Summary
Since the diagnosis of cryptococcosis is challenging in low prevalence settings, uncovering predictive factors can improve early diagnosis and timely treatment. The aim of the study was to relate clinical outcomes to predictive variables for the presence of cryptococcosis. A retrospective case-control study matching by collection date, age, and gender at a 1:2 ratio (55 cases and 112 controls) was performed in case patients diagnosed with Cryptococcus infection at the University of Colorado Hospital between 2000 and 2017 (n=167). A bivariate and a forward, stepwise multivariable logistic regression model was performed to identify predictors of cryptococcosis. In an adjusted multivariable model; cryptococcal infection was significantly associated with the presence of respiratory symptoms, hyponatremia, lung disease, or corticosteroids. Additionally; cryptococcal meningitis was associated with headaches, corticosteroids, or increased CSF protein. Conversely, a reduced risk of cryptococcosis was associated with hypertension or peripheral monocytosis. Cryptococcal meningitis lead to subsequent hearing impairment (16% vs. 4% (control), p=0.013), muscle weakness (40 vs. 20%, p=0.021), cognitive deficits (33% vs. 6%, p=0.0001) or any adverse outcome (84% vs. 29%, p=0.0001). We uncovered novel clinical predictors for the presence of cryptococcal infection or cryptococcal meningitis. This study in patients at a low prevalence US medical center underscores the importance of early diagnosis in this population.
Keywords: Cryptococcosis, cryptococcal meningitis, Cryptococcus neoformans, Fungemia, risk factors
Introduction
Cryptococcal meningitis upholds a large global impact with nearly 223,000 new cases each year and results in 181,100 deaths among HIV infected individuals [1]. Internationally, cryptococcal meningitis causes 15% of all AIDS-related deaths with an estimated mortality of approximately 20%. In the United States, cryptococcal meningitis causes about 3,400 hospitalizations every year [2]. Despite diminishing incidence in the United States due to an increase in the use of antiretroviral drugs, cryptococcal disease maintains a high mortality rate [2]. Cryptococcosis is also now associated with new hematologic cancer chemotherapies and its incidence among HIV-negative patients approaches those of patients with HIV [3, 4]. When a patient becomes infected with Cryptococcus, it commonly manifests in one of two ways, cryptococcal meningitis or pulmonary cryptococcosis. Disseminated infection is associated with higher mortality [5]. The risk of dissemination or neuroinvasion increase in immunosuppressed patients with HIV infection with or without AIDS, solid organ transplantation, systemic lupus erythematosus (SLE), malignancy, sarcoidosis, or cirrhosis [6–10]. Cryptococcal meningitis is associated with the presence of fever, headaches, altered mental status (AMS), weight loss, or high dose corticosteroids [6, 11–13]. However, the clinical presentation of cryptococcosis in immunocompromised patients is often atypical, overlaps with other medical conditions, and in low prevalence locations results in a delayed diagnosis. This delay often leads to an increased risk of neurologic sequela [14]. Identification of clinical risk factors for cryptococcosis and neuroinvasive disease may allow more accurate, and timely prediction of the disease among immunosuppressed patients with non-specific symptoms in low prevalent settings. Early diagnosis should prevent the development of neurological complications. Finally, clinical outcomes and neurologic sequela among survivors of cryptococcal meningitis are poorly characterized. The aim of this study was to perform a matched case-control analysis in patients with or without cryptococcal infection to assess clinical predictors for cryptococcosis and to describe adverse clinical outcomes in patients with cryptococcal meningitis.
Patients/Methods
Ethics statement
The present investigation is in health insurance portability and accountability act (HIPAA) compliance according to the Colorado Multiple Institutional Review Board (COMIRB) at University of Colorado Denver. Analysis of clinical data has been performed under an approved protocol (COMIRB Protocol 15-1340).
