Abstract
Glioblastoma in adults, and medulloblastoma and pineoblastoma that mainly affect children, are aggressive brain tumors. The survival for patients with glioblastoma remains dismal. While the cure rate for medulloblastoma exceeds 70%, this figure has stagnated over the past few decades and survivors still contend with significant long-term debilitating side effects. The prognosis for pineoblastoma is age-dependent, with little chance of a cure for children younger than three years. More effective molecularly targeted strategies are urgently required to treat these cancers. Hyper-activation of epidermal growth factor receptor (EGFR) signaling is characteristic of several different classes of human cancers, including a subset of glioblastoma and medulloblastoma. This has provided the impetus for the development of a suite of EGFR pathway blockers, including second generation irreversible inhibitors, such as dacomitinib. We have developed a comprehensive drug evaluation pipeline, including in vitro interaction analyses and orthotopic xenograft mouse models, to address the efficacy of drugs for brain tumor treatment, enabling the exclusion of potentially ineffective treatments and prioritization of truly beneficial novel treatments for clinical trial. We used this system to examine the effects of dacomitinib as a single agent, or in combination with conventional chemotherapeutics, on the growth of human adult and pediatric brain tumor cell lines. Dacomitinib inhibited EGFR or EGFRvIII activity in vitro in all three tumor types tested, and as a single agent induced a modest increase in survival time for mice bearing glioblastoma, which accurately predicted human clinical trial data. For pediatric medulloblastoma, dacomitinib blocked EGFR/HER signalling in orthotopic xenografts and extended median survival as a single agent, however was antagonistic when used in combination with standard frontline medulloblastoma chemotherapies. The findings caution against the use of dacomitinib for pediatric brain tumor clinical trials.
Abbreviations: 4HPC, 4-hydroperoxycyclophosphamide; CC3, cleaved caspase-3; CI, combination index; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; EGFRvIII, constitutively active mutant form of the epidermal growth factor receptor; SMO, ND2:SmoA1 transgenic mouse
Introduction
Glioblastoma and medulloblastoma are the most common types of malignant brain tumor affecting adults and children, respectively. Although there has been significant progress in understanding the molecular pathogenesis of these tumor types, this has yet to translate to improved outcomes. Glioblastoma continues to have a dismal prognosis in both adults and children [1], [2] and while the cure rate for medulloblastoma exceeds 70% [3], this survival rate has stagnated over the past few decades at a level well below that of other childhood cancers, such as leukemia [4]. Moreover, survivors still contend with significant long-term debilitating side effects. Pineoblastoma is a rare and aggressive tumor of the pineal gland, which mainly affects children. The molecular biology of this disease remains inadequately understood and the prognosis is variable depending on age; with infants having little chance of a cure, while children over the age of three years treated with radiotherapy have survival outcomes similar to medulloblastoma [5]. Standard of care frontline treatment for glioblastoma includes surgery, radiotherapy and temozolomide chemotherapy [6], while for medulloblastoma and pineoblastoma, surgery and craniospinal radiotherapy are generally combined with multiple DNA alkylators and the tubulin inhibitor, vincristine [7]. Improved outcomes for brain tumor patients depend on the development of more effective targeted therapies that not only increase survival, but also reduce treatment related side-effects, particularly for pediatric patients.
The human epidermal growth factor (EGF) family of receptor tyrosine kinases consists of four members, commonly referred to as EGFR, ERBB2, ERBB3 and ERBB4. The four proteins function as homo- or heterodimers, and interact with a variety of EGFR family ligands to regulate diverse aspects of cell growth and development in a context specific manner. Hyper-activation of EGFR signaling linked to amplification, overexpression or mutation of the EGFR family genes plays a critical role in driving the initiation and progression of several common classes or subtypes of human cancers [8], [9]. For this reason, the development of new drugs and therapeutic strategies aimed at blocking EGFR signaling in cancer cells has been pursued for many years, and continues to be a major focus of research laboratories world-wide. In the context of human brain tumors, aberrant EGFR signaling has been linked to the pathogenesis of a subset of glioblastoma and medulloblastoma. Approximately 40% of glioblastomas are associated with EGFR amplification and overexpression, and in ~60% of these cases EGFR amplification is associated with deletion of exons 2-7 (referred to as the EGFRvIII mutation) [10]. First generation EGFR inhibitors, such as erlotinib and gefitinib, which bind reversibly to EGFR have been disappointing for the treatment of glioblastoma for various reasons including pathway redundancy, the development of resistance through downstream mutations, aberrant receptor dimerization, and difficulties crossing the blood brain barrier [11]. Overexpression of ERBB2 and/or ERBB4 occurs in a subset of medulloblastoma, although the prognostic significance of these phenomena remain controversial [12]. Earlier studies [13], [14], [15], [16], [17] reported poorer outcomes associated with overexpression of ERBB2 alone, or in combination with ERBB4; however, the clinical significance and efficacy of EGFR/ERBB inhibitors for the treatment of human medulloblastoma has not been comprehensively assessed.
Dacomitinib (PF299804, Pfizer) is a second-generation pan-ERBB inhibitor that irreversibly and selectively binds to the ATP binding pockets of EGFR, ERBB2 and ERBB4 at low nanomolar affinities [18]. These second-generation inhibitors are considered clinically superior to their reversible predecessors because they may block the activity of multiple receptors simultaneously and for a longer duration, while maintaining at least partial activity in the presence of mutant receptors that render first generation inhibitors ineffective [19]. Animal studies for dacomitinib have demonstrated encouraging bio-availability (>50%) and half-life (>12 hours) [20]. Recent pre-clinical data suggest that dacomitinib effectively blocked the growth of EGFR amplified and/or EGFR mutant glioblastoma cells in vitro and in intracranial xenografts [21], [22]. Moreover, dacomitinib has shown promise for the treatment of non-small cell lung cancer, squamous cell carcinoma of the head and neck, and in a subset of glioblastomas [23], [24], [25], [26]. The effects of dacomitinib on medulloblastoma or pineoblastoma cell growth have not been assessed.
As part of our efforts to identify more effective drugs for the treatment of brain tumors, we developed a comprehensive drug evaluation pipeline, in which the efficacy of new drugs for the treatment of brain tumors can be determined, enabling the exclusion of potentially ineffective treatments and prioritization of truly beneficial novel treatments for clinical trial. Using this system, we examined the effects of dacomitinib as a single agent, or in combination with conventional chemotherapeutics currently used in the clinic, on the growth of human adult and pediatric brain tumor cells in vitro and in vivo.
Materials and Methods
Cell Lines and Culture Conditions
The human glioblastoma cell line, U87MG, and the human medulloblastoma cell line Daoy, were purchased from the American Type Culture Collection. U87MG cells were transduced with retrovirus to express green fluorescent protein (GFP) and a puromycin acetyltransferase/luciferase fusion protein (pacLuc2) using the retroviral expression constructs MSCV-ires-GFP and MSCV-ires-pacLuc2 (U87.Luc2). U87MG cells were also transduced to express pacLuc2, GFP, and a constitutively active form of the EGF receptor (EGFRvIII) using the retroviral expression constructs MSCV-ires-pacLuc2 and MSCV-EGFRvIII-ires-GFP (U87vIII.Luc2). Retroviral constructs were kindly provided by Drs Suzanne Baker and Richard Williams of St Jude Children’s Research Hospital (Memphis, TN, USA). Daoy cells were also retrovirally-transduced to express luciferase using MSCV-ires-pacLuc2 (Daoy.Luc2). Medulloblastoma and glioblastoma cells were cultured in DMEM (Gibco) supplemented with Glutamax (Invitrogen), and 10% fetal bovine serum (FBS). The human patient-derived medulloblastoma and pineoblastoma cell lines, PER547, PER452 and PER453 were gifted from Prof Ursula Kees [27], and retrovirally-transduced to express luciferase using MSCV-ires-pacLuc2 (547.Luc2, 452.Luc2 and 453.Luc2). These cells were cultured in RPMI (Gibco) supplemented with Glutamax, 1 mM sodium pyruvate (Invitrogen), non-essential amino acids (Invitrogen), 50 μM 2-mercaptoethanol (Sigma-Aldrich) and 10% FBS. Short-term GBM6 cells [28] were not modified by retrovirus and were cultured in KnockOut DMEM/F12 supplemented with Glutamax and StemPro Neural Supplement (all Gibco). Growth factors (recombinant human EGF and basic FGF, Shenandoah Biotechnology) were added at 20 ng/mL. Cells were cultured at 37°C in 5% CO2 for all experiments.
