Abstract
Background
The cardiac autonomic nervous system plays a crucial role in genesis and development of atrial fibrillation (AF) through the G protein signal transduction pathway. Therefore, intervening in the G protein signal transduction pathway may be a new “selective drug” method to regulate autonomic nerve activity to prevent vagally-mediated AF.
Material/Methods
Seventeen adult beagles were randomized into 3 groups: shame-operation control group (group A, n=5), empty vector gene control group (group B, n=6), and Gαi2ctp gene experimental group (group C, n=6). Group A was injected with normal saline into the anterior atrial wall, and group B and group C animals were injected with recombinant adenovirus with empty vector or Gαi2ctp vector in the same region. AF was induced by the method of rapid atrial pacing in groups B and C. To determine the clinical effect of vagal modulation, the effective refractory periods (ERP) and field action potential duration (FAPD) were evaluated by electrophysiological study. The expression levels of tyrosine hydroxylase (TH) and choline acetyl transferase (CHAT) in different parts were determined with immunohistochemistry.
Results
After successful Gαi2ctp gene transfer, in group B, the ERP and FAPD significantly decreased (P<0.05), and TH and CHAT expression observably increased (P<0.05), while those differences were absent between groups A and C (P>0.05).
Conclusions
Recombinant adenovirus-mediated overexpression of Gαi2ctp in canine myocardial cells can interfere with the activity of the vagus nerve, reverse the development and progression of electrical remodeling, and reduce the incidence of AF.
MeSH Keywords: Atrial Fibrillation, Atrial Remodeling, Autonomic Nervous System, GTP-Binding Protein Regulators
Background
Atrial fibrillation (AF) is a common tachyarrhythmia in clinical practice; it causes great harm to patients, and while stroke remains the most common complication, heart failure is the second [1–3]. Even more unfortunately, current clinical treatments such as drugs or catheter ablation achieve few demonstrable effects [4,5]. The cardiac autonomic nervous system is activated in AF, inducing autonomic nervous remodeling and electrical remodeling, which, in turn, aggravates AF [6,7]. Some studies proposed that low-level vagus nerve stimulation (LL-VNS) can inhibit the occurrence and persistence of AF through decreasing the cardiac autonomic nervous remodeling [8–11], and speculated that this anti-arrhythmia was associated with inhibitory vagus nerve and sympathetic activity. G protein features in autonomic nervous signal transduction pathway, and can regulate autonomic nervous activity [12]. Thus, the objective of this study was to find an optimal gene therapy that interferes in the G protein signal transduction pathway for AF prevention and treatment.
Material and Methods
Experimental animals
The study protocol was approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Xinjiang Medical University, China (Permit Number: IACUC-20121122005), and conformed to guidelines developed by the Association for Assessment and Accreditation of Laboratory Care (AAALAC).
The 17 adult healthy beagle dogs were randomly divided into 3 groups: the sham-operation control group (group A, n=5), the empty vector gene control group (group B, n=6), and the Gαi2ctp gene experimental group (group C, n=6). Our study was conducted with 17 healthy beagle dogs (mean body weight, 12.0±1.5 kg; aged 1–2 years) provided by the First Affiliated Hospital of Xinjiang Medical University Animal Center. The dogs were anesthetized with ketamine (20 mg/kg) and underwent thoracic surgery at the 4th intercostal rib on the left. Based on the methods described by Arora et al. [8], a 4-ml solution prepared from 500 μl of normal saline was injected into the anterior atrial wall following thoracotomy in the sham group (group A), while a 4-ml solution containing 500 μl of recombinant adenovirus with empty vector was administered to the group B dogs, and a 4-ml solution containing 500 μl of recombinant adenovirus with Gαi2ctp vector was administered to the group C dogs. Injections were performed at multiple injection sites (6–8 equidistant sites that were 0.5–1 cm apart), with 0.5 ml injected per site.
