Supplemental Digital Content is available in the text.
Keywords: brain ischemia, extracellular vesicles, magnetic resonance imaging, stroke, white matter
Abstract
Background and Purpose—
Recent work from our group suggests that human neural stem cell–derived extracellular vesicle (NSC EV) treatment improves both tissue and sensorimotor function in a preclinical thromboembolic mouse model of stroke. In this study, NSC EVs were evaluated in a pig ischemic stroke model, where clinically relevant end points were used to assess recovery in a more translational large animal model.
Methods—
Ischemic stroke was induced by permanent middle cerebral artery occlusion (MCAO), and either NSC EV or PBS treatment was administered intravenously at 2, 14, and 24 hours post-MCAO. NSC EV effects on tissue level recovery were evaluated via magnetic resonance imaging at 1 and 84 days post-MCAO. Effects on functional recovery were also assessed through longitudinal behavior and gait analysis testing.
Results—
NSC EV treatment was neuroprotective and led to significant improvements at the tissue and functional levels in stroked pigs. NSC EV treatment eliminated intracranial hemorrhage in ischemic lesions in NSC EV pigs (0 of 7) versus control pigs (7 of 8). NSC EV–treated pigs exhibited a significant decrease in cerebral lesion volume and decreased brain swelling relative to control pigs 1-day post-MCAO. NSC EVs significantly reduced edema in treated pigs relative to control pigs, as assessed by improved diffusivity through apparent diffusion coefficient maps. NSC EVs preserved white matter integrity with increased corpus callosum fractional anisotropy values 84 days post-MCAO. Behavior and mobility improvements paralleled structural changes as NSC EV–treated pigs exhibited improved outcomes, including increased exploratory behavior and faster restoration of spatiotemporal gait parameters.
Conclusions—
This study demonstrated for the first time that in a large animal model novel NSC EVs significantly improved neural tissue preservation and functional levels post-MCAO, suggesting NSC EVs may be a paradigm changing stroke therapeutic.
Food and Drug Administration approved therapies for stroke (tissue-type plasminogen activator and endovascular thrombectomy) are currently only available to a small subpopulation of stroke victims.1,2 After a litany of failed treatments, assessment by the Stem Cell Emerging Paradigm in Stroke consortium meetings identified major needs, including (1) a regenerative therapy, and (2) testing in translational animal models more reflective of human pathology.3,4 Similarly, the Stroke Therapy Academic Industry Roundtable encouraged (1) testing in higher-order gyrencephalic species, (2) evaluating clinically relevant routes of administration, and (3) longitudinal behavior assessment.5,6 These recommendations prompted our therapeutic evaluation of intravenously administered human neural stem cell extracellular vesicles (NSC EVs) in a translational pig ischemic stroke model.
One of the most promising emerging therapeutics capable of addressing the need for a neuroprotective and regenerative therapy are extracellular vesicles (EVs) sourced from stem cells cultures.7 EVs are heterogeneous populations of both 50 to 1000 nm plasma membrane–shed microvesicles, and 40 to 150 nm exosomes derived from the endocytic pathway. These EVs are enriched in transmembrane proteins, bioactive lipids, and microRNAs and are produced by virtually all cell types.8,9 Recently, the therapeutic potential of these cell signaling vesicles has been explored from several cell sources and for varied applications.10 The vast majority of previously reported neural injury studies evaluating stem cell–derived EVs have used mesenchymal stem cell (MSC)–derived EVs.11–13 However, in vivo biodistribution of EVs is highly dependent on cell source, suggesting EVs will display specific biodistribution patterns in vivo reflecting their parent cell line.14 We compared the neuroprotective and regenerative properties of NSC EVs versus isogenically derived MSC EVs in a mouse thromboembolic stroke model. MSC EV treatments trended toward decreasing stroke lesion volume whereas NSC EVs significantly decreased lesion size, preserved motor function, and improved episodic memory.15 These findings collectively warrant further rigorous testing of NSC EVs in a secondary pig ischemic stroke model.
Following the Stem Cell Emerging Paradigm in Stroke and Stroke Therapy Academic Industry Roundtable committees’ recommendations, NSC EV therapeutic benefits should be extensively tested using clinically relevant routes of administration, treatment regimen, and end points in a large animal model of ischemic stroke. The porcine permanent middle cerebral artery occlusion (MCAO) model possesses several advantages, including brain anatomy and physiology comparable to humans.16–18 Both human and porcine brains are gyrencephalic and are composed of >60% white matter (WM) while rodent brains are lissencephalic and are composed of <10% WM.19–22 These similar attributes in cytoarchitecture are critically important as WM is highly vulnerable to pathological processes that follow ischemic stroke.22 Because pigs are of similar body size to humans and their brains are only 7.5× smaller than human brains, compared with the 650× smaller rodent brain, pigs are a more direct assessment of dosing in a preclinical model.18 These similarities in brain composition, cytoarchitecture, and size collectively support the use of a pig ischemic stroke model to better predict outcomes between preclinical rodent models and human clinical trials.
The objectives of this study were to evaluate the therapeutic potential of NSC EVs through magnetic resonance imaging (MRI) at 1 and 84 days post-MCAO and to longitudinally assess changes in motor function via gait analysis and open field testing. In this study, we present for the first time, evidence NSC EVs promote extensive tissue and functional level recovery in a large animal preclinical stroke model.