InVitro Drug Sensitivity Assays
Dacomitinib (Pfizer or Eurasian Chemicals) and the activated form of cyclophosphamide, 4-hydroperoxycyclophosphamide (4HPC; Toronto Research Chemicals) were dissolved in DMSO (Sigma-Aldrich). Vincristine sulfate was supplied in saline (Hospira). Cells (5,000/well) were plated in 384 well plates using a Microlab NIMBUS (Hamilton). Drug dilutions were prepared in DMSO (or saline in the case of vincristine) and further diluted in media prior to addition to cells in a combination array matrix. Cells were treated for 72 hours and incubated with alamar blue (0.6 mM resazurin, 1 mM potassium hexacyanoferrate (II) trihydrate, 1 mM potassium hexacyanoferrate (III), 2.5% methylene blue (all from Sigma-Aldrich)) for the final 6 hours of treatment. Fluorescence was detected using a Biotek Synergy Mx with excitation/emission wavelengths of 570 nm and 590 nm, respectively. Raw fluorescence data were normalized to the fluorescence measured in the DMSO control, and expressed as a percentage of control. The ED50 (effective dose resulting in 50% survival) and the factor of cells affected (Fa) were interpolated from a best-fit dose-response curve determined using Prism v7 (GraphPad Software). CompuSyn was used to determine drug interactions [29].
Protein Extraction and Immunoblotting
U87.Luc2, U87vIII.Luc2, Daoy.Luc2 and 452.Luc2 cells were washed twice in phosphate buffered saline (PBS) and starved in medium containing 0.1% FBS for 3.5 hours prior to the addition of increasing concentrations of dacomitinib. U87.Luc2 and U87vIII.Luc2 cells were treated with 400 nM, 1 or 4 μM dacomitinib. Daoy.Luc2 and 452.Luc2 cells treated with 6.25, 25, 100 or 400 nM dacomitinib. Thirty minutes after the addition of dacomitinib, EGFR was stimulated by the addition of 20 ng/ml recombinant human EGF (Shenandoah Biotechnology). GBM6 cells were treated with 500 nM dacomitinib overnight prior to harvest. Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors (Roche). Immunoblotting was performed with specific primary antibodies, followed by horseradish peroxidase-conjugated secondary antibodies (GE Healthcare), detection using Supersignal West Dura (Pierce) and visualization in a BioRad Chemidoc. Antibodies used were phosphorylated EGFR (p-EGFR) Y1173 (Cell Signaling #4407, 1:1000), p-EGFR Y1068 (Cell Signaling #2234, 1:1000), total EGFR (Cell Signaling #4267, 1:1000), phosphorylated AKT (p-AKT) S473 (Cell Signaling #9271, 1:1000), pan AKT (Cell Signaling #4691, 1:1000), phosphorylated ERK1/2 (pERK1/2) T202/Y204 (Cell Signaling #9101, 1:1000), total ERK1/2 (Cell Signaling #9102, 1:1000), PTEN (Cell Signaling #9559, 1:1000), and β-actin (Sigma-Aldrich #A1978, 1:1000).
Immunofluorescence
U87vIII.Luc2 cells were seeded onto glass slides and cultured overnight in medium containing 10% FBS. Cells were treated with DMSO (0.01%) or 1 μM dacomitinib for 16 hours prior to fixation in 4% paraformaldehyde in PBS. Cells were blocked and permeabilized with PBS containing 0.1% Triton X-100 and 10% normal goat serum (Vector Laboratories) at room temperature for 1 hour. Cells were further incubated with an anti-phosphorylated EGFR Y1173 primary antibody (Cell Signaling #4407, 1:200) followed by a Cy3-conjugated anti-rabbit secondary antibody (Jackson Immunoresearch, 1:400). Nuclei counterstained with DAPI and slides coverslipped using VectorShield HardSet mounting medium (Vector Laboratories). Wide-field epifluorescence images were taken using a Nikon Ti Eclipse and NIS Elements software (Nikon).
Animals, Intracranial Implantation and Bioluminescence Imaging
All animal experiments were approved by the Animal Ethics Committee of the Telethon Kids Institute and performed in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes. The ND2:SmoA1 transgenic mouse (the SMO mouse) was a generous gift from James Olson (Fred Hutchinson Cancer Research Centre, Seattle, USA) and has been previously described [30], [31].
For orthotopic xenografts, cells were suspended in matrigel (BD Biosciences) (106 cells in a total volume of 5 μL) and implanted into the right cerebral hemisphere of 10-12 week old athymic female mice (Balb/c nude, Animal Resources Centre, Perth, Western Australia) using a Hamilton syringe. For survival studies, tumor size was monitored weekly by bioluminescence using an IVIS Spectrum (Caliper). Once tumors were established (as determined by bioluminescence) mice were treated as indicated in the text. Kaplan–Meier tumor-free survival curves were generated using Prism v7 (GraphPad Software) based on the number of days post-implantation until tumors caused morbidity requiring euthanasia.
InVivo Drug Administration
Cyclophosphamide (Endoxan, Baxter) was dissolved in saline and delivered intraperitoneally (150 mg/kg). Vincristine (Hospira) was purchased as a pre-prepared solution in saline and delivered intraperitoneally (0.5 mg/kg). Dacomitinib (Pfizer) was dissolved in 50 mM sodium lactate pH 4 (Sigma-Aldrich) and delivered by oral gavage (30 mg/kg or 50 mg/kg as indicated).
Immunohistochemistry (IHC)
Mouse brain tissue was fixed in 4% paraformaldehyde in PBS overnight and embedded in paraffin. Tissue sections (5 μm) underwent microwave antigen retrieval in a citrate buffer before immunostaining with the following antibodies: Ki-67 (Leica #NCL-Ki67p, 1:5000), cleaved caspase-3 (CC3) (BD #559565, 1:500), p-EGFR Y1173 (Cell Signaling #4407, 1:250), phosphorylated ErbB4 (pErbB4) Y984 (Cell Signaling #3790, 1:50), or total ErbB4 (Santa Cruz #SC-283, 1:200). Sections were developed using biotinylated secondary antibodies, followed by detection with an Elite ABC kit and NovaRED peroxidase substrate, then counterstained with Gill’s hematoxylin (all from Vector Laboratories). Ki67- and CC3-positive cells were quantified using a Nuance spectral unmixing camera and InForm Tissue Finder software (Perkin Elmer). Quantification of phosphorylated and total ErbB4 was performed using particle analysis in ImageJ [32].
Statistical Analyses
Unpaired two-tailed Student’s t tests were used to evaluate the statistical significance between treatment groups as applicable. Kaplan-Meier survival curves were compared using the log-rank (Mantel-Cox) test. All statistical analyses were performed using GraphPad Prism v7, with P < .05 considered significant.
Results
Dacomitinib Inhibits EGFR Signaling in Brain Tumor Cell Line Models
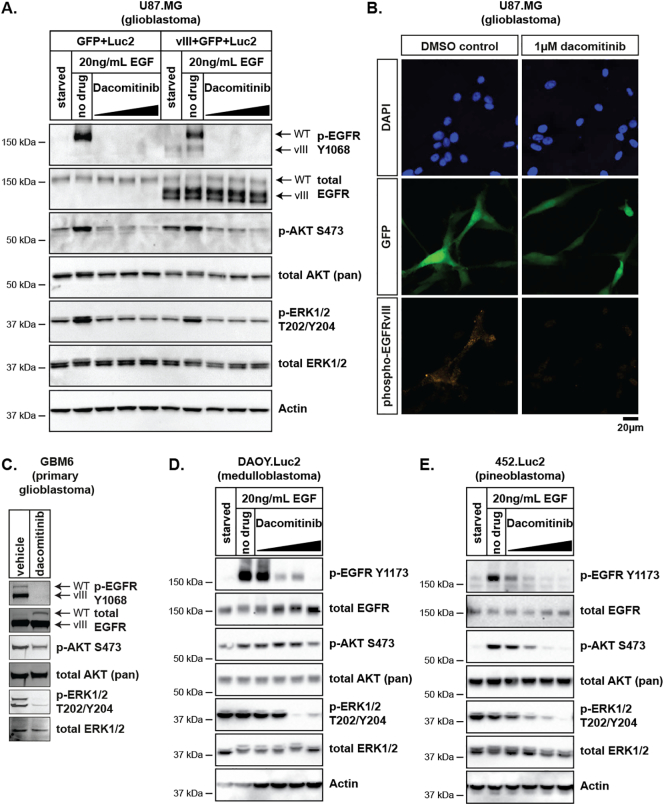
Upon ligand binding, EGFR receptors form homodimers or heterodimers which leads to activation of the intracellular tyrosine kinase domain. This in turn activates downstream signaling cascades, including the phosphoinositide 3-kinase, AKT and ERK1/2 pathways [33], [34]. To examine the effects of dacomitinib on EGFR signaling in glioblastoma, medulloblastoma and pineoblastoma cell lines, we assessed the expression and/or phosphorylation of EGFR and components of downstream pathways by immunoblot. In U87.Luc2 cells, full length EGFR was detectable with minimal phosphorylated EGFR (Y1173), whereas in U87vIII.Luc2 cells both full length EGFR and EGFRvIII were expressed, and phosphorylated EGFR (Y1173) was detectable in vehicle-treated cells (Figure 1A). Treatment with dacomitinib significantly reduced EGFRvIII phosphorylation (Y1173) at doses ranging from 0.4-4 μM. Inhibition of EGFRvIII phosphorylation was also observed by immunofluorescence in U87vIII.Luc2 cells exposed to dacomitinib in vitro (Figure 1B). Phosphorylation of downstream EGFR effectors AKT (S473) and ERK1/2 (T202/Y204) was also assessed in the U87.Luc2 and U87vIII.Luc2 cells (Figure 1A). Dacomitinib treatment decreased phosphorylation of both kinases, relative to EGF-stimulated/DMSO-treated controls, indicating that both receptor activity and downstream signaling was inhibited in glioblastoma cells. Similarly, in a short-term culture of GBM6 patient-derived glioblastoma cells that also harbor EGFR amplification and vIII-mediated activation [35], dacomitinib effectively blocked EGFR phosphorylation, and downstream AKT and ERK1/2 activation (Figure 1C).