Establishing the acute AF model
At 7 days after revival, we opened the chest of each dog again. We sewed 10-bipolar electrodes into the left atrial appendage, left and right atria, and pulmonary veins to make pacing and record electrophysiological data. The blood oxygen saturation, rhythm and rate of heart, arterial blood pressure, and other vital signs were monitored in the experiment. In groups B and C, AF was induced by repetitive 1200-Hz burst pacing of the left atrial appendage at 2×threshold for 6 h, while group A was not stimulated. AF was induced by S1S1 method, which intermittently stimulated the vagus nerve (S1–S1=300 ms) by suprathreshold stimulation for 30 s. AF was considered to be induced successfully if an irregular 500 beats/min heart rate with an irregular atrioventricular conduction could be maintained for more than 5 s. Each part was repeated 5 times. All of the fast pacing and parameters recording were finished by a multi-conductive physiological recorder (Lead-7000 EP Control, Sichuan Jinjiang, China).
Recording of electrophysiological data
The ERP was defined as the longest interval with S1S2 method until failing to response (S1–S1=300 ms, S1: S2=8: 1, V=2× Threshold). The pacing was suspended at baseline, 1 h, 3 h, and 6 h, when measured.
Tissue samples (about 5–8 mm2) were collected from the anterior wall of the atria, immersed in 4°C oxygenated Tyrode’s solution, and placed onto an MEA electrode. After connecting the MEA electrode to the TCO2 thermal control and data collection system, electrocardiac signals were amplified 1200 times at 1060-AMP and recorded using Mc-Rack software. The field action potential duration (FAPD) was graphed based on the changes in peak amplitude, and the sequence of action potential was indicated by the difference in color and voltage in the diagram.
Immunohistochemistry studies
When the fast pacing finished, the fresh anterior right ganglionated plexi (ARGP) were routinely processed and fixed in 4% paraformaldehyde, stored in 70% ethanol, and paraffin-embedded. Then, the samples were cut into 5-um sections for histological evaluation. Rabbit polyclonal anti-TH (ab93291, Abcam, UK) and Cat polyclonal anti-CHAT (AB144P, Millipore, USA) were used to stain cardiac nerves. The positive staining area density, which was analyzed by Image-Pro Plus 6.0, was defined as the ratio of positive staining area to entire detection area (um2/mm2) to describe vagus nerve activity and sympathetic nerve activity. Three fields were selected for observation with a ×40 eyepiece in each section with highest levels of positive density. The mean density was defined as the positive area density of that section.
Biochemical analysis
Fresh myocardial tissues (200 mg) were collected and homogenized in RIPA buffer. The tissue homogenates were centrifuged, and the supernatants (20 μl) were collected. Protein samples were mixed with 5× loading buffer, separated by SDS-PAGE for 45 min, transferred to polyvinylidene fluoride (PVDF) membranes, and blocked with Western blocking buffer (5% skim milk powder in 100 ml PBST) for 60 min. Each membrane was incubated overnight with 10 ml of primary antibodies directed against Gαi2ctp (1: 4000), M2R (1: 2000), or β-actin (1: 5000) at 4°C. After washing primary antibodies twice for about 20 min at room temperature, membranes were incubated with 10 ml of alkaline phosphatase-labeled anti-rabbit, anti-mouse, and anti-goat secondary antibodies for 2 h at room temperature. Substrate solution was added to the membranes for protein visualization, and when purple bands were clearly visible, the reaction was stopped. Proteins were quantified using Image-ProPlus (version 4.1) software.
Statistical analysis
The mean ERP and FAPD values at different time points were analyzed using analysis of variance for repeated measurements. Comparisons of TH and CHAT protein levels were performed by one-way ANOVA, while pair-wise comparisons between multiple groups were analyzed using the LSD method. P-values <0.05 were considered statistically significant.
Results
In the process of the experiment, the blood pressure of the animals remained stable and the oxyhemoglobin saturation remained above 90%. No signs of heart failure due to rapid pacing were observed.
The effect of Gαi2ctp gene overexpression on basic electrophysiological study
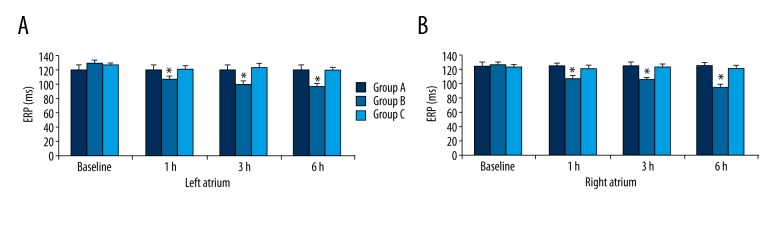
The ERP were measured at baseline, 1 h, 3 h, and 6 h. In group B compared to groups A and C, the ERP was clearly shortened (P<0.05), but these differences were absent between groups A and C (P>0.05). In group B, the ERP values gradually decreased over time and reached a minimum at 6 h (P<0.05), but these changes disappeared in groups A and C (P>0.05) (Figure 1).