Materials and Methods
Data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design
The overarching aim of these studies were to evaluate NSC EV efficacy as a potential acute stroke therapy in a preclinical, biologically relevant porcine MCAO model of ischemic stroke. End points were selected to evaluate tissue and functional level changes in response to treatment. We used a split plot experimental design, where all treatment groups were conducted within 1 day to control for and reduce experimental variation. The sample size for this study was determined by a power calculation based on our previously published work using the pig MCAO model with lesion volume changes by MRI being the primary end point.23 The power analysis was calculated using a 2-tailed ANOVA test, α=0.05, and an 80% power of detection, effect size of 1.19, and a SD of 44.63. Initially, 14 pigs were randomly assigned to the treated and control groups. However, because of high mortality rates within the control group, 2 additional pigs were added to the control group for a total 9 pigs in the control group and 7 pigs in the treated group (physiological data and mortality information included in Tables I and II in the online-only Data Supplement, respectively). Although a greater percentage of NSC EV pigs survived relative to control pigs, there were no statistically significant survival rate differences between treatment groups (Figure I in the online-only Data Supplement). Ischemic stroke was induced by a blinded surgeon, and EVs were delivered as single use aliquots by investigators. To control for potential day effects, 1 treated and 1 control pig were assigned to each surgical day except for 1 surgical day in which the 2 additional control pig surgeries were performed. Because of the timing of the first treatment, it was not possible to show proof of identical lesion sizes before NSC EV administration or account for progression rate of lesions. However, a 1-way ANOVA and post hoc Tukey–Kramer pair-wise test comparing the lesion volumes of pigs within each treatment group between the first and second half of the study resulted in no significant difference (treated P=0.9994, nontreated P=0.7804). This consistency in lesion volumes suggests that there was no significant difference in time-dependent variables, including the effect of surgical procedures during the course of the study. All end points and functional measurements were prospectively planned and underwent unblinded analysis. Predefined exclusion criteria from all end points included instances of infection in the injury site, self-inflicted injuries that required euthanasia, inability to thermoregulate, uncontrolled seizure activity, and respiratory distress. One control pig was excluded from MRI collection because of postoperative complications and premature death (Table II in the online-only Data Supplement). Data collection from 1 treated pig was retrospectively excluded from all assessments because of a Trueperella (Arcanobacterium) pyogenes abscess and was determined to be the result of the surgery by pathologists and veterinarians. No outliers were removed from the data.
Results
NSC EV Manufacture Consistently Produced Biologically Active and Reproducible Vesicles
EVs were harvested from NSC basal culture medium according to standard production protocol and with reproducible size profile with >90% of EVs under 200 nm in diameter as determined by Nanosight (methods in the online-only Data Supplement).15 To determine cellular uptake of NSC EVs, a critical component of EV function, uptake of DiI-labeled NSC EVs was evaluated using an interferometric technique known as spatial light interference microscopy.24 Time lapse imaging (18-hour time point shown) indicated NSC EVs were taken up by cells and were visualized while being transported within the cell (Figure 1A–1C; Movie I in the online-only Data Supplement). NSC EVs may ultimately exert their efficacy through uptake by various cell types when in circulation. NSC EVs were analyzed using a commercially available MACSPlex exosome kit and displayed a consistent EV marker profile (Figure 1D). Along with the recently published physical size evaluation, these data supported a consistent profile and bioactivity of NSC EVs derived from separate purifications.15
Figure 1.
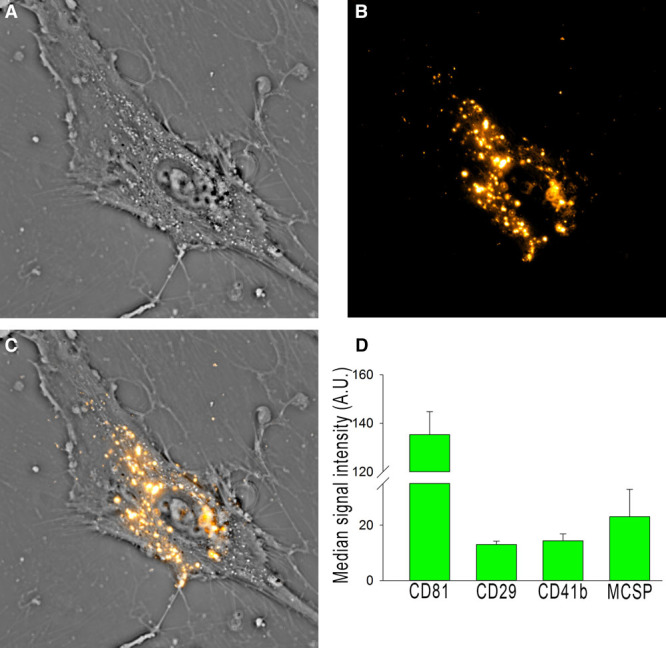
Neural stem cell–derived extracellular vesicle (NSC EV) manufacture produces biologically active, consistent vesicles. DiI-labeled vesicles (B) were added into the culture medium of human umbilical mesenchymal stem cells (A) and imaged for 24 hours (A–C). Vesicles are taken up by the cells (C) and can be seen being actively transported within the cell. Flow cytometry is routinely used for batch analysis of NSC EVs (using the commercially available MACSPlex kit) and indicates NSC EVs have a consistent marker profile (D).
