in any case, but our study reinforces that prompt attention should be given to any new neurological sign or symptom in this vulnerable group.
Strengths of our study include its large size, comprehensive capture of delivery admissions in the states sampled, and geographic, racial and ethnic diversity. Matching by presence and severity of HDP removed a potential confounding factor, since preeclampsia and related conditions are associated with both infections and peripartum stroke. Furthermore, restricting our exposure of interest to infections present on admission (rather than hospital-acquired) reduces the likelihood that these infections could be a complication of stroke rather than a risk factor.
Our study has important limitations. First, administrative datasets are prone to coding errors, leading to possible over- or underestimation of stroke diagnoses. While the traditional stroke diagnostic codes we employed have been shown to have high sensitivity, specificity, and positive predictive value in rigorous validation studies,37 questions have been raised about the accuracy of pregnancy-specific stroke diagnostic codes (ICD-9 674.0x);38 however, these codes have been employed in many prior administrative database studies,8,16,39–41 and at our own institution, we found the 674.0x codes to have a positive predictive value of 80.4%.15 Furthermore, the cumulative incidence of stroke diagnoses during delivery hospitalization in our sample was 16.3 per 100,000 deliveries, a rate which is consistent with a recent meta-analysis showing the overall incidence of pregnancy-associated stroke (including antepartum, delivery, and postpartum admissions) to be 30 per 100,000 deliveries.1 Second, some women may have been misclassified in terms of presence and severity of HDP; however, these diagnostic codes have been shown to have 94.5% positive predictive value when coded during inpatient hospitalizations.42 The potential of misclassification for infection identification is an additional limitation. Diagnostic codes we employed for infections have variable sensitivity and specificity.43 In an effort to mitigate the limitations of ICD-9 code use for detecting infections, algorithms, multiple ICD-9 codes, and combinations of ICD-9 codes are effective ways of identifying an infection from administrative data. We recognize that this could influence the sensitivity and specificity of infection diagnosis; however, the misclassification in the identification of infections through these methods would be non-differential and would bias our results towards the null. Also, we were unable to assess for medications such as antibiotic use, which could have affected the risk of stroke. Lastly, pregnancy-specific stroke codes specify timing of the stroke (before, during or after delivery) but do not specify stroke subtype (ischemic versus hemorrhagic); conversely, non-pregnancy specific codes can better characterize stroke subtype but provide no information on timing in relation to delivery. These limitations, common to most large administrative dataset-based studies, highlight the need for prospective studies of pregnancy cohorts44 to meticulously track maternal neurological outcomes, both during the delivery admission and postpartum. Our results should be interpreted with caution and viewed as exploratory and hypothesis-generating.
Conclusion
Infections present on admission increased risk of stroke diagnosis during delivery admissions in women with and without hypertensive disorders of pregnancy. The results were driven by genitourinary infections and sepsis, which were associated with considerably higher risk than infections overall. Infections may be an under-recognized precipitant of peripartum stroke.
Supplementary Material
Acknowledgments
Sources of Funding: Dr. Miller is supported by a National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS) StrokeNet Training Core fellowship (U10 NS086728). Dr. Boehme is supported by grants from the NIH NINDS (R03 NS101417) and NIH National Institute on Minority Health and Health Disparities (R21 MD012451). Dr. Friedman is supported by a career development award (K08HD082287) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. Ms. Gallo received support from Pfizer for research training in epidemiology and public health.
Footnotes
Disclosures: Dr. Elkind receives compensation for providing consultative services for Abbott and Boehringer-Ingelheim; receives compensation for expert testimony related to stroke from Hi-Tech Pharmaceuticals (dimethylamylamine), Merck/Organon (Nuvaring), and Auxilium (testosterone); receives royalties from UpToDate for chapters related to stroke; receives study medication in kind but no personal compensation from the BMS-Pfizer Alliance and laboratory assay support but no personal compensation from Roche for a clinical trial of atrial cardiopathy. Dr. Elkind’s institution, Columbia University, receives compensation through service agreements with Medtronic for Dr. Elkind’s effort on clinical trials related to cardiac monitoring and with Biogen for a clinical trial of natalizumab for stroke. He serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. Ms. Gallo is employed by Pfizer. Pfizer had no input on the study design or the manuscript.