Figure 1.
ERP at different time points in the 3 groups. * P<0.05 compared with baseline, ERP – effective refractory period.
In the empty vector gene control group, the field action potential duration (FAPD) of atrial tissues at different time was observed in the microelectrode array (MEA) detection map, which indicated the transmission sequence of electric shock from bottom left to upper right (Figure 2A). The frequency of excitation was significantly increased and disordered, with inconsistent rhythm and rate. During signal transduction, significant changes in voltage were observed as indicated by disappearance of the step-wise voltage changes and the presence of signals with inconsistent amplitude and direction (Figure 2B). Further analysis of the electrocardiac signal revealed disorganized shapes of different colors and shades (Figure 2C). The FAPD of left atrial tissues in the 3 groups were 76.84±4.25 ms, 62.35±3.18 ms, and 78.13±7.18 ms. The FAPD of right atrial tissues in the 3 groups were 82.35±6.23 ms, 59.75±3.54 ms, and 81.36±5.32 ms. Compared to groups A and C, the FAPD of group B was significantly decreased (P<0.05), while that difference was absent between groups A and C (P>0.05) (Figure 2D).
Figure 2.
MEA detection in atrium. (A) Left atrial tissues MEA detection in group B; (B) recorded single-window graph of MEA detection for left atrial tissues in group B; (C) electrocardiac signal analysis of left atrial tissues; (D) FAPD comparison for right or left atrial tissue MEA detection potential. MEA – microelectrode array; FAPD – field action potential duration. * P<0.05 compared with group B.
Histology
Ganglionated plexi, which are an important element of the epicardial neural network, feature in occurrence and persistence of AF [13]. In this experiment, TH and CHAT protein expression levels in ARGP were measured by immuohistochemical staining. The niger-brown-staining areas were positive ganglion cells and unpigmented areas were negative ganglion cells, while the blue-staining areas were cores (Figure 3A–3C).
Figure 3.
ARGP CHAT levels shown by immunohistochemistry staining. (A–C) ARGP CHAT staining in sham group (group A), empty vector gene control group (group B), and Gαi2ctp gene experimental group (group C). The niger-brown-staining areas were positive ganglion cells and unpigmented areas were negative ganglion cells, while the blue-staining areas were cores. (D) TH and CHAT expression levels in ARGP. * P<0.05 compared with group B.
The CHAT expression levels of ARGP in groups A, B and C were (39.35±6.18) ×1000 um2/mm2, (87.35±7.63) ×1000 um2/mm2, (42.50±7.64) ×1000um2/mm2.
The TH expression levels of ARGP in groups A, B and C were (41.25±7.63) ×1000 um2/mm2, (81.24±6.72) ×1000 um2/mm2, (39.45±6.57) ×1000um2/mm2.
Compared to groups A and C, the expression levels of TH and CHAT observably increased in group B (P<0.05), but that difference was absent between groups A and C (P>0.05) (Figure 3D).
Biochemical analysis
As shown in Figure 4, protein expression of Gαi2ctp was observed in all parts of the atrium, and Gαi2ctp protein expression compared to β-actin expression could be calculated.
Figure 4.

The electrophoretic band of Gαi2ctp protein.
Although the level of Gαi2ctp protein expression was slightly different between different parts of the atrium and pulmonary vein in group C (P>0.05) (Figure 5A), the atrial Gαi2ctp protein expression was significantly higher in group C compared to that in group A and group B (P<0.05) (Figure 5B).
Figure 5.
Different parts of Gαi2ctp protein expression of the atrium and the pulmonary veins. (A) Level of Gαi2ctp protein expression of different parts of the atrium and pulmonary vein in group C. (B) Atrial Gαi2ctp protein expression in the 3 groups. * P<0.05 compared with group C.