NSC EVs Decreased Lesion Volume and Mitigated Cerebral Swelling 1-Day Post-MCAO
To confirm ischemic stroke 1-day post-MCAO, MRI T2-weighted fluid-attenuated inversion recovery and diffusion-weighted imaging sequences were assessed and exhibited territorial hyperintense lesions characteristic of an edematous injury (Figure 2A, white arrows). Hypointense lesions observed on corresponding apparent diffusion coefficient (ADC) maps confirmed areas of restricted diffusion indicative of cytotoxic edema (Figure 2A, white arrows), thus confirming permanent cauterization of the middle cerebral artery resulted in ischemic stroke. T2-weighted sequences at 1-day post-MCAO revealed characteristic hyperintense lesions indicative of acute ischemic stroke (Figure 2B). To account for the space-occupying effect of brain edema, edema-corrected lesion volume was calculated using T2-weighted and corresponding ADC maps revealing a significant (P<0.05) decrease in edema-corrected lesion volume in NSC EV–treated pigs when compared with controls (6.0±1.4 versus 10.7±1.4 cm3, respectively, Figure 2C). T2-weighted– based results also indicated significantly (P≤0.01) decreased swelling of the affected ipsilateral hemisphere resulting in a less pronounced midline shift in NSC EV–treated pigs relative to control pigs 1-day post-MCAO (113.7%±2.6% versus 126.8%±3.4%, respectively; Figure 2B and 2D). Despite these acute changes, there were no significant differences in lesion volume or brain atrophy between treatment groups 84 days post-MCAO (Figure III in the online-only Data Supplement). The occurrence of intracranial hemorrhage (ICH) was also substantially reduced in NSC EV–treated pigs relative to controls (0 of 7 and 7 of 8 pigs, respectively, Figure 2B, white arrows; Figure II in the online-only Data Supplement).
Figure 2.
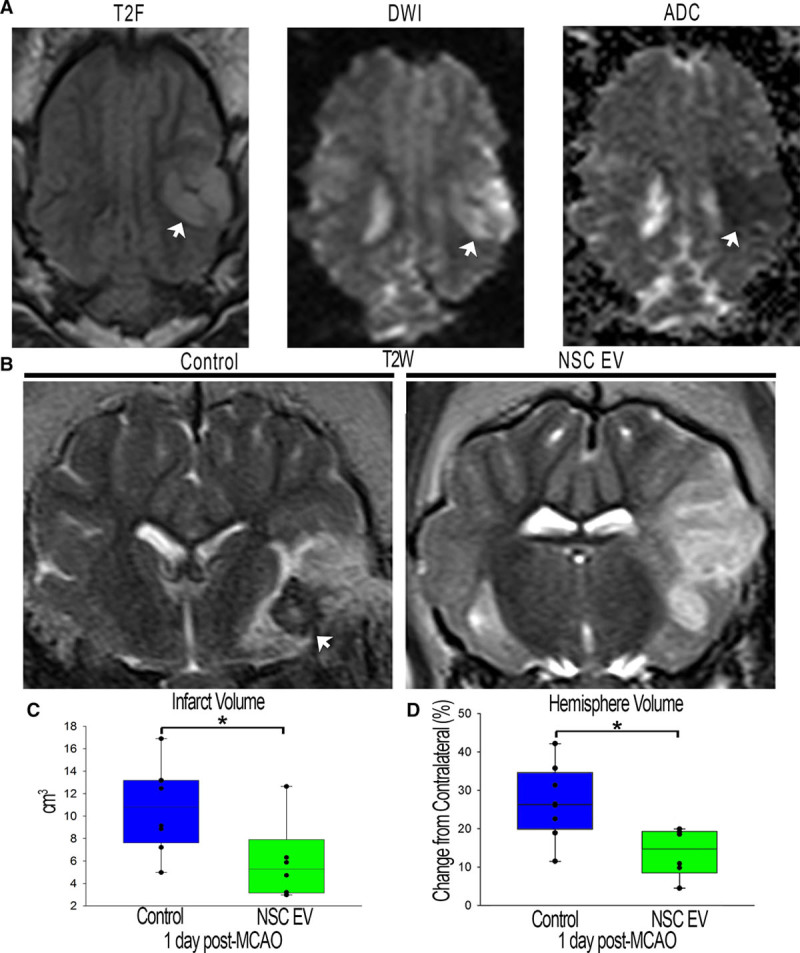
Neural stem cell–derived extracellular vesicle (NSC EV) treatment decreases intracranial hemorrhage, lesion volume, and hemispheric swelling 1-day post-middle cerebral artery occlusion (MCAO). T2-weighted (T2W) and diffusion-weighted imaging (DWI) sequences revealed territorial hyperintense lesions characteristic of an edematous injury (A, white arrows). Hypointense lesions observed on corresponding apparent diffusion coefficient (ADC) maps confirmed areas of restricted diffusion indicative of cytotoxic edema (A, white arrow). These resulting hallmarks demonstrated permanent cauterization of the ventral aspect of the middle cerebral artery resulted in bona fide, repeatable ischemic stroke in all pigs. NSC EV–treated pigs exhibited a reduced incidence of intracranial hemorrhage (B, white arrows). NSC EV–treated pigs also demonstrated a significant (P<0.05) decrease in edema-corrected lesion volume when compared with control pigs at 1-day post-MCAO (6.0±1.4 vs 10.7±1.4 cm3, respectively; C) and a significantly (P<0.01) lower percent increase in hemisphere volume resulting in a less pronounced midline shift relative to control pigs at 1-day post-MCAO (113.77%±2.571% vs 126.83%±3.41% respectively; D). *Significant difference between treatment groups.
