Abstract
Background
Different abdominal symptoms may signal cancer, but their role is unclear.
Aim
To examine associations between abdominal symptoms and subsequent cancer diagnosed in the abdominal region.
Design and setting
Prospective cohort study comprising 493 GPs from surgeries in Norway, Denmark, Sweden, Scotland, Belgium, and the Netherlands.
Method
Over a 10-day period, the GPs recorded consecutive consultations and noted: patients who presented with abdominal symptoms pre-specified on the registration form; additional data on non-specific symptoms; and features of the consultation. Eight months later, data on all cancer diagnoses among all study patients in the participating general practices were requested from the GPs.
Results
Consultations with 61 802 patients were recorded and abdominal symptoms were documented in 6264 (10.1%) patients. Malignancy, both abdominal and non-abdominal, was subsequently diagnosed in 511 patients (0.8%). Among patients with a new cancer in the abdomen (n = 251), 175 (69.7%) were diagnosed within 180 days after consultation. In a multivariate model, the highest sex- and age-adjusted hazard ratio (HR) was for the single symptom of rectal bleeding (HR 19.1, 95% confidence interval = 8.7 to 41.7). Positive predictive values of >3% were found for macroscopic haematuria, rectal bleeding, and involuntary weight loss, with variations according to age and sex. The three symptoms relating to irregular bleeding had particularly high specificity in terms of colorectal, uterine, and bladder cancer.
Conclusions
A patient with undiagnosed cancer may present with symptoms or no symptoms. Irregular bleeding must always be explained. Abdominal pain occurs with all types of abdominal cancer and several symptoms may signal colorectal cancer. The findings are important as they influence how GPs think and act, and how they can contribute to an earlier diagnosis of cancer.
Keywords: cancer, early diagnosis, general practice, proportional hazard models, symptoms
INTRODUCTION
Approximately half of cancers documented and diagnosed in Norway are located within the abdominal cavity1 and abdominal symptoms (such as pain or bloating) may be associated with cancer. A previous article described a cohort in which approximately 10% of adult patients, consecutively consulting in general practice in six north European countries, presented with abdominal symptoms. For almost 5% of those who were symptomatic, the symptoms were associated with cancers in the abdomen.2
GPs play an active role in the diagnostic process for the majority of patients diagnosed with cancer.3–5 In population studies and clinical practice, the high frequency of abdominal symptoms contrasts with the relatively rare diagnosis of a cancer in the abdomen;6,7 this can make appropriate referral to more specialised services challenging. Much of the recent literature relating to primary care has concentrated on elucidating symptoms of more-common cancers such as colorectal cancer;8,9 this not only reflects the importance of those cancers that occur more often, but also the challenge of collecting sufficient numbers of patients who have cancers that are less common. As such, in addition to studying specific cancers, it may be helpful to study all cancers located in one anatomical region that present with similar symptom clusters.
This article, based on a prospective patient cohort study, focuses on new cancer diagnoses in the abdominal region that are diagnosed within 180 days of the index consultation. It aimed to analyse the extent to which various abdominal symptoms are associated with new cancers of the abdomen and, hence, how predictive specific abdominal symptoms are of such cancers.
METHOD
Setting
Details of the method, including variables of interest, power calculations, and data analysis techniques, have been described elsewhere.2 The study was carried out in primary care practices in Norway, Denmark, Sweden, the Netherlands, Belgium, and Scotland. Overall, 493 participating GPs were recruited through academic institutions active in the Cancer and Primary Care Research International Network (Ca-PRI, www.ca-pri.org/index.php).
How this fits in
A patient with cancer can present with no classical signs or symptoms. However, different abdominal symptoms have varying levels of association with abdominal cancer. Three symptoms relating to irregular bleeding, that is, rectal bleeding, unexpected genital bleeding, and macroscopic haematuria, are highly likely to lead to a cancer diagnosis unless an alternative diagnosis can be confirmed. Abdominal pain can be a presenting symptom for all types of cancers of the abdomen, no matter how common or rare they are. In a common cancer such as colorectal cancer, almost all the investigated symptoms are potentially relevant. Several combinations of symptoms may initiate suspicion of cancer in the abdomen.
Initial registration
Between 25 February 2011 and 27 July 2011 inclusive, participating GPs registered consecutive consultations held with patients aged ≥16 years, over a period of 10 working days. GPs recorded sex and date of birth for all patients, and certain abdominal symptoms if they were mentioned during the consultation. If abdominal symptoms were recorded, GPs were asked to complete additional symptom-related questions, including those on non-specific symptoms selected from medical literature related to cancer (this can be viewed on the UiT Open Research Data website: ‘Appendix1_ExampleForm’; http://dx.doi.org/10.18710/75C5TA).
No patients were contacted; only the individual GP knew the identity of the patient.
Follow-up
Participating GPs consented to provide data on all cancer diagnoses, new or recurring, that occurred after the consultation date for any of their patients for whom they had recorded data during consultation. Eight months after these consultations, each GP was asked to report all such patients on a standardised proforma (this can be viewed on the UiT Open Research Data website: ‘Appendix2_BJGP_Follow_up’; http://dx.doi.org/10.18710/75C5TA). It was reasoned that a cancer present during the initial consultation would usually have presented and been diagnosed within 6 months, supported, for instance, by a study on ovarian cancer.10 This interval of 6 months is also short enough to increase the probability that a recorded symptom has something to do with an as-yet-undiagnosed cancer.11 With the additional 2 months before completed proformas were collected, the authors assumed that all hospital reports about cancers diagnosed during the 6-month interval had reached the GPs.
GPs used electronic records to identify patients and to provide anonymised information about those registered during the initial study period who were diagnosed with cancer during the follow-up period, regardless of whether they had presented with symptoms during the initial survey. Sex, date of birth, GP identifier, and date of consultation were used to identify patients. Two reminders were sent to GPs. The last patient reported as having cancer was diagnosed in April 2012.
