Abstract
Dramatic increases in obesity and diabetes have occurred worldwide over the past 30 years. Some investigators have suggested that these increases may be due, in part, to increased added sugars consumption. Several scientific organizations, including the World Health Organization, the Scientific Advisory Council on Nutrition, the Dietary Guidelines Advisory Committee 2015, and the American Heart Association, have recommended significant restrictions on upper limits of sugars consumption. In this review, the scientific evidence related to sugars consumption and its putative link to various chronic conditions such as obesity, diabetes, heart disease, nonalcoholic fatty liver disease, and the metabolic syndrome is examined. While it appears prudent to avoid excessive calories from sugars, the scientific basis for restrictive guidelines is far from settled.
Keywords: added sugars, diabetes, fructose, heart disease, high fructose corn syrup, obesity, sucrose
INTRODUCTION
While it is generally recognized that sugars should not be consumed in excessive amounts, controversies exist concerning the appropriate upper limit for sugars consumption. Sugars are very common in the food supply and are consumed both as a naturally occurring component of many foods and as an ingredient added to foods during processing, preparation, or at the table.
Simple sugars include monosaccharides and disaccharides. The most common dietary monosaccharides are fructose, glucose, and galactose. Fructose occurs naturally, along with similar amounts of glucose, in many fruits and vegetables and in the sweetener high-fructose corn syrup (HFCS). Glucose is also a polymeric building block of dietary starches, maltodextrins, and corn syrup. The most common disaccharide is sucrose (glucose bonded to fructose), which occurs predominantly in sugar cane and sugar beets, with smaller amounts found in honey, fruits, vegetables, and nuts. Other common disaccharides are the lactose (glucose bonded to galactose) found naturally in milk products and the maltose (glucose bonded to glucose) found in malt from germinating grains such as barley.
Sugars may also be classified as “naturally occurring” or “added.” Added sugars are defined as sugars or syrups added to foods during processing or preparation, including those sugars and syrups added at the table. It should be noted that the World Health Organization (WHO) focused specifically on the intake of free sugars, defined as “monosaccharides and disaccharides added to foods by the manufacturer, a cook, or a consumer and sugars naturally present in honey, syrups, fruit juices, and fruit juice concentrates.”1 The WHO made the distinction that free sugars were different from the intrinsic sugars found in whole fresh fruits and vegetables, and emphasized that its guidance does not apply to consumption of intrinsic sugars.
This review will focus largely on added sugars, with a particular emphasis on sucrose and HFCS, which are the most common sources of added sugars in the human diet. Some attention will also be paid to free fructose and glucose, even though neither is consumed alone to any appreciable degree in the human diet. A great deal of research, however, has focused on these monosaccharides.
Recently, the WHO issued guidance recommending an upper limit of free sugars at 10% of calories, with an ultimate goal of reducing sugars consumption to 5% of calories.1 This recommendation was based on the analysis of data related to added sugars and obesity and dental caries. The WHO rated the evidence favoring a role for added sugars in adult obesity as “moderate” and in child obesity as “low.” Evidence for a role in dental caries was rated as moderate within cohort studies among adults when the limit was at 10% of total energy but was rated as low among national populations. The WHO rated the relationship as “low quality” when the limit was at 5% of total energy. The WHO concluded that the 10% limit was a strong recommendation, despite moderate- or lower-quality evidence, and the 5% limit a conditional one.
The Scientific Advisory Council on Nutrition in England followed roughly the same reasoning and recommended similar restrictions for upper limits of calories from sugars at 10%, with the goal of ultimately achieving an even lower threshold of 5%.2 This recommendation is 50% lower than the current mean added sugars consumption in the United States.3
The American Heart Association has recommended even more stringent restrictions on calories from added sugars, with a suggested upper limit of no more than 150 kcal of added sugars per day for the average adult male and no more than 100 kcal of added sugars per day for the average adult female.4 These guidelines were based on the concept of “discretionary calories” advanced by the 2005 Dietary Guidelines Advisory Committee (2005 DGAC) and concern that added sugars above these levels would constitute a predominant source of energy in this component of the diet. The concept of discretionary calories was developed for the 2005 DGAC document and was introduced to help people meet all of their nutritional requirements while avoiding excess total energy intake. It should be noted that the concept of discretionary calories is no longer widely used. Although this concept was an attempt to provide consumers with greater flexibility in their dietary patterns, it was not advanced by the 2010 Dietary Guidelines Advisory Committee (2010 DGAC) since there was evidence that consumers viewed discretionary calories as a requirement.
In addition, the American Heart Association cited evidence – largely from epidemiologic studies – suggesting that intake of sugars, in general, and intake of fructose-containing sugars, in particular, could lead to an increase in risk factors for a variety of chronic diseases, including obesity, diabetes, and cardiovascular disease.4 Although the American Heart Association’s Scientific Statement recognized that prospective trial data were limited, the organization relied heavily on observational studies, particularly those assessing higher intakes of sugar-sweetened beverages (SSBs). In a 2000-kcal diet, the American Heart Association recommendations reside at approximately the 10th percentile population consumption level,3 meaning that 90% of Americans are consuming more added sugars at the current time than recommended by the American Heart Association.
