Abstract
Background
Sleep disordered breathing (SDB) is common in pregnancy, but there are limited data on predictors.
Objectives
The objective of this study was to develop predictive models of SDB during pregnancy.
Study Design
Nulliparous women completed validated questionnaires to assess for symptoms related to snoring, fatigue, excessive daytime sleepiness insomnia and restless leg syndrome. These included questions regarding the timing of sleep and sleep duration, work schedules (e.g., shift work, night work), sleep positions, and previously diagnosed sleep disorders. Frequent snoring was defined as self-reported snoring ≥3 days per week. Participants underwent in-home portable sleep studies for SDB assessment in early (6–15 weeks’) and mid-pregnancy (22–31 weeks’). SDB was characterized using an apnea hypopnea index (AHI) that included all apneas, plus hypopneas with ≥3% oxygen desaturation. For primary analyses, an AHI ≥5 events/hour was used to define SDB. Odds ratios and 95% confidence intervals (CIs) were calculated for predictor variables. Predictive ability of the logistic models was estimated using area under the receiver-operating-characteristic curves, along with sensitivities, specificities, and positive and negative predictive values and likelihood ratios.
Results
Among 3705 women who were enrolled, data were available for 3,264 and 2,512 women in early and mid-pregnancy, respectively. The corresponding prevalence of SDB was 3.6% and 8.3%. At each time point in gestation, frequent snoring, chronic hypertension, greater maternal age, BMI, neck circumference, and systolic blood pressure were most strongly associated with an increased risk of SDB. Logistic regression models that included current age, BMI, and frequent snoring predicted SDB in early pregnancy, SDB in mid-pregnancy, and new onset SDB in mid-pregnancy with 10-fold cross-validated AUCs of 0.870, 0.838, and 0.809. We provide a supplement with expanded tables, integrated predictiveness and classification curves, and an Excel predicted probability calculator.
Conclusion(s)
Among nulliparous pregnant women, logistic regression models with just three variables (i.e., age, BMI, and frequent snoring) achieved good prediction of prevalent and incident SDB. These results can help with screening for SDB in the clinical setting and for future clinical treatment trials.
Keywords: home sleep test, methods, pregnancy, sleep-disordered breathing, sleep, prediction
Sleep disordered breathing (SDB), predominantly obstructive sleep apnea, occurs in 10 to 32% of pregnancies (varying with population, diagnostic method, and definition of SDB).1–3 SDB is a risk factor for pregnancy-related complications including gestational hypertension, preeclampsia, and gestational diabetes mellitus (GDM).4–6 The presence of SDB is also associated with an increased risk for cardiomyopathy, venous thromboembolism, anesthetic complications, severe maternal morbidity, and maternal mortality.7 Neonatal complications such as growth restriction8,9 and preterm delivery10 are also increased. There is therefore considerable interest in identifying pregnant women with SDB.
SDB is challenging to identify even in a general population, because of limited specificity of SDB symptoms.11 Women are less likely than men to report sleepiness as a symptom and are more likely to report fatigue and insomnia.11,12 Questionnaires which have been validated in the general population, are poorly predictive in pregnant women.2,13 Although overnight polysomnography is the gold standard for diagnosis, the cost and inconvenience preclude widespread use14. Thus, it is desirable to identify pregnant women at increased risk for SDB before obtaining such polysomnography.
A number of studies have attempted to identify predictors of SDB in pregnancy. These studies have been limited by their sample size, limited study population or method of testing for SDB.3,15 In this study, we sought to develop clinically-feasible prediction models for SDB in a large cohort of nulliparous pregnant women undergoing objective testing for SDB.
Methods
Participants in this study were enrolled in the Sleep Disordered Breathing Substudy of the: Nulliparous Pregnancy Outcomes: Monitoring Mothers-to-Be (nuMoM2b) study. Details of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b) parent16 and Sleep Disordered Breathing Substudy methods have been published previously.17 For the nuMoM2b parent study, which was managed by an independent data-coordinating center, women were recruited at eight clinical sites. Women were eligible for participation in the parent study if they were nulliparous (no prior delivery at 20 weeks of gestation or greater) and had a viable singleton pregnancy at the time of screening (6–136 weeks of gestation). Participants from the parent study were offered enrollment into the Sleep Disordered Breathing Substudy. The primary aim of the sleep substudy was to evaluate SDB during pregnancy as a risk factor for the development of hypertensive disorders of pregnancy and gestational diabetes.5 Women were excluded for the following conditions: current SDB treated with continuous positive airway pressure treatment, severe asthma requiring continuous oral steroid therapy for more than 14 days, or a condition requiring oxygen supplementation. After enrollment, participants received routine prenatal care. During study visits, trained research coordinators obtained longitudinal clinical measurements per protocol.17
Questionnaires
Sleep questionnaires were completed by all participants in the parent study at the first (6–136 weeks of gestation) and third study visits (22–296 weeks). These included questions regarding the timing of sleep and sleep duration, work schedules (e.g., shift work, night work), sleep positions, and previously diagnosed sleep disorders. Frequent snoring was defined as self-reported snoring ≥3 days per week. Questions from validated sleep questionnaires that were collected included: 1) the Berlin Questionnaire (a screening instrument designed to identify adults likely to have OSA through a series of questions pertaining to snoring behavior and wake-time sleepiness);18 2) the Epworth Sleepiness Scale (a measurement of daytime sleep propensity, using estimates of the likelihood of dozing off or falling asleep in 8 different sedentary situations);19,20 3) the International Restless Legs Syndrome Study Group diagnostic criteria for restless legs syndrome;21 and 4) the Women’s Health Initiative Insomnia Rating Scale (quantifying insomnia symptom severity).22,23
Home Sleep Testing
Participants in the nuMoM2b Sleep Disordered Breathing Substudy underwent in-home sleep apnea testing using a six-channel monitor that was self-applied by the participant for a single night at two time points: early pregnancy at 6–156 weeks of gestation (within 2 weeks following nuMoM2b study Visit 1) and mid-pregnancy at 22–316 weeks (within 2 weeks following Visit 3). Sleep study data were downloaded at the study site and electronically transmitted to a central sleep reading center where data were scored by individuals masked to all other study data. A description of the scoring and quality control protocol has been previously published.17 Sleep studies were scored using the alternative definitions of the American Academy of Sleep Medicine.24 SDB was characterized using an apnea hypopnea index (AHI) that included all apneas, plus hypopneas with ≥3% oxygen desaturation
Statistical Analysis
AHI was analyzed as a dichotomous variable with an AHI ≥5 defining SDB.25 Separate models were developed to predict the following outcomes: 1) SDB at Visit 1, 2) SDB at Visit 3, and 3) new-onset SDB at Visit 3 for those without SDB at Visit 1. Eighty-six potential predictors of interest were specified a priori and are summarized in the supplemental materials (Table S1 – descriptive statistics; and Table S2 – single-variable associations with SDB). These were determined by reviewing the published literature in the pregnant and non-pregnant populations, and were included if they were considered well-established risk factors.1,14,26–28
The potential predictors of interest were further evaluated by the members of the writing group which included experts in sleep medicine (P.Z., S.R.), maternal fetal medicine (J.L., F.F., R.S.), biostatistics (M.K., B.C., C.P.). Sixteen candidate variables for predictive modelling were selected from the potential predictors of interest based on the writing group’s consensus and statistical factors: p<0.15 for association with SDB for at least one outcome; magnitude of observed odds ratios; avoidance of sparse numbers of SDB events in predictor response categories (where pooling was not possible) for each outcome, avoidance of potential collinearity among predictor variables, and ease and reliability of measurement in a clinical setting. Chosen candidate predictors are shown in Tables 1 and 2, which are subset from Tables S1 and S2.