NSC EVs Promoted Increased Diffusivity and WM Integrity 1 and 84 Days Post-MCAO
Cerebral diffusivity was evaluated using diffusion-weighted imaging sequences and derived ADC maps. Signal void, consistent with restricted diffusion and indicative of cytotoxic edema, was quantified (Figure 3A, white arrows). Mean ADC values in the affected ipsilateral hemisphere were compared with the contralateral hemisphere with calculated percent changes closer to zero being more similar to normal tissue. NSC EV–treated pigs exhibited a significantly (P<0.01) reduced percent change in ADC values when compared with control pigs 1-day post-MCAO (−18.7%±2.6% versus −32.3%±1.5%, respectively; Figure 3C). To assess long-term changes in WM integrity, the corpus callosum was examined 84 days post-MCAO. Changes in fractional anisotropy in the affected ipsilateral hemisphere were again compared with the contralateral hemisphere. Fractional anisotropy maps depicted a decrease in the corpus callosum of the ipsilateral hemisphere of control pigs 84 days post-MCAO (Figure 3B, white arrow) while NSC EV–treated pigs exhibited a significantly (P<0.01) lower percent decrease in fractional anisotropy values (−13.9%±3.2% versus −43.3%±6.7%, respectively; Figure 3D). Collectively, MRI results offered compelling evidence NSC EV treatment provided neuroprotection and promoted tissue level recovery by decreasing cerebral lesion volume, swelling, incidence of ICH, and preserving diffusivity and WM integrity.
Figure 3.
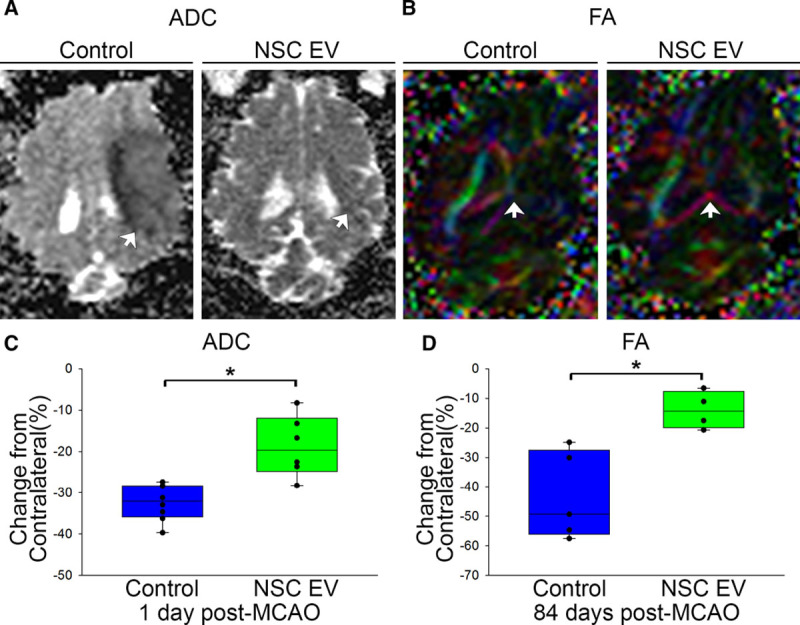
Neural stem cell–derived extracellular vesicle (NSC EV) treatment promotes increased diffusivity of ischemic lesions and preserved white matter integrity of the corpus callosum. Apparent diffusion coefficient (ADC) maps derived from diffusion-weighted imaging (DWI) sequences revealed signal void indicative of restricted diffusion and cytotoxic edema. NSC EV–treated pigs exhibited a significantly (P<0.01) lower percent decrease in ADC values relative to control pigs 1-day post-MCAO (−18.72%±2.55% vs −32.35%±1.54%, respectively; A, C, white arrows), suggesting improved diffusivity in ischemic lesions. Color-coded fractional anisotropy (FA) maps depicted territorial changes in the corpus callosum 84-days post-MCAO. NSC EV–treated pigs exhibited a significantly (P<0.01) lower percent decrease in FA values (−13.94%±3.18% vs −43.29%±6.65%, respectively; B, D, white arrows) when compared with control pigs, suggesting preserved white matter integrity. *Significant difference between treatment groups.
NSC EVs Resulted in Increased Motor Activity and Exploratory Behavior
Exploratory behavior and motor activity pre- and post-MCAO were assessed by open field testing. NSC EV–treated pigs did not significantly decrease their distance traveled while control pigs were less active, compared with pre-MCAO time points (113.6±12.0 versus 42.0±12.7 m; P<0.01; Figure 4A). Interestingly, longitudinal analysis at 84 days post-MCAO revealed NSC EV–treated pigs exhibited a significant increase in distance traveled compared with their pre-MCAO time points (107.3±9.9 versus 217.0±29.6 m; P<0.05); however, this trend was not observed in control pigs. Together, these findings suggest NSC EVs preserved normal exploratory behaviors and motor activity post-MCAO.