The Institute of Medicine has established an upper limit of added sugars of 25% of total calories.5 This guidance was based on an extensive review of the scientific literature. The Institute of Medicine did not find any adverse effects related to added sugars below this level, with the exception of dental caries, but was concerned that micronutrient dilution might occur above 25% of calories from added sugars. As such, in contrast to guidelines provided by the other organizations, the Institute of Medicine’s 25% does not constitute a recommendation per se, but rather an upper limit not to exceed. These guidelines were also utilized in the Dietary Guidelines for Americans, 2010 (2010 DGA),6 which also recommended that individuals limit their consumption of added sugars as part of an overall strategy to lower caloric intake and fight obesity. This recommendation was highlighted in the report of the Advisory Committee on the Dietary Guidelines for Americans, 2010.7 The 2015 Dietary Guidelines Advisory Committee (2015 DGAC) recommended a maximum of 10% of total calories from added sugars per day on the basis of studies that compared highest intake with lowest intake and the risk of preventing weight gain, cardiovascular disease, and type 2 diabetes.8 The Dietary Guidelines for Americans, 2015–20209 followed the recommendations of the 2015 DGAC, recommending a maximum 10% of total calories from added sugars per day.
In 2011, the European Food Safety Authority issued a scientific opinion on fructose, stating that “consumption of fructose leads to a lower blood glucose rise than consumption of sucrose or glucose,” while noting that high intakes of fructose (set at >25% of total energy) were shown to lead to metabolic complications such as dyslipidemia, insulin resistance, and increased visceral adiposity.10
Thus, a controversy exists about defining the appropriate upper levels of consumption of added sugars. Issues related to the proper upper limit of added sugars carry nutritional, public health, and public policy implications. The purpose of the current review is to explore contemporary scientific studies, particularly randomized controlled trials, systematic reviews, and meta-analyses, to assess the scientific basis for recommended upper limits of sugars consumption. The authors wish to emphasize that this review article conveys their personal points of view and is not mandated by a governmental agency, a nongovernmental organization, or a scientific society. Rather, each of the authors has conducted extensive research in the area of sugars and health, which forms the basis and substance of this collaborative review.
SUGARS DIGESTION AND METABOLISM
After ingestion, sugars are delivered into the intestinal lumen. From a physiological point of view, this step in digestion is the only one that differs between digestion of sucrose and that of HFCS: when HFCS is ingested, the glucose and fructose molecules are already in their free form and are ready to be absorbed by their specific transporters. In contrast, sucrose needs first to be cleaved, as only monosaccharides can be absorbed by enterocytes. Several sucrose enzymes in the brush border of the gut are responsible for the hydrolysis of sucrose into its two moieties, glucose and fructose, the quantitatively most important being Enzyme Commission (EC) 3.2.1.48, sucrose alpha-glucohydrolase. When equimolar amounts of fructose are administered as either sucrose or HFCS, the kinetics of fructose and glucose absorption after ingestion of sucrose and HFCS is similar, indicating that sucrose hydrolysis is not rate-limiting.11
As mentioned above, natural sources of fructose include mainly fruits, honey, and to some extent vegetables. A large portion of daily sugars intake, however, consists of either sucrose extracted from cane or beets or HFCS added at some stage during food preparation.12 The food structure and matrix modulate gastric emptying, which will subsequently influence the absorption rate of intestinal sugars and affect the overall postprandial metabolic response (glycemic index, for instance). Apart from that, fructose and glucose entering the enterocytes are metabolized identically, irrespective of their original food source. In this sense, the concept of added sugars has little nutritional relevance.13
In intestinal cells, glucose is absorbed at the luminal side of the enterocytes by the sodium-glucose cotransporter 1. Glucose is then transferred to the blood through a facilitative glucose transporter, glucose transporter 2 (GLUT2), at the basolateral side of the enterocyte. The simultaneous transport of sodium and glucose allows use of the sodium luminal–intracellular sodium gradient to drive glucose absorption, eventually against its own concentration gradient. This “secondary active” transport relies on the gradient of sodium actively generated by the sodium/potassium–adenosine triphosphatase pump. The same transport system operates for glucose and galactose. In contrast, fructose is absorbed at the luminal side of the enterocyte by a facilitative transporter, glucose transporter 5 (GLUT5), and at the basolateral side by GLUT2.
After being absorbed into the portal blood, glucose and fructose will have markedly different fates because glucose can be used directly as an energy substrate by all human cells, while fructose cannot. Glucose is indeed the predominant energy source for human metabolism, and all cells express specific glucose transporters and glucose-metabolizing enzymes. There are various facilitative glucose transporters (which constitute the GLUT family), each with different transport kinetics and specific expression, depending on the cell function. GLUT1 and GLUT3 are constitutively present in the plasma membrane of neurons, glial cells, and red blood cells. Because of their low Km (≈1 mM) for glucose, they allow for relatively constant glucose uptake throughout the range of fasting and postprandial glycemia. GLUT2 is expressed in pancreatic β-cells, liver cells, and some central nervous system neurons; it is characterized by a high Km for glucose (ca 10 mM) and, hence, is responsible for the variation in cell glucose uptake according to changes in glycemia. GLUT2 can therefore confer glucose-sensing properties to GLUT-expressing cells. GLUT4 is expressed mainly in insulin-sensitive cells (ie, skeletal muscle and adipose tissue, with the exception of liver). It has a low Km for glucose, and the insulin-dependent glucose transport in GLUT4-expressing cells is triggered by the translocation of GLUT4 receptors from an intracellular pool to the cell surface.14
Once inside the cell, glucose is first phosphorylated into glucose-6-phosphate by a hexokinase, and to fructose-1,6-bisphosphate by the enzyme phosphofructokinase. Fructose-1,6-bisphosphate is further converted into triose phosphates and, finally, into pyruvate, which can ultimately be used for mitochondrial oxidation and energy release as adenosine triphosphate (ATP). All cells of the organism express at least one member of the GLUT family, one of several isoforms of hexokinase, and glycolytic enzymes. Glucose transport and metabolism are tightly regulated by several mechanisms, including insulin levels, as well as by the glucose concentration itself and the intracellular ATP and citrate concentrations. This ensures an appropriate use of glucose according to the cell need and the blood glucose level.