References
- 1.Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert LR, Bushnell C, et al. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. International Journal of Stroke. 2017;12:687–697. doi: 10.1177/1747493017723271. [DOI] [PubMed] [Google Scholar]
- 2.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol [Internet] 2005;106:509–516. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 3.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31:1274–1282. doi: 10.1161/01.str.31.6.1274. [DOI] [PubMed] [Google Scholar]
- 4.Sharshar T, Lamy C, Mas JL. Incidence and causes of strokes associated with pregnancy and puerperium. A study in public hospitals of Ile de France. Stroke in Pregnancy Study Group. Stroke. 1995;26:930–936. doi: 10.1161/01.str.26.6.930. [DOI] [PubMed] [Google Scholar]
- 5.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768–774. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaigobin C, Silver FL. Stroke and pregnancy. Stroke. 2000;31:2948–2951. doi: 10.1161/01.str.31.12.2948. [DOI] [PubMed] [Google Scholar]
- 7.Skidmore FM, Williams LS, Fradkin KD, Alonso RJ, Biller J. Presentation, etiology, and outcome of stroke in pregnancy and puerperium. J Stroke Cerebrovasc Dis. 2001;10:1–10. doi: 10.1053/jscd.2001.20977. [DOI] [PubMed] [Google Scholar]
- 8.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstetrics & Gynecology. 2015;125:124–131. doi: 10.1097/AOG.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salonen Ros H, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology. 2001;12:456–460. doi: 10.1097/00001648-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Elkind MSV. Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51–57. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]
- 11.Hills NK, Sidney S, Fullerton HJ. Timing and number of minor infections as risk factors for childhood arterial ischemic stroke. Neurology. 2014;83:890–897. doi: 10.1212/WNL.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkind MSV, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, et al. Herpesvirus Infections and Childhood Arterial Ischemic Stroke: Results of the VIPS Study. Circulation. 2016;133:732–741. doi: 10.1161/CIRCULATIONAHA.115.018595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 14.Gao H, Yang BJ, Jin LP, Jia XF. Predisposing factors, diagnosis, treatment and prognosis of cerebral venous thrombosis during pregnancy and postpartum: a case-control study. Chinese medical journal. 2011;124:4198–4204. [PubMed] [Google Scholar]
- 15.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert LR, Marshall RS, et al. Risk Factors for Pregnancy-Associated Stroke in Women With Preeclampsia. Stroke. 2017;48:1752–1759. doi: 10.1161/STROKEAHA.117.017374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ros HS, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? The American Journal of Obstetrics & Gynecology. 2002;186:198–203. doi: 10.1067/mob.2002.119177. [DOI] [PubMed] [Google Scholar]
- 17.Nourollahpour Shiadeh M, Riahi SM, Adam I, Saber V, Behboodi Moghadam Z, Armon B, et al. Helicobacter pylori infection and risk of preeclampsia: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2017:1–8. doi: 10.1080/14767058.2017.1378331. [DOI] [PubMed] [Google Scholar]
- 18.Easter SR, Cantonwine DE, Zera CA, Lim K-H, Parry SI, McElrath TF. Urinary tract infection during pregnancy, angiogenic factor profiles, and risk of preeclampsia. American Journal of Obstetrics and Gynecology. 2016;214:387e1–7. doi: 10.1016/j.ajog.2015.09.101. [DOI] [PubMed] [Google Scholar]
- 19.Minassian C, Thomas SL, Williams DJ, Campbell O, Smeeth L. Acute maternal infection and risk of pre-eclampsia: a population-based case-control study. PLoS One. 2013;8:e73047. doi: 10.1371/journal.pone.0073047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Defining the Medicare Severity Diagnosis Related Groups (MS-DRGs), Version 34.0. Centers for Medicare and Medicaid Services; [Accessed November 15, 2017]. https://www.cms.gov/ICD10Manual/version34-fullcode-cms/fullcode_cms/Defining_the_Medicare_Severity_Diagnosis_Related_Groups_(MS-DRGs)_PBL-038.pdf. [Google Scholar]
- 21.Introduction to the HCUP State Inpatient Databases. Healthcare Cost and Utilization Project; [Accessed December 5, 2017]. https://www.hcup-us.ahrq.gov/sidoverview.jsp. [Google Scholar]