Patients and data collection
We collected data on all patients with a culture that grew Cryptococcus or with positive serum antigens from any specimen (pleural, CSF, blood, synovial, bronchoalveolar lavage, peritoneal fluid, and tissue) that were recorded at the University of Colorado Hospital microbiology laboratory between January 2000 and February 2017. After collecting all negative serum cryptococcal antigen results from the microbiology lab during the same period; controls were randomly chosen patients with a matched to cases by specimen collection date, age, and gender at a 1:2 (case: control) ratio. If controls had CSF studies, we verified that the CSF cryptococcal antigen was also negative. Controls were patients evaluated in both the hospital and ambulatory settings where clinicians had suspicious for cryptococcal infection and test was ordered to rule out the disease. Controls share common associated risk factors and they came from the same community that University of Colorado hospital serves. Cryptococcus infection was identified through Immuno-Mycologics Inc. (IMMY, OK) serum and CSF cryptococcal antigen tests (CrAg® LFA —Cryptococcal Antigen Lateral Flow Assay) using semi-quantitative enzyme immunoassay. Cryptococcal meningitis was defined as a positive cryptococcal CSF antigen study or positive CSF culture or a positive blood cryptococcal culture with endophthalmitis or known history of cryptococcal meningitis. Medical reports were accessed to collect clinical and laboratory variables for all patients. The following data were retrospectively collected: demographics (gender, race, age, place of birth, place of residence, and occupation); symptoms (constitutional, headaches, altered mental status, respiratory abnormalities, fever, and others), medical history (smoking, lung disease, diabetes mellitus, hypertension, lupus, malignancy, sarcoidosis, cirrhosis, HIV infection, solid organ transplant, use of calcineurin inhibitors or corticosteroids, and prednisone dose); HIV (time since diagnosis, history of HIV antiretroviral drug resistance, antiretroviral therapy, CD4 count, and viral load); transplant (type and time since transplant); vital signs at collection times (systolic blood pressure, diastolic blood pressure, weight, pulse, temperature, and BMI); absence of presence of cryptococcal meningitis, laboratory results (complete blood cell count, comprehensive metabolic panel, baseline renal function, lumbar puncture opening pressure, serum cryptococcal antigen, cerebrospinal fluid (CSF) cryptococcal antigen, CSF culture, blood culture, CSF cell count, CSF glucose, and CSF protein), and outcomes of cryptococcal infection: immune reconstitution syndrome (IRS), treatment regimen, death and attributable death, cognitive deficits, muscle weakness, speech difficulties, hearing impairment, MRI imaging results; use of ventriculoperitoneal shunts (VPS), new onset cryptococcal infection, and relapse.
Definitions
A detailed description of the definition of each variable is already published [15]. For a direct access please see Appendix A.
Statistical analysis
We used data from 167 patients which included 55 patients with Cryptococcus infection and 112 controls without infection. Statistical analyses were performed using STATA software, version 12.1 (StataCorp, College Station, Texas, USA). The means and standard deviations for continuous variables were calculated. For categorical variables, frequencies and percentages were calculated. We initially performed a bivariate analysis for dichotomous outcomes variables using chi-squared, and the Fisher exact test for dichotomous and nominal independent variables respectively. For interval independent variables, we used the t-test. For the multivariable analysis, we selected variables associated with outcome with a p-value <=0.05, and variables known to be associated with the outcome or known to be confounders. We removed collinear variables or variables with a significant number of missing data. For incorporation of variables, we transformed nominal and ordinal variables to dichotomous variables. We determined if interval variables followed a normal distribution by performing the Shapiro-Wilk test. If the data were not normally distributed, the variable was transformed into a dichotomous variable. Final selected variables were included in a multivariable, forward, stepwise logistic regression model. A parallel conditional logistic regression model was run for comparison. Multivariable analysis was done for all cases vs. controls and separately for cases with meningitis vs. controls. Post-estimation models’ performance outputs were evaluated for sensitivity, specificity, and area under the receiver operator characteristic (ROC) curve.
Results
Clinical characteristics of patients with cryptococcosis
We evaluated a total of 55 patients with Cryptococcus infection and 112 controls without infection (Table 1). The median age for the cases was 54.3 years. Patients were predominantly male, Caucasian, and held an outdoor occupation. Constitutional symptoms predominated in cases and controls. Headaches (37% vs. 15.2%, p=0.002) and respiratory symptoms (32.7% vs. 13.4%, p=0.003) were more frequently present in patients with cryptococcosis. The most prevalent comorbidities among patients with cryptococcal infection were HIV infection (44.4% vs. 35.7%, p=0.279), corticosteroid use (28.3% vs. 16.4%, p=0.076), malignancy (25.5% vs. 18.8%; p=0.317), active smoking (20.0% vs. 24.1%; p=0.751), transplant status (18.9% vs. 18.8%; p=0.986), and lung disease (18.2% vs. 4.45%; p=0.004). The most common condition among controls was the diagnosis of altered mental status, most of which were diagnosed with transient metabolic or medication-related encephalopathy. Patients with cryptococcal infection had slightly lower systolic blood pressures (119.8 vs. 127.9 mm Hg; p=0.025) and had normal heart rates, temperatures, and body mass indices. Laboratory data in patients were significant for normal white blood counts (WBC), hemoglobin (Hb), and platelets counts. Compared to controls, patients with Cryptococcosis had lower monocyte counts (0.48 vs. 0.64 × 109/L; p=0.049), relative hyponatremia (134.8 vs. 137.3 mmol/L; p=0.004) and normal kidney and liver function tests. Compared to controls, patients had increase cerebrospinal fluid (CSF), opening pressure (27.3 vs. 21.1 cm H2O; p=0.232), elevated CSF WBC (121.5 vs. 32.7 × 106/L; p=0.011), and more predominantly neutrophilic CSF CBC composition (51.2%). In patients with cryptococcal meningitis, CSF monocytes were markedly increased (30.6 vs. 6.4 × 106/L; p=0.034) and CSF protein was larger. Cases were characterized by the presence of hypoglycorrhachia (CSF glucose 41.5 vs. 66 mg/dl; p=0.0001). Approximately 15% of patients had meningeal enhancement on the MRI result. As reported previously, our data confirm the lack of systemic response, lack of meningeal enhancement on MRI images in most of the cases, and possible SIADH related hyponatremia in case patients.