The authors distinguished between abdominal and non-abdominal types of cancer. In the abdominal group, all cancers of the digestive organs, female genital organs (except cancer of the vulva as this may be considered as both a skin cancer and a gynaecological cancer, and the location is not within what most doctors associate with the abdomen), and urinary organs including the testis, were included. Carcinoids, lymphomas, soft-tissue cancers, endocrine tumours, and generalised metastatic cancer were included if they showed any abdominal sign or symptom.
Data analysis
Statistical analyses were performed using SPSS (version 22) and Stata (version 14). The χ2 test was used to examine differences between groups. Association between symptoms and cancer was expressed as Cox hazard ratio (HR), in addition to sensitivity/specificity and likelihood ratio (LR). A positive predictive value (PPV) was presented with age and sex subgroups.
The LR in the study expresses the likelihood of a symptom being present and cancer subsequently being diagnosed, compared with the absence of a cancer diagnosis at follow-up. HR expresses the hazard for cancer being diagnosed when a patient had presented with an abdominal symptom, compared with when no symptom had been presented. The reference group in the Cox analyses were patients without abdominal symptoms. Age was the timescale variable for Cox analyses; entry time was age at consultation and exit time was age at cancer diagnosis or end of follow-up (30 April 2012); some GPs returned reports only after a few weeks. Age adjustment is inherent in the model.
The HR was calculated for single symptoms and for combinations of symptoms. In the multivariate analyses, the HR was calculated in models in which the most frequent symptoms and combinations of symptoms were adjusted for sex. Due to interaction, separate models were applied for each symptom, and for combinations of symptoms. The proportional hazards assumption was neither rejected for patients diagnosed within 180 days nor for all patients with new abdominal cancer. Therefore, although the main analyses were for patients with new abdominal cancer diagnosed within 180 days, sensitivity analyses were performed for the more numerous group of all patients with a new abdominal cancer.
Interaction analyses were also performed for age and each symptom; no such interactions were found. Cox analyses included only patients with a new cancer. Sensitivity analyses also were undertaken for all patients with a new cancer, that is, abdominal or non-abdominal and regardless of diagnostic interval.
The level of significance was set at 0.05.
RESULTS
Patient profile and cancers detected
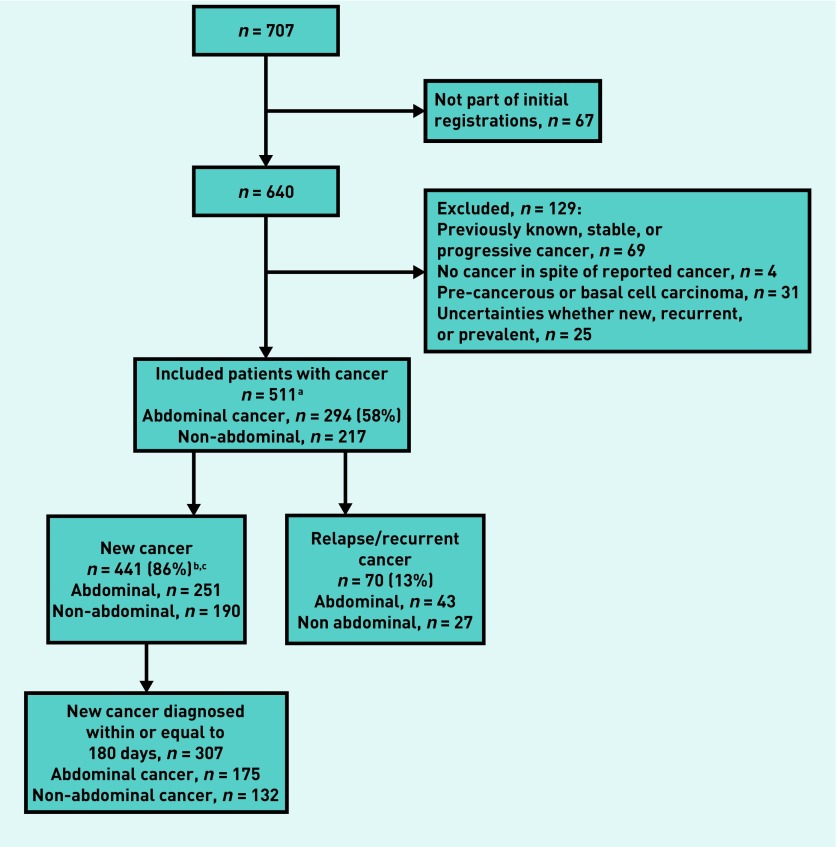
Data on the sex and age of patients are shown in Table 1. After corrections for multiple consultations, 61 802 patients were included in the cohort. Follow-up forms indicated that 640 patients with cancer were seen by 315 GPs; after exclusions, 511 patients were included (Figure 1). Of those 441 patients who had a new cancer, 251 (56.9%) had abdominal and 190 had non-abdominal cancers. In total, 175 patients of those 251 were diagnosed within 6 months of their GP consultation. Results presented in this article focus on these 175 patients. The higher proportion of males compared with females in the group with abdominal cancers (P<0.001) was consistent with Norwegian population-based data.1
Table 1.