Although both fructose and glucose are transported into hepatocytes by the same GLUT2 facilitative transporter, fructose metabolism differs markedly from that of glucose at several levels. First, in contrast to glucose, fructose does not increase glycemia and, hence, does not stimulate insulin release. Second, fructose has a low affinity for hexokinases and, hence, will be metabolized almost exclusively in a limited set of organs that express specific fructose-metabolizing enzymes. These enzymes catalyze the initial steps of fructose metabolism, ie, fructolysis: Fructokinase, or ketohexokinase, is responsible for the phosphorylation of fructose into fructose 1-phosphate; aldolase B catalyzes the cleavage of fructose 1-phosphate into glyceradehyde and dihydroxyacetone phosphate; and finally, triokinase phosphorylates glyceraldehyde to glyceraldehyde-3-phosphate. In contrast to glycolysis, which is tightly regulated by intracellular ATP at the level of phosphofructokinase, fructolysis is not regulated, with the consequence that triose phosphate will be generated according to the amount of fructose entering the hepatocytes, irrespective of the cell’s energy need.15 As triose phosphates are common intermediates to glycolysis, they can be diverted into the following pathways: (1) synthesis of acetyl-coenzyme A, followed by mitochondrial oxidation of coenzyme A; (2) conversion into glucose and lactate, which can be either released into the systemic circulation for further utilization by extrahepatic tissues or used to replenish hepatic glycogen stores; or (3) synthesis of fatty acids, through the de novo lipogenesis pathway. This last route, however, is costly in energy and occurs mainly when fructose consumption is abnormally high.16
Fructose-metabolizing enzymes are synthesized predominantly in duodenal and jejunal enterocytes and in hepatocytes. The gut and the liver together remove most ingested fructose, with the consequence that blood fructose levels increase very little, even after ingestion of large amounts of fructose. Fructose-metabolizing enzymes are also expressed in the kidney, possibly to ensure the removal of whatever fructose escaped first-pass splanchnic uptake. Although many cell types express the specific GLUT5 fructose transporter, it is doubtful they metabolize fructose to any great extent, owing to the low affinity of fructose for hexokinases and to the extremely low amount of fructose found in the systemic circulation under physiological conditions.
It should be noted that some of the abnormalities associated with fructose metabolism, such as hypertriglyceridemia and hepatic insulin resistance, may be merely a reflection of the specific pathways of fructose metabolism rather than markers of disease.
ADDED SUGARS CONSUMPTION IN THE UNITED STATES
Composition
As defined by US Department of Agriculture (USDA), added sugars are those sugars and syrups added to foods during processing or preparation, including sugars and syrups added at the table.
While the composition of added sugars consumed – or predigested – is most important when considering the functional attributes each sugar confers to foods and beverages, it is the digested composition that enters the bloodstream and is most important in predicting metabolic effects. Table 1 compares the as-consumed vs postdigestion compositions of common added sugars. The action of hydrolytic enzymes during digestion releases free fructose and glucose from sucrose, and free glucose from the range of oligosaccharides found in honey and corn syrup. Although sucrose, HFCS, and honey may differ somewhat in composition before digestion, their fructose and glucose ratios are quite similar afterward, explaining their nearly equivalent effects on metabolic processes.
Table 1.
Comparison of sugars: consumed vs post digestion
| Added sugars | Percentage of total sugars | |||
|---|---|---|---|---|
| Sucrose | Fructose | Glucose | Glucose oligosaccharides | |
| Consumed | ||||
| Sucrose varieties | 96–99.3 | 0.006–3 | 0.007–2 | |
| Total inverted sugar | 6 | 47 | 47 | |
| Honeya | 4 | 50 | 42 | 4 |
| HFCS-42 | 42 | 53 | 5 | |
| HFCS-55 | 55 | 42 | 3 | |
| Fructose | 100 | |||
| Dextrose (glucose) | 100 | |||
| Corn syrup | 100 (varies by product) | |||
| Post digestion | ||||
| Sucrose varieties | 50 | 50 | ||
| Total inverted sugar | 50 | 50 | ||
| Honey | 52 | 48 | ||
| HFCS-42 | 42 | 58 | ||
| HFCS-55 | 55 | 45 | ||
| Fructose | 100 | |||
| Dextrose (glucose) | 100 | |||
| Corn syrup | 100 | |||
Abbreviation: HFCS, high-fructose corn syrup.
aAverage sugars profile of 4 varieties of honey produced in the United States.
Prior concerns about differences in SSB composition contributing unexpected fructose to the diet17,18 were unfounded19,20 and likely due to the use of improper and unverified methodology.
Consumption
Sugars consumption data in the United States are available from two independent and corroborating sources: dietary intake estimates using the National Health and Nutrition and Examination Survey (NHANES) database, an extensive relation of health and nutritional status (collected via 24-hour food recalls), and industry commodity production records collected annually by USDA Economic Research Service (ERS) (food availability, adjusted for loss).