Table 1.
Characteristics of the nuMoM2b Sleep Breathing Study Participants at Visit 1 (60 to 136 weeks’ gestation) and Visit 3 (220 to 296 weeks’ gestation)
| Candidate predictor variables | Visit 1 N = 3264 |
Visit 3 N = 2512 |
|---|---|---|
|
| ||
| Maternal age at Visit 1, in years | ||
| Mean (standard deviation) | 26.8 (5.6) | 27.0 (5.4) |
| Category: n (%) | ||
| 13–21 | 679 (20.8) | 463 (18.4) |
| 22–35 | 2,365 (72.5) | 1,888 (75.2) |
| >35 | 220 (6.7) | 161 (6.4) |
| Maternal race/ethnicity: n (%) | ||
| White, Non-Hispanic | 1,971 (60.4) | 1,590 (63.3) |
| Black, Non-Hispanic | 416 (12.7) | 287 (11.4) |
| Hispanic | 598 (18.3) | 424 (16.9) |
| Asian | 123 (3.8) | 92 (3.7) |
| Other | 156 (4.8) | 119 (4.7) |
| Education status reported at Visit 1: n (%) | ||
| Less than high school | 222 (6.8) | 150 (6.0) |
| Completed high school or GED | 410 (12.6) | 276 (11.0) |
| Some college | 685 (21.0) | 517 (20.6) |
| Associate or technical degree | 360 (11.0) | 281 (11.2) |
| Completed college | 911 (27.9) | 756 (30.1) |
| Degree work beyond college | 676 (20.7) | 532 (21.2) |
| Smoked during 3 months before became pregnant: n (%) | ||
| Yes | 564 (17.3) | 412 (16.4) |
| No | 2,698 (82.7) | 2,099 (83.6) |
| Chronic hypertension: n (%) | ||
| Yes | 70 (2.2) | 57 (2.3) |
| No | 3,065 (97.8) | 2,418 (97.7) |
| Hypothyroidism: n (%) | ||
| Yes | 172 (5.5) | 136 (5.6) |
| No | 2,958 (94.5) | 2,301 (94.4) |
| Family history* of diabetes: n (%) | ||
| Yes | 697 (22.4) | 546 (22.2) |
| No | 2,411 (77.6) | 1,909 (77.8) |
| Family history* of heart disease: n (%) | ||
| Yes | 260 (11.6) | 214 (12.2) |
| No | 1,972 (88.4) | 1,543 (87.8) |
| Family history* of hypertension: n (%) | ||
| Yes | 1,017 (45.7) | 807 (46.1) |
| No | 1,210 (54.3) | 945 (53.9) |
| BMI at Visit 1, in kg/m2 | ||
| Mean (standard deviation) | 26.4 (6.4) | 26.4 (6.3) |
| Category: n (%) | ||
| <25 | 1,690 (52.4) | 1,317 (53.1) |
| 25 to <30 | 805 (25.0) | 591 (23.8) |
| 30 to <35 | 380 (11.8) | 311 (12.5) |
| ≥35 | 349 (10.8) | 263 (10.6) |
| BMI at Visit 3, in kg/m2 | ||
| Mean (standard deviation) | -- | 29.2 (6.1) |
| Category: n (%) | ||
| <25 | -- | 644 (26.2) |
| 25 to <30 | -- | 961 (39.1) |
| 30 to <35 | -- | 444 (18.1) |
| ≥35 | -- | 407 (16.6) |
| Neck circumference at Visit 1, in cm | ||
| Mean (standard deviation) | 32.9 (3.0) | 32.9 (3.0) |
| Category: n (%) | ||
| <34 | 2,095 (67.0) | 1,636 (67.5) |
| 34 to 36.5 | 697 (22.3) | 535 (22.1) |
| >36.5 | 335 (10.7) | 252 (10.4) |
| Systolic blood pressure at Visit 1, in mm Hg | ||
| Mean (standard deviation) | 109.1 (10.7) | 109.0 (10.7) |
| Category: n (%) | ||
| <140 | 3,190 (99.4) | 2,460 (99.4) |
| ≥140 | 19 (0.6) | 14 (0.6) |
| Systolic blood pressure at Visit 3, in mm Hg | ||
| Mean (standard deviation) | -- | 110.7 (10.9) |
| Category: n (%) | ||
| <140 | -- | 2,465 (99.4) |
| ≥140 | -- | 16 (0.6) |
| Frequent snoring** in 4 weeks before Visit 1: n (%) | ||
| Yes | 409 (18.1) | 317 (17.7) |
| No | 1,854 (81.9) | 1,475 (82.3) |
| Frequent snoring** in 4 weeks before Visit 3: n (%) | ||
| Yes | -- | 471 (25.7) |
| No | -- | 1,361 (74.3) |
Father, mother, brother, sister, half-brother or half-sister.
Snoring ≥3 days per week
Table 2.