Number of patients by sex and age group, for all patients and different subgroups of patients
| 16–29 years, n (%) | 30–54 years, n (%) | 55–74 years, n (%) | ≥75 years, n (%) | Total, n | Age, years | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | Median | Range | 25th–75th percentile | ||||||
| All patients | 8457 (13.7) | 23 144 (37.4) | 19 983 (32.3) | 10 218 (16.5) | 61 802 | 53 | 54 | 16–102 | 38–68 |
| Male | 2931 (12.2) | 8365 (35.0) | 8689 (36.3) | 3943 (16.5) | 23 928 | 55 | 56 | 16–102 | 41–69 |
| Female | 5526 (14.6) | 14 779 (39.0) | 11 294 (29.8) | 6275 (16.6) | 37 874 | 53 | 52 | 16–101 | 37–68 |
|
| |||||||||
| Patients with symptoms | 907 (14.5) | 2261 (36.1) | 1992 (31.8) | 1104 (17.6) | 6264 | 54 | 53 | 16–100 | 38–70 |
| Male | 236 (10.7) | 767 (34.9) | 792 (36.1) | 401 (18.3) | 2196 | 56 | 57 | 16–100 | 42–70 |
| Female | 671 (16.5) | 1494 (36.7) | 1200 (29.5) | 703 (17.3) | 4068 | 53 | 52 | 16–100 | 36–69 |
|
| |||||||||
| Patients with new cancer | 2 (0.5) | 62 (14.1) | 193 (43.8) | 184 (41.7) | 441 | 69 | 71 | 28–96 | 60–80 |
| Male | 0 (0) | 25 (12.5) | 91 (45.5) | 84 (42.0) | 200 | 70 | 71 | 35–94 | 62–79 |
| Female | 2 (0.8) | 37 (15.4) | 102 (42.3) | 100 (41.5) | 241 | 69 | 70 | 28–96 | 59–80 |
|
| |||||||||
| Patients with new cancer diagnosed within 180 days | 2 (0.7) | 47 (15.3) | 130 (42.3) | 128 (41.7) | 307 | 69 | 71 | 28–96 | 59–80 |
| Male | 0 (0) | 21 (15.0) | 61 (43.6) | 58 (41.4) | 140 | 69 | 72 | 35–94 | 60–79 |
| Female | 2 (1.2) | 26 (15.6) | 69 (41.3) | 70 (41.9) | 167 | 69 | 71 | 28–96 | 59–80 |
|
| |||||||||
| Patients with new abdominal cancer diagnosed within 180 days | 1 (0.6) | 23 (13.1) | 74 (42.3) | 77 (44.0) | 175 | 70 | 73 | 28–96 | 60–80 |
| Male | 0 (0) | 12 (12.6) | 43 (45.3) | 40 (42.1) | 95 | 70 | 72 | 42–94 | 59–79 |
| Female | 1 (1.3) | 11 (13.8) | 31 (38.8) | 37 (46.3) | 80 | 70 | 74 | 28–96 | 61–81 |
Figure 1.
Inclusion and exclusion of patients with cancer. aMost patients had a histological verification. The few remaining patients had other convincing evidence of cancer. bOne patient had two new cancers, colon cancer (counted here), plus squamous cell carcinoma of lung discovered in hospital. Lung cancer discovered incidentally during work-up. cOne patient had one new (prostate) and one recurrent (bladder) cancer. The prostate cancer has been counted here, because this was the new cancer.
Profile of symptoms
Table 2 shows that of 175 patients with a new abdominal cancer diagnosed within 180 days, 76 (43.4%) had abdominal symptoms and 39 (22.3%) had multiple abdominal symptoms. For patients with no cancer, 10.0% had abdominal symptoms and 4.5% multiple abdominal symptoms. Patients with a new non-abdominal cancer had abdominal symptoms in 12.6% of cases (data not shown). This was similar to results for patients with abdominal symptoms but no cancer.
Table 2.
Association between symptoms and new abdominal cancer for patients diagnosed with new abdominal cancer within 180 days after consultation, N = 61 337a
| Patients with cancer | Measures of association | Diagnostic probability (PPV) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All n = 175 | Male n= 95 | Female n= 80 | No cancer n= 61 162 | All LR | 95% CI | Male LR | Female LR | All sensitivity | 95% CI | All specificity | All PPV, % | 95% CI | Male PPV, % | Female PPV, % | ≤54 years PPV, % | 55–74 years PPV, % | ≥75 years PPV, % | |
| Abdominal symptoms | ||||||||||||||||||
| Abdominal pain, upper part | 31 | 15 | 16 | 2046 | 5.3 | 3.8 to 7.4 | 5.2 | 5.7 | 17.7 | 12.4 to 24.2 | 96.7 | 1.5 | 1.0 to 2.1 | 2.0 | 1.2 | 0.8 | 1.7 | 3.8 |
| Abdominal pain, lower part | 20 | 8 | 12 | 2084 | 3.4 | 2.2 to 5.1 | 3.1 | 3.9 | 11.4 | 7.1 to 17.1 | 96.6 | 1.0 | 0.6 to 1.5 | 1.2 | 0.8 | 0.3 | 1.4 | 2.3 |
| Constipation | 16 | 6 | 10 | 676 | 8.2 | 5.1 to 13.2 | 7.2 | 10.0 | 9.1 | 5.3 to 14.4 | 98.9 | 2.3 | 1.3 to 3.7 | 2.8 | 2.1 | 1.0 | 3.0 | 3.8 |
| Diarrhoea | 8 | 3 | 5 | 1103 | 2.5 | 1.3 to 5.0 | 1.7 | 3.5 | 4.6 | 2.0 to 8.8 | 98.2 | 0.7 | 0.3 to 1.4 | 0.7 | 0.7 | 0.7 | 0.3 | 1.7 |
| Distended abdomen, bloating | 17 | 8 | 9 | 1011 | 5.9 | 3.7 to 9.3 | 5.7 | 6.4 | 9.7 | 5.8 to 15.1 | 98.3 | 1.7 | 1.0 to 2.6 | 2.2 | 1.3 | 1.3 | 1.2 | 3.9 |