TABLE 1.
Clinical characteristics of controls, patients with cryptococcosis, and cryptococcal meningitis
| Count (%)/Mean ± SD | ||||||
|---|---|---|---|---|---|---|
| Patient characteristics | N | Controls (n=112) |
Total Cases (n=55) |
P-values | CNS Cases (n=32 |
P-values |
| Demographics | ||||||
| Gender (Male) | 167 | 88 (78.6%) | 45 (81.8%) | 0.624 | 29 (90.6%) | 0.123 |
| Race (White) | 149 | 71 (72.5%) | 33 (64.7%) | 0.446 | 20 (64.5%) | 0.570 |
| Age | 167 | 55.5 ± 14.6 | 54.3 ± 14.4 | 0.608 | 50.2 ± 13.5 | 0.068 |
| Symptoms | ||||||
| Constitutional | 167 | 77 (68.8%) | 41 (74.6%) | 0.44 | 26 (81.3%) | 0.167 |
| Headaches | 167 | 17 (15.2%) | 20 (37%) | 0.002 | 18 (56.3%) | 0.0001 |
| Altered mental status | 167 | 43 (38.3%) | 10 (18.2%) | 0.008 | 8 (25%) | 0.162 |
| Respiratory | 167 | 15 (13.4%) | 18 (32.7%) | 0.003 | 4 (12.5%) | 0.895 |
| Other symptoms | 167 | 86 (76.8%) | 38 (69.1%) | 0.285 | 23 (71.9%) | 0.568 |
| Fever | 162 | 17 (15.2%) | 11 (22%) | 0.289 | 9 (31%) | 0.05 |
| Past Medical History | ||||||
| Smoking (Current) | 167 | 27 (24.1%) | 11 (20%) | 0.751 | 8 (25%) | 0.902 |
| Lung Disease | 167 | 5 (4.45%) | 10 (18.2%) | 0.004 | 3 (9.4%) | 0.285 |
| Diabetes Mellitus type 2 | 167 | 23 (20.5%) | 9 (16.4%) | 0.520 | 5 (15.6%) | 0.536 |
| Malignancy | 167 | 21 (18.8%) | 14 (25.5%) | 0.317 | 4 (12.5%) | 0.410 |
| Cirrhosis | 167 | 3 (2.7%) | 6 (11.1%) | 0.027 | 4 (12.5%) | 0.023 |
| HIV | 166 | 40 (35.7%) | 24 (44.4%) | 0.279 | 18 (56.3%) | 0.037 |
| Transplant | 165 | 21 (18.8%) | 10 (18.9%) | 0.986 | 6 (18.8%) | 1.000 |
| Corticosteroid | 163 | 18 (16.4%) | 15 (28.3%) | 0.076 | 11 (34.4%) | 0.026 |
| Sarcoidosis | 167 | 1 (0.9%) | 1 (1.8%) | 0.605 | 1 (3.1%) | 0.341 |
| Calcineurin inhibitors | 165 | 17 (15.2%) | 8 (15.1%) | 0.989 | 5 (15.6%) | 0.951 |
| Hypertension | 167 | 46 (41.1%) | 13 (23.6%) | 0.027 | 7 (21.9%) | 0.047 |
| Vitals signs | ||||||
| SBP (mm Hg) | 164 | 127.9 ± 22.1 | 119.8 ± 19.9 | 0.025 | 115.2 ± 18.3 | 0.004 |
| Pulse | 162 | 90.0 ± 18.1 | 87.5 ± 21.9 | 0.439 | 92.3 ± 20.0 | 0.550 |
| Temperature | 162 | 37.4 ± 5.8 | 37.05 ± 1.0 | 0.711 | 37.2 ± 1.2 | 0.870 |
| Hypothermia | 162 | 11 (9.82%) | 3 (6%) | 0.424 | 2 (6.9%) | 0.628 |
| BMI | 101 | 25.7 ± 5.9 | 25.9 ± 4.4 | 0.873 | 26.7 ±4.8 | 0.659 |
| Laboratory Data | ||||||
| WBC (4.0–11.1 × 109/L) | 164 | 7.4 ± 5.9 | 7.3 ± 6.4 | 0.921 | 7.7 ± 7.8 | 0.829 |
| Hemoglobin (14.3–18.1 g/dl) | 164 | 12.6 ± 3.3 | 12.3 ± 2.6 | 0.560 | 11.7 ± 2.4 | 0.184 |
| Platelets (150–400 × 109/L) | 163 | 215.3 ± 138.4 | 199.2 ± 111.8 | 0.459 | 197.4 ± 134.1 | 0.527 |
| Monocytes (0.2–0.9 × 109/L) | 162 | 0.64 ± 0.53 | 0.48 ± 0.36 | 0.049 | 0.46 ± 0.35 | 0.079 |
| Na (133–145 mmol/L) | 163 | 137.3 ± 4.1 | 134.8 ± 6.3 | 0.004 | 133.8 ± 7.2 | 0.001 |