Discussion
Electrophysiological analysis confirmed that this technique prolonged the ERP of the atrium and pulmonary vein, and reduced the field potential of the tissue. Histology indicated that recombinant adenovirus-mediated overexpression of Gαi2ctp reduced the expression of TH and CHAT in ARGP. At the molecular level, this gene transfer method can increase the expression of Gαi2ctp in myocardial cells in the atrium. In summary, this method can reverse the development and progression of autonomic nervous remodeling to reduce the incidence of AF.
Autonomic remodeling during AF
Studies in animal models and humans have confirmed that abnormal autonomic innervation is associated with AF. In a canine model of AF, Jayachandran et al. showed that the density of the sympathetic nerve was increased and it played a role in the induction and maintenance of AF following myocardial infarction [14–17]. Moreover, Ng et al. reported enhanced sympathetic and parasympathetic nerve growth in the left atrium in a canine model of heart failure and AF [18,19], whereas Choi et al. found that AF was triggered by endogenous cardiac discharges in dogs [20]. Similar findings were observed in human AF. In summary, these studies demonstrated that the changes in the autonomic nervous system during the development and maintenance of AF stemmed from autonomic remodeling.
Norepinephrine is the main neurotransmitter used by the sympathetic nervous system, and tyrosine hydroxylase (TH) is the rate-limiting enzyme in the biosynthesis of norepinephrine. On the other hand, acetylcholine is the main vagal neurotransmitter, and choline acetyltransferase (CHAT) is the rate-limiting enzyme in the biosynthesis of acetylcholine. In the present study, the levels of TH and CHAT were significantly higher in group B than in group A, indicating enhanced sympathetic and vagal activities during RAP-induced AF, which is consistent with the findings reported in previous studies. However, Gαi2ctp transduction in group C resulted in significantly reduced TH and CHAT levels compared with group B but not with group A, indicating that overexpression of Gαi2ctp decreased vagal activity and reversed nerve remodeling in AF.
Intervention of autonomic remodeling can reverse electrical remodeling in AF
Several studies have elucidated the effect of intervention of autonomic remodeling on electrical remodeling in AF. Sheng et al. demonstrated that low-level vagus nerve stimulation inhibited cardiac autonomic activity and prevented electrical remodeling in acute AF [21]. Similarly, Dietrich et al. showed that subcutaneous low-level stimulation of the vagus nerve reversed electrical remodeling in a canine model of RAP-induced AF [22,23]. Both studies demonstrated that inhibition of vagal activity reversed atrial electrical remodeling.
In the present study, atrial ERP shortened and dispersion clearly increased in group B compared to group A, indicating that the model of electrical remodeling was successfully built in AF [24,25]. In group C compared to group B but not group A, ERP was significantly prolonged and dispersion was apparently decreased, indicating that overexpression of Gαi2ctp decreased vagal activity and reversed electrical remodeling in AF.
Inhibitory mechanism of Gαictp gene overexpression against electrical remodeling in AF
In previous studies, it was shown that vagal activity is increased in AF [26,27]. Vagal activity is dependent on the spatial heterogeneity of acetylcholine, which is synthesized from acetyl-CoA and choline by the action of CHAT. Acetylcholine mainly accumulates in the synaptic knob of cholinergic nerves, and, upon release, it binds to choline receptors to continue signal propagation [28]. The M2 muscarinic receptor is the predominant choline receptor expressed on myocardial cells [29], which, upon binding with acetylcholine, activates the breakdown of Gαi/oβγ into Gαi/o and Gβγ. Since Gαi/o can lead to attenuation of L-type calcium channels and inwardly rectifying potassium channels (If), while Gβγ can activate acetylcholine-sensitive potassium channels (IαCh), which eventually results in delayed signal conduction in the sinus node and atrioventricular (AV) node, and shortened atrial ERP with increased dispersion, the latter of which acts as the substrate for recurrent and increased induction of AF [30–32].
High Gαi2 expression in the AV node inhibited basal AV conduction, prolonged the duration of AV node conduction, and reduced the ventricular rate during AF in the absence of a complete heart block [33,34] because given that Gαi2 is structurally similar to Gαi, it can competitively inhibit the binding between Gαi and M2R, interfere with the degradation of the Gαi/oβγ trimer, and block signal transduction, thereby leading to the absence of free Gαi/o and Gβγ, prolonged atrial ERP with reduced dispersion, and lower AF induction [35–37]. Therefore, it is believed that this is the mechanism by which Gαi2ctp (Gαi2 peptide) overexpression reduced vagal activity and prevented the development and progression of AF.