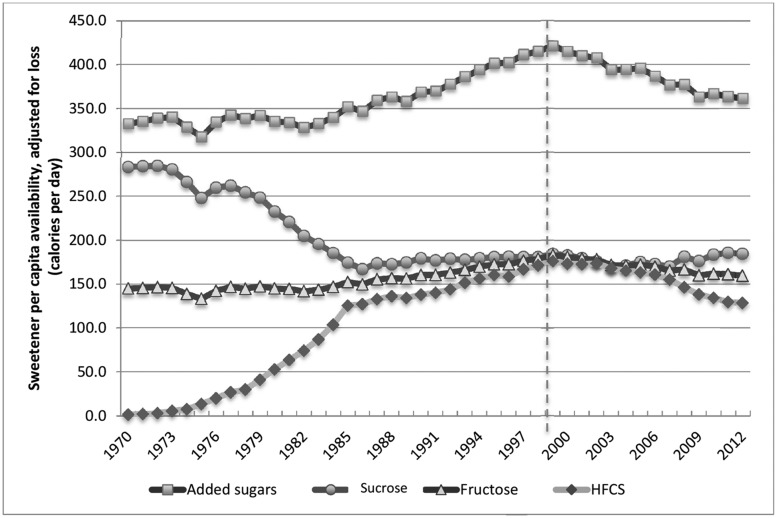
In independent cross-sectional studies using the NHANES database, Welsh et al.,21 Yang et al.,22 and Marriott et al.23 concluded that energy from added sugars in the United States has been in steady decline since the 1994–1998 survey. Historical data on sugars production have been collected by USDA ERS since the early 1900s. Apparent consumption of sucrose increased 40% between 1910 and 1921 but remained constant over the following 50 years, except for supply interruptions during World War II.24 The USDA ERS now makes available contemporary commodity production figures adjusted for losses from transportation and warehousing, food spoilage, cooking loss, and plate waste that better estimate actual per capita intake.25 Loss-adjusted per capita availability trends for various added sugars over the past 40 years – since the introduction of HFCS – are compared in Figure 1. Apparent consumption of added sugars increased by 27% during the 30-year period from 1970 to 1999; however, the subsequent substantial decline returned much of that gain. The net increase in energy from added sugars over the past 4 decades was approximately 29 kcal/d.
Figure 1.
Comparison of trends in per capita availability (loss-adjusted) of nutritive sweeteners
Carden and Carr26 and White24 independently analyzed the USDA ERA Loss-Adjusted Food Availability Database to conclude that total fructose availability did not materially increase from 1970 to 2012 and was, therefore, unlikely to have played a causative role in the increased prevalence of obesity and associated diseases. As illustrated in Figure 1, trends in consumption of the two predominant fructose-containing sweeteners, sucrose and HFCS, were mirror images: the increase in HFCS was largely offset by the decline in sucrose. Since sucrose and HFCS both comprise roughly equal amounts of glucose and fructose, the net effect on fructose consumption over this period was minor.
In summary, dietary intake estimates and commodity production records both report a rise in added sugars consumption that peaked around the turn of the 21st century and was followed by a downward trend that extends to the present. Americans are consuming less added sugars today than 15 years ago. Though reasons for the decline are various – greater health consciousness among consumers, reduced consumption of SSBs and increased consumption of bottled water, and improved quality of reduced-calorie foods and beverages – the downward trend is clear.
SUGARS CONSUMPTION AND OBESITY
The world is in the midst of a pandemic of obesity. Recent estimates suggest more than 66 million American adults are obese and an additional 74 million are overweight.27 The problem of obesity is truly global. In European countries, obesity rates range from 8% to 30%28,29 and are even higher in South America, Australia, the Middle East, and Polynesia.28 According to the WHO, there are currently 1.1 billion obese individuals worldwide; if current trends continue, it is estimated there will be 1.5 billion obese individuals worldwide by 2015 and 2.16 billion by 2030.30
The rapid increase in obesity and associated health costs has stimulated research on a variety of nutritional factors that might be associated with weight gain and obesity. The putative association between fructose-containing sugars and obesity has prompted many investigators to focus specifically on whether the consumption of these sugars contributes disproportionately to weight gain.
Prospective cohort studies
Several prospective cohort studies have assessed the relation between intake of total sugars and body weight. Neither individual cohort studies using energy-adjusted and -unadjusted models nor a WHO-commissioned systematic review and meta-analysis of prospective cohort studies using energy-adjusted models found an association between total sugars and body weight when comparing the highest with the lowest level of intake.31 The same was seen when random-effects models were used to assess intakes of important sources of added sugars like cakes, pastries, and sweets.31 A consistent relation, however, has been observed between SSBs and body weight or risk of overweight/obesity. The WHO-commissioned systematic review and meta-analysis showed a significant association between SSBs and risk of overweight/obesity in children and weight gain in adults31 when the highest level of SSB exposure was compared with the lowest level by pooling data from energy-adjusted models. This adjustment controls for excess energy intake from sugars or other sources that may be consumed along with or apart from SSBs. Other systematic reviews and meta-analyses32,33 have not found an association between SSBs and body mass index when using energy-adjusted models. However, when energy-unadjusted models were used, a significant positive association was seen between SSBs and body mass index in these same systematic reviews and meta-analyses.32,34 Associations were also not seen at intermediate levels of exposure that were below the 50th percentile for added sugars or fructose intake in the United States.21,35 The implication is that the special association between SSBs and weight gain is mediated by energy and may be attributable to the energy rather than the sugars per se. Other explanations may relate to the observation that liquid calories are more poorly compensated than solid calories36 or that SSBs represent a marker of an unhealthy lifestyle in prospective cohort studies.37 Both explanations remain open questions and argue against a unique role of sugars.
Controlled trials
Numerous randomized controlled trials of the effect of sugars consumption on body weight have been performed. These have been recently analyzed by 4 different groups who performed systematic reviews and meta-analyses of these published trials.31,32,38,39 As already indicated in this review, these studies have typically not found that sugars in isocaloric exchange for other carbohydrates create a unique risk of obesity. Many of these trials, however, suffered from recruitment of too few subjects (≤100) and interventions of relatively short duration (≤1 year).