| Increased belching, flatulence | 9 | 5 | 4 | 489 | 6.4 | 3.4 to 12.2 | 7.1 | 6.0 | 5.1 | 2.4 to 9.5 | 99.2 | 1.8 | 0.8 to 3.4 | 2.8 | 1.3 | 2.4 | 1.1 | 1.4 |
| Acid regurgitation | 10 | 5 | 5 | 669 | 5.2 | 2.9 to 9.6 | 5.3 | 5.4 | 5.7 | 2.8 to 10.3 | 98.9 | 1.5 | 0.7 to 2.7 | 2.1 | 1.1 | 0.9 | 2.6 | 1.1 |
| Rectal bleeding | 15 | 4 | 11 | 385 | 13.6 | 8.3 to 22.3 | 6.8 | 21.6 | 8.6 | 4.9 to 13.7 | 99.4 | 3.8 | 2.1 to 6.0 | 2.7 | 4.4 | 2.1 | 4.5 | 6.8 |
| Unexpected genital bleeding | 3 | 0 | 3 | 195 | 5.4 | 1.7 to 16.7 | – | 7.7 | 1.7 | 0.4 to 4.9 | 99.7 | 1.5 | 0.3 to 4.4 | – | 1.6 | – | 8.1 | – |
| Macroscopic haematuria | 6 | 4 | 2 | 141 | 15.7 | 6.8 to 34.5 | 13.1 | 14.4 | 3.4 | 1.3 to 7.3 | 99.8 | 4.1 | 1.5 to 8.7 | 5.0 | 3.0 | – | 5.2 | 7.9 |
| Increased urinary frequency | 10 | 6 | 4 | 732 | 4.8 | 2.6 to 8.8 | 6.2 | 3.8 | 5.8 | 2.8 to 10.3 | 98.8 | 1.3 | 0.6 to 2.5 | 2.4 | 0.8 | – | 1.6 | 3.3 |
| Other abdominal problems | 18 | 9 | 9 | 1116 | 5.6 | 3.6 to 8.8 | 5.7 | 5.8 | 10.3 | 6.2 to 15.8 | 98.2 | 1.6 | 0.9 to 2.5 | 2.2 | 1.2 | 0.3 | 2.0 | 4.4 |
| 1 abdominal symptom only | 37 | 14 | 23 | 3354 | ||||||||||||||
| >1 abdominal symptom | 39 | 19 | 20 | 2748 | 5 | 3.8 to 6.6 | 5.2 | 5.1 | 22.3 | 16.4 to 29.2 | 95.5 | 1.4 | 1.0 to 1.9 | 2.0 | 1.1 | 0.6 | 1.9 | 3.1 |
| At least 1 abdominal symptom | 76 | 33 | 43 | 6102 | 4.4 | 3.7 to 5.2 | 3.9 | 5.1 | 43.4 | 36.0 to 51.1 | 90.0 | 1.2 | 1.0 to 1.5 | 1.5 | 1.1 | 0.3 | 1.6 | 3.2 |
| No symptom | 99 | 62 | 37 | 55060 | ||||||||||||||
| Non-specific symptoms when at least one abdominal symptom has been recorded | ||||||||||||||||||
| Lack of appetite | 18 | 6 | 12 | 853 | 7.4 | 4.7 to 11.5 | 4.8 | 10.4 | 10.3 | 6.2 to 15.8 | 98.6 | 2.1 | 1.2 to 3.2 | 1.9 | 2.2 | 0.9 | 2.9 | 3.7 |
| Unusual tiredness | 17 | 5 | 12 | 812 | 7.3 | 4.6 to 11.6 | 4.9 | 10.1 | 9.7 | 5.8 to 15.1 | 98.7 | 2.1 | 1.2 to 3.3 | 1.9 | 2.1 | 1.1 | 1.6 | 4.6 |
| Involuntary weight loss | 11 | 5 | 6 | 304 | 12.7 | 7.1 to 22.7 | 10.9 | 14.8 | 6.3 | 3.2 to 11.0 | 99.5 | 3.5 | 1.8 to 6.2 | 4.2 | 3.1 | 2.0 | 0.9 | 7.8 |
| >1 non-specific symptom | 13 | 4 | 9 | 381 | 11.6 | 6.8 to 19.7 | 7.4 | 17.1 | 7.4 | 4.0 to 12.4 | 99.4 | 3.3 | 1.8 to 5.6 | 2.9 | 3.5 | 2.0 | 1.7 | 6.6 |
| Any non-specific symptom | 29 | 10 | 19 | 1500 | 6.8 | 4.8 to 9.5 | 4.9 | 9.0 | 16.6 | 11.4 to 22.9 | 97.5 | 1.9 | 1.3 to 2.7 | 1.9 | 1.9 | 0.7 | 2.2 | 4.2 |
Among patients with at least one abdominal symptom, non-specific symptoms occurred in 29 (38.2%) of the 76 patients with cancer, and in 1500 (24.6%) of the 6102 patients without cancer (Table 2). There was no statistical difference in the recording of non-specific symptoms between patients with abdominal and non-abdominal types of cancer (data not shown).
Measures of association and predictive value of abdominal symptoms in relation to new abdominal cancer
The LR was slightly higher for females than males for most symptoms (Table 2). Any abdominal symptom had a sensitivity of 43.4%, and >1 abdominal symptom a sensitivity of 22.3%. The three symptoms indicating irregular bleeding, that is, rectal bleeding, unexpected genital bleeding, and macroscopic haematuria, had higher specificity than the other symptoms, ranging from 99.4% to 99.8% (Table 2).
Three symptoms reached the cancer referral guideline PPV threshold of 3% in England:12 macroscopic haematuria, rectal bleeding, and involuntary weight loss (Table 2). In the oldest age groups, several symptoms reached this threshold. The highest PPVs were for unexpected genital bleeding (8.1% in the 55–74 years age group) and macroscopic haematuria (7.9% for patients aged ≥75 years) (Table 2).
Table 3 shows the HRs for all single symptoms investigated in a multivariate model, with patients without symptoms as the reference group. Symptoms included upper abdominal pain, lower abdominal pain, constipation, and rectal bleeding, while the remaining single symptoms were grouped together in one variable. The highest HR for a single symptom was for rectal bleeding (HR 19.1, 95% confidence interval [CI] = 8.7 to 41.7). For ≥3 abdominal symptoms, the HR was 14.0 (95% CI = 9.1 to 21.6). The HR for males was 1.8 (95% CI = 1.4 to 2.5), indicating that, if symptoms were recorded, the probability of cancer being diagnosed was higher in males.