| Creatinine (0.7–1.3 mg/dl) | 158 | 2.6 ± 9.1 | 1.3 ± 0.86 | 0.288 | 1.40 ± 0.95 | 0.463 |
| Ca (8.6–10.3 mg/dl) | 156 | 9.3 ± 1.1 | 9.4 ± 0.54 | 0.544 | 9.32 ± 0.55 | 0.850 |
| Albumin (3.5–5.7 g/dl) | 158 | 3.3 ± 0.96 | 3.1 ± 0.75 | 0.171 | 3.1 ± 0.73 | 0.245 |
| ALT (7–52 U/L) | 157 | 35.9 ± 38.8 | 35.4 ± 35.5 | 0.935 | 43.7 ± 43.2 | 0.353 |
| OP (<20 cm H2O) | 27 | 21.1± 11.6 | 27.3 ± 13.4 | 0.232 | 28.2 ± 13.3 | 0.178 |
| CSF WBC (0–5 × 106/L) | 104 | 32.7 ± 72.1 | 121.5 ± 271.6 | 0.011 | 153.8 ± 298.9 | 0.002 |
| CSF Neutrophils | 78 | 8.0 ± 30.1 | 62.3 ± 176.7 | 0.047 | 78.9 ± 196.5 | 0.019 |
| CSF Lymphocytes | 92 | 25.8 ± 64.2 | 28.3 ± 43.9 | 0.839 | 35.6 ± 47.0 | 0.485 |
| CSF Monocytes | 92 | 6.4 ± 19.6 | 30.6 ± 82.7 | 0.034 | 38.8 ± 91.9 | 0.011 |
| CSF glucose (40-70 mg/dl) | 101 | 66.0 ± 28.7 | 41.5 ± 21.3 | 0.000 | 35.7 ± 19.4 | 0.0001 |
| CSF protein (15-45 mg/dl) | 102 | 61.6 ± 60.1 | 99.9 ± 80.9 | 0.008 | 115.9 ± 84.2 | 0.0007 |
| MRI meningeal enhancement | 107 | 30 (37.5%) | 4 (14.8%) | 0.029 | 3 (16.7%) | 0.091 |
| Outcomes | ||||||
| Death | 167 | 17 (15.2%) | 16 (29.1%) | 0.034 | 9 (28.1%) | 0.093 |
| Opportunistic Infections | 124 | 1 (1.3%) | 10 (21.8%) | 0.000 | 8 (30.8%) | 0.0001 |
| Ventriculo-peritoneal Shunt | 125 | 0 (0%) | 2 (4.3%) | 0.066 | 2 (7.4%) | 0.015 |
| Cognitive deficits | 163 | 7 (6.3 %) | 10 (19.6%) | 0.010 | 10 (33.3%) | 0.0001 |
| Hearing Impairment | 167 | 4 (3.6%) | 5 (9.1%) | 0.138 | 5 (15.6%) | 0.013 |
| Incoherent speech | 167 | 11 (9.8%) | 1 (1.8%) | 0.060 | 1 (3.1%) | 0.227 |
| Muscle weakness | 167 | 23 (20.5%) | 18 (32.7%) | 0.085 | 13 (40.6%) | 0.021 |
Predictors of cryptococcal Infection
In a multivariable analysis, the following variables were found to be statistically significantly associated with cryptococcosis at the 0.05 alpha level (table 2): symptoms of headaches (OR: 11.09, 95% CI: 3.42 – 35.94; p=0.0001), respiratory complaints (OR: 7.47, 95% CI: 2.39 – 23.35; p=0.001); history of lung disease (OR: 5.33, 95% CI: 1.11-25.65; p=0.037), corticosteroids use (OR: 4.57, 95% CI: 1.37-15.19; p=0.013), and hyponatremia at presentation (OR: 9.53, 95% CI: 2.57-35.35); p=0.001). Conversely, hypertension (OR: 0.24, 95% CI: 0.08 – 0.74; p=0.013), and peripheral monocytosis (OR: 0.14, 95% CI: 0.03 – 0.67; p=0.014) were associated with absence of a diagnosis of cryptococcosis. Although altered mental status (OR: 0.21, 95% CI: 0.07 – 0.66; p=0.007) was significantly present in controls compared to patients, this sign commonly triggers physicians to order the cryptococcal serum antigen test. The multivariable model had a sensitivity of 67.4%, specificity of 89.9%, positive predictive value of 75%, and a negative predictive value of 86.0% in classifying a patient as having Cryptococcosis. A conditional logistic regression model displayed very similar results.