Statistics for Candidate Predictor Variables for Visit 1 (60 to 136 weeks’ gestation) and Visit 3 (220 to 296 weeks’ gestation): Numbers and Proportions of Participants with Sleep Disordered Breathing (SDB; AHI ≥5), Means by SDB Status, and Odds Ratios for Associations between Candidate Predictor Variables and SDB
| Candidate predictor variables | Visit 1 N = 3264 |
Visit 3 N = 2512 |
New Onset at Visit 3 N = 2258* | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Descriptive Statistic |
Odds Ratio** (95% CI) |
Descriptive Statistic |
Odds Ratio** (95% CI) |
Descriptive Statistic | Odds Ratio** (95% CI) | |
| Total: n (%) SDB | 115 (3.5) | 207 (8.2) | 132 (5.8) | |||
| Maternal age at Visit 1, in years | ||||||
| Mean (standard deviation) | ||||||
| SDB | 29.7 (5.9) | 1.7 (1.4, 2.0) | 29.8 (5.5) | 1.7 (1.5, 2.0) | 29.8 (5.1) | 1.8 (1.5, 2.1) |
| no SDB | 26.7 (5.6) | reference | 26.7 (5.3) | reference | 26.7 (5.3) | reference |
| P-value | <.0001 | <.0001 | <.0001 | |||
| Category: n (%) SDB | ||||||
| 13–21 | 11 (1.6) | 0.5 (0.2, 0.9) | 13 (2.8) | 0.3 (0.2, 0.5) | 8 (1.9) | 0.3 (0.1, 0.6) |
| 22–35 | 82 (3.5) | reference | 162 (8.6) | reference | 107 (6.3) | reference |
| >35 | 22 (10.0) | 3.1 (1.9, 5.1) | 32 (19.9) | 2.6 (1.7, 4.0) | 17 (12.6) | 2.1 (1.2, 3.7) |
| P-value | <.0001 | <.0001 | <.0001 | |||
| Maternal race: n (%) SDB | ||||||
| White Non-Hispanic | 66 (3.3) | reference | 124 (7.8) | reference | 83 (5.8) | reference |
| Black Non-Hispanic | 22 (5.3) | 1.6 (1.0, 2.6) | 32 (11.1) | 1.5 (1.0, 2.2) | 17 (7.1) | 1.2 (0.7, 2.1) |
| Hispanic | 15 (2.5) | 0.7 (0.4, 1.3) | 25 (5.9) | 0.7 (0.5, 1.2) | 17 (4.3) | 0.7 (0.4, 1.2) |
| Asian | 6 (4.9) | 1.5 (0.6, 3.5) | 14 (15.2) | 2.1 (1.2, 3.9) | 9 (10.7) | 1.9 (0.9, 4.0) |
| Other | 6 (3.8) | 1.2 (0.5, 2.7) | 12 (10.1) | 1.3 (0.7, 2.5) | 6 (5.6) | 1.0 (0.4, 2.3) |
| P-value | 0.1738 | 0.0126 | 0.2131 | |||
| Education status reported at Visit 1: n (%) SDB | ||||||
| Less than high school | 8 (3.6) | reference | 8 (5.3) | reference | 3 (2.3) | reference |
| Completed high school or GED | 18 (4.4) | 1.2 (0.5, 2.9) | 17 (6.2) | 1.2 (0.5, 2.8) | 8 (3.3) | 1.4 (0.4, 5.5) |
| Some college | 28 (4.1) | 1.1 (0.5, 2.5) | 36 (7.0) | 1.3 (0.6, 2.9) | 23 (4.9) | 2.2 (0.6, 7.4) |
| Associate or technical degree | 19 (5.3) | 1.5 (0.6, 3.5) | 39 (13.9) | 2.9 (1.3, 6.3) | 24 (9.6) | 4.5 (1.3, 15.3) |
| Completed college | 24 (2.6) | 0.7 (0.3, 1.6) | 66 (8.7) | 1.7 (0.8, 3.6) | 45 (6.6) | 3.0 (0.9, 9.8) |
| Degree work beyond college | 18 (2.7) | 0.7 (0.3, 1.7) | 41 (7.7) | 1.5 (0.7, 3.2) | 29 (6.0) | 2.7 (0.8, 9.1) |
| P-value | 0.1459 | 0.0067 | 0.0227 | |||
| Smoked during 3 months before became pregnant: n (%) SDB | ||||||
| Yes | 30 (5.3) | 1.7 (1.1, 2.6) | 48 (11.7) | 1.6 (1.1, 2.3) | 28 (8.0) | 1.5 (1.0, 2.3) |
| No | 85 (3.2) | reference | 159 (7.6) | reference | 104 (5.5) | reference |
| P-value | 0.0120 | 0.0064 | 0.0685 | |||
| Chronic hypertension: n (%) SDB | ||||||
| Yes | 9 (12.9) | 4.2 (2.0, 8.6) | 18 (31.6) | 5.5 (3.1, 9.8) | 11 (24.4) | 5.5 (2.7, 11.1) |
| No | 105 (3.4) | reference | 188 (7.8) | reference | 121 (5.6) | reference |
| P-value | 0.0001 | <.0001 | <.0001 | |||
| Hypothyroidism: n (%) SDB | ||||||
| Yes | 11 (6.4) | 1.9 (1.0, 3.7) | 22 (16.2) | 2.3 (1.4, 3.7) | 14 (11.8) | 2.3 (1.3, 4.1) |
| No | 101 (3.4) | reference | 178 (7.7) | reference | 114 (5.5) | reference |
| P-value | 0.0443 | 0.0007 | 0.0058 | |||
| Family history*** of diabetes: n (%) SDB | ||||||
| Yes | 42 (6.0) | 2.1 (1.4, 3.2) | 62 (11.4) | 1.7 (1.2, 2.3) | 36 (7.6) | 1.5 (1.0, 2.2) |
| No | 70 (2.9) | reference | 137 (7.2) | reference | 90 (5.2) | reference |
| P-value | 0.0001 | 0.0018 | 0.0480 | |||
| Family history*** of heart disease: n (%) SDB | ||||||
| Yes | 20 (7.7) | 2.3 (1.4, 3.8) | 26 (12.1) | 1.6 (1.0, 2.5) | 13 (7.3) | 1.3 (0.7, 2.3) |
| No | 69 (3.5) | reference | 124 (8.0) | reference | 81 (5.8) | reference |
| P-value | 0.0015 | 0.0452 | 0.4298 | |||
| Family history*** of hypertension: n (%) SDB | ||||||
| Yes | 53 (5.2) | 1.8 (1.2, 2.9) | 87 (10.8) | 1.7 (1.2, 2.4) | 56 (7.8) | 1.8 (1.2, 2.8) |
| No | 35 (2.9) | reference | 62 (6.6) | reference | 37 (4.4) | reference |
| P-value | 0.0058 | 0.0018 | 0.0051 | |||
| BMI at Visit 1, in kg/m2 | ||||||
| Mean (standard deviation) | ||||||
| SDB | 36.7 (8.3) | 2.9 (2.5, 3.4) | 33.4 (7.6) | 2.5 (2.2, 2.8) | 31.1 (6.2) | 2.0 (1.7, 2.2) |
| no SDB | 26.0 (6.0) | reference | 25.8 (5.8) | reference | 25.7 (5.8) | reference |
| P-value | <.0001 | <.0001 | <.0001 | |||
| Category: n (%) SDB | ||||||
| <25 | 8 (0.5) | reference | 28 (2.1) | reference | 24 (2.0) | reference |
| 25 to <30 | 12 (1.5) | 3.2 (1.3, 7.8) | 41 (6.9) | 3.4 (2.1, 5.6) | 35 (6.4) | 3.5 (2.0, 5.9) |
| 30 to <35 | 33 (8.7) | 20.0 (9.2, 43.7) | 60 (19.3) | 11.0 (6.9, 17.6) | 38 (14.6) | 8.6 (5.0, 14.6) |
| ≥35 | 60 (17.2) | 43.7 (20.7, 92.2) | 76 (28.9) | 18.7 (11.8, 29.6) | 34 (16.8) | 10.1 (5.9, 17.5) |
| P-value | <.0001 | <.0001 | <.0001 | |||
| BMI at Visit 3, in kg/m2 | ||||||
| Mean (standard deviation) | ||||||
| SDB | -- | -- | 35.7 (6.7) | 2.5 (2.2, 2.8) | 33.9 (6.0) | 2.0 (1.8, 2.3) |
| no SDB | -- | -- | 28.6 (5.7) | reference | 28.5 (5.7) | reference |
| P-value | -- | -- | <.0001 | <.0001 | ||
| Category: n (%) SDB | ||||||
| <25 | -- | -- | 8 (1.2) | reference | 7 (1.2) | reference |
| 25 to <30 | -- | -- | 37 (3.9) | 3.2 (1.5, 6.9) | 31 (3.5) | 3.1 (1.3, 7.0) |
| 30 to <35 | -- | -- | 54 (12.2) | 11.0 (5.2, 23.4) | 39 (10.0) | 9.5 (4.2, 21.5) |
| ≥35 | -- | -- | 103 (25.3) | 26.9 (13.0, 56.0) | 54 (16.5) | 16.8 (7.5, 37.4) |
| P-value | -- | -- | <.0001 | <.0001 | ||
| Neck circumference at Visit 1, in cm | ||||||
| Mean (standard deviation) | ||||||
| SDB | 36.8 (4.6) | 2.8 (2.3, 3.3) | 35.5 (3.9) | 2.3 (2.0, 2.6) | 34.6 (2.7) | 1.8 (1.6, 2.2) |
| no SDB | 32.8 (2.8) | reference | 32.7 (2.8) | reference | 32.6 (2.7) | reference |
| P-value | <.0001 | <.0001 | <.0001 | |||