- 22.Averill R, Goldfield N, Hughes JS, Bonazelli J, McCullough EC, Steinbeck BA, Mullin R, Tang AM. All Patient Refined Diagnosis Related Groups (APR-DRGs) Healthcare Cost and Utilization Project; [Accessed December 6, 2017]. https://www.hcup-us.ahrq.gov/db/nation/nis/APR-DRGsV20MethodologyOverviewandBibliography.pdf. [Google Scholar]
- 23.HCUP Comorbidity Software. Healthcare Cost and Utilization Project; [Accessed February 20, 2018]. www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. [Google Scholar]
- 24.Elkind MSV, Cole JW. Do common infections cause stroke? Semin Neurol. 2006;26:88–99. doi: 10.1055/s-2006-933312. [DOI] [PubMed] [Google Scholar]
- 25.Fullerton HJ, Hills NK, Elkind MSV, Dowling MM, Wintermark M, Glaser CA, et al. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS study. Neurology. 2015;85:1459–1466. doi: 10.1212/WNL.0000000000002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grau AJ, Urbanek C, Palm F. Common infections and the risk of stroke. Nature Reviews Neurology. 2010;6:681–694. doi: 10.1038/nrneurol.2010.163. [DOI] [PubMed] [Google Scholar]
- 27.Miller EC, Elkind MSV. Infection and Stroke: an Update on Recent Progress. Curr Neurol Neurosci Rep. 2016;16:2. doi: 10.1007/s11910-015-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meijer WJ, van Noortwijk AGA, Bruinse HW, Wensing AMJ. Influenza virus infection in pregnancy: a review. Acta Obstet Gynecol Scand. 2015;94:797–819. doi: 10.1111/aogs.12680. [DOI] [PubMed] [Google Scholar]
- 29.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and Infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol. 2013;2013:752852. doi: 10.1155/2013/752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO recommendations for prevention and treatment of maternal peripartum infections. World Health Organization; [Accessed December 5, 2017]. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/peripartum-infections-guidelines/en/ [PubMed] [Google Scholar]
- 32.Bonet M, Ota E, Chibueze CE, Oladapo OT. Routine antibiotic prophylaxis after normal vaginal birth for reducing maternal infectious morbidity. Cochrane Database Syst Rev. 2017;11:CD012137. doi: 10.1002/14651858.CD012137.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014:CD007892. doi: 10.1002/14651858.CD007892.pub4. [DOI] [PubMed] [Google Scholar]
- 34.Swank ML, Wing DA, Nicolau DP, McNulty JA. Increased 3-gram cefazolin dosing for cesarean delivery prophylaxis in obese women. The American Journal of Obstetrics & Gynecology. 2015;213:415e1–8. doi: 10.1016/j.ajog.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Tita ATN, Szychowski JM, Boggess K, Saade G, Longo S, Clark E, et al. Adjunctive Azithromycin Prophylaxis for Cesarean Delivery. N Engl J Med. 2016;375:1231–1241. [Google Scholar]
- 36.Eppes C. Management of Infection for the Obstetrician/Gynecologist. Obstet Gynecol Clin North Am. 2016;43:639–657. doi: 10.1016/j.ogc.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 38.Sigakis MJG, Leffert LR, Mirzakhani H, Sharawi N, Rajala B, Callaghan WM, et al. The Validity of Discharge Billing Codes Reflecting Severe Maternal Morbidity. Anesthesia & Analgesia. 2016;123:731–738. doi: 10.1213/ANE.0000000000001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leffert LR, Clancy CR, Bateman BT, Cox M, Schulte PJ, Smith EE, et al. Patient Characteristics and Outcomes After Hemorrhagic Stroke in Pregnancy. Circ Cardiovasc Qual Outcomes. 2015;8:S170–S178. doi: 10.1161/CIRCOUTCOMES.115.002242. [DOI] [PubMed] [Google Scholar]
- 40.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MSV. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370:1307–1315. doi: 10.1056/NEJMoa1311485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke. 2011;42:2564–2570. doi: 10.1161/STROKEAHA.110.610592. [DOI] [PubMed] [Google Scholar]
- 42.Palmsten K, Huybrechts KF, Kowal MK, Mogun H, Hernandez-Diaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiol Drug Saf. 2014;23:646–655. doi: 10.1002/pds.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barber C, Lacaille D, Fortin PR. Systematic Review of Validation Studies of the Use of Administrative Data to Identify Serious Infections. Arthritis Care & Research. 2013;65:1343–1357. doi: 10.1002/acr.21959. [DOI] [PubMed] [Google Scholar]
- 44.Haas DM, Parker CB, Wing DA, Parry S, Grobman WA, Mercer BM, et al. A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b) The American Journal of Obstetrics & Gynecology. 2015;212:539.e1–539.e24. doi: 10.1016/j.ajog.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.