Table 2.
Multivariable analysis for cryptococcal infection predictors.
| Characteristic | Odds Ratio (95% Confidence Intervals) |
p-value |
|---|---|---|
| Headaches | 11.09 (3.42 – 35.94) | 0.0001 |
| Respiratory Symptoms | 7.47 (2.39 – 23.35) | 0.001 |
| Hyponatremia | 9.53 (2.57 – 35.35) | 0.001 |
| Lung disease | 5.33 (1.11 – 25.65) | 0.037 |
| Altered mental status | 0.21 (0.07 – 0.66) | 0.007 |
| Corticosteroids | 4.57 (1.37 – 15.19) | 0.013 |
| Smoking | 0.46 (0.14 – 1.45) | 0.185 |
| Diabetes Mellitus | 0.41 (0.11 – 1.53) | 0.186 |
| Hypertension | 0.24 (0.08 – 0.74) | 0.013 |
| Monocytosis | 0.14 (0.03 – 0.67) | 0.014 |
Predictors of cryptococcal meningitis disease
In a multivariable analysis, the following variables were found to be statistically significantly associated with cryptococcal meningitis at the 0.05 alpha level (table 3): Headaches (OR: 49.15, 95% CI: 3.91 – 618.08; p=0.003), corticosteroids use (OR: 96.46, 95% CI: 4.82 – 1932.37; p=0.003), and increased CSF protein (OR: 30.55, 95% CI: 2.39 – 390.85; p=0.009). History of hypertension associated with a reduced risk of having cryptococcal meningitis (OR: 0.012, 95% CI: 0.0004 -0.44; p=0.016). The multivariable model had a sensitivity of 83.3%, specificity of 95.6%, positive predictive value of 87%, and a negative predictive value of 94.2% in classifying a patient as having cryptococcal meningitis.
Table 3.
Multivariable analysis for cryptococcal meningitis predictors.
| Characteristic | Odds Ratio (95% Confidence Intervals) |
p-value |
|---|---|---|
| Decrease CSF glucose | 11.12 (0.98 – 126.28) | 0.052 |
| Headaches | 49.15 (3.91 – 618.08) | 0.003 |
| Increase CSF protein | 30.55 (2.39 – 390.85) | 0.009 |
| Hyponatremia | 4.42 (0.57 – 34.15) | 0.155 |
| HIV infection | 7.26 (0.67 – 78.64) | 0.118 |
| SBP | 0.96 (0.90 – 1.01) | 0.118 |
| Hypertension | 0.012 (0.0004 –0.44) | 0.016 |
| Corticosteroids | 96.46 (4.82 – 1932.37) | 0.003 |
CSF: Cerebrospinal fluid; HIV: Human immunodeficiency virus; SBP: Systolic blood pressure.
Outcomes and adverse clinical outcomes in cryptococcal meningitis
Cryptococcal meningitis resulted in hearing impairment (16% vs. 4%, p=0.013), muscle weakness (40 vs. 20%, p=0.021), cognitive deficits (33% vs. 6%, p=0.0001) or any adverse outcome (84% vs. 29%, p=0.0001) more frequently than controls (Figure 1). Hydrocephalus with the need for a ventriculoperitoneal shunt (7.4%) and death (28.1%), were frequent in this cohort of patients with cryptococcal meningitis. Relapses occurred in 23% of the cases. Stroke arose as a complication in 33% of the patients with cryptococcal meningitis.