| Category: n (%) SDB | ||||||
| <34 | 23 (1.1) | reference | 62 (3.8) | reference | 50 (3.3) | reference |
| 34 to 36.5 | 32 (4.6) | 4.3 (2.5, 7.5) | 73 (13.6) | 4.0 (2.8, 5.7) | 50 (10.6) | 3.5 (2.3, 5.2) |
| >36.5 | 56 (16.7) | 18.1 (11.0, 29.8) | 65 (25.8) | 8.8 (6.0, 12.9) | 27 (13.9) | 4.7 (2.9, 7.8) |
| P-value | <.0001 | <.0001 | <.0001 | |||
| Systolic blood pressure at Visit 1, in mm Hg | ||||||
| Mean (standard deviation) | ||||||
| SDB | 116.5 (13.3) | 1.9 (1.6, 2.3) | 115.0 (11.8) | 1.8 (1.6, 2.1) | 114.0 (11.3) | 1.7 (1.4, 2.0) |
| no SDB | 108.8 (10.5) | reference | 108.5 (10.4) | reference | 108.5 (10.4) | reference |
| P-value | <.0001 | <.0001 | <.0001 | |||
| Category: n (%) SDB | ||||||
| <140 | 107 (3.4) | reference | 197 (8.0) | reference | 126 (5.7) | reference |
| ≥140 | 5 (26.3) | 10.3 (3.6, 29.1) | 6 (42.9) | 8.6 (3.0, 25.1) | 4 (36.4) | 9.5 (2.7, 32.8) |
| P-value | <.0001 | <.0001 | 0.0004 | |||
| Systolic blood pressure at Visit 3, in mm Hg | ||||||
| Mean (standard deviation) | ||||||
| SDB | -- | -- | 116.6 (11.7) | 1.8 (1.5, 2.0) | 115.4 (10.8) | 1.6 (1.4, 1.9) |
| no SDB | -- | -- | 110.2 (10.6) | reference | 110.1 (10.6) | reference |
| P-value | -- | -- | <.0001 | <.0001 | ||
| Category: n (%) SDB | ||||||
| <140 | -- | -- | 201 (8.2) | reference | 131 (5.9) | reference |
| ≥140 | -- | -- | 4 (25.0) | 3.8 (1.2, 11.7) | 1 (8.3) | 1.5 (0.2, 11.3) |
| P-value | -- | -- | 0.0230 | 0.7224 | ||
| Frequent snoring**** in 4 weeks before Visit 1: n (%) SDB | ||||||
| Yes | 35 (8.6) | 3.9 (2.5, 6.2) | 64 (20.2) | 4.0 (2.8, 5.7) | 39 (14.3) | 3.6 (2.3, 5.5) |
| No | 43 (2.3) | reference | 87 (5.9) | reference | 59 (4.4) | reference |
| P-value | <.0001 | <.0001 | <.0001 | |||
| Frequent snoring**** in 4 weeks before Visit 3: n (%) SDB | ||||||
| Yes | -- | -- | 106 (22.5) | 6.5 (4.6, 9.2) | 67 (16.8) | 6.1 (4.0, 9.2) |
| No | -- | -- | 58 (4.3) | reference | 40 (3.2) | reference |
| P-value | -- | -- | <.0001 | <.0001 | ||
Participants with AHI <5 at V1.
Odds ratios for continuous predictors are computed by logistic regression for a 1-standard deviation difference.
Father, mother, brother, sister, half-brother or half-sister.
Snoring ≥3 days per week
Forward selection using logistic regression with p <0.15 to enter was used to reduce the candidate list for each outcome. Among candidate predictors, body mass index (BMI), systolic blood pressure, and frequent snoring were collected at both Visit 1 and Visit 3. Of these, only the values measured through Visit 1 were used to model SDB at Visit 1, and for Visit 3 predictions, we used only the Visit 3 measurements to avoid potential collinearity with measurements from Visit 1. Also, the latter reflected the goal of identifying measures that are readily available, acknowledging that many pregnant women initiate prenatal care after the Visit 1 time interval, and thus would not have these measurements from early pregnancy.
After forward selection, further reduction in the number of predictors was pursued to identify parsimonious and easy to apply models without appreciable loss of predictive performance; these reduced models contained age, BMI, and frequent snoring for each outcome. These models were then examined for non-linearity. Significant quadratic effects for BMI (p=0.0003, p<0.0001, p<0.0001) were found for Visit 1, Visit 3, and Visit 3 new-onset SDB, but their inclusion resulted in observations with undue leverage on the regression. Consequently, because BMI was highly skewed at both visits, we sought a Box-Cox power transformation29 to better normalize the BMIs for the reduced models. Using the maximum likelihood procedure30 in SAS PROC TRANSREG, we identified λ = −1.25 as a reasonable power parameter. We computed the power transformed BMI variables as tBMI = (BMIλ – 1) / λg, where g = (geometric mean BMI)(λ–1) on the BMI values for the models at Visit 1, Visit 3, and new-onset SBD at Visit 3. The geometric means for BMI were 25.8, 28.6, and 28.3. Quadratic terms for BMI became nonsignificant (p=0.86, 0.37, and 0.11) after power transformation. Interactions for frequent snoring with tBMI and with age were nonsignificant (all p>0.22), as was the main effect of weeks of gestation at the sleep assessment (all p>0.20).
Calibration of the final parsimonious models was assessed using the Hosmer-Lemeshow goodness-of-fit test.31 The models were then internally validated with 10-fold cross-validation to reduce model optimism.32 Data for a given outcome were randomly divided into 10 subsets (folds); for a given subset, predicted probabilities were calculated based on fitting the model to the 90% not in the subset, and this was repeated for each subset. These predicted probabilities were combined across subsets and performance measures were generated. Classification was assessed using area under the curve (AUC) for the receiver-operating-characteristic (ROC) curve, and the following test characteristics were calculated at selected specificities: predicted probability cutoff, proportion of participants exceeding the cutoff, sensitivity, positive and negative predictive values (PPV, NPV), and positive and negative likelihood ratios (LR+, LR-).
In the logistic regression models, continuous predictors were standardized to zero mean and unit standard deviation so that resulting odds ratios pertain to a one standard deviation difference in the predictor. Except as otherwise stated, all tests were performed at a nominal significance level of α = 0.05. All single degree-of-freedom tests were two-sided. No correction was made for multiple comparisons. In a given regression model, participants with missing values for one or more predictors were excluded. Analyses were conducted using SAS 9.4 (SAS Institute, Inc.). This study was approved by the institutional review board at each center, and all women provided informed written consent prior to enrollment.