Table 3.
Association between some important abdominal symptoms and new abdominal cancer diagnosed within 180 days after consultation, expressed as hazard ratio, N = 61 337a
| Symptom | New abdominal cancer diagnosed within 180 days, n= 175 | Male, n= 95 | Female, n= 80 | No cancer, n= 61 162 | HR | 95% CI | HR male | HR female |
|---|---|---|---|---|---|---|---|---|
| No abdominal symptom | 99 | 62 | 37 | 55 060 | 1.0 | Ref | 1.0 | 1.0 |
| Abdominal pain, upper part, single symptom | 5 | 1 | 4 | 663 | 4.8 | 1.9 to 11.8 | 1.9 | 8.5 |
| Abdominal pain, lower part, single symptom | 5 | 2 | 3 | 608 | 5.8 | 2.4 to 14.3 | 3.8 | 9.1 |
| Constipation, single symptom | 3 | 0 | 3 | 141 | 6.8 | 2.1 to 21.8 | 17.3 | |
| Rectal bleeding, single symptom | 7 | 0 | 7 | 191 | 19.1 | 8.7 to 41.7 | 49.5 | |
| Any other single abdominal symptoms, grouped together | 17 | 11 | 6 | 1751 | 4.7 | 2.8 to 7.9 | 4.9 | 4.3 |
| Two abdominal symptoms | 12 | 8 | 4 | 1574 | 4.6 | 2.5 to 8.5 | 5.6 | 3.5 |
| ≥3 abdominal symptoms | 27 | 11 | 16 | 1174 | 14.0 | 9.1 to 21.6 | 10.2 | 21.1 |
| Male versus female sex | 1.8 | 1.4 to 2.5 |
Multivariate Cox analysis. The model includes mutually exclusive groups (one patient cannot be part of more than one group) containing selected single symptoms, any other remaining symptoms grouped together, combinations of two symptoms, and of ≥3 symptoms. Patients without symptoms are the reference group. HR = hazard ratio. HR is shown for single symptoms and for multiple symptoms, regardless of whether there were also non-specific symptoms. LR = likelihood ratio. PPV = positive predictive value. Ref = reference.
Table 4 shows the HRs for different combinations of symptoms. The reference group was patients without symptoms. The HR is shown for combinations of two symptoms — at least one of which was upper or lower abdominal pain — regardless of whether there were additional symptoms. The highest HR was for all combination categories containing upper abdominal pain and rectal bleeding (HR 64.2 (95% CI = 26.9 to 153.1).
Table 4.
Sex-adjusted hazard ratios for the most important combinations of two symptoms, with or without additional symptomsa, N = 61 337
| Symptom combinations | New abdominal cancer diagnosed within 180 days, n= 175 | Male, n= 90 | Female, n= 85 | No cancer, n= 61 162 | HR | 95% CI | HR, male | HR, female |
|---|---|---|---|---|---|---|---|---|
| No abdominal symptoms | 99 | 62 | 37 | 55 060 | 1.0 | Ref | 1.0 | 1.0 |
| Abdominal pain, upper part + lower part | 7 | 3 | 4 | 543 | 8.1 | 3.7 to 17.6 | 6.7 | 11.1 |
| Abdominal pain, upper part + constipation | 7 | 4 | 3 | 194 | 22.2 | 10.1 to 48.5 | 19.8 | 26.1 |
| Abdominal pain, upper part + diarrhoea | 5 | 3 | 2 | 361 | 11.5 | 4.6 to 28.8 | 17.6 | 8.8 |
| Abdominal pain, upper part + distended abdomen | 11 | 5 | 6 | 458 | 15.0 | 8.0 to 28.3 | 12.4 | 19.9 |
| Abdominal pain, upper part + increased belching | 9 | 5 | 4 | 263 | 23.2 | 11.4 to 46.7 | 21.1 | 26.1 |
| Abdominal pain, upper part + acid regurgitations | 8 | 4 | 4 | 440 | 13.3 | 6.3 to 27.6 | 16.6 | 12.9 |
| Abdominal pain, upper part + rectal bleeding | 6 | 3 | 3 | 47 | 64.2 | 26.9 to 153.1 | 57.5 | 100.5 |
| Abdominal pain, upper part + other abdominal problem | 7 | 3 | 4 | 200 | 22.3 | 10.2 to 48.8 | 19.6 | 24.2 |
| Abdominal pain, lower part + constipation | 7 | 3 | 4 | 296 | 12.6 | 5.8 to 27.5 | 9.2 | 19.7 |
| Abdominal pain, lower part + distended abdomen | 6 | 3 | 3 | 449 | 7.9 | 3.4 to 18.0 | 7.1 | 9.6 |
| Abdominal pain, upper part + lack of appetite | 14 | 6 | 8 | 457 | 17.2 | 9.7 to 30.5 | 14.9 | 22.4 |
| Abdominal pain, upper part + unusual tirednesss | 10 | 4 | 6 | 342 | 16.8 | 8.6 to 32.8 | 13.4 | 23.3 |
| Abdominal pain, upper part + unexpected weight loss | 5 | 3 | 2 | 130 | 21.6 | 8.6 to 54.2 | 30.8 | 18.2 |
Criteria for analyses: all combinations to have at least 50 patients presenting with that combination and at least five cases of cancer with that combination. Multivariate Cox analyses, with each row representing one separate model. Patients without symptoms as reference group. Because the combinations are with or without additional symptoms, some of them may occur in more than one model. HR = hazard ratio. Ref = reference.