Several recent trials have also explored issues of either sugars as part of a hypocaloric diet or various dosages of sugars as related to weight change. In a randomized controlled trial reported by Lowndes et al.,40 10% of calories were consumed as either HFCS or sucrose and compared with 20% of calories from either of these sugars. Individuals consumed these added sugars over a 10-week free-living period as part of a eucaloric diet. While there was an overall body weight increase of slightly less than 1 kg when the entire cohort of 65 individuals was evaluated, none of the individual weight gains achieved significance within group, and there was no difference between 10% and 20% of calories from added sugars. The same group of investigators compared 8% of calories from either sucrose or HFCS to 18% of calories from either HFCS or sucrose and 30% of calories from HFCS or sucrose in a randomized controlled trial involving 355 subjects (165 males, 190 females) who completed a 10-week intervention in a free-living environment.41 While, once again, there was an increase in weight of slightly less than 1 kg for the entire cohort, there was no difference in weight gain when different levels of added sugars from either sucrose or HFCS were compared. However, there were significant differences in body weight, body mass index, and fat mass between the sugars levels, suggesting that the dose, rather than the type of sugar, plays an important role. Similar dose–response results were found by another study, which compared only different levels of HFCS and did not include a sucrose arm.42
Several systematic reviews and meta-analyses have pooled the totality of the evidence from randomized and nonrandomized controlled trials on the effect of chronic feeding with fructose-containing sugars on body weight. Different trial designs have been used to isolate the effect of sugars from that of energy. These include “substitution” trials, in which added fructose-containing sugars are compared with other macronutrient sources (usually starch or other sugars) under energy-matched conditions; hypercaloric “addition” trials, in which fructose-containing sugars supplement a diet with excess energy compared with the same diet alone without the excess energy; and “subtraction” trials, in which energy from fructose-containing sugars (usually in the form of SSBs) is reduced by displacing it with water and/or noncaloric sweeteners or eliminating it altogether from the background diet. Fructose-containing sugars did lead to the expected weight gain in hypercaloric addition trials31,32,38,39 and to the expected weight loss (with some exceptions in children) in subtraction trials.31,32,38,39 Fructose-containing sugars did not affect weight in substitution trials.31,39 Thus, the effects of fructose-containing sugars were not different from those of other macronutrients (mainly starch), as long as the comparisons remained matched for calories.
Although these different trial designs have been useful in isolating the effect of energy as opposed to the effect of any special metabolic, endocrine, or neuroendocrine response to sugars, they do not allow for real world compensation since participants are required to consume the full dose of sugars. A more ecologically valid way to assess whether sugars, more than other forms of energy, promote overconsumption leading to weight gain under free-living conditions is an ad libitum design, in which fructose-containing sugars are freely replaced with other sources of energy in the diet without any strict control of the amount of replacement of the sugars or the background diet. The CArbohydrate Ratio Management in European National diets (CARMEN) trial43 is the largest and longest trial to date that uses an ad libitum design to assess the effect of sugars on weight gain. It compared the effects on body weight of an ad libitum high-fructose-containing sugars diet (29% energy from sugars), an ad libitum high-complex-carbohydrate diet, and an ad libitum higher-fat control diet in 398 obese adults over 6 months. Both the ad libitum high-sugars diet and ad libitum high-complex-carbohydrate diet resulted in lost weight compared with the ad libitum higher-fat control diet. There was no significant difference between the ad libitum high-sugars diet and the ad libitum high-complex-carbohydrate diet, although there was a nonsignificant tendency for greater weight loss with the latter. Similar results were found in a randomized trial of 46 participants with metabolic syndrome who followed the same protocol over 6 months.43 These trials show that, under free-living conditions, it is possible to lose weight while following an ad libitum high-sugars diet and that a simple strategy to freely replace energy from fructose-containing sugars with other sources of energy in the diet, especially from fat, offers no clear advantages for weight loss.
Thus, based on higher-quality evidence from systematic reviews and meta-analyses of randomized controlled trials and individual randomized controlled trials, there does not appear to be any difference with regard to weight gain when sugars are consumed as part of a controlled isocaloric diet, regardless of the dose. Of note, most of these studies were performed during relatively short periods of time (<1 year) and under controlled conditions. The small weight gain reported in some randomized control trials suggests that individuals may have had difficulty incorporating added sugars into their normal eating patterns, which further suggests that these changes are a result of added calories rather than the sugars per se.
ENERGY-REGULATING HORMONES
Research comparing the effects of 25% of calories from fructose with 25% of calories from glucose as part of a eucaloric diet on energy-regulating hormones that included insulin, leptin, and ghrelin suggested that fructose created less increase of insulin, less increase of leptin, and less depression of ghrelin than glucose, thereby creating an environment that could lead to overconsumption of calories.45 Subsequent research reported by Melanson et al.,46 who investigated individuals given 25% of calories as normally consumed sugars (ie, either sucrose or HFCS), showed all of these differences disappearing. An additional research trial reported by Yu et al.,47 who compared 8%, 18%, and 30% of calories from either fructose or sucrose or HFCS in 138 individuals, showed no differences in leptin or ghrelin at baseline or post testing. The area under the curve for insulin and leptin increased over the course of the intervention, but there was no difference between the various dosages of added sugars. There was no change in area under the curve or active ghrelin in the entire cohort, and no difference between the groups. These data support the conclusion that there is no difference in these energy-regulating hormones when 8%, 18%, and 30% of calories from added sugars are compared and no differences between the effects of HFCS and sucrose on these parameters within the normal range of human consumption.