Results
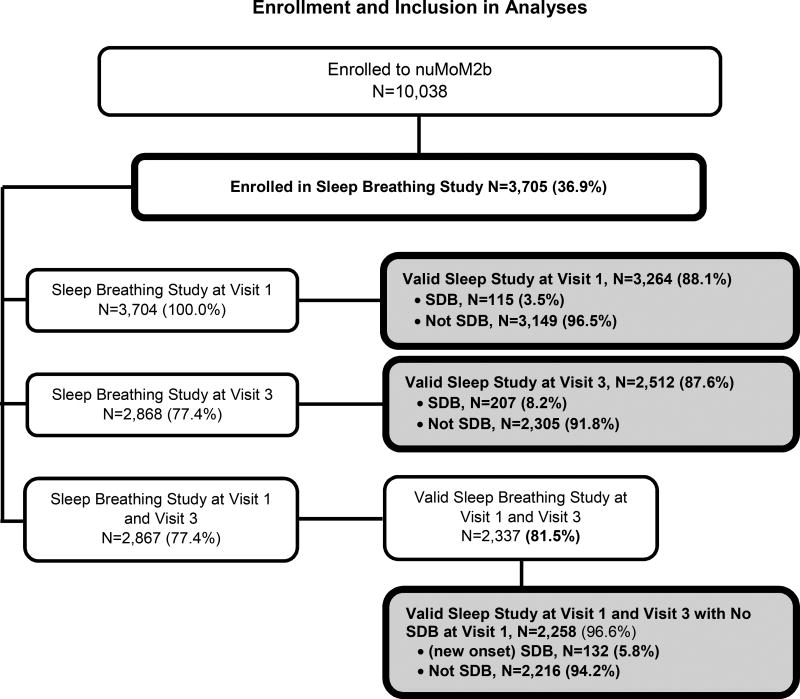
Among the 10,038 participants of the nuMom2b parent study, 3,705 women enrolled in the Sleep Disordered Breathing Substudy between March 2011 and September 2013. Enrollment data are presented in Figure 1. Among the 3,704 women who attempted a Visit 1 sleep study, 88.1% generated data of quality sufficient to be interpretable.17 The Visit 3 sleep study was attempted by 2,868 women and 96.6% met criteria for data. Among those with successful Visit 1 studies, 3.5% had SDB. At Visit 3, 8.2% had SDB and 5.2% had new-onset SDB. Among the 837 women without a Visit 3 sleep study (one participant had a Visit 3 study but no Visit 1 study), 65 had a preterm delivery (35 at <20 weeks and 30 at >20 weeks) before the sleep study could be completed, 137 missed the study visit (including the sleep assessment), and 635 had a Visit 3 but did not choose to perform the sleep study. In planning the study, it was assumed and considered in sample size estimations that 25% of women would decline the second sleep study13; thus, the participation rate in the Visit 3 sleep study was greater than expected.
Figure 1.
Flowchart showing enrollment in the Sleep Disordered Breathing Study within the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be (nuMoM2b) and inclusion in modelling for prediction of SDB at the first study visit and the third study visit, and new onset SDB at the third study visit.
Descriptive statistics for candidate predictors by study visit are presented in Table 1. Characteristics were similar between participants with sleep studies at Visit 1 and Visit 3. The prevalence of frequent snoring increased from 18.1% to 25.7% between Visit 1 and Visit 3. Single-variable associations between candidate variables and SDB outcomes are presented in Table 2. All candidate variables had significant associations with at least one of the three outcomes. (Similar tables including all the potential predictors of interest are found in Supplemental tables S1 and S2.).
Table 3 shows results of forward logistic selection. Visit 3 BMI, maternal age, and frequent snoring captured most of the predictive power for Visit 3 SDB and new-onset Visit 3 SDB, with AUCs at entry of 0.838 and 0.812. Accordingly, they were adopted provisionally as predictors for the parsimonious models. Age and BMI at Visit 1 BMI predicted Visit 1 SDB with an AUC at entry of 0.869. We added frequent snoring at Visit 1 to this provisional set for Visit 1 SDB; this did not improve the AUC but provided consistency with the Visit 3 models. In forward selection, participants were excluded if they were missing data in any of the candidate predictors. For these provisional models, the number of participants excluded for missing predictors was 40 (1.2%), 57 (2.2%), and 50 (2.2%) for the three outcomes.
Table 3.
Summary of logistic modelling of sleep disordered breathing (AHI>5) with forward selection (p<0.15 to enter) for the first study visit (V1), third study visit (V3), and new onset SDB at the third study visit (V3). Frequent snoring was defined as snoring ≥3 days per week during the 4 weeks prior to the visit.
| Step | Variable entered | P-value at entry |
AUC after inclusion in model |
P-value in final selection model |
|---|---|---|---|---|
| Sleep disordered breathing at V1*: | ||||
| 1 | BMI (kg/m2) at V1 | <0.0001 | 0.8594 | <0.0001 |
| 2 | Age (years) at V1 | <0.0001 | 0.8691 | <0.0001 |
| 3 | Education | 0.0173 | 0.8713 | 0.0343 |
| 4 | Race/ethnicity | 0.0740 | 0.8725 | 0.0928 |
| 5 | Neck circumference at V1 (cm) | 0.0862 | 0.8725 | 0.0826 |
| 6 | Family history of heart disease | 0.1071 | 0.8752 | 0.1107 |
| Sleep disordered breathing at V3**: | ||||
| 1 | BMI (kg/m2) at V3 | <0.0001 | 0.8016 | <0.0001 |
| 2 | Age (years) at V3 | <0.0001 | 0.8257 | <0.0001 |
| 3 | Frequent snoring at V3 | <0.0001 | 0.8379 | <0.0001 |
| 4 | Race/ethnicity | 0.0056 | 0.8416 | 0.0077 |
| 5 | Education | 0.0113 | 0.8474 | 0.0116 |
| 6 | History of hypothyroidism | 0.0182 | 0.8498 | 0.0169 |
| 7 | Family history of diabetes mellitus | 0.0170 | 0.8520 | 0.0222 |
| New onset sleep disordered breathing at V3***: | ||||
| 1 | BMI (kg/m2) at V3 | <0.0001 | 0.7606 | <0.0001 |
| 2 | Frequent snoring at V3 | <0.0001 | 0.7907 | <0.0001 |
| 3 | Age (years) at V3 | <0.0001 | 0.8119 | <0.0001 |
| 4 | Family history of diabetes mellitus | 0.0219 | 0.8154 | 0.0190 |
| 5 | History of hypothyroidism | 0.0166 | 0.8191 | 0.0280 |
| 6 | Education | 0.0461 | 0.8279 | 0.0520 |
Variables not selected for SDB at V1 were: smoking during 3 months before became pregnant, chronic hypertension, hypothyroidism, family history of diabetes, family history of hypertension, systolic blood pressure at V1, and frequent snoring in the 4 weeks before V1.