Figure 4.
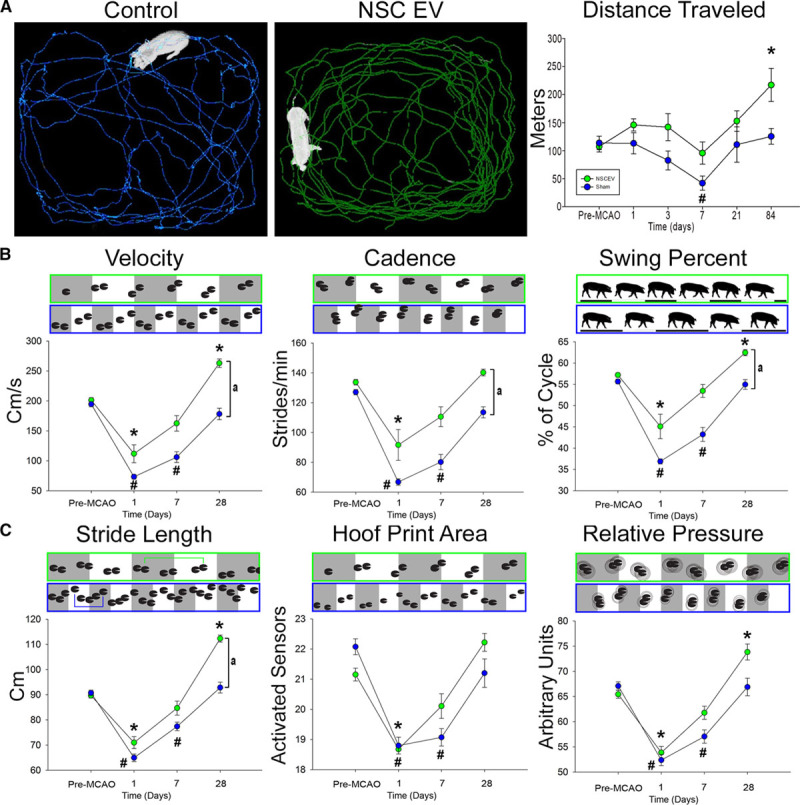
Neural stem cell–derived extracellular vesicle (NSC EV) treatment results in increased motor activity and improved recovery of spatiotemporal gait parameters. Ethovision XT tracking software was used during open-field testing to automatically assess differences in distance traveled between treatment groups; representative 10-min movement tracings shown for control (A, blue) and NSC EV treated (A, green) pigs. Control pigs experienced a significant decrease in distance traveled at 7-days post-middle cerebral artery occlusion (MCAO) while treated pigs did not. Both groups increased distance traveled over 28 days; however, treated pigs traveled significantly further than their pre-MCAO distance while control pigs did not. At 1-day post-MCAO, NSC EV–treated and control pigs exhibited significant decreases in temporal gait parameters, including velocity, cadence, and swing percent pigs (B). By 7-days post-MCAO, NSC EV–treated pigs recovered these parameters while control pigs did not recover until 28 days. At 28 days, the NSC EV–treated pigs performed significantly better in velocity, cadence, and swing percent than control pigs. Differences in spatial gait parameters were also noted between NSC EV–treated and control pigs in terms of stride length, hoof print area, and relative pressure (C). By 7-days post-MCAO, NSC EV–treated pigs had recovered from deficits in stride length, hoof print area, and relative pressure, whereas control pigs remained impaired. In addition, NSC EV–treated pigs performed significantly better in terms of stride length when compared with control pigs at the same time point. *, #Significant (P<0.01) difference between pre- and post-MCAO time points. a indicates significant (P<0.01) difference between treatment groups.
NSC EV Treatment Led to Faster and Improved Recovery of Spatiotemporal Gait Parameters
In addition to exploratory activity, there were several key differences in measured temporal gait parameters between treatment groups. Velocity (distance traveled/second), cadence (strides/min), and swing percent of cycle (percentage of 1 full gait cycle in which the contralateral hindlimb was in the noncontact phase) significantly decreased 1-day post-MCAO for both NSC EV–treated and control pigs. However, by 7 days post-MCAO, NSC EV–treated pigs recovered when compared with pre-MCAO performance (Figure 4B). In contrast, control pigs’ deficits in velocity, cadence, and swing percent of cycle persisted through 7 days post-MCAO. By 28 days post-MCAO, NSC EV–treated pigs exhibited a significant increase in temporal gait parameters relative to control pigs, thus demonstrating substantial improvement.
Similar functional outcomes in spatial gait parameters were also observed. Stride length (distance between consecutive hoof prints of the contralateral forelimb), hoof print area (measured by the number of activated sensors of the contralateral forelimb), and total scaled pressure (the sum of peak pressure values recorded from each activated sensor by a hoof during contact) decreased similarly in both groups 1-day post-MCAO (Figure 4C). However, NSC EV–treated pigs recovered by 7 days post-MCAO while control pigs remained significantly impaired at the same time points for these spatial parameters, indicating faster recovery.