Table 5 shows the distribution of recorded symptoms for the main types of new abdominal cancers, diagnosed within 180 days. The proportion of symptomatic cases varies for different cancers; there is a considerable variety of symptoms for most cancers, which is most pronounced in colorectal cancer, that is, colon cancer or rectal cancer. Abdominal pain was present in all individual cancer types. The three irregular bleeding symptoms were notable in that they each related strongly to cancer in one type of organ:
of 15 patients diagnosed with a new abdominal cancer who had reported rectal bleeding, 14 had either colon or rectal cancer;
of three patients with a new abdominal cancer who reported unexpected genital bleeding, two had uterine body cancer and one had cervical cancer; and
of six patients with a new abdominal cancer and macroscopic haematuria, three had bladder cancer and one had renal cancer. One patient had uterine body cancer.
Table 5.
Symptoms recorded at consultation in the main types of new abdominal cancer, diagnosed within 180 days after consultation
| Patients with cancera | Oesophagal cancer | Stomach cancer | Pancreatic cancer | Primary hepatic cancer | Biliary cancer | Colon cancer | Rectal cancer | Cervical cancer | Uterine body cancer | Ovarian cancer | Renal cancer | Bladder cancer | Prostate cancer | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | 175 | 5 | 6 | 9 | 7 | 3 | 37 | 17 | 6 | 8 | 4 | 12 | 17 | 34 |
| Hereof patients with symptom(s) recorded at consultation, n | 76 | 3 | 5 | 2 | 3 | 1 | 22 | 12 | 2 | 3 | 3 | 4 | 4 | 8 |
| Abdominal symptoms | ||||||||||||||
| Abdominal pain, upper part | 31 | 2 | 4 | 2 | 1 | 1 | 7 | 5 | 1 | 1 | 2 | 2 | ||
| Abdominal pain, lower part | 20 | 1 | 6 | 3 | 1 | 1 | 2 | 1 | 2 | |||||
| Constipation | 16 | 1 | 1 | 6 | 4 | 1 | 1 | |||||||
| Diarrhoea | 8 | 4 | 2 | 1 | ||||||||||
| Distended abdomen, bloating | 17 | 1 | 2 | 6 | 4 | 1 | 1 | |||||||
| Increased belching, flatulence | 9 | 1 | 2 | 1 | 2 | 2 | 1 | |||||||
| Acid regurgitation | 10 | 2 | 1 | 1 | 4 | 1 | 1 | |||||||
| Rectal bleeding | 15 | 1 | 8 | 6 | ||||||||||
| Unexpected genital bleeding | 3 | 1 | 2 | |||||||||||
| Macroscopic haematuria | 6 | 1 | 1 | 3 | ||||||||||
| Increased urinary frequency | 10 | 1 | 1 | 1 | 1 | 2 | 3 | |||||||
| Other abdominal problems | 18 | 3 | 3 | 2 | 5 | 1 | 1 | 2 | ||||||
| Non-specific symptoms when at least one abdominal symptom has been recorded | ||||||||||||||
| Lack of appetite | 18 | 2 | 1 | 1 | 1 | 4 | 2 | 1 | 1 | |||||
| Unusual tiredness | 17 | 2 | 1 | 1 | 1 | 5 | 2 | 1 | 1 | 2 | ||||
| Involuntary weight loss | 11 | 1 | 1 | 1 | 3 | 2 | 1 | 1 | ||||||
The total number of patients in this column may be higher than the sum of the other columns because a few rare or more undetermined types of abdominal cancer have not been included in the table.
In all, 20 of the 24 patients with a new abdominal cancer who had irregular bleeding, or 22 of the 24 if we count the cervical and the renal cancer, had cancers typically associated with such bleeding. This degree of specificity was not observed for other symptoms; for example, only six of 16 patients with a new abdominal cancer who had constipation and six of 20 patients with a new abdominal cancer who had lower abdominal pain had colon cancer.
DISCUSSION
Summary
Abdominal symptoms were associated with a new cancer in the abdominal region; the strength of these associations confirms the importance of responding appropriately to abdominal symptoms presenting in primary care.13–15 However, a high proportion of diagnoses of a new cancer in the abdomen did not feature such symptoms during consultations in the months before diagnosis. This duality underlines the need for a high index of suspicion and use of clinical judgement in all phases of an illness episode. Symptoms are important but, alone, have insufficient sensitivity and specificity to underpin cancer diagnostic decisions. The diagnostic importance of PPV is considerable: ‘low’ values may still prompt action, illustrated by the lowering of the threshold to 3% in referral guidelines for England,12 and additional information can increase or decrease a symptom’s contribution to the PPV,16–18 as demonstrated by the higher PPVs for certain symptoms in higher age groups.
This study also provides important information on the relative significance of different types of symptoms: there was a high HR for rectal bleeding as a single symptom and the three bleeding symptoms showed high specificity for certain types of cancer. In addition, the broad range of cancers affected by abdominal pain and the variety of symptoms in colorectal cancer are important findings. Three or more abdominal symptoms increased the probability of cancer, as did some combinations of symptoms, especially upper abdominal pain with rectal bleeding and upper abdominal pain with a non-specific symptom.
Symptoms related to sex-specific types of cancer, such as ovarian and prostate cancer, could be part of the explanation for sex differences if they were highly associated with abdominal symptoms; however, both of these cancers were relatively symptom poor in another study with cross-sectional registration before diagnosis.19
The higher proportion of abdominal cancer in males is largely due to the high number of prostate cancers, and the fact that the most common cancer in females — breast cancer — is not located within the abdominal cavity.
Strengths and limitations
The prospective nature of the follow-up ensured that neither the patient nor the GP knew about the cancer diagnosis at the time of symptom registration, reducing the risk of the bias that is often inherent in retrospective studies. However, symptoms presenting before diagnosis but after the initial consultation do not show in the cross-sectional data from the consultations, and thus do not contribute to the sensitivity figures reported. Consecutive patients were registered, with no selection bias; all common abdominal symptoms were investigated.