Figure 1.
Dacomitinib inhibits EGFR activity and downstream signaling in glioblastoma, medulloblastoma and pineoblastoma cells. Immunoblot analyses of (A) U87.GFP.Luc2 and U87.vIII.Luc2 (C) GBM6 (D) Daoy.Luc2 and (E) 452.Luc2 cells using the indicated antibodies. Cells were treated with increasing concentrations of dacomitinib and protein was harvested as described in the methods. (B) U87MG glioblastoma cells were transduced with a retrovirus to drive expression of EGFRvIII, GFP and pacLuc2. Cells were cultured in the absence (DMSO) or presence of dacomitinib and phosphorylation of EGFRvIII was determined by immunofluorescence for phosphorylated EGFR (Y1173) (phospho-EGFRvIII, orange). Staining for GFP (green) confirms cells in the dacomitinib treated sample were successfully transduced. Nuclei were counterstained with DAPI (blue). Scale bar applies to all images.
The effect of dacomitinib in pediatric brain tumor cell cultures was consistent with these data. Dacomitinib potently inhibited full length EGFR phosphorylation in the Daoy.Luc2 medulloblastoma cell line (Figure 1D) and 452.Luc2 pineoblastoma cell line (Figure 1E). Moreover, AKT and ERK1/2 phosphorylation was also significantly reduced in both cell lines. Overall, these data indicate that dacomitinib inhibits EGFR phosphorylation in multiple brain tumor cell lines resulting in inhibition of downstream pathways in both pediatric brain tumor cells and adult glioblastoma cells.
Dacomitinib Inhibits ERBB Signaling in Orthotopic Brain Tumor Xenografts
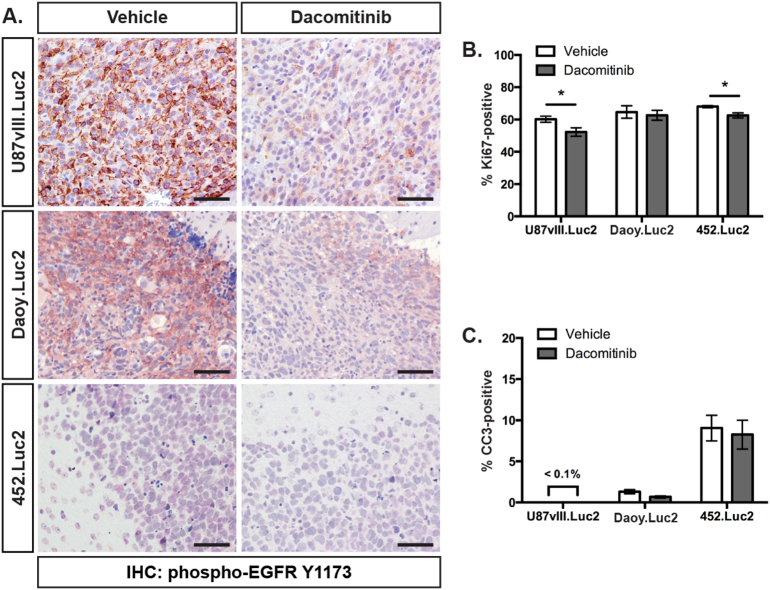
Dacomitinib potently inhibited EGFR signaling in vitro. To assess whether dacomitinib could inhibit EGFR signaling in vivo, mice carrying U87vIII.Luc2 glioblastoma, Daoy.Luc2 medulloblastoma or 452.Luc2 pineoblastoma intracranial xenografts were treated by oral gavage with 30 mg/kg dacomitinib. Twenty-four hours after treatment, brain tissue was harvested and examined immunohistochemically. Phosphorylated EGFR (Y1173) was reduced in dacomitinib-treated mice bearing U87vIII.Luc2 and Daoy.Luc2 tumors compared to vehicle controls, consistent with successful drug penetration of the tumor and subsequent receptor inhibition (Figure 2A). Phosphorylated EGFR levels were very low in 452.Luc2 pineoblastoma tumors, making receptor inhibition difficult to assess in these mice.
Figure 2.
Dacomitinib inhibits EGFR phosphorylation and reduces proliferation in brain tumor xenografts. (A) Representative sections of U87vlll.Luc2 glioblastoma, Daoy.Luc2 medulloblastoma and 452.Luc2 pineoblastoma tumors from vehicle- or dacomitinib-treated animals stained for phosphorylated EGFR (Y1173) (brown) and counterstained with hematoxylin (blue). Scale bar indicates 50 μm. The percentage of tumor cells positive for (B) Ki67 or (C) cleaved caspase-3 (CC3) was determined from U87vIII.Luc2, Daoy.Luc2 and 452.Luc2 xenografts after treatment with vehicle (white) or dacomitinib (grey). Bars show mean ± SEM. n = 4-7 mice per group. * P < .05.
The effects of dacomitinib treatment on tumor cell proliferation and apoptosis (measured by Ki67- and CC3-positivity, respectively) were also examined immunohistochemically 24 hours after treatment. At this time-point, a small but statistically significant decrease in proliferation was observed in dacomitinib-treated mice with U87vIII.Luc2 and 452.Luc2 xenografts (Figure 2B), with no significant increase in apoptosis observed in any tumors (Figure 2C).
Dacomitinib Treatment Delays Brain Tumor Growth and Improves Survival
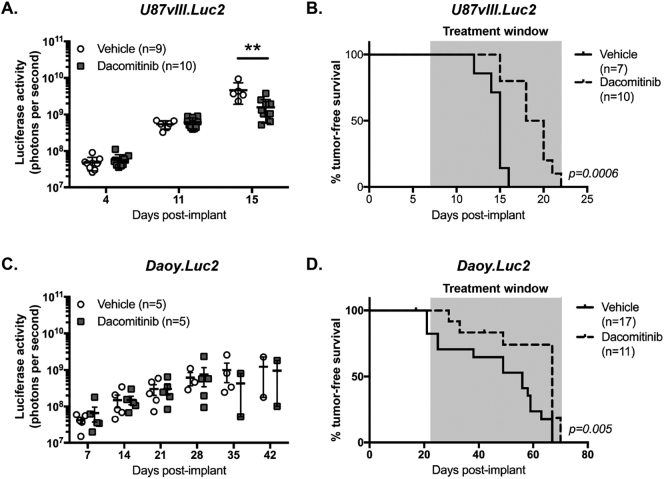
In vivo expression, and dacomitinib-induced inhibition, of exogenous EGFRvIII and endogenous EGFR were clearly observed in the U87vIII.Luc2 and Daoy.Luc2 models, respectively. Therefore, we assessed the impact of dacomitinib on the overall survival of these glioblastoma and medulloblastoma models. Orthotopic U87vIII.Luc2 glioblastoma xenografts were established in nude mice and treatment with dacomitinib (30 mg/kg) or vehicle (sodium lactate) was given thrice weekly. Bioluminescence assessment of tumor growth demonstrated that dacomitinib-treated glioblastomas were significantly smaller than control tumors at day 15 post-implant (Figure 3A). Consistent with the significantly reduced bioluminescence, Kaplan-Meier survival analyses revealed that dacomitinib significantly extended the lifespan of mice bearing U87vIII.Luc2 glioblastoma xenografts compared with controls (Figure 3B).