Figure 1. Adverse clinical outcomes for patients with cryptococcal meningitis vs. controls.
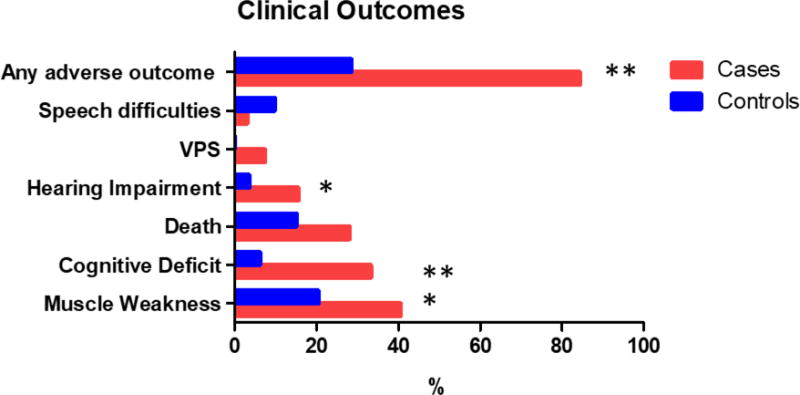
VPS: ventriculoperitoneal shunt. *p-value<0.05; **p-value<0.005
Discussion
We uncovered clinical symptoms such as headaches, respiratory complaints, history of pulmonary disease, and corticosteroids as strong predictors of cryptococcosis in a low prevalence tertiary care US medical center. These factors applied to a cohort of patients with a significant proportion of immunocompromised conditions such as HIV infection, malignancy, or transplantation. Furthermore, hypoglycorrhachia, increased CSF protein, and corticosteroids use at presentation was associated with increased likelihood of cryptococcal meningitis compared to controls. Patients with cryptococcosis often present with nonspecific symptoms —as shown here— and they usually do not display a systemic inflammatory response. Therefore, the above predictors may be useful tools to identify patients with failure to thrive or subacute symptoms who are at increased risk for cryptococcosis. We also described severe adverse outcomes in survivors with cryptococcal meningitis. These meningitis survivors are more likely to develop hearing impairment, muscle weakness, and cognitive deficits compared to other conditions that cause altered mental status. Previous cohorts have shown rates up to 45% of long-term neurological deficits following cryptococcal meningitis, which included cognitive impairment, vision loss, ataxia, deafness, and seizures [14]. Strokes — seen at a high rate in our cohort—are a complication of cryptococcal meningitis associated vasculopathy and is rarely described [16]. Strokes in this setting affect the basal ganglia and is a component of secondary neurologic deficits.
Limited research currently exists on predictive factors of cryptococcal meningitis in North America, and specifically in the Mountain West Region. This study confirmed previous recognized common risk factors for cryptococcosis: organ transplant, HIV infection, corticosteroid use, and malignancy. In contrast to previous studies, the presence of fever [6, 11], altered mental status [17], or constitutional symptoms, were unpredictive of cryptococcosis or meningitis in our cohort. We also did not observe other host-related factors that have been previously reported like smoking, diabetes, and ethnicity [18–21] with Cryptococcus infection. This may describe a true absence of these associations or associations may have been missed due to small sample size.
We are unfamiliar with potential protective clinical factors that reduce the risk of cryptococcal infection. Some studies have suggested a protective role for calcineurin inhibitors (CNI) for CNS invasion in solid organ transplant recipients [22], and some in vitro data exhibited CNI antifungal activity [23, 24]. In our cohort, we did not observe a protective role of CNI for cryptococcal meningitis. Conversely, the presence of a diagnosis of hypertension and monocytosis seem to confer a protective role for cryptococcal infection. We hypothesized that a common anti-hypertensive medication —calcium channel blockers— may possess anticryptococcal properties. Calcium channel blockers strongly enhance in vitro phagosome maturation, fungicidal activity, and reduced cryptococcal intracellular replication [25]. Peripheral monocytes are recruited by brain tissue in cases of CNS infections and play a role on Cryptococcus neuroinvasion [26]. This may explain the association between CSF monocytes and the presence of cryptococcal meningitis (table 1). Its protective mechanism role in cryptococcal infection merits further analysis.
There are several limitations in this study. The retrospective selection of data limits the reliability of the predictors and limits the number of variables analyzed. To those that were collected, some selection bias may exist as cases were collected by different observers. Missing data occurred in medical records. We employed measures to reduced biases by randomly selecting cases and controls at a 1:2 ratio and matching for age, gender, and sample collection dates.