The authors believe HRs give a complex, but also rather complete and precise, estimate of the association between symptoms and cancer. Sensitivity analyses for all 251 patients with a new abdominal cancer — which included those whose cancers were diagnosed >180 days after the consultation — resulted in HRs that were slightly lower than in Tables 3 and 4. This adds weight to the assumption that symptoms recorded >180 days before diagnosis may be less related to the subsequent cancer. Sensitivity analyses of all patients diagnosed with a new cancer consistently gave slightly higher or lower HRs in the expected direction, increasing the reliability of the estimates.
Comparison with existing literature
It has been shown previously that abdominal symptoms commonly precede diagnoses of abdominal cancers.14,19 This study provides a more detailed description of different symptoms located in the abdominal region. Such symptoms should alert clinicians to the possibility of abdominal cancers, without it being forgotten that they may also act as lower-risk symptoms20 in relation to other cancers.
Higher risk of cancer in males who have symptoms compared with females who have similar symptoms, and the cumulative effect of multiple symptoms, are consistent with findings from a primary care-based, colorectal cancer study by Lawrenson et al.21 Hippisley-Cox and Coupland9,22 also used a large primary care database and included information from the patient’s medical history and on anaemia, as well as combining different abdominal symptoms and constructing diagnostic algorithms. They analysed HRs and found values not dissimilar to the ones resulting from this study, with especially high values for haematuria in relation to renal tract cancer, and rectal bleeding in relation to colorectal cancer.
Some of the symptoms studied here have been shown in previous studies to have higher PPV for specified types of cancer23,24 and specific age groups; this is consistent with the findings presented here. Referral guidelines may be interpreted and applied differently in different GP practices, and GP knowledge of PPV values may have an impact on reducing variation in referral thresholds.25
Colorectal cancer is common and has been studied in primary care more than most other types of cancer. Hamilton20,26 found that fewer than half of patients with colorectal cancer experienced rectal bleeding and emphasised the important role of ‘low-risk-but-not-no-risk‘ symptoms, which are typically less likely to lead to a fast-track referral in order to speed up a diagnosis.
Non-specific symptoms have been shown elsewhere to be associated with rectal bleeding in patients with colorectal cancer,27 and this was the case for several of the patients with rectal bleeding and colorectal cancer in the results presented here. In line with this, a study from Sweden found that rectal bleeding combined with either diarrhoea, constipation, change in bowel habit, or abdominal pain is a predictor of non-metastatic colorectal cancer; this confirmed that there is a window of opportunity for GPs to have a positive impact on patient prognosis.28
Non-specific symptoms have been shown to have poor association with cancer if occurring alone,29 but their importance in terms of diagnosis can increase if they occur in combination with alarm symptoms; the findings presented here concur with this.
Several studies on the relationship between symptoms and cancer have used a longer observation period, commonly 12 months21 or 24 months.9 The authors’ reasons for choosing 6 months have been explained in the Method section previously, and the sensitivity analyses performed seem to suggest that this was a wise approach, giving more precise HRs.
Implications for research and practice
A patient with abdominal cancer can present in a range of ways — they may be asymptomatic or they may have multiple symptoms. Almost all reasons for the consultation require further questioning and examination before the GP may suspect cancer and refer appropriately. This study is relevant for real-life consultations in primary care because all investigated symptoms in this study were associated with an abdominal cancer; however, different symptoms were related to cancer in different ways. The three symptoms that involved bleeding had particularly high specificity for the individual cancer type most associated with that symptom. Any of these three irregular bleeding symptoms should, therefore, lead to further investigation or referral unless a benign cause can be determined. Even then, bleeding haemorrhoids, for example, do not exclude a more proximal cancer or polyp; likewise, urinary infection with haematuria may mask bladder cancer. The case of uterine body cancer with recorded haematuria should remind GPs that uterine bleeding in rare cases may appear as haematuria to the patient, with a finding of blood in the urine.
Abdominal pain as a presenting symptom showed sensitivity for both common and not-so-common types of abdominal cancer and should not be ignored, in spite of having a lower specificity than other symptoms. For colorectal cancer, almost all investigated symptoms warrant investigation. Several combinations of symptoms call for GPs to demonstrate increased vigilance.
This study adds further prospective data to inform cancer diagnostic processes in primary care, and encourages continued primary care research about symptoms and cancer.
Acknowledgments
The authors sincerely thank the GPs in six different countries who collected the patient data, along with Peter Vedsted, Lisbeth Ellegaard, and Børge Hart for important work related to planning and data collection; Josef Schwarz, Marjolein Truyers, Victoria Hammersley, Sara Dorsman, and Marianne Heshusius for help with data collection; Knut Hansen for converting files with consultations into files with patients; and Tom Wilsgaard for help with power calculations.
Funding
This study was funded by the Norwegian Research Council (project no. 202663). The Department of Community Medicine, UiT The Arctic University of Norway, contributed a salary for L Ellegaard who assisted with collecting patient data.