SUGARS AND RISK FACTORS FOR HEART DISEASE
There have been no randomized controlled trials exploring the impact of added sugars on heart disease per se. Studies in this area have typically focused on risk factors for heart disease.
Prospective cohort studies
A number of prospective cohort studies have assessed the relation of the intake of total sugars with the risk of hypertension and coronary heart disease (CHD). A systematic review and meta-analysis of the available prospective cohort studies showed no association between total fructose and incident hypertension in energy-adjusted models when comparing the highest with the lowest levels of exposure.48 There was even a modest protective association seen at intermediate intakes.48 Individual large prospective cohort studies from the United States and Europe have also failed to show an association between total sugars and incident CHD.49–52 The large National Institutes of Health (NIH)–AARP Diet and Health Study also failed to show an adverse association of total sugars, sucrose, or fructose with cardiovascular mortality in 353 751 men and women aged 50 to 71 years after up to 13 years of follow-up using energy-adjusted models.53 It should be noted that, in a recent systematic review and meta-analysis of prospective cohort studies, Jayalath et al.54 found an association between fructose-containing SSBs and risk of hypertension. The authors caution, however, that there is a need for high-quality randomized trials to assess the role of SSBs in the development of hypertension and its complications.
Yang et al.22 reported that risk of cardiovascular disease was 1.30 for those who consumed 10% to 24.9% of calories from added sugars and 2.75 for those who consumed 25% or more of calories from added sugars (≈10% of the population) when compared with those who consumed less than 10% of added calories from sugars. These investigators, utilizing NHANES data, also reported that the percentage of daily calories from added sugars increased from 15.7% in 1988–1994 to 16.8% in 1999–2004 but decreased to 14.9% in 2005–2010.
A protective association was even seen for sucrose or fructose from solid foods.53 As far as can be determined, there are no prospective cohort studies that have assessed the association between total sugars and incident stroke.
On the other hand, a positive association between added sugars and CHD mortality was shown in the NHANES when energy-unadjusted models were used to compare the highest level of exposure with the lowest level over almost 15 years of follow-up.22 This observation, however, may relate more to the categorization of added sugars than the added sugars per se. The larger NIH-AARP Diet and Health Study failed to show a positive association of added sugars with cardiovascular mortality, observing that a signal for harm was restricted to added sugars from liquid foods. The opposite association (protective) was seen between added sugars from solid foods and cardiovascular mortality.53 Systematic reviews and meta-analyses of prospective cohort studies have shown a relatively consistent positive association between SSBs and the risk of hypertension and CHD when using energy-adjusted models to compare the highest with the lowest level of exposure.54,55 These associations, however, do not hold at intermediate levels of exposure, with a dose–response relationship seen for CHD only at the highest level of exposure, and pooled analyses have not shown a significant association with stroke.55 These findings, however, appear directly related to the most important source of added sugars in NHANES: SSBs. Why SSBs appear to be the special case in these analyses again remains an open question and argues against a unique role of sugars per se.
Lipids
Diets with greater than 20% of calories from simple sugars have been associated with increases in triglycerides.55–58 It is for this reason that the American Heart Association lists reduction in fructose-containing sugars as one potential mechanism for lowering triglycerides.59 Recent studies in this area, however, have yielded conflicting results.60,61 It has been argued that fructose consumption, particularly at levels above 20% of calories, increases triglycerides, and it has been suggested this might be an appropriate upper limit of fructose consumption to be considered.62
Controlled trials
Some randomized controlled trials have reported that sugars consumption resulted in various dyslipidemias in human participants. Stanhope et al.,63 using an acute model in which 25% of energy consumption from fructose was compared with 25% from glucose, showed increases in triglycerides in the fructose-consuming group.63 Other investigators, including Marckmann,64 Raben et al.,65 Teff et al.,45 and Maersk et al.,66 have also reported increases in total cholesterol and/or low-density lipoprotein cholesterol (LDL-C) in participants consuming either sucrose or HFCS.
In contrast, the study by Lowndes et al.,41 which compared HFCS or sucrose at 8%, 18%, and 30% of energy (equivalent to 4%, 9%, and 15% of energy from fructose) over a 10-week free-living randomized controlled trial, showed no increases in total cholesterol and no differences between the 3 different levels of sugars. Low-density lipoprotein cholesterol also did not change over this 10-week period, nor were there differences between the 3 different levels of sugars. In the overall cohort, triglycerides rose approximately 10% over the course of the 10-week period. However, there were no differences between the 3 different levels of sugars with regard to triglycerides. Thus, it would appear that there are no differences between these 3 levels of sugars consumption with regard to effects on lipids, and no differences between HFCS and sucrose. In contrast, another study that administered 0%, 10%, 17.5%, and 25% of energy requirements as HFCS for 15 days showed dose-dependent increases in both fasting and postprandial plasma triglycerides, LDL-C, and apolipoproteins B and C-III.42 Discrepancies between these studies may result from differences in study design or food intake from other sources. Several systematic reviews and meta-analyses have pooled the data from the available controlled feeding trials investigating the effect of fructose consumption on lipids. Pooled analyses did not show an effect on fasting or postprandial triglycerides, total cholesterol, LDL-C, or high-density lipoprotein cholesterol (HDL-C) in substitution trials, in which fructose is compared with other macronutrient sources (usually starch or glucose) under energy-matched conditions.61,67,68 A dose threshold, however, was shown for fasting triglycerides (≥100 g/d across participants with different metabolic phenotypes67 and >60 g/d in people with type 2 diabetes68) and for postprandial triglycerides (≥50 g/d) across participants with different metabolic phenotypes.67 But these thresholds have not been reproduced in updated systematic reviews and meta-analyses.61,69 A more reproducible signal for harm was seen under conditions in which fructose provides excess calories. Fructose was shown to increase fasting or postprandial triglycerides and apolipoprotein B in addition trials, in which fructose-supplemented diets with excess energy were compared with the same diets without the excess energy.69 In the absence of a consistent signal for harm of fructose in isocaloric substitution trials, the adverse effect of fructose on lipids in the addition trials appears to be attributable more to the excess calories than to fructose as a substrate.