Variables not selected for SDB at V3 were: smoking during 3 months before became pregnant, chronic hypertension, family history of heart disease, family history of hypertension, neck circumference at V1, and systolic blood pressure at V3,
Variables not selected for new onset SDB at V3 were: race/ethnicity, smoking during 3 months before became pregnant, chronic hypertension, family history of heart disease, family history of hypertension, neck circumference at V1, and systolic blood pressure at V3,
The provisional parsimonious models were then checked as described in the methods. Each of the three final parsimonious models included (transformed) BMI, maternal age, and frequent snoring. AUCs were 0.875, 0.841, and 0.816, and lack of fit was nonsignificant (p=0.18, 0.98, and 0.39), indicating reasonable calibration (lack of bias) for the regressions.
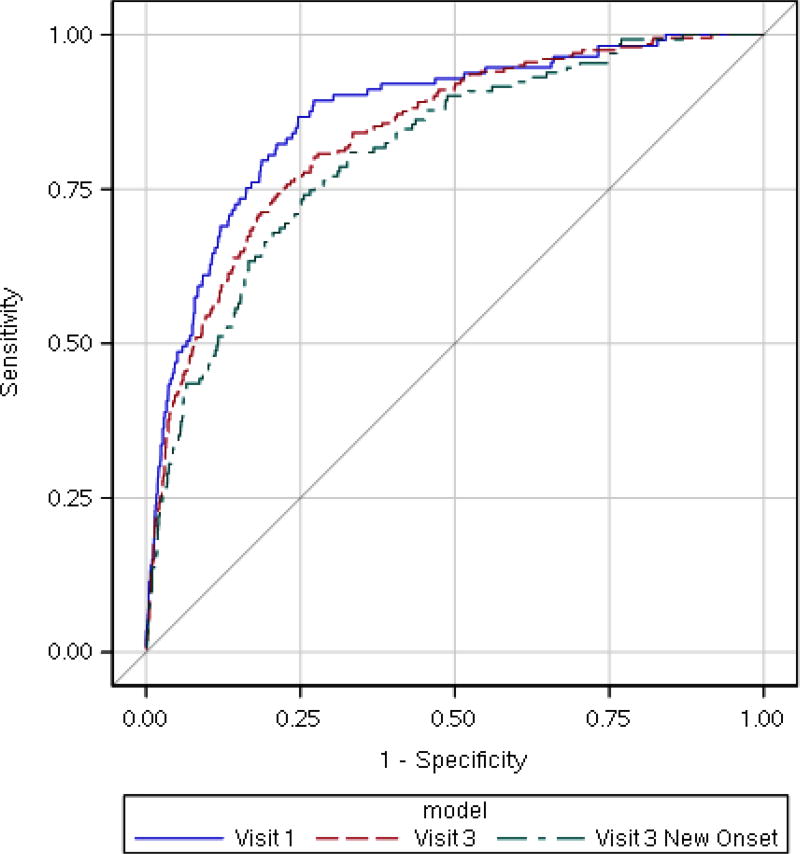
Figure 2 shows 10-fold cross-validated ROC curves for the final parsimonious models. The AUCs for Visit 1, Visit 3, and new-onset Visit 3 were 0.870, 0.838, and 0.809, which were only slightly smaller than the non-validated AUCs. Table 4 provides 10-fold cross-validated performance statistics for these models at selected specificities. Predicted probability cutoffs corresponding to 90% specificity were ≥0.082 for SDB at Visit 1, ≥0.170 at Visit 3, and ≥0.128 for new onset at Visit 3. Testing using these cutoffs, which are each about twice the overall prevalence, would involve referring 11.8%, 13.6%, and 12.1% of patients for objective testing and would yield sensitivities of 61.1%, 54.5%, and 45.8%. Testing with lower specificity would provide greater sensitivity but would involve cutoff probabilities near to or below the overall prevalence; this may be appropriate for designing a screening procedure for a clinical trial.
Figure 2.
Ten-fold cross-validated receiver operating characteristic (ROC) curves for the final parsimonious models for prediction of SDB at the first study visit (transformed BMI at V1, maternal age at V1, and frequent snoring at V1; AUC=0.870), SDB at the third study visit (transformed BMI at V3, maternal age at V3, and frequent snoring at V3; AUC=0.838), and new onset SDB at the third study visit (also transformed BMI at V3, maternal age at V3, and frequent snoring at V3; AUC=0.809). BMI was transformed using a Box-Cox power transformation with λ = −1.25 (see methods). Frequent snoring was defined as snoring ≥3 days per week during the 4 weeks prior to the visit.
Table 4.
Ten-fold cross-validated performance of final parsimonious models for prediction of SDB at the first study visit (V1), third study visit (V3), and new onset SDB at the third study visit (V3) at selected specificities. Predictor variables are transformed BMI at V1, maternal age at V1, and frequent snoring at V1 for SDB at V1; and transformed BMI at V3, maternal age at V3, and frequent snoring at V3 for both SDB and new onset SDB at V3. BMI was transformed using a Box-Cox power transformation with λ = −1.25 (see methods). Frequent snoring was defined as snoring ≥3 days per week during the 4 weeks prior to the visit.
| SDB model |
nominal specificity |
predicted probability cutoff |
percentage of participants |
Sens | Spec | PPV | NPV | LR+ | LR- |
|---|---|---|---|---|---|---|---|---|---|
| V1 | 50% | ≥0.008 | 51.5% | 92.9% | 50.0% | 6.3% | 99.5% | 1.86 | 0.14 |
| 55% | ≥0.010 | 46.6% | 92.0% | 55.0% | 6.9% | 99.5% | 2.05 | 0.14 | |
| 60% | ≥0.013 | 41.8% | 92.0% | 60.0% | 7.7% | 99.5% | 2.30 | 0.13 | |
| 65% | ≥0.017 | 36.9% | 90.3% | 65.1% | 8.6% | 99.5% | 2.58 | 0.15 | |
| 70% | ≥0.022 | 32.1% | 89.4% | 70.0% | 9.8% | 99.5% | 2.98 | 0.15 | |
| 75% | ≥0.029 | 27.1% | 86.7% | 75.0% | 11.2% | 99.4% | 3.47 | 0.18 | |
| 80% | ≥0.038 | 22.1% | 80.5% | 80.0% | 12.8% | 99.1% | 4.03 | 0.24 | |
| 85% | ≥0.055 | 17.0% | 72.6% | 85.0% | 15.0% | 98.8% | 4.84 | 0.32 | |
| 90% | ≥0.082 | 11.8% | 61.1% | 90.0% | 18.2% | 98.5% | 6.11 | 0.43 | |
| 95% | ≥0.139 | 6.5% | 46.9% | 95.0% | 25.5% | 98.0% | 9.41 | 0.56 | |
| V3 | 50% | ≥0.031 | 53.4 % | 91. 6% | 50. 0% | 14. 1% | 98. 5% | 1.8 3 | 0.1 7 |
| 55% | ≥0.037 | 48.6% | 89.1% | 55.0% | 15.1% | 98.3% | 1.98 | 0.20 | |
| 60% | ≥0.045 | 43.8% | 86.1% | 60.0% | 16.2% | 98.0% | 2.15 | 0.23 | |
| 65% | ≥0.053 | 39.0% | 84.2% | 65.0% | 17.7% | 97.9% | 2.41 | 0.24 | |
| 70% | ≥0.065 | 34.1% | 80.7% | 70.0% | 19.5% | 97.6% | 2.69 | 0.28 | |
| 75% | ≥0.080 | 29.2% | 76.7% | 75.0% | 21.6% | 97.3% | 3.07 | 0.31 | |
| 80% | ≥0.101 | 24.2% | 71.3% | 80.0% | 24.2% | 96.9% | 3.57 | 0.36 | |
| 85% | ≥0.126 | 19.0% | 63.9% | 85.0% | 27.7% | 96.3% | 4.27 | 0.42 | |
| 90% | ≥0.170 | 13.6% | 54.5% | 90.0% | 32.8% | 95.7% | 5.45 | 0.51 | |
| 95% | ≥0.258 | 8.0% | 41.6% | 95.0% | 42.9% | 94.8% | 8.37 | 0.61 | |
| new onset V3 | 50% | ≥0.027 | 52.4 % | 90. 1% | 50. 0% | 10. 2% | 98. 8% | 1.8 0 | 0.2 0 |
| 55% | ≥0.031 | 47.4% | 86.3% | 55.0% | 10.8% | 98.4% | 1.92 | 0.25 | |
| 60% | ≥0.036 | 42.5% | 83.2% | 60.0% | 11.6% | 98.3% | 2.08 | 0.28 | |
| 65% | ≥0.042 | 37.7% | 80.9% | 65.0% | 12.7% | 98.2% | 2.31 | 0.29 | |
| 70% | ≥0.050 | 32.8% | 77.1% | 70.0% | 14.0% | 98.0% | 2.57 | 0.33 | |
| 75% | ≥0.061 | 27.8% | 72.5% | 75.0% | 15.5% | 97.7% | 2.90 | 0.37 | |
| 80% | ≥0.075 | 22.7% | 66.4% | 80.0% | 17.3% | 97.4% | 3.32 | 0.42 | |
| 85% | ≥0.094 | 17.4% | 56.5% | 85.0% | 19.2% | 96.9% | 3.77 | 0.51 | |
| 90% | ≥0.128 | 12.1% | 45.8% | 90.0% | 22.5% | 96.3% | 4.60 | 0.60 | |
| 95% | ≥0.187 | 6.7% | 33.6% | 95.0% | 29.9% | 95.8% | 6.77 | 0.70 |
Sens = sensitivity, Spec = specificity, PPV = positive, predictive value, NPV = negative predictive value, LR+ = positive likelihood ratio, LR− = negative likelihood ratio.