Figure 3.
Dacomitinib treatment delays glioblastoma tumor growth and improves survival in glioblastoma and medulloblastoma xenograft models. Mice with orthotopic xenografts of U87vIII.Luc2 or Daoy.Luc2 were treated with sodium lactate (vehicle) or 30 mg/kg dacomitinib. Mice were serially monitored using bioluminescence imaging and tumor-free survival since implantation was determined. (A) Luciferase activity detected from U87vIII.Luc2-bearing animals treated with vehicle (white circles) or dacomitinib (grey squares). Bars show mean ± SEM, number of mice (n) per group is shown. Differences were compared using a Student’s t-test and a significant difference was observed between the groups at day 15 (** P < .01). (B) Kaplan-Meier survival curves of U87vIII.Luc2-bearing mice treated with vehicle (solid line) or dacomitinib (dashes). Number of mice (n) per group is shown. The difference between each group was determined using a log-rank test and the significance (P) is indicated. (C) Luciferase signal detected from mice with Daoy.Luc2 xenografts treated with vehicle (white circles) or dacomitinib (grey squares). Bars show mean ± SEM, number of mice (n) per group is shown. No significant differences between the groups were observed. (D) Kaplan-Meier survival curves of Daoy.Luc2-bearing animals treated with vehicle (solid line) or dacomitinib (dashes). Number of mice (n) per group is shown. The differences between each group were determined using a log rank test and the significance (P) is indicated. The time during which mice received treatment is shown by the shaded area in (B) and (D).
The effect of dacomitinib treatment (30 mg/kg, thrice weekly) on survival was also assessed in mice harboring Daoy.Luc2 xenografts. However, in this model no difference was observed in the bioluminescence between control and dacomitinib-treated mice (Figure 3C). Overall survival of animals was also assessed, and although an initial delay was observed, as reflected by the log-rank test, there was no difference in end-point (Figure 3D).
Cyclophosphamide Enhances Delivery of Dacomitinib to Mouse Medulloblastomas
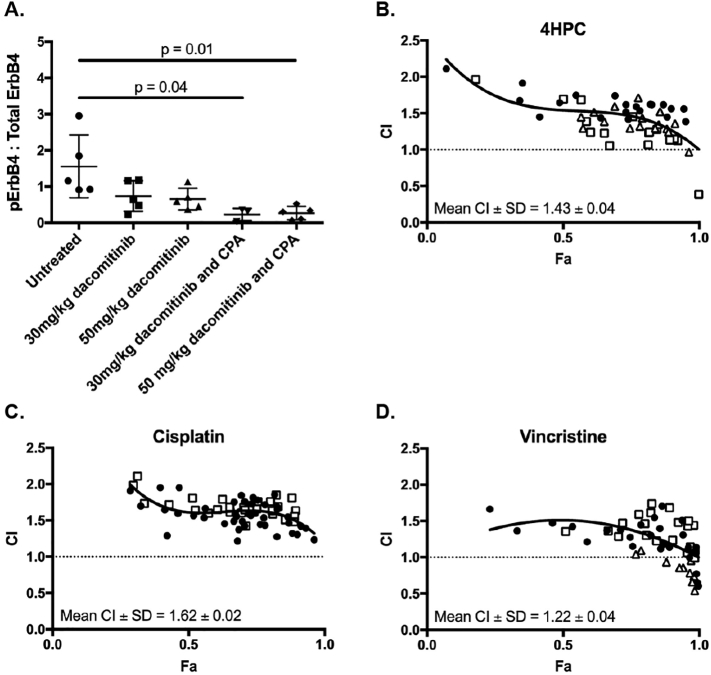
Intracranial implantation of cells can disrupt the blood-brain barrier, and although sufficient time was allowed for the surgical wound to heal prior to drug administration, it remained possible that the surgical process could facilitate access of dacomitinib to the tumor. To investigate whether dacomitinib was able to cross an intact blood-brain tumor barrier, we utilized the SMO mouse, which spontaneously develops medulloblastoma due to overexpression of activated Smoothened (SMOA1) in the mouse cerebellum [30], [31]. Unlike the intracranial implant models where the xenografts exhibited uniform levels of EGFR expression, the amounts of phosphorylated Egfr varied significantly between individual untreated SMO mice (Supplementary Figure 1), making it difficult to determine the effect of dacomitinib on Egfr activity immunohistochemically. Instead, total ErbB4 expression was found to be more consistent in tumors across multiple animals (Supplementary Figure 2), therefore the ratio of phosphorylated ErbB4 to total ErbB4 for each SMO medulloblastoma was determined to assess the ability of dacomtinib to penetrate brain tumors, and inhibit receptor activity. Tissue was harvested 24 hours after drug treatment. The data revealed that although ErbB4 phosphorylation (Y984) appeared reduced in SMO medulloblastomas treated with 30 or 50 mg/kg dacomitinib, these results were not statistically significant (Figure 4A and Supplementary Figure 2). The difference in both survival outcomes and receptor inhibition between medulloblastoma tumors (Daoy and SMO) and glioblastomas (U87MG) treated with dacomitinib alone raises the possibility that the blood-tumor barrier is different in these different tumor types.
Figure 4.
Dacomitinib reduces ERBB4 phosphorylation in vivo but antagonizes conventional medulloblastoma chemotherapeutics in vitro. (A) SMO mice harboring medulloblastomas were treated with vehicle, dacomitinib (doses indicated) or a combination of dacomitinib and cyclophosphamide (CPA, 150 mg/kg). Tumors were stained for phosphorylated or total ErbB4, stain intensity determined using ImageJ, and the ratio of phosphorylated:total ErbB4 was determined. Shown is mean ± SEM, n = 5 mice per group. Groups were compared using a Student’s t-test (* P < .05). In vitro interactions between dacomitinib and (B) 4-hydroperoxycyclophosphamide (4HPC), (C) cisplatin and (D) vincristine were determined using CompuSyn. Graphs show the combination index (CI) values versus factor affected (Fa) values, where values above or below the dashed line indicate antagonism or synergism, respectively. Mean CI ± standard deviation are shown. Closed circles, open squares and open triangles indicate replicate experiments.
We hypothesized that the blood-tumor barrier in medulloblastoma could be made more permeable to dacomitinib by the co-administration of a chemotherapeutic agent commonly used in the clinical treatment of medulloblastoma. Therefore, SMO mice were treated with either 30 or 50 mg/kg dacomitinib in combination with 150 mg/kg cyclophosphamide (CPA). In both combination-treated groups, a significant reduction in phosphorylated ErbB4 levels compared to vehicle-treated mice was observed (P = .04 and P = .01, Figure 4A). These data suggest that although dacomitinib may not be able to cross an intact blood-tumor barrier at sufficient levels to effectively inhibit ErbB receptor signaling when administered alone, impairment of the barrier, either by physical disruption (such as intracranial implantation or craniotomy), or increased permeabilization caused by chemotherapeutics (such as cyclophosphamide), appears to facilitate penetration of the drug into brain tumors.
Given these findings, we investigated whether the combination of dacomitinib and cyclophosphamide was effective against medulloblastoma cells in vitro and in vivo. This is particularly of interest in the pre-clinical assessment of novel treatments for pediatric cancer, since in pediatric clinical trials new drugs are often evaluated in combination with existing chemotherapeutics [36].
Dacomitinib Antagonizes Conventional Chemotherapy for Medulloblastoma
Cyclophosphamide, vincristine and cisplatin are widely used to treat medulloblastoma [7]. We initially investigated the in vitro interactions between dacomitinib and these three drugs using well-established drug interaction assays [37]. Daoy.Luc2 medulloblastoma cells were cultured in the presence of increasing concentrations of dacomitinib and either 4HPC, vincristine or cisplatin in a matrix array. Cell survival was assessed by alamar blue assay and results were analyzed with CompuSyn software, which calculates a combination index (CI) value for each drug pair. CI values greater than one indicate that the drugs antagonize each other, a CI value of one indicates additivity, and values below one indicate a synergistic interaction. For all combinations tested, dacomitinib antagonized all three of the drugs used in medulloblastoma treatment in vitro (Figure 4B-D).