The effects are harder to interpret for total sugars. A recent systematic review and meta-analysis of randomized controlled trials of the effect of total sugars on fasting triglycerides, total cholesterol, LDL-C, and HDL-C showed mixed results.70 Total sugars showed small unfavorable effects on fasting triglycerides, total cholesterol, and LDL-C but favorable effects on HDL-C in isocaloric substitution trials. A lack of effect, however, on total cholesterol, LDL-C, and HDL-C was seen under more free-living conditions in ad libitum trials. Although these discrepant findings raise questions about the importance of any lipid effects, it is difficult to draw conclusions either way. In addition to the true ad libitum trials included in this review, ad libitum trials were defined so as to also include addition and subtraction trials. Updated analyses of lipid effects will be useful for understanding the role of total sugars in lipid control.
Blood pressure
It has been argued that fructose-containing sugars may affect blood pressure, although results in humans have been variable. Some trials report increases in blood pressure,71,72 while others do not.73 Johnson et al.74 have proposed a theoretical mechanism to explain why fructose-containing sugars may increase blood pressure. According to this theory, metabolism of fructose in the liver results in large amounts of ATP being consumed. The ATP is then degraded to adenosine monophosphate (AMP) and, ultimately, to uric acid, which, according to this theory, can lead to endothelial dysfunction and increased blood pressure. Subsequent studies have not confirmed this hypothesis.75,76
Controlled trials
A number of studies have explored the link between fructose-containing sugars and blood pressure. These studies have been subject to systematic review and meta-analysis by Ha et al.77 These investigators reviewed a total of 15 trials of blood pressure and fructose-containing sugars, including 13 isocaloric and 2 hypercaloric trials. Their overall conclusion was that fructose, in isocaloric exchange for other carbohydrates, significantly decreased diastolic blood pressure and mean arterial pressure but had no effect on systolic blood pressure. In addition, the randomized controlled trials of hypercaloric fructose in comparison with other carbohydrates found no overall increase in arterial blood pressure. These investigators concluded that fructose did not appear to lead to any increased added risk of elevated blood pressure when compared with other carbohydrates; they did suggest, however, that longer and larger trials were needed.
In their recent randomized trial involving 355 individuals who consumed either 8%, 18%, or 30% of calories from sucrose or HFCS, Angelopoulos et al.78 found no changes in systolic blood pressure and no differences between any of the sugars types or levels of consumption. Diastolic blood pressure also did not change, nor were there any differences between sugars types or levels of sugars. Thus, it would appear that there are no differences in the 3 levels of consumption of sugars with regard to increases in blood pressure.
A systematic review and meta-analysis was conducted to synthesize evidence on the effect of fructose on blood pressure in the available controlled feeding trials.77 There was no evidence of harm in the substitution trials, in which added fructose-containing sugars were compared with other carbohydrates (starch or other sugars) under energy-matched conditions. Fructose was even shown to decrease diastolic blood pressure and mean arterial pressure under calorie-matched conditions. Fructose also failed to increase blood pressure in hypercaloric addition trials, in which diets with excess energy were supplemented with fructose-containing sugars and compared with the same diets alone without the excess energy. The data from the hypercaloric addition trials, however, were quite limited: there were only 2 trials, which makes it difficult to draw strong conclusions.
Inconsistent results were found for total sugars. A recent systematic review and meta-analysis of randomized controlled trials studying the effect of total sugars on blood pressure showed no increase in either systolic or diastolic blood pressure in isocaloric substitution trials.70 Although an increase was observed for diastolic blood pressure in ad libitum trials, drawing a firm conclusion was complicated because of the varying definitions of ad libitum trials, which included true ad libitum trials, addition trials, and subtraction trials.
SUGARS AND INSULIN RESISTANCE OR DIABETES
Type 2 diabetes has increased dramatically worldwide in the past 20 years.79 It has been estimated that 6.4% of the world population is currently diabetic, with predictions the incidence will rise to 7.7% by the year 2030.80 Insulin resistance typically precedes diabetes by 10 to 20 years and is the major risk factor for diabetes.81,82 The dramatic increase in diabetes has paralleled an increase in the prevalence of obesity worldwide. This, in turn, has raised interest in the nutritional aspects of both diabetes and obesity. Considerable attention has been focused on consumption of fructose-containing sugars as playing a possible role in promoting type 2 diabetes.
Several epidemiologic studies have linked consumption of SSBs to increased risk of diabetes.83–86 Two recent ecological studies have linked the rise in fructose availability (either from sucrose or HFCS) to the increased prevalence of diabetes both in the United States and around the world.87,88 Animal studies89–92 and human trials,63 particularly those in which fructose was overfed beyond normal population intakes,63 have also supported this association. Higher-quality evidence from prospective cohort studies, systematic reviews, meta-analyses, and randomized controlled trials generally has not supported the link between consumption of fructose-containing sugars and the development of type 2 diabetes.