Integrated predictiveness and classification curves, along with orientation and illustrative examples, are found in the Supplement (Figures S1, S2, and S3). These show the relationships between predicted probability cutoff, proportion of participants at or above the cutoff, and the cross-validated true positive fraction (sensitivity) and false positive fraction (1 minus specificity).31 Also in the Supplement are expanded versions of Tables 1 and 2 including all the potential predictors of interest and an Excel predicted probability calculator based on the final models, which were internally but not externally validated.
Discussion
In the clinical setting, it is important to have an inexpensive, easy-to-use and rapid tool that can be used to identify women at risk for SDB. We developed predictive models using only three variables – current maternal age, BMI, and frequent snoring – to predict objectively measured SDB in early and mid-pregnancy, including new-onset SDB in mid-pregnancy. These predictors are easily obtainable and assessment of frequent snoring involves the simple questions, “In the last 4 weeks, have you snored?” and if so, “Have you snored 3 or more times a week?”
In nuMoM2b, overnight home sleep apnea testing provided objective assessments of SDB on 3,264 nulliparous pregnant women at 6–156 weeks of gestation (within 2 weeks following nuMoM2b study Visit 1) and 2,512 at 22–316 weeks (within 2 weeks following Visit 3).
This study is the largest to date to prospectively evaluate the risk factors for objectively measured SDB during pregnancy. As others have reported, the Berlin and Epworth Sleepiness Scales, tools, which have been developed and used in the adult nonpregnant population, have low sensitivity and specificity, and are poorly predictive of SDB in pregnant women.27,33,34 Our model presents a simplified option for identifying these women.
Our findings are similar to those of Facco et al.27 Using a cohort of 100 women in early pregnancy, Facco et al. devised a scoring system which used four variables to predict SDB: chronic hypertension, age, BMI, and self- reported frequent snoring.27 In that group, the weighted model predicted objectively-measured OSA with a sensitivity of 86% (95% CI: 66– 95%) and a specificity of 74% (62–83%). Our study has the added benefit of examining mid-pregnancy as well as early pregnancy. One of the oft cited limitations of existing cross sectional studies is the inability to differentiate between pre-existing SDB detected during pregnancy and new-onset SDB in pregnancy.27,33 Our large sample size and longitudinal study design allowed us the opportunity to address the occurrence and predictors of new-onset SDB in mid-pregnancy. It is also notable that BMI and not pregnancy weight gain was predictive of SDB and new onset SDB. While other studies were not powered to evaluated pregnancy weight gain, their findings stressed the importance of BMI as a predictor.3,4,27
Pien et al. also found an increased prevalence of SDB over the course of pregnancy in a group of women who underwent in-laboratory polysomnography, and concluded that age and BMI were the predominant risk factors for SDB regardless of the trimester of pregnancy.3 Our findings are also consistent with observations in the general population, in which the predictors of age, BMI, and frequent snoring are most predictive of SDB.35
As in other reports, excessive daytime sleepiness, a traditionally reported symptom of SDB was not predictive of SDB in this population. The sole such variable which was associated with SDB was the self-reported “nodding off while driving” but reported numbers were small, and the associations did not meet criteria for inclusion into the candidate list. Our findings are consistent with other reports2,27,33 and highlight the difficulty of identifying SDB.36 One exception was self-reported frequent snoring. While most cases of SDB in early pregnancy were predicted by age and BMI, later in pregnancy, the presence of self-reported frequent snoring was associated with the ascertainment of more cases of SDB. The prevalence of self-reported snoring increases over the course of pregnancy, as does the prevalence of SDB, 1,33,37 as we also found in our study. Our results agree with the findings of Shah and colleagues who specifically examined the value of considering symptoms such as fatigue and insomnia as predictor of SDB in women, and we also found that a simpler equation, including age, BMI and snoring frequency provided the best prediction equation.35
The strengths of this study are the prospective design, longitudinal assessment of exposures and outcomes in both early and mid-pregnancy, and the sample size. The use of a central sleep reading center allowed for uniform interpretation of sleep data. However, when interpreting our data, a few limitations should be considered. This was a cohort of mostly young healthy women. As such, the prevalence of SDB may have been lower than in a community-based sample. Given this limitation, our model may underestimate the probability of SDB in a cohort with more multiparous and older women. While the gold standard for diagnosis of SDB is overnight polysomnography,38 the cost and inconvenience of laboratory overnight polysomnography precluded its use in such a large study. Home sleep apnea testing is an acceptable alternative for diagnosis in select populations and has been used increasingly in the general population.38 However, because total sleep time is usually overestimated during home sleep testing and arousals precipitated by disordered breathing events are not detected (since EEG signals are not usually recorded), AHI may be underestimated. In our study we used a cutoff of AHI ≥5 as abnormal and participants underwent a single night of home sleep testing. One night of sleep may not be truly representative as there has been reported night to night variation in the severity of sleep apnea.39 Furthermore, the AHI may not identify individuals with airflow limitation, or upper airway resistance syndrome, who despite a low AHI may experience symptoms and adverse health outcomes.