The average survival time of cryptococcal meningitis is less than 3 months from diagnosis, and commonly patients do not survive past the first month of illness [20]. Targeted screening is an effective prevention method in immunocompromised patients at risk for cryptococcal infection. This enables early detection, a better chance of survival, and decreased neurological complications. We have uncovered clinical predictors associated with cryptococcosis among immunocompromised patients in a low prevalence US center.
A larger prospective study can confirm and refine our findings. Factors associated with a reduced risk of infection, such as hypertension and monocytosis deserve further follow up studies. Cryptococcal infection is the third most common invasive fungal infection among immunocompromised hosts [27]. It leads to significant mortality and disabling neurologic sequelae. Clinical tools to improve the timely recognition of the infection will likely result in early diagnosis and treatment and improved outcomes.
Acknowledgments
No funding agencies had any role in the preparation, review, or approval of this manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the University of Colorado Denver. J.D.B receives research support from the University of Colorado Neurosciences Institute and NINDS 1R01NS097729.
Appendix A: Definitions
Occupation was recorded as written in the history and physical (H&P) report. An occupation was labeled outdoor if it was performed primarily in the open air. Symptoms and past medical history were recorded as written in the initial H&Ps (by medicine residents, attending physicians and/or sub-specialties consults notes). Abstracted constitutional symptoms included weakness, weight loss, fatigue, fever, myalgias, night sweats, and malaise. Recorded respiratory symptoms included cough, shortness of breath, congestion, sore throat, and chest pain. Other recorded symptoms included rash, pruritus, and gastrointestinal (GI) complaints (diarrhea, flank pain, hematochezia, nausea, vomiting). Fever was defined in the initial H&P as temperature >37.7 °C. Smoking history was considered current or any former use of tobacco. Lung diseases included chronic obstructive pulmonary disease (COPD), pulmonary embolism, pneumonia, bronchiectasis, obstructive sleep apnea and lung neoplasm. Malignancy included current hematologic or solid organ neoplasms. Prednisone dose was calculated in milligrams and for those on non-prednisone steroids an equivalence converter was used to calculate the corresponding prednisone dose. HIV resistance was defined as written in the HIV provider history note or by the presence of any major mutations on a standard HIV genotype. Cryptococcus infection was identified through Immuno-Mycologics Inc. (IMMY, OK) serum and CSF cryptococcal antigen tests (CrAg® LFA —Cryptococcal Antigen Lateral Flow Assay) using semi-quantitative enzyme immunoassay. Confirmation was done through regular fungal culture. These tests unfortunately cannot distinguish the species or the genotype of the isolate. Blood cultures were processed using the BD BACTEC 9240 automated culturing system. CD4 count, viral load, and laboratory data were obtained at the time of diagnosis with the cryptococcal infection. IRS was defined per previous published guided criteria [13]. Cryptococcal meningitis was defined as a positive cryptococcal CSF antigen study or positive CSF culture or a positive blood cryptococcal culture with endophthalmitis or known history of cryptococcal meningitis. Standard treatment for cryptococcal meningitis was defined as at least 14 days of amphotericin B plus flucytosine. Cryptococcus attributable death was defined as mortality with Cryptococcus infection considered to be the direct cause of death. Residual cognitive deficits were speech, hearing impairment, muscle weakness or gait abnormalities documented in follow-up clinical assessments more than three months after the episode of cryptococcal meningitis. New onset infection was defined as first episode of cryptococcal infection. Relapse was any episode of recurrence of infection following clinical and microbiological pathogen control. Systolic blood pressure, diastolic blood pressure, weight, pulse, temperature, and BMI were recorded as written in medical records on date of positive cryptococcal infection diagnosis. MRI was recorded as normal or abnormal results, if abnormal, result was also recorded. Place of birth and place of residency was recorded as written in the medical records. Place of residency was also recorded as urban or rural.
Footnotes
DR ANDRÉS F. HENAO-MARTÍNEZ (Orcid ID: 0000-0001-7363-8652)
Conflicts of Interest:
No conflict of interest was reported by the authors.