Prospective cohort studies
A number of prospective cohort studies have assessed the relation of sugars with the risk of diabetes. A systematic review and meta-analysis of prospective cohort studies showed that neither total sugars nor fructose-containing sugars had an unfavorable association with incident type 2 diabetes.93 There was also no association with important food sources of fructose-containing sugars, such as sweets and cakes94 and pure fruit juice,95 while a protective association for fruits95 was found in individual prospective cohort studies and in systematic reviews and meta-analyses of prospective cohort studies. Again, the one exception was SSBs. Separate systematic reviews and meta-analyses of prospective cohort studies showed associations between SSBs in the form of sodas96 or fruit drinks95 and diabetes when comparing the highest with the lowest levels of exposure. None of the included studies, however, showed significant associations at levels of exposure that were below the 50th percentile for intakes of added sugars or fructose in the United States.21,35 These analyses also preferentially pooled energy-unadjusted models. This lack of adjustment complicates interpretation, making it difficult to separate the association of SSBs from that of energy (especially where SSBs may have important collinearity with other highly palatable caloric foods). The association with SSBs may be mediated more by energy intake, poor compensation of liquid calories (although this does not explain the lack of association with pure fruit juice), and/or collinearity effects from other aspects of an unhealthy lifestyle.
Controlled trials
There are no randomized controlled trials examining whether sugars consumption in subjects with normal glycemia results in diabetes or prediabetes. However, there are studies that have examined surrogate measures of diabetes risk. These studies have generated mixed results. Most have demonstrated that sucrose or fructose had no adverse effects on fasting glucose, insulin levels, or postprandial glucose.41,45,66,92,97–101 Inconsistent results have been reported when direct markers of insulin sensitivity were measured, regardless of whether the sugar was fructose or sucrose.63,99–102
In a randomized controlled trial involving 352 overweight/obese men and women, Lowndes et al.41 explored the impact of consuming 8%, 18%, or 30% of calories from either HFCS or sucrose and reported no increases in risk factors for diabetes, including fasting glucose or insulin resistance as computed by the homeostatic model of assessment.41
A series of systematic reviews and meta-analyses have been conducted to synthesize the effects of fructose on blood glucose and insulin regulation in the available controlled feeding trials.103 There was no evidence of an adverse effect of fructose on glycemic control, fasting insulin, or markers of whole-body and hepatic insulin sensitivity in substitution trials, in which added fructose-containing sugars were compared with other carbohydrates (starch or other sugars) under energy-matched conditions. On the contrary, fructose was shown to lead to clinically meaningful improvements in glycemic control as assessed by glycated blood proteins (equivalent to a 0.57% reduction in hemoglobin A1c [HbA1c], which exceeds the US Federal Drug Administration threshold of 0.3% for the development of new oral antihyperglycemic agents) in people with and without diabetes. Fructose was found to have unfavorable effects, however, on fasting glucose, insulin, and markers of whole-body and hepatic insulin sensitivity in hypercaloric addition trials. The lack of harm, and even the benefit seen, in isocaloric substitution trials suggests that the signal for harm seen in the hypercaloric addition trials is again explained by the excess calories rather than the fructose.
THE METABOLIC SYNDROME
Metabolic syndrome is a constellation of findings represented by dysregulated glucose handling, dyslipidemia, hypertension, and abdominal obesity. The prevalence of metabolic syndrome varies widely, depending on the various criteria used to define it. However, the prevalence has increased dramatically over the past 20 years. Estimates using criteria from the National Cholesterol Education Program (ATP-IV) suggest that up to 36% of the population in the United States show signs of metabolic syndrome.104
Several investigators have suggested that the metabolism of fructose-containing sugars may create the environment to increase the risk of metabolic syndrome. Johnson et al.74 have proposed a mechanism through which fructose metabolism results in the consumption of ATP, which is degraded into AMP and, ultimately, to uric acid and excess fatty acids. According to this theory, these fatty acids result in insulin resistance, fatty infiltration of the liver, and the hallmarks of metabolic syndrome, including glucose intolerance, dyslipidemia, and hypertension.
Prospective cohort studies
As far as can be determined, there are no prospective cohort studies that have assessed the relation of total sugars, sucrose, or fructose with the risk of metabolic syndrome. Evidence is available for SSBs as a proxy for added sugars. A systematic review and meta-analysis of prospective cohort studies showed a positive association between SSBs and incident metabolic syndrome when the highest and the lowest levels of exposure were compared.96 None of the included studies, however, showed significant associations at levels of exposure below the 50th percentile for intakes of added sugars or fructose in the United States.21,35 These analyses also preferentially pooled data from energy-unadjusted models, making it difficult, once again, to disentangle the effect of SSBs from that of energy.
Controlled trials
Several randomized controlled trials have come to different conclusions about the components of metabolic syndrome. In a 4-week study of a high-fructose diet containing 1.5 g of fructose per kilogram of body weight, Lé et al.85 did not find changes in insulin sensitivity, although triglycerides were increased. In contrast, Silbernagel et al.101 found that insulin sensitivity decreased in a 4-week high-fructose (150 g/d) diet in healthy humans. Maersk et al.66 reported that individuals who consumed SSBs at 1 L/d (slightly above the average consumption of added sugars) increased liver fat storage and visceral fat, both of which are risk factors for metabolic syndrome. Stanhope et al.63 studied overweight and obese individuals who were given 25% of calories from either fructose or glucose and reported that fructose at this level increased visceral adiposity and led to dyslipidemia.
In contrast, in their study of 352 individuals who consumed 8%, 18%, or 30% of calories from either sucrose or HFCS, Lowndes et al.41 did not show increases in glucose, uric acid, or systolic or diastolic blood pressure, nor was there a change in fasting glucose