Discussion
This pivotal study presents the first experimental evidence that intravenous administration of NSC EVs improved tissue and functional level outcomes in a translational porcine ischemic stroke model while adhering to the Stem Cell Emerging Paradigm in Stroke and Stroke Therapy Academic Industry Roundtable committee recommendations for developing and testing novel stroke therapeutics.3–6,25,26 NSC EV intervention led to significant decreases in lesion volume, which has never been observed before in EV-related neural injury studies and has been considered a key biomarker for recovery.12,13,27,28 Although EVs were harvested from human NSC EVs, no overt negative immune responses were detected in the porcine model. These data support our recently published data in a thromboembolic mouse model where the injury response to stroke was dampened while augmenting a reparative systemic response favoring macrophage polarization toward anti-inflammatory M2 cells, increasing regulatory T cells (Treg) cells, and decreasing proinflammatory T helper 17 (TH17) cells.15 In addition, NSC EV therapy led to preserved diffusivity and sustained WM integrity, which strongly correlates with improvements in executive function, cognitive decline, and sensorimotor deterioration, as well as decreased hemispheric swelling and ICH incidence, which are intimately associated with stroke patient morbidity.22,29–33
Significant decreases in hemispheric swelling and decreased incidence of ICH indicated NSC EV treatment not only preserved cellular integrity in the ischemic site but also preserved the integrity of microvessels and associated capillary beds 1-day post-MCAO. A recent study of MSC EVs post-MCAO reported increased vascular remodeling in the ischemic boundary zone of rats.12 The NSC EV marker profile (Figure 1D) indicated consistent presence of integrins, including integrin β-1 (CD29) and integrin α 2b (CD41b). Integrin β-1 is known to mediate cell-to-cell and cell-to-matrix interactions and regulate cell migration.34,35 Similarly, integrin α-2b is a receptor known to bind a variety of ligands leading to rapid platelet aggregation, as well as positive regulation of leukocyte migration and megakaryocyte differentiation.36,37 In addition, blockade of integrin α-2b (CD41) increases ICH incidence and mortality after transient MCAO in a dose-dependent manner.38 By altering the processes of coagulation and vascular function, intravenously administered NSC EVs may protect the integrity of the blood–brain barrier through inherent intercellular signaling components. Although the exact molecular mechanism of action is currently unknown, whether dependent on one or multiple EV components or direct action at the systemic level or on the brain directly, these data, in addition to our published rodent study, support that NSC EVs are biologically active and elicit a positive neuroprotective response in vivo in both rodent and large animal preclinical stroke models.15
A frequently used predictive indicator of patient prognosis is acute lesion volume because of the high correlation between neurological deficits and long-term functional outcomes.39–42 Although multiple MSC EV–related rodent models of stroke have observed improvements in tissue and functional recovery, the extent of neural protection seen with NSC EV treatment is unprecedented. Previous rodent stroke studies assessing the efficacy of MSC EVs showed no changes in lesion volume.12,13,27,28 In comparison, our recently published data indicated an ≈35% reduction in lesion volume in the mouse thromboembolic stroke model. Comparatively, our data in the porcine model possessed a significant 44% decrease in lesion volume at 1-day post-MCAO, suggesting NSC EVs are potentially more protective and thus more therapeutically relevant than MSC EVs.
Restoring motor function in patients with stroke is critical for improvement in quality of life and is a robust measure of therapeutic potential.43–47 Most patients with stroke exhibit hemiparesis with correlative asymmetries, decreased velocity, stride length, and other spatiotemporal parameters, therefore it was vital to determine whether these cellular benefits resulted in functional benefits at the organismal level.48,49 To date, no exosome efficacy study has performed a comprehensive assessment of changes in gait function poststroke. Previous studies have relied on gross measurements (foot fault tests, rotorod), which do not account for fine motor changes in gait as do relative pressure, swing percent, and stride length.11,12 In this study, we found significant changes and decreased recovery time in these and other translational parameters that are critical readouts for human patients. The pig is also likely a more representative model of human gait changes poststroke when compared with rodents, despite being a quadruped, as weight (pigs in this study were between 72 and 104 kg), limb, and body length are more comparable to humans and are more similarly affected by biomechanical forces generated during normal movement.
In this study, we have demonstrated that NSC EVs are a potent biological treatment that positively impact both molecular and functional outcomes poststroke while abiding by Stem Cell Emerging Paradigm in Stroke and Stroke Therapy Academic Industry Roundtable committee recommendations for rigorously developing and testing therapeutics. NSC EVs in our porcine ischemic stroke model exhibited a multifactorial effect leading to decreased lesion volume, hemispheric swelling, and ICH while also promoting diffusivity, WM integrity, and functional performance in a large animal model with similar cerebral architecture and WM composition to humans. As an effective treatment in both rodent and porcine stroke models, NSC EVs possess inherent biological characteristics suitable for translation into human stroke therapeutics.