In conclusion, our results demonstrate that SDB among pregnant women can be predicted in early pregnancy and mid-pregnancy (prevalent and incident SDB) by simple models including current age, BMI, and self-reported frequent snoring. Our models performed well, with AUCs above 0.8. Our findings are important for the practicing clinician who seeks to identify pregnant women at high risk for SDB. Our findings can also help inform the screening of women for future studies of SDB in pregnancy.
Supplementary Material
Acknowledgments
We would like to acknowledge Aaron Laposky, PhD of the National Heart, Lung, and Blood Institute (NHLBI) for his contributions to this manuscript, and Georgiy Bobashev, PhD of RTI International for consultation on the prediction analyses.
Supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart Lung and Blood Institute: U10 HD063036, Research Triangle Institute; U10 HD063072, Case Western Reserve University; U10 HD063047, Columbia University; U10 HD063037, Indiana University; U10 HD063041, Magee-Women’s Hospital; U10 HD063020, Northwestern University; U10 HD063046, University of California– Irvine; U10 HD063048, University of Pennsylvania; and U10 HD063053, University of Utah.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Comments and views of the authors do not necessarily represent views of the NIH.
Disclosures: None of the authors have financial disclosures
References
- 1.Antony KM, Agrawal A, Arndt ME, et al. Obstructive sleep apnea in pregnancy: reliability of prevalence and prediction estimates. Journal of Perinatology. 2014;34(8):587–593. doi: 10.1038/jp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockhart EM, Ben Abdallah A, Tuuli MG, Leighton BL. Obstructive Sleep Apnea in Pregnancy: Assessment of Current Screening Tools. Obstetrics & Gynecology. 2015;126(1):93–102. doi: 10.1097/AOG.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 3.Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69(4):371–377. doi: 10.1136/thoraxjnl-2012-202718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–1092. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facco FL, Parker CB, Reddy UM, et al. Association Between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obstet Gynecol. 2017;129(1):31–41. doi: 10.1097/AOG.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y-H, Kang J-H, Lin C-C, Wang I-T, Keller JJ, Lin H-C. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. American journal of obstetrics and gynecology. 2012;206(2):136. doi: 10.1016/j.ajog.2011.09.006. e131–136. e135. [DOI] [PubMed] [Google Scholar]
- 7.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep. 2014;37(5):843. doi: 10.5665/sleep.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung AM, Wilson DL, Lappas M, et al. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One. 2013;8(7):e68057. doi: 10.1371/journal.pone.0068057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pamidi S, Marc I, Simoneau G, et al. Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax. 2016;71(8):719–725. doi: 10.1136/thoraxjnl-2015-208038. [DOI] [PubMed] [Google Scholar]
- 10.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136. doi: 10.1016/j.ajog.2011.09.006. e131–135. [DOI] [PubMed] [Google Scholar]
- 11.Weaver EM, Kapur V, Yueh B. Polysomnography vs self-reported measures in patients with sleep apnea. Archives of Otolaryngology-Head & Neck Surgery. 2004;130(4):453–458. doi: 10.1001/archotol.130.4.453. [DOI] [PubMed] [Google Scholar]
- 12.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: clinical features. Sleep. 2002;25(4):409–416. [PubMed] [Google Scholar]
- 13.Tantrakul V, Numthavaj P, Guilleminault C, et al. Performance of screening questionnaires for obstructive sleep apnea during pregnancy: A systematic review and meta-analysis. Sleep Medicine Reviews. 36:96–106. doi: 10.1016/j.smrv.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Sleep Apnea Task Force of the American Academy of Sleep M. Clinical Guideline for the Evaluation, Management and Long-term Care of Obstructive Sleep Apnea in Adults. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson DL, Walker SP, Fung AM, O’Donoghue F, Barnes M, Howard M. Can we predict sleep-disordered breathing in pregnancy? The clinical utility of symptoms. Journal of Sleep Research. 2013;22(6):670–678. doi: 10.1111/jsr.12063. [DOI] [PubMed] [Google Scholar]
- 16.Haas DM, Parker CB, Wing DA, et al. A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b) Am J Obstet Gynecol. 2015;212(4):539. doi: 10.1016/j.ajog.2015.01.019. e531–539 e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facco FL, Parker CB, Reddy UM, et al. NuMoM2b Sleep-Disordered Breathing study: objectives and methods. Am J Obstet Gynecol. 2015;212(4):542. doi: 10.1016/j.ajog.2015.01.021. e541–542 e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of internal medicine. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 21.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep medicine. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 22.Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosomatic medicine. 2005;67(1):98–104. doi: 10.1097/01.psy.0000151743.58067.f0. [DOI] [PubMed] [Google Scholar]
- 23.Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):123–136. doi: 10.1037/1040-3590.15.2.123. [DOI] [PubMed] [Google Scholar]
- 24.Zhao YY, Weng J, Mobley DR, et al. Effect of Manual Editing of Total Recording Time: Implications for Home Sleep Apnea Testing. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2017;13(1):121–126. doi: 10.5664/jcsm.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus C, Vaughn B. Rules, Terminology and Technical Specifications. Darien, Illinois: American Academy of Sleep Medicine; 2012. The AASM manual for the scoring of sleep and associated events. [Google Scholar]
- 26.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 27.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8(4):389–394. doi: 10.5664/jcsm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosmer DW., Jr . Applied logistic regression. Vol John Wiley & Sons; 2000. [Google Scholar]
- 30.Bradley AP. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern recognition. 1997;30(7):1145–1159. [Google Scholar]
- 31.Box GE, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society Series B (Methodological) 1964:211–252. [Google Scholar]
- 32.Draper NR, Smith H. Applied regression analysis. John Wiley & Sons; 2014. [Google Scholar]
- 33.Tantrakul V, Sirijanchune P, Panburana P, et al. Screening of obstructive sleep apnea during pregnancy: differences in predictive values of questionnaires across trimesters. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2015;11(2):157. doi: 10.5664/jcsm.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivarez SA, Maheshwari B, McCarthy M, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol. 2010;202(6):552. doi: 10.1016/j.ajog.2009.12.008. e551–557. [DOI] [PubMed] [Google Scholar]
- 35.Shah N, Hanna DB, Teng Y, et al. Sex-Specific Prediction Models for Sleep Apnea From the Hispanic Community Health Study/Study of Latinos. Chest. 2016;149(6):1409–1418. doi: 10.1016/j.chest.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mindell JA, Cook RA, Nikolovski J. Sleep patterns and sleep disturbances across pregnancy. Sleep medicine. 2015;16(4):483–488. doi: 10.1016/j.sleep.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487. doi: 10.1016/j.ajog.2012.08.034. e481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White LH, Lyons OD, Yadollahi A, Ryan CM, Bradley TD. Night-to-night Variability in Obstructive Sleep Apnea Severity: Relationship to Overnight Rostral Fluid Shift. J Clin Sleep Med. 11(2):149–156. doi: 10.5664/jcsm.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.