References
- 1.Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17:873–81. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS ONE. 2013;8:e56269. doi: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamilos G, Lionakis MS, Kontoyiannis DP. Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways. Clin Infect Dis. 2017 doi: 10.1093/cid/cix687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George IA, Spec A, Powderly WG, Santos CAQ. Comparative Epidemiology and Outcomes of HIV, Non-HIV Non-Transplant and Organ Transplant Associated Cryptococcosis: A Population-Based Study. Clin Infect Dis. 2017 doi: 10.1093/cid/cix867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS ONE. 2013;8:e60431. doi: 10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baddley JW, Perfect JR, Oster RA, et al. Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis. 2008;27:937–43. doi: 10.1007/s10096-008-0529-z. [DOI] [PubMed] [Google Scholar]
- 7.Wang LR, Barber CE, Johnson AS, Barnabe C. Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis. Semin Arthritis Rheum. 2014;44:325–30. doi: 10.1016/j.semarthrit.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Cancelli I, Merlino G, Serafini A, Valente M, Gigli GL. Sarcoidosis as risk factor for cryptococcal meningitis in an apparently immunocompetent patient. Neurol Sci. 2008;29:33–5. doi: 10.1007/s10072-008-0856-y. [DOI] [PubMed] [Google Scholar]
- 9.Lin YY, Shiau S, Fang CT. Risk factors for invasive Cryptococcus neoformans diseases: a case-control study. PLoS One. 2015;10:e0119090. doi: 10.1371/journal.pone.0119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henao-Martinez AF, Beckham JD. Cryptococcosis in solid organ transplant recipients. Curr Opin Infect Dis. 2015;28:300–7. doi: 10.1097/QCO.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 11.Hung MS, Tsai YH, Lee CH, Yang CT. Pulmonary cryptococcosis: Clinical, radiographical and serological markers of dissemination. Respirology. 2008;13:247–51. doi: 10.1111/j.1440-1843.2007.01202.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein E, Rambo ON. Cryptococcal Infection Following Steroid Therapy. Ann Intern Med. 1962;56:114–20. doi: 10.7326/0003-4819-56-1-114. [DOI] [PubMed] [Google Scholar]
- 13.Olson PE, Earhart KC, Rossetti RJ, Newton JA, Wallace MR. Smoking and risk of cryptococcosis in patients with AIDS. JAMA. 1997;277:629–30. [PubMed] [Google Scholar]
- 14.Aye C, Henderson A, Yu H, Norton R. Cryptococcosis-the impact of delay to diagnosis. Clin Microbiol Infect. 2016;22:632–5. doi: 10.1016/j.cmi.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Henao-Martinez AF, Gross L, McNair B, et al. Risk Factors for Cryptococcal Meningitis: A Single United States Center Experience. Mycopathologia. 2016;181:807–14. doi: 10.1007/s11046-016-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosario M, Song SX, McCullough LD. An unusual case of stroke. Neurologist. 2012;18:229–32. doi: 10.1097/NRL.0b013e31825bbf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osawa R, Alexander BD, Lortholary O, et al. Identifying predictors of central nervous system disease in solid organ transplant recipients with cryptococcosis. Transplantation. 2010;89:69–74. doi: 10.1097/TP.0b013e3181bcda41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorvillo F, Beall G, Turner PA, Beer VL, Kovacs AA, Kerndt PR. Incidence and factors associated with extrapulmonary cryptococcosis among persons with HIV infection in Los Angeles County. AIDS. 1997;11:673–9. doi: 10.1097/00002030-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Khan ZU. Smoking, melanization, and cryptococcosis: is there a connection? J Clin Microbiol. 2006;44:1207. doi: 10.1128/JCM.44.3.1207.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuang YM, Ku SC, Liaw SJ, et al. Disseminated Cryptococcus neoformans var.grubii infections in intensive care units. Epidemiol Infect. 2010;138:1036–43. doi: 10.1017/S0950268809990926. [DOI] [PubMed] [Google Scholar]
- 21.Lin KH, Chen CM, Chen TL, et al. Diabetes Mellitus is Associated with Acquisition and Increased Mortality in HIV-uninfected Patients with Cryptococcosis: A Population-Based Study. J Infect. 2016 doi: 10.1016/j.jinf.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Davis JA, Horn DL, Marr KA, Fishman JA. Central nervous system involvement in cryptococcal infection in individuals after solid organ transplantation or with AIDS. Transpl Infect Dis. 2009;11:432–7. doi: 10.1111/j.1399-3062.2009.00424.x. [DOI] [PubMed] [Google Scholar]
- 23.Steinbach WJ, Reedy JL, Cramer RA, Jr, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5:418–30. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- 24.Kontoyiannis DP, Lewis RE, Alexander BD, et al. Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: correlation with outcome in solid organ transplant recipients with cryptococcosis. Antimicrob Agents Chemother. 2008;52:735–8. doi: 10.1128/AAC.00990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samantaray S, Correia JN, Garelnabi M, Voelz K, May RC, Hall RA. Novel cell-based in vitro screen to identify small-molecule inhibitors against intracellular replication of Cryptococcus neoformans in macrophages. Int J Antimicrob Agents. 2016;48:69–77. doi: 10.1016/j.ijantimicag.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–7. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12:220–9. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]