Acknowledgments
We thank Simon R. Platt, DVM, who performed the pig permanent occlusion surgeries, as well as Caroline Jackson, Justin Sharma, Austin Passaro, and Viviana Martinez who were involved with various aspects of the extracellular vesicle manufacturing process, pig gait/behavioral testing, and figure preparation. We also thank Tracey Stice for project management guidance. We also thank Julie Nelson at the UGA CTEGD Flow Cytometry Core and Phi Optics for use of the spatial light interference microscopy system.
Sources of Funding
This work was supported by ArunA Biomedical, Inc, National Institute of Neurological Disorders and Stroke grant R43NS103596, National Institute of Neurological Disorders and Stroke grant R01NS093314, Science and Technology Center Emergent Behaviors of Integrated Cellular Systems grant no. CBET-0939511, and the Georgia Research Alliance.
Disclosures
Drs Webb and Stice have submitted a patent filing on the neural stem cell–derived extracellular vesicles, and this technology is licensed from the University of Georgia Research Foundation by ArunA Biomedical, Inc. All authors affiliated with Aruna Biomedical, Inc own equity in the company. The other authors report no conflicts.
Supplementary Material
Footnotes
R.L. Webb and E.E. Kaiser contributed equally.
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.117.020353/-/DC1.
References
- 1.Cheng NT, Kim AS. Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist. 2015;5:101–109. doi: 10.1177/1941874415583116. doi: 10.1177/1941874415583116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle K, Joundi RA, Aviv RI. An historical and contemporary review of endovascular therapy for acute ischemic stroke. Neurovasc Imaging. 2017;3:1.. [Google Scholar]
- 3.Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem cell therapies as an emerging paradigm in stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 4.Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L STEPS Participants. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 2011;42:825–829. doi: 10.1161/STROKEAHA.110.601914. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- 5.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. STAIR Group. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M STAIR VI Consortium. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40:2594–2600. doi: 10.1161/STROKEAHA.109.552554. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basso M, Bonetto V. Extracellular vesicles and a novel form of communication in the brain. Front Neurosci. 2016;10:127. doi: 10.3389/fnins.2016.00127. doi: 10.3389/fnins.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. doi: 10.1016/j.neuint.2016.08.003. doi: 10.1016/j.neuint.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model [published online ahead of print December 28, 2017]. Transl Stroke Res. doi: 10.1007/s12975-017-0599-2. doi: 10.1007/s12975-017-0599-2. https://link.springer.com/article/10.1007/s12975-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platt SR, Holmes SP, Howerth EW, Duberstein KJJ, Dove CR, Kinder HA, et al. Development and characterization of a Yucatan miniature biomedical pig permanent middle cerebral artery occlusion stroke model. Exp Transl Stroke Med. 2014;6:5. doi: 10.1186/2040-7378-6-5. doi: 10.1186/2040-7378-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duberstein KJ, Platt SR, Holmes SP, Dove CR, Howerth EW, Kent M, et al. Gait analysis in a pre- and post-ischemic stroke biomedical pig model. Physiol Behav. 2014;125:8–16. doi: 10.1016/j.physbeh.2013.11.004. doi: 10.1016/j.physbeh.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Imai H, Konno K, Kubota C, Seki K, Puentes S, et al. Experimental investigation of encephalomyosynangiosis using gyrencephalic brain of the miniature pig: histopathological evaluation of dynamic reconstruction of vessels for functional anastomosis. Laboratory investigation. J Neurosurg Pediatr. 2009;3:488–495. doi: 10.3171/2008.6.PEDS0834. doi: 10.3171/2008.6.PEDS0834. [DOI] [PubMed] [Google Scholar]
- 20.Kuluz JW, Prado R, He D, Zhao W, Dietrich WD, Watson B. New pediatric model of ischemic stroke in infant piglets by photothrombosis: acute changes in cerebral blood flow, microvasculature, and early histopathology. Stroke. 2007;38:1932–1937. doi: 10.1161/STROKEAHA.106.475244. doi: 10.1161/STROKEAHA.106.475244. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Imai H, Konno K, Miyagishima T, Kubota C, Puentes S, et al. Experimental model of lacunar infarction in the gyrencephalic brain of the miniature pig: neurological assessment and histological, immunohistochemical, and physiological evaluation of dynamic corticospinal tract deformation. Stroke. 2008;39:205–212. doi: 10.1161/STROKEAHA.107.489906. doi: 10.1161/STROKEAHA.107.489906. [DOI] [PubMed] [Google Scholar]