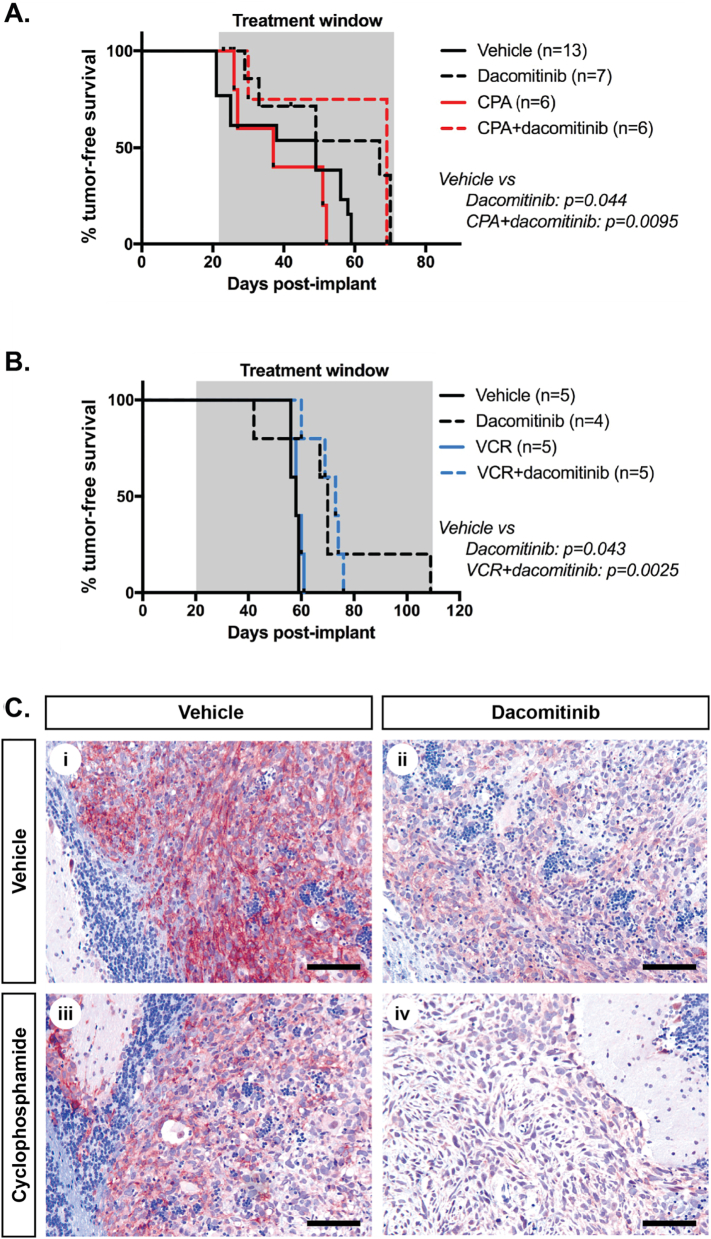
However, based on our findings in SMO medulloblastomas, where cyclophosphamide treatment increased the penetration of dacomitinib into tumors, and enhanced receptor inhibition, we wanted to assess whether the interactions determined from in vitro experiments were consistent in vivo. As the combination of dacomitinib with cyclophosphamide or vincristine had the lowest average CI values of the three combinations tested, cohorts of mice bearing intracranial medulloblastoma xenografts were treated with vehicle, or dacomitinib (30 mg/kg), cyclophosphamide (150 mg/kg) or vincristine (0.5 mg/kg) alone or in combination, and survival data were generated (Figure 5, A and B). Mice in the cyclophosphamide cohort received dacomitinib thrice weekly and/or cyclophosphamide once weekly, while mice in the vincristine cohort were treated every alternate week with dacomitinib twice per week and vincristine once per week. Consistent with Figure 3, a slight improvement in animal survival was observed in mice treated with dacomitinib alone. Notably, the conventional chemotherapeutics, cyclophosphamide and vincristine, did not impact survival in this model, and survival of mice treated with the drug combinations were also not increased compared with mice treated with dacomitinib alone. Due to this, we could not confirm if dacomitinib acted antagonistically in vivo with either cyclophosphamide or vincristine. Importantly, it was evident that combination treatment did not improve survival over the dacomitinib-treated mice, indicating a lack of synergy. Animals treated with both drug combinations exhibited increased weight loss, suggestive of drug-related toxicity, preventing additional experiments. Assessment of EGFR phosphorylation by immunohistochemistry certainly indicated that dacomitinib was active in both single drug-treated, and combination-treated animals (Figure 5C). Quantitation of phosphorylated EGFR staining intensity in Daoy xenografts (Supplementary Figure 3) suggested that dacomitinib was more active in combination-treated mice, consistent with our findings from the SMO medulloblastomas. The fact that this did not result in any additional survival benefit suggests that there likely exists some antagonism between cyclophosphamide and dacomitinib in vivo. Proliferation and apoptosis were also assessed, but the percentages of cells containing Ki67 or cleaved caspase 3 remained unchanged in both single agent and combination-treated animals compared with vehicle treated mice (data not shown).
Figure 5.
Dacomitinib does not synergize with conventional chemotherapy for medulloblastoma in vivo. (A) Survival of Daoy.Luc2-bearing animals treated with vehicle (solid black line), dacomitinib (30 mg/kg, black dashes), cyclophosphamide (CPA, 150 mg/kg, solid red line) or a combination of dacomitinib and cyclophosphamide at the same dosages (red dashes). (B) Survival of mice with Daoy.Luc2 xenografts treated with vehicle (solid black line), dacomitinib (30 mg/kg, black dashes), vincristine (VCR, 0.5 mg/kg, solid blue line) or a combination of dacomitinib and vincristine at the same dosages (blue dashes). Number of mice per group (n) is indicated, and significance values (P, log-rank test) are shown for groups that differed from vehicle-treated animals. (C) Representative sections of Daoy.Luc2 tumors from animals treated with (i) vehicle, (ii) dacomitinib (30 mg/kg), (iii) cyclophosphamide (150 mg/kg) or (iv) a combination of dacomitinib (30 mg/kg) and cyclophosphamide (150 mg/kg) stained for p-EGFR (Y1173) (brown) and counterstained with hematoxylin (blue). Scale equals 100 μm.
To confirm these findings in an additional medulloblastoma mouse model, we studied dacomitinib in ERBB2-expressing PER547 medulloblastoma cells [38]. Similar to Daoy medulloblastoma cells, dacomitinib was observed to behave antagonistically with cisplatin in vitro, and was only additive with 4HPC. Furthermore, when 547.Luc2 orthotopic xenografts were immunohistochemically examined from mice treated with dacomitinib, no changes in tumor cell proliferation, nor apoptosis, were observed. In contrast, the conventional chemotherapeutic cyclophosphamide induced significant reductions in proliferating cells, and increased numbers of apoptotic cells in these tumors. Notably, the combination of dacomitinib with cyclophosphamide did not significantly alter proliferation or apoptosis, compared to tumors treated with cyclophosphamide alone (Supplementary Figure 4).
Dacomitinib Does Not Enhance Conventional Chemotherapy in Pineoblastoma
Pineoblastomas in children are rare, and due to limited proven clinical treatment options, patients are treated using medulloblastoma protocols. However, despite histological similarities between these two tumor types, pineoblastoma patients experience poorer survival outcomes compared to children with medulloblastoma. EGFR expression in supratentorial primitive neuroectodermal tumors, including pineoblastoma, has been reported to correlate with poor five-year survival outcomes, warranting the investigation of mechanisms to block EGFR-mediated signalling [39]. Therefore, despite modest effects of dacomitinib in glioblastoma and medulloblastoma, we examined the effects of dacomitinib in combination with several conventional chemotherapeutics using the 452.Luc2 patient-derived pineoblastoma cells, as well as a second pineoblastoma cell line (453.Luc2) derived from the same patient but following disease relapse post-chemotherapy [27]. Despite the two cultures being derived from the same patient, the results of in vitro drug interaction assays testing dacomitinib with 4HPC or cisplatin were inconsistent, with synergy or additivity observed in 452.Luc2 cells and synergy observed in 453.Luc2 cells (Supplementary Figure 5). However, a synergistic effect of dacomitinib with cyclophosphamide was not evident in vivo for either tumor. Mice harboring orthotopic xenografts of 452.Luc2 and 453.Luc2 were treated with vehicle, dacomitinib, cyclophosphamide or a combination of dacomitinib with cyclophosphamide, then proliferation and apoptosis were assessed using immunohistochemistry. No change in proliferation was observed in any treatment group, and dacomitinib also failed to induce apoptosis when used alone. Apoptosis appeared increased in both tumor models following treatment with chemotherapy, but this was not enhanced by co-administration of dacomtinib, even in 453.Luc2 tumors (Supplementary Figure 6). Due to these findings, the impact of dacomitinib on overall animal survival was not investigated in these models.
Discussion
EGFR pathway inhibitors continue to be assessed for their potential to treat a range of different major tumor classes, particularly those of which the majority are linked to aberrant ERBB pathway activation (such as head and neck cancers), or others which include discrete subtypes characterized by overexpression and/or mutations affecting EGFR family members (such as non-small-cell lung cancer, ERBB2/HER2+ breast cancer, classical glioblastoma). However, the therapeutic potential of EGFR pathway inhibitors for the treatment of pediatric brain tumors has received less attention [2], [40].
Our study adds to the body of research investigating the pan-ERBB inhibitor dacomitinib for the treatment of glioblastoma [26], and here we have extended the analysis to pediatric brain tumors. Our analysis revealed that the drug inhibited EGFR and EGFRvIII activity in vitro in all three tumor types tested. As a single agent, in vivo treatment with dacomitinib resulted in a statistically significant but only clinically modest improvement in survival rates for mice bearing glioblastoma orthotopic xenografts and a statistically significant, but not clinically relevant survival advantage for mice bearing medulloblastoma orthotopic xenografts, given there was no difference in the total length of animal survival. Importantly, our data for glioblastoma are highly consistent with the results of a recently reported Phase II clinical trial of dacomitinib for the treatment of recurrent glioblastoma in adults with EGFR amplification, which revealed limited single agent activity [26]. The trial results showed that across all patients (with or without EGFRvIII) progression-free survival was only 10.6% at six months (median 2.6 months); however, five patients were progression-free at 6 months and four remained progression-free at 12 months with one patient experiencing complete response. A comparison of the genetic features of the glioblastomas that did and did not respond to dacomitinib treatment would be essential to interpret the general lack of efficiency, as well as better understand potential biomarkers that predict response to treatment. For example, previously published pre-clinical data suggest that dysregulation of signalling downstream of EGFR, such as PTEN loss, impedes sensitivity to dacomitinib [21], [41]. In contrast, we showed that dacomitinib was able to down-regulate AKT activity in PTEN-mutant U87MG cells with or without EGFRvIII. Moreover, in vivo, dacomitinib treatment induced a modest increase in survival despite PTEN deficiency. Another Phase II study in adults with recurrent glioblastoma is ongoing, although they are no longer recruiting participants [42], [43]. Our data argue that careful preclinical assessment of therapeutic response should be considered, if not essential, in order to avoid the unnecessary burden and expense of a clinical trial not likely to significantly impact patient outcomes.
There are fewer examples of clinical trials targeting ERBB receptors in pediatric brain tumor patients. A limited number of early phase clinical trials involving first generation EGFR pathway inhibitors, such as erlotinib and lapatinib [2], [40], [44], suggested that these drugs were well tolerated in children with medulloblastoma, although they were of limited clinical effectiveness, possibly due to low tumor penetration of the drug [40]. Our analysis of the Daoy medulloblastoma cell line were initially encouraging, and suggested that dacomitinib as a single agent effectively inhibited EGFR signaling in vitro and in vivo, as well as increased survival in mice bearing Daoy orthotopic xenografts. However, the absence of a difference in the bioluminescence between control and dacomitinib-treated mice, combined with no difference in the total length of animal survival, strongly suggest that the clinical impact would likely be insignificant, especially since EGFR/ERBB4 activation are probably not essential drivers of growth in Daoy xenografts. This is in contrast to the U87.vIII glioblastoma xenograft model where EGFR activation is the principal driver of tumor proliferation and where the effects of dacomitinib were more pronounced pre-clinically, but notably still of limited efficacy in the clinic [26]. Moreover, dacomitinib antagonized standard front-line medulloblastoma chemotherapies such as cyclophosphamide, vincristine and cisplatin. This was corroborated in a second medulloblastoma xenograft model (PER547), where no significant impact on proliferation or apoptosis were observed with dacomitinib treatment alone or in combination with cyclophosphamide. Taken together these data, indicate that dacomitinib should not be a candidate to consider for upfront therapy for pediatric medulloblastoma. These data are of important consideration in the clinical setting when selecting tumor types most likely to benefit from dacomitinib treatment.
The role for the EGFR pathway in the pathogenesis of pineoblastoma has not been investigated to date. Our data, albeit limited, would suggest that while dacomitinib demonstrated pathway inhibition in vitro in these cells, the in vivo data suggest that EGFR pathway inhibitors are highly unlikely to be an effective treatment option for this disease, alone or in combination with cytotoxic chemotherapy, despite clinical data available that suggests EGFR expression is associated with poorer survival outcomes [39].
In assessing the efficacy of dacomitinib for the treatment of the three distinct brain tumor types, we have developed a comprehensive pre-clinical pipeline to test novel chemotherapeutic drugs for these diseases. This pipeline interrogates the effects of novel compounds from the level of signaling pathways and effects on cell viability in vitro through to in vivo target inhibition, and assessment of effects on tumor growth and survival. Furthermore, our approach allows robust testing, not only of single agents, but also of combinations of these drugs with current chemotherapeutics, which is necessary, particularly in the up-front pediatric pre-clinical setting. In conclusion, the limited efficacy of dacomitinib in a Phase II clinical trial in adult patients with recurrent glioblastoma with deregulated EGFR are highly consistent with our pre-clinical data and highlight the value of rigorous testing in pre-clinical pipelines such as ours prior to conducting clinical trials. To this end, our findings do not support the use of dacomitinib in clinical trials for the predominantly pediatric tumors, medulloblastoma or pineoblastoma.
Acknowledgements
This work was supported by the NHMRC [grant number APP1033720], a Pfizer IIR grant, The Adventurers, and Cure Brain Cancer Foundation. NGG is supported by the Raine Medical Research Foundation and Cancer Council of Western Australia. We acknowledge The Adventurers and Bright Blue: The Police Commissioner’s Fund for Sick Kids for supporting the purchase of equipment for the work, and infrastructure funding from the Telethon Kids Institute. All authors met the International Committee for Medical Journal Editors authorship criteria and retained full control of the manuscript content. Editorial support in the form of scientific writing was provided by Peter Dallas. Expert technical assistance with immunoblotting was provided by Brooke Strowger.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2018.02.004.
Appendix A. Supplementary data
Supplementary Figure 1 Levels of phosphorylated EGFR are inconsistent across medulloblastomas in SMO mice. Representative sections of medulloblastomas from untreated SMO mouse brains. Sections were stained with hematoxylin and eosin (H&E) or for phosphorylated EGFR (Y1173) (brown) and counterstained with hematoxylin (blue). Scale bars represent 50 µm.
Supplementary Figure 2 Dacomitinib-induced reduction in phosphorylated ErbB4 is enhanced by combined treatment with cyclophosphamide. Representative sections of medulloblastoma tissue from individual SMO mice treated with (A) vehicle, (B) 30 mg/kg dacomitinib, (C) 50 mg/kg dacomitinib, (D) 30 mg/kg dacomitinib and 150 mg/kg cyclophosphamide, or (E) 50 mg/kg dacomitinib and 150 mg/kg cyclophosphamide. Tissue was harvested 24 hours after treatment and stained with hematoxylin and eosin (H&E), total ErbB4 (brown) or phosphorylated ErbB4 (Y984) (pErbB4, brown). IHC sections were counterstained with hematoxylin (blue), and scale bars (100 µm) apply to all images in each panel.
Supplementary Figure 3 Dacomitinib inhibits EGFR phosphorylation in Daoy medulloblastoma xenografts in vivo. Daoy.Luc2 tumors from mice treated with vehicle, dacomitinib, cyclophosphamide or a combination of dacomitinib and cyclophosphamide were stained for phosphorylated EGFR (Y1068). The intensity of the stain was quantified using a spectral unmixing camera and InForm software (Perkin Elmer). Scores were defined as follows: negative (-, green), low (+, yellow), medium (++, orange) or high (+++, red) intensity staining. n = 3-4 mice per treatment, with up to four representative sections counted per tumor. The proportion of tumour cells in each category is shown.
Supplementary Figure 4 Dacomitinib does not synergize with conventional chemotherapeutics in 547.Luc2 medulloblastoma cells in vitro, nor in orthotopic xenografts. (A) Expression of EGFR and ERBB2 in 547.Luc2 medulloblastoma cells or xenografts (right) were determined by immunoblot for the antibodies indicated from in vitro cultured cells, or orthotopic xenografts and compared to A431 (stimulated with EGF, Cell Signaling #5634) and NIH3T3 cells as positive controls for EGFR and ERBB2, respectively. NIH3T3 and PER547 cells were starved overnight in 0.1% FBS (starved), or stimulated with 10% FBS or human neuregulin (hNRG, Cell Signaling #5218). Antibodies for ERBB2 and phospho-ERBB2 Y1221/2 were from Cell Signaling (#2242 and #2243, respectively). The interactions between dacomitinib and (B) 4HPC or (C) cisplatin were determined in 547.Luc2 cells using CompuSyn. Graphs show the combination index (CI) values versus factor affected (Fa) values, where values above or below the dashed line indicate antagonism or synergism, respectively. Mean CI ± standard deviation are shown. Different symbols indicate replicate experiments. 547.Luc2 intracranial tumors from mice treated with vehicle, dacomitinib, cyclophosphamide or a combination of dacomitinib and cyclophosphamide were stained for (D) Ki67 or (E) cleaved caspase 3 to measure proliferation and apoptosis, respectively. Positively staining cells were quantified as a percentage of all tumor cells from four different fields of view per tumor, from four individual mice per group. The mean from each mouse is shown as symbols, the bar represents the mean of the group, and error bars represent the SEM. Significant differences between the groups determined using an unpaired t-test are shown (P).
Supplementary Figure 5 Dacomitinib can synergize with cytotoxic chemotherapy in pineoblastoma cells in vitro. The interactions between dacomitinib and 4HPC (left), or cisplatin (right) were investigated in 452.Luc2 (top) or 453.Luc2 (bottom) pineoblastoma cells using CompuSyn. Graphs show the combination index (CI) values versus factor affected (Fa) values, where values above or below the dashed line indicate antagonism or synergism, respectively. Mean CI ± standard deviation (SD) are shown. Different symbols indicate replicate experiments.
Supplementary Figure 6 Dacomitinib does not reduce proliferation or enhance apoptosis in pineoblastoma xenografts. Intracranial xenografts derived from 452.Luc2 (top) or 453.Luc2 (bottom) patient-derived pineoblastoma cells were treated with vehicle (grey circles), 150 mg/kg cyclophosphamide (red squares), 30 mg/kg dacomitinib (blue triangles) or 30 mg/kg dacomitinib with 150 mg/kg cyclophosphamide (green diamonds) and immunohistochemistry was used to detect Ki67-positive (left) or cleaved caspase 3-positive (right) cells. The percentage of positive tumor cells from four different fields of view per tumor was counted, from five individual mice per group. The mean from each mouse is shown as symbols, the bar represents the mean of the group, and error bars represent the SEM. No statistically significant differences between the groups were observed.
References
- 1.Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, Grabenbauer GG, Ochsenbein AF, Simon M, Dietrich PY. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 2.Jakacki RI, Hamilton M, Gilbertson RJ, Blaney SM, Tersak J, Krailo MD, Ingle AM, Voss SD, Dancey JE, Adamson PC. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children's Oncology Group Phase I Consortium Study. J Clin Oncol. 2008;26:4921–4927. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric brain tumors: Innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol. 2015;33:2986–2998. doi: 10.1200/JCO.2014.59.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leary SE, Olson JM. The molecular classification of medulloblastoma: driving the next generation clinical trials. Curr Opin Pediatr. 2012;24:33–39. doi: 10.1097/MOP.0b013e32834ec106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mynarek M, Pizer B, Dufour C, van Vuurden D, Garami M, Massimino M, Fangusaro J, Davidson T, Gil-da-Costa MJ, Sterba J. Evaluation of age-dependent treatment strategies for children and young adults with pineoblastoma: analysis of pooled European Society for Paediatric Oncology (SIOP-E) and US Head Start data. Neuro Oncol. 2017;19:576–585. doi: 10.1093/neuonc/now234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 7.Gottardo NG, Gajjar A. Chemotherapy for malignant brain tumors of childhood. J Child Neurol. 2008;23:1149–1159. doi: 10.1177/0883073808321765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alaoui-Jamali MA, Morand GB, da Silva SD. ErbB polymorphisms: insights and implications for response to targeted cancer therapeutics. Front Genet. 2015;6:17. doi: 10.3389/fgene.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholas MK, Lukas RV, Jafri NF, Faoro L, Salgia R. Epidermal growth factor receptor - mediated signal transduction in the development and therapy of gliomas. Clin Cancer Res. 2006;12:7261–7270. doi: 10.1158/1078-0432.CCR-06-0874. [DOI] [PubMed] [Google Scholar]
- 11.Padfield E, Ellis HP, Kurian KM. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front Oncol. 2015;5:5. doi: 10.3389/fonc.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waage IS, Vreim I, Torp SH. C-erbB2/HER2 in human gliomas, medulloblastomas, and meningiomas: a minireview. Int J Surg Pathol. 2013;21:573–582. doi: 10.1177/1066896913492196. [DOI] [PubMed] [Google Scholar]
- 13.Gilbertson R, Wickramasinghe C, Hernan R, Balaji V, Hunt D, Jones-Wallace D, Crolla J, Perry R, Lunec J, Pearson A. Clinical and molecular stratification of disease risk in medulloblastoma. Br J Cancer. 2001;85:705–712. doi: 10.1054/bjoc.2001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57:3272–3280. [PubMed] [Google Scholar]
- 15.Ray A, Ho M, Ma J, Parkes RK, Mainprize TG, Ueda S, McLaughlin J, Bouffet E, Rutka JT, Hawkins CE. A clinicobiological model predicting survival in medulloblastoma. Clin Cancer Res. 2004;10:7613–7620. doi: 10.1158/1078-0432.CCR-04-0499. [DOI] [PubMed] [Google Scholar]
- 16.Bal MM, Das Radotra B, Srinivasan R, Sharma SC. Does c-erbB-2 expression have a role in medulloblastoma prognosis? Indian J Pathol Microbiol. 2006;49:535–539. [PubMed] [Google Scholar]
- 17.Gajjar A, Hernan R, Kocak M, Fuller C, Lee Y, McKinnon PJ, Wallace D, Lau C, Chintagumpala M, Ashley DM. Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol. 2004;22:984–993. doi: 10.1200/JCO.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 19.Ou SH. Second-generation irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs): a better mousetrap? A review of the clinical evidence. Crit Rev Oncol Hematol. 2012;83:407–421. doi: 10.1016/j.critrevonc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales AJ, Hook KE, Althaus IW, Ellis PA, Trachet E, Delaney AM, Harvey PJ, Ellis TA, Amato DM, Nelson JM. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2008;7:1880–1889. doi: 10.1158/1535-7163.MCT-07-2232. [DOI] [PubMed] [Google Scholar]
- 21.Zahonero C, Aguilera P, Ramirez-Castillejo C, Pajares M, Bolos MV, Cantero D, Perez-Nunez A, Hernandez-Lain A, Sanchez-Gomez P, Sepulveda JM. Preclinical test of dacomitinib, an irreversible EGFR inhibitor, confirms its effectiveness for glioblastoma. Mol Cancer Ther. 2015;14:1548–1558. doi: 10.1158/1535-7163.MCT-14-0736. [DOI] [PubMed] [Google Scholar]
- 22.Greenall SA, Donoghue JF, Gottardo NG, Johns TG, Adams TE. Glioma-specific domain IV EGFR cysteine mutations promote ligand-induced covalent receptor dimerization and display enhanced sensitivity to dacomitinib in vivo. Oncogene. 2015;34:1658–1666. doi: 10.1038/onc.2014.106. [DOI] [PubMed] [Google Scholar]
- 23.Ramalingam SS, O'Byrne K, Boyer M, Mok T, Janne PA, Zhang H, Liang J, Taylor I, Sbar EI, Paz-Ares L. Dacomitinib versus erlotinib in patients with EGFR-mutated advanced nonsmall-cell lung cancer (NSCLC): pooled subset analyses from two randomized trials. Ann Oncol. 2016;27:423–429. doi: 10.1093/annonc/mdv593. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Batty KM, Crowe PJ, Goldstein D, Yang JL. The potential of panHER inhibition in cancer. Front Oncol. 2015;5:2. doi: 10.3389/fonc.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HS, Kim SM, Kim H, Pyo KH, Sun JM, Ahn MJ, Park K, Keam B, Kwon NJ, Yun HJ. Phase II clinical and exploratory biomarker study of dacomitinib in recurrent and/or metastatic esophageal squamous cell carcinoma. Oncotarget. 2015;6:44971–44984. doi: 10.18632/oncotarget.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sepúlveda-Sánchez JM, Ángeles Vaz MA, Balañá C, Gil-Gil M, Reynés G, Gallego Ó, Martínez-García M, Vicente E, Quindós M, Luque R. Phase II trial of dacomitinib, a pan–human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro-Oncology. 2017;19:1522–1531. doi: 10.1093/neuonc/nox105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kees UR, Biegel JA, Ford J, Ranford PR, Peroni SE, Hallam LA, Parmiter AH, Willoughby ML, Spagnolo D. Enhanced MYCN expression and isochromosome 17q in pineoblastoma cell lines. Genes Chromosomes Cancer. 1994;9:129–135. doi: 10.1002/gcc.2870090209. [DOI] [PubMed] [Google Scholar]
- 28.Sarkaria JN, Carlson BL, Schroeder MA, Grogan P, Brown PD, Giannini C, Ballman KV, Kitange GJ, Guha A, Pandita A. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC, Martin N. ComboSyn Inc; New Jersey: 2005. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values. [Google Scholar]
- 30.Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, Russell TL, Ellenbogen RG, Bernstein ID, Beachy PA. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 31.Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzler S, Pullar B, Hansen S, Knoblaugh SE, Lee D, Eberhart CG. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 2008;68:1768–1776. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]
- 32.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987;26:1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- 34.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 35.Pandita A, Aldape KD, Zadeh G, Guha A, James CD. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- 36.Gottardo NG, Hansford JR, McGlade JP, Alvaro F, Ashley DM, Bailey S, Baker DL, Bourdeaut F, Cho YJ, Clay M. Medulloblastoma Down Under 2013: a report from the third annual meeting of the International Medulloblastoma Working Group. Acta Neuropathol. 2014;127:189–201. doi: 10.1007/s00401-013-1213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 38.Holthouse DJ, Dallas PB, Ford J, Fabian V, Murch AR, Watson M, Wong G, Bertram C, Egli S, Baker DL. Classic and desmoplastic medulloblastoma: complete case reports and characterizations of two new cell lines. Neuropathology. 2009;29:398–409. doi: 10.1111/j.1440-1789.2008.00989.x. [DOI] [PubMed] [Google Scholar]
- 39.Cole B, Rudzinski E, Anderson M, Bloom K, Lee A, Leary S. TB-037 - Immunohistochemical detection of therapeutic targets in malignant pediatric brain tumors. Neuro-Oncology. 2014;16:i137–i145. [Google Scholar]
- 40.Fouladi M, Stewart CF, Blaney SM, Onar-Thomas A, Schaiquevich P, Packer RJ, Goldman S, Geyer JR, Gajjar A, Kun LE. A molecular biology and phase II trial of lapatinib in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Neuro-Oncol. 2013;114:173–179. doi: 10.1007/s11060-013-1166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Shah K. Multiple lesions in receptor tyrosine kinase pathway determine glioblastoma response to pan-ERBB inhibitor PF-00299804 and PI3K/mTOR dual inhibitor PF-05212384. Cancer Biol Ther. 2014;15:815–822. doi: 10.4161/cbt.28585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ClinicalTrialsgov Bethesda (MD): National Library of Medicine (US) 2010. NCT01112527. PF-00299804 in adult patients with relapsed/recurrent glioblastoma. [Google Scholar]
- 43.ClinicalTrialsgov Bethesda (MD): National Library of Medicine (US) 2012. NCT01520870. Safety and Efficacy of PF-299804 (Dacomitinib), a Pan-HER irreversible inhibitor, in patients with recurrent glioblastoma with EGFR amplification or presence of EGFRvIII mutation. A Phase II CT. [Google Scholar]
- 44.Fouladi M, Stewart CF, Blaney SM, Onar-Thomas A, Schaiquevich P, Packer RJ, Gajjar A, Kun LE, Boyett JM, Gilbertson RJ. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28:4221–4227. doi: 10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Levels of phosphorylated EGFR are inconsistent across medulloblastomas in SMO mice. Representative sections of medulloblastomas from untreated SMO mouse brains. Sections were stained with hematoxylin and eosin (H&E) or for phosphorylated EGFR (Y1173) (brown) and counterstained with hematoxylin (blue). Scale bars represent 50 µm.
Supplementary Figure 2 Dacomitinib-induced reduction in phosphorylated ErbB4 is enhanced by combined treatment with cyclophosphamide. Representative sections of medulloblastoma tissue from individual SMO mice treated with (A) vehicle, (B) 30 mg/kg dacomitinib, (C) 50 mg/kg dacomitinib, (D) 30 mg/kg dacomitinib and 150 mg/kg cyclophosphamide, or (E) 50 mg/kg dacomitinib and 150 mg/kg cyclophosphamide. Tissue was harvested 24 hours after treatment and stained with hematoxylin and eosin (H&E), total ErbB4 (brown) or phosphorylated ErbB4 (Y984) (pErbB4, brown). IHC sections were counterstained with hematoxylin (blue), and scale bars (100 µm) apply to all images in each panel.
Supplementary Figure 3 Dacomitinib inhibits EGFR phosphorylation in Daoy medulloblastoma xenografts in vivo. Daoy.Luc2 tumors from mice treated with vehicle, dacomitinib, cyclophosphamide or a combination of dacomitinib and cyclophosphamide were stained for phosphorylated EGFR (Y1068). The intensity of the stain was quantified using a spectral unmixing camera and InForm software (Perkin Elmer). Scores were defined as follows: negative (-, green), low (+, yellow), medium (++, orange) or high (+++, red) intensity staining. n = 3-4 mice per treatment, with up to four representative sections counted per tumor. The proportion of tumour cells in each category is shown.
Supplementary Figure 4 Dacomitinib does not synergize with conventional chemotherapeutics in 547.Luc2 medulloblastoma cells in vitro, nor in orthotopic xenografts. (A) Expression of EGFR and ERBB2 in 547.Luc2 medulloblastoma cells or xenografts (right) were determined by immunoblot for the antibodies indicated from in vitro cultured cells, or orthotopic xenografts and compared to A431 (stimulated with EGF, Cell Signaling #5634) and NIH3T3 cells as positive controls for EGFR and ERBB2, respectively. NIH3T3 and PER547 cells were starved overnight in 0.1% FBS (starved), or stimulated with 10% FBS or human neuregulin (hNRG, Cell Signaling #5218). Antibodies for ERBB2 and phospho-ERBB2 Y1221/2 were from Cell Signaling (#2242 and #2243, respectively). The interactions between dacomitinib and (B) 4HPC or (C) cisplatin were determined in 547.Luc2 cells using CompuSyn. Graphs show the combination index (CI) values versus factor affected (Fa) values, where values above or below the dashed line indicate antagonism or synergism, respectively. Mean CI ± standard deviation are shown. Different symbols indicate replicate experiments. 547.Luc2 intracranial tumors from mice treated with vehicle, dacomitinib, cyclophosphamide or a combination of dacomitinib and cyclophosphamide were stained for (D) Ki67 or (E) cleaved caspase 3 to measure proliferation and apoptosis, respectively. Positively staining cells were quantified as a percentage of all tumor cells from four different fields of view per tumor, from four individual mice per group. The mean from each mouse is shown as symbols, the bar represents the mean of the group, and error bars represent the SEM. Significant differences between the groups determined using an unpaired t-test are shown (P).
Supplementary Figure 5 Dacomitinib can synergize with cytotoxic chemotherapy in pineoblastoma cells in vitro. The interactions between dacomitinib and 4HPC (left), or cisplatin (right) were investigated in 452.Luc2 (top) or 453.Luc2 (bottom) pineoblastoma cells using CompuSyn. Graphs show the combination index (CI) values versus factor affected (Fa) values, where values above or below the dashed line indicate antagonism or synergism, respectively. Mean CI ± standard deviation (SD) are shown. Different symbols indicate replicate experiments.
Supplementary Figure 6 Dacomitinib does not reduce proliferation or enhance apoptosis in pineoblastoma xenografts. Intracranial xenografts derived from 452.Luc2 (top) or 453.Luc2 (bottom) patient-derived pineoblastoma cells were treated with vehicle (grey circles), 150 mg/kg cyclophosphamide (red squares), 30 mg/kg dacomitinib (blue triangles) or 30 mg/kg dacomitinib with 150 mg/kg cyclophosphamide (green diamonds) and immunohistochemistry was used to detect Ki67-positive (left) or cleaved caspase 3-positive (right) cells. The percentage of positive tumor cells from four different fields of view per tumor was counted, from five individual mice per group. The mean from each mouse is shown as symbols, the bar represents the mean of the group, and error bars represent the SEM. No statistically significant differences between the groups were observed.