In this study, atrial Gαi2ctp protein expression was significantly higher in group C compared to that in group A and group B. Although the level of Gαi2ctp protein expression was slightly different between different parts of the atrium and pulmonary vein, these differences were not statistically significant, indicating that the Gαi2ctp gene was successfully transduced into the canine atrium. Furthermore, comparison with the control groups demonstrated that the results found in group C were solely caused by overexpression of the Gαi2ctp gene in the atrium, as the effects of surgery and gene transduction were excluded in group A and group B, respectively.
Our study provides an effective genetic target for cardiac ablation at the molecular level for the effective treatment of AF. However, our study has some limitations. The model of AF induced by left atrial appendage fast pacing is different from human AF. The effects of Gαi2ctp gene overexpression in dogs with untreated AF might be different from that in patients receiving antiarrhythmic drugs. Therefore, further investigations are needed.
Conclusions
Gαi2ctp gene overexpression may be a new “selective drug” method to regulate autonomic nerve activity to prevent vagally-mediated AF.
Footnotes
Source of support: Natural Science Foundation of China (NSFC81660071)
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: A systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–98. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 3.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639–54. doi: 10.1038/nrcardio.2014.118. [DOI] [PubMed] [Google Scholar]
- 5.LaPar DJ, Speir AM, Crosby IK, et al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014;98(2):527–33. doi: 10.1016/j.athoracsur.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Park HW, Shen MJ, Lin SF, et al. Neural mechanisms of atrial fibrillation. Curr Opin Cardiol. 2012;27:24–28. doi: 10.1097/HCO.0b013e32834dc4e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksu, et al. Vagal denervation in atrial fibrillation ablation: A comprehensive review. Anatol J Cardiol. 2017;18(2):142–48. doi: 10.14744/AnatolJCardiol.2017.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherlag BJ, Nakagawa H, Jackman WM, et al. Non-pharmacological, non-ablative approaches for the treatment of atrial fibrillation: experimental evidence and potential clinical implications. J Cardiovasc Transl Res. 2011;4(1):35–41. doi: 10.1007/s12265-010-9231-5. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Jiang Z, He Y, et al. Continuous vagal nerve stimulation affects atrial neural remodeling and reduces atrial fibrillation inducibility in rabbits. Cardiovasc Pathol. 2015;24(6):395–98. doi: 10.1016/j.carpath.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Stavrakis S, Humphrey MB, Scherlag BJ, et al. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65(9):867–75. doi: 10.1016/j.jacc.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Yu L, Zhou X, et al. Low-level vagus nerve stimulation: An important therapeutic option for atrial fibrillation treatment via modulating cardiac autonomic tone. Int J Cardiol. 2015;199:437–38. doi: 10.1016/j.ijcard.2015.07.083. [DOI] [PubMed] [Google Scholar]
- 12.Arora R. Recent insights into the role of the autonomic nervous system in the creation of substrate for atrial fibrillation: Implications for therapies targeting the atrial autonomic nervous system. Circ Arrhythm Electrophysiol. 2012;5(4):850–59. doi: 10.1161/CIRCEP.112.972273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Sun J, Zhang L, et al. Intermittent low-level vagosympathetic nerve trunk stimulation inhibits ganglionated plexi activity to prevent atrial fibrillation. Int J Clin Exp Med. 2015;8(4):5094–102. [PMC free article] [PubMed] [Google Scholar]
- 14.Shen MJ, Choi EK, Tan AY, et al. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. 2011;9:30–39. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- 15.Jayachandran JV, Sih HJ, Winkle W, et al. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–91. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Hong SY, Wi J, et al. Catheter ablation of atrial fibrillation raises the plasma level of NGF-β which is associated with sympathetic nerve activity. Yonsei Med J. 2015;56(6):1530–37. doi: 10.3349/ymj.2015.56.6.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould PA, Yii M, McLean C, et al. Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:821–29. doi: 10.1111/j.1540-8159.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 18.Ng J, Villuendas R, Cokic I, et al. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: Creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–96. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Y, Wei C, Liu L, et al. Atrial fibrillation increases sympathetic and parasympathetic neurons in the intrinsic cardiac nervous system. Pacing Clin Electrophysiol. 2014;37(11):1462–69. doi: 10.1111/pace.12450. [DOI] [PubMed] [Google Scholar]