- 22.Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci. 2008;28:1479–1489. doi: 10.1523/JNEUROSCI.5137-07.2008. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker EW, Platt SR, Lau VW, Grace HE, Holmes SP, Wang L, et al. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke pig model. Sci Rep. 2017;7:10075. doi: 10.1038/s41598-017-10406-x. doi: 10.1038/s41598-017-10406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mir M, Kim T, Majumder A, Xiang M, Wang R, Liu SC, et al. Label-free characterization of emerging human neuronal networks. Sci Rep. 2014;4:4434. doi: 10.1038/srep04434. doi: 10.1038/srep04434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher M Stroke Therapy Academic Industry Roundtable. Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003;34:1539–1546. doi: 10.1161/01.STR.0000072983.64326.53. doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- 26.Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. STAIR VII Consortium. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- 27.Otero-Ortega L, Laso-García F, Gómez-de Frutos MD, Rodríguez-Frutos B, Pascual-Guerra J, Fuentes B, et al. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci Rep. 2017;7:44433. doi: 10.1038/srep44433. doi: 10.1038/srep44433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lövblad KO, Baird AE, Schlaug G, Benfield A, Siewert B, Voetsch B, et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol. 1997;42:164–170. doi: 10.1002/ana.410420206. doi: 10.1002/ana.410420206. [DOI] [PubMed] [Google Scholar]
- 30.Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized MRI stroke protocol: comparison with CT in hyperacute intracerebral hemorrhage. Stroke. 1999;30:765–768. doi: 10.1161/01.str.30.4.765. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad AS, Satriotomo I, Fazal J, Nadeau SE, Doré S. Considerations for the optimization of induced white matter injury preclinical models. Front Neurol. 2015;6:172. doi: 10.3389/fneur.2015.00172. doi: 10.3389/fneur.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jokinen H, Gouw AA, Madureira S, Ylikoski R, van Straaten EC, van der Flier WM, et al. LADIS Study Group. Incident lacunes influence cognitive decline: the LADIS study. Neurology. 2011;76:1872–1878. doi: 10.1212/WNL.0b013e31821d752f. doi: 10.1212/WNL.0b013e31821d752f. [DOI] [PubMed] [Google Scholar]
- 33.Srikanth V, Beare R, Blizzard L, Phan T, Stapleton J, Chen J, et al. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40:175–180. doi: 10.1161/STROKEAHA.108.524355. doi: 10.1161/STROKEAHA.108.524355. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell. 2005;9:663–673. doi: 10.1016/j.devcel.2005.09.012. doi: 10.1016/j.devcel.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, et al. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J Biol Chem. 2003;278:34605–34616. doi: 10.1074/jbc.M303159200. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- 36.Ma YQ, Qin J, Plow EF. Platelet integrin alpha(IIb)beta(3): activation mechanisms. J Thromb Haemost. 2007;5:1345–1352. doi: 10.1111/j.1538-7836.2007.02537.x. doi: 10.1111/j.1538-7836.2007.02537.x. [DOI] [PubMed] [Google Scholar]
- 37.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat Cell Biol. 2002;4:E97–E100. doi: 10.1038/ncb0402-e97. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 38.Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 39.Borsody M, Warner Gargano J, Reeves M, Jacobs B MASCOTS Insula-Stroke Substudy Group. Infarction involving the insula and risk of mortality after stroke. Cerebrovasc Dis. 2009;27:564–571. doi: 10.1159/000214220. doi: 10.1159/000214220. [DOI] [PubMed] [Google Scholar]
- 40.Huisa BN, Neil WP, Schrader R, Maya M, Pereira B, Bruce NT, et al. Clinical use of computed tomographic perfusion for the diagnosis and prediction of lesion growth in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:114–122. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.020. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiemanck SK, Kwakkel G, Post MW, Prevo AJ. Predictive value of ischemic lesion volume assessed with magnetic resonance imaging for neurological deficits and functional outcome poststroke: a critical review of the literature. Neurorehabil Neural Repair. 2006;20:492–502. doi: 10.1177/1545968306289298. doi: 10.1177/1545968306289298. [DOI] [PubMed] [Google Scholar]
- 42.Tong DC, Yenari MA, Albers GW, O’Brien M, Marks MP, Moseley ME. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<6.5 hour) ischemic stroke. Neurology. 1998;50:864–870. doi: 10.1212/wnl.50.4.864. [DOI] [PubMed] [Google Scholar]
- 43.Nascimento LR, de Oliveira CQ, Ada L, Michaelsen SM, Teixeira-Salmela LF. Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic review. J Physiother. 2015;61:10–15. doi: 10.1016/j.jphys.2014.11.015. doi: 10.1016/j.jphys.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Hak L, Houdijk H, van der Wurff P, Prins MR, Beek PJ, van Dieën JH. Stride frequency and length adjustment in post-stroke individuals: influence on the margins of stability. J Rehabil Med. 2015;47:126–132. doi: 10.2340/16501977-1903. doi: 10.2340/16501977-1903. [DOI] [PubMed] [Google Scholar]
- 45.Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech. 2010;43:2348–2355. doi: 10.1016/j.jbiomech.2010.04.027. doi: 10.1016/j.jbiomech.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolan KJ, Yarossi M, Mclaughlin P. Changes in center of pressure displacement with the use of a foot drop stimulator in individuals with stroke. Clin Biomech (Bristol, Avon) 2015;30:755–761. doi: 10.1016/j.clinbiomech.2015.03.016. doi: 10.1016/j.clinbiomech.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 47.De Nunzio A, Zucchella C, Spicciato F, Tortola P, Vecchione C, Pierelli F, et al. Biofeedback rehabilitation of posture and weightbearing distribution in stroke: a center of foot pressure analysis. Funct Neurol. 2014;29:127–134. [PMC free article] [PubMed] [Google Scholar]
- 48.Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38:2309–2314. doi: 10.1161/STROKEAHA.106.475483. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- 49.Patterson KK, Parafianowicz I, Danells CJ, Closson V, Verrier MC, Staines WR, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89:304–310. doi: 10.1016/j.apmr.2007.08.142. doi: 10.1016/j.apmr.2007.08.142. [DOI] [PubMed] [Google Scholar]