Ethical approval
The Regional Committee for Medical and Health Research Ethics of Northern Norway approved the survey protocol (ref. 2010/1056-4). Ethical approval was given thereafter in the other five participating countries. No patients were contacted; only the individual GP knew the identity of the patient.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Cancer Registry of Norway. Institute of Population-Based Cancer Research. Cancer in Norway 2016 Cancer incidence, mortality, survival and prevalence in Norway. https://www.kreftregisteret.no/globalassets/cancer-in-norway/2016/cin-2106.pdf (accessed 13 Feb 2018)
- 2.Holtedahl K, Vedsted P, Borgquist L, et al. Abdominal symptoms in general practice: frequency, cancer suspicions raised, and actions taken by GPs in six European countries. Cohort study with prospective registration of cancer. Heliyon. 2017;3(6):e00328. doi: 10.1016/j.heliyon.2017.e00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allgar VL, Neal RD. General practitioners’ management of cancer in England: secondary analysis of data from the National Survey of NHS Patients — cancer. Eur J Cancer Care. 2005;14(5):409–416. doi: 10.1111/j.1365-2354.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 4.Demagny L, Holtedahl K, Bachimont J, et al. General practitioners’ role in cancer care: a French–Norwegian study. BMC Res Notes. 2009;2:200. doi: 10.1186/1756-0500-2-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin G, Berendsen A, Crawford SM, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16(12):1231–1272. doi: 10.1016/S1470-2045(15)00205-3. [DOI] [PubMed] [Google Scholar]
- 6.Elnegaard S, Andersen RS, Pedersen AF, et al. Self-reported symptoms and healthcare seeking in the general population — exploring ‘The Symptom Iceberg’. BMC Public Health. 2015;15:685. doi: 10.1186/s12889-015-2034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtedahl KA. Diagnosis of cancer in general practice: a study of delay problems and warning signals of cancer, with implications for public cancer information and for cancer diagnostic strategies in general practice. Munin open research archive, UiT The Arctic University of Norway; 1991. http://hdl.handle.net/10037/2325 (accessed 13 Feb 2018) [Google Scholar]
- 8.Astin M, Griffin T, Neal RD, et al. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011. DOI: https://doi.org/10.3399/bjgp11X572427. [DOI] [PMC free article] [PubMed]
- 9.Hippisley-Cox J, Coupland C. Identifying patients with suspected colorectal cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract. 2012. DOI: https://doi.org/10.3399/bjgp12X616346. [DOI] [PMC free article] [PubMed]
- 10.Mayor S. Abdominal symptoms are poor at predicting ovarian cancer, study finds. BMJ. 2010;340:c581. [Google Scholar]
- 11.Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–1267. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence. Suspected cancer: recognition and referral. NG12. London: NICE; 2015. https://www.nice.org.uk/guidance/ng12 (accessed 13 Feb 2018) [PubMed] [Google Scholar]
- 13.Holtedahl KA. The value of warning signals of cancer in general practice. Scand J Prim Health Care. 1987;5(3):140–143. doi: 10.3109/02813438709013994. [DOI] [PubMed] [Google Scholar]
- 14.Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. 2007;334(7602):1040. doi: 10.1136/bmj.39171.637106.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton W. Cancer diagnosis in primary care. Br J Gen Pract. 2010. DOI: https://doi.org/10.3399/bjgp10X483175. [DOI] [PMC free article] [PubMed]
- 16.Holtedahl KA. Probability revision in general practice: occult blood in stool in patients with indigestion, and daily smoking in patients who cough. Allgemeinmedizin. 1990;19:35–38. https://munin.uit.no/handle/10037/12453 (accessed 28 Mar 2018). [Google Scholar]
- 17.Lyratzopoulos G, Vedsted P, Singh H. Understanding missed opportunities for more timely diagnosis of cancer in symptomatic patients after presentation. Br J Cancer. 2015;112(Suppl 1):S84–S91. doi: 10.1038/bjc.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donker GA, Wiersma E, van der Hoek L, Heins M. Determinants of general practitioner’s cancer-related gut feelings — a prospective cohort study. BMJ Open. 2016;6(9):e012511. doi: 10.1136/bmjopen-2016-012511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheel BI, Holtedahl K. Symptoms, signs, and tests: the general practitioner’s comprehensive approach towards a cancer diagnosis. Scand J Prim Health Care. 2015;33(3):170–177. doi: 10.3109/02813432.2015.1067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton W. The CAPER studies: five case–control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101(Suppl 2):S80–S86. doi: 10.1038/sj.bjc.6605396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrenson R, Logie J, Marks C. Risk of colorectal cancer in general practice patients presenting with rectal bleeding, change in bowel habit or anaemia. Eur J Cancer Care. 2006;15(3):267–271. doi: 10.1111/j.1365-2354.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 22.Hippisley-Cox J, Coupland C. Identifying patients with suspected renal tract cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract. 2012. DOI: https://doi.org/10.3399/bjgp12X636074. [DOI] [PMC free article] [PubMed]
- 23.Shapley M, Mansell G, Jordan JL, Jordan KP. Positive predictive values of ≥5% in primary care for cancer: systematic review. Br J Gen Pract. 2010. DOI: https://doi.org/10.3399/bjgp10X515412. [DOI] [PMC free article] [PubMed]
- 24.Bruyninckx R, Buntinx F, Aertgeerts B, Van Casteren V. The diagnostic value of macroscopic haematuria for the diagnosis of urological cancer in general practice. Br J Gen Pract. 2003;53(486):31–35. [PMC free article] [PubMed] [Google Scholar]
- 25.Burton CD, McLernon DJ, Lee AJ, Murchie P. Distinguishing variation in referral accuracy from referral threshold: analysis of a national dataset of referrals for suspected cancer. BMJ Open. 2017;7(8):e016439. doi: 10.1136/bmjopen-2017-016439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton W. Five misconceptions in cancer diagnosis. Br J Gen Pract. 2009. DOI: https://doi.org/10.3399/bjgp09X420860. [DOI] [PMC free article] [PubMed]
- 27.Wauters H, Van Casteren V, Buntinx F. Rectal bleeding and colorectal cancer in general practice: diagnostic study. BMJ. 2000;321(7267):998–999. doi: 10.1136/bmj.321.7267.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewing M, Naredi P, Zhang C, Månsson J. Identification of patients with non-metastatic colorectal cancer in primary care: a case-control study. Br J Gen Pract. 2016. DOI: https://doi.org/10.3399/bjgp16X687985. [DOI] [PMC free article] [PubMed]
- 29.Ingebrigtsen SG, Scheel BI, Hart B, et al. Frequency of ‘warning signs of cancer’ in Norwegian general practice, with prospective recording of subsequent cancer. Fam Pract. 2013;30(2):153–160. doi: 10.1093/fampra/cms065. [DOI] [PubMed] [Google Scholar]