- 20.Choi EK, Shen MJ, Han S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–23. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng X, Scherlag BJ, Yu L, et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vago sympathetic nerve stimulation. J Am Coll Cardiol. 2011;57(5):563–71. doi: 10.1016/j.jacc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Scherlag BJ, Li S, et al. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: A non-invasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10(3):428–35. doi: 10.1016/j.hrthm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich S, Smith J, Scherzinger C, et al. [A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI]. Biomed Tech (Berl) 2008;53:104–11. doi: 10.1515/BMT.2008.022. [in German] [DOI] [PubMed] [Google Scholar]
- 24.Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: Clinical insights and scientific opportunities. Europace. 2010;12:160–72. doi: 10.1093/europace/eup328. [DOI] [PubMed] [Google Scholar]
- 25.Fan J, Zou L, Cui K, Woo K, et al. Atrial overexpression of angiotensin-converting enzyme 2 improves the canine rapid atrial pacing-induced structural and electrical remodeling. Fan, ACE2 improves atrial substrate remodeling. Basic Res Cardiol. 2015;110(4):45. doi: 10.1007/s00395-015-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gollob MH. Cardiac connexins as candidate genes for idiopathic atrial fibrillation. Curr Opin Cardiol. 2006;21:155–58. doi: 10.1097/01.hco.0000221574.95383.6f. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 28.Oberhauser V, Schwertfeger E, Rutz T, et al. Acetylcholine release in human heart atrium: influence of muscarinic autoreceptors, diabetes, and age. Circulation. 2001;103:1638–43. doi: 10.1161/01.cir.103.12.1638. [DOI] [PubMed] [Google Scholar]
- 29.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–53. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 30.Zuberi Z, Birnbaumer L, Tinker A. The role of inhibitory heterotrimeric G proteins in the control of in vivo heart rate dynamics. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1822–30. doi: 10.1152/ajpregu.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hippe HJ, Lüdde M, Schnoes K, et al. Competition for Gβγ dimers mediates a specific crosstalk between stimulatory and inhibitory G protein α subunits of the adenylyl cyclase in cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(6):459–69. doi: 10.1007/s00210-013-0876-x. [DOI] [PubMed] [Google Scholar]
- 32.Sebastian S, Ang R, Abramowitz J, et al. The in vivo regulation of heart rate in the murine sinoatrial node by stimulatory and inhibitory heterotrimeric G proteins. Am J Physiol Regul Integr Comp Physiol. 2013;305(4):R435–42. doi: 10.1152/ajpregu.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donahue JK, Heldman AW, Fraser H, et al. Focal modification of electrical conduction in the heart by viral gene transfer. Nat Med. 2000;6(12):1395–98. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 34.Lugenbiel P, Thomas D, Kelemen K, et al. Genetic suppression of Gαs protein provides rate control in atrial fibrillation. Bas Res Cardiol. 2012;107(3):265. doi: 10.1007/s00395-012-0265-5. [DOI] [PubMed] [Google Scholar]
- 35.Aistrup GL, Cokic I, Ng J, et al. Targeted nonviral gene-based inhibition of Gα(i/o)-mediated vagal signaling in the posterior left atrium decreases vagal-induced atrial fibrillation. Heart Rhythm. 2011;8(11):1722–29. doi: 10.1016/j.hrthm.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aistrup GL, Villuendas R, Ng J, et al. Targeted G-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovasc Res. 2009;83(3):481–92. doi: 10.1093/cvr/cvp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichiyama S, Nemoto R, Tanabe H, Haga T. Interaction of the muscarinic acetylcholine receptor M2 subtype with G protein Gα(i/o) isotypes and Gβγ subunits as studied with the maltose-binding protein-M2-Gα(i/o) fusion proteins expressed in Escherichia coli. J Biochem. 2014;156(5):259–72. doi: 10.1093/jb/mvu036. [DOI] [PubMed] [Google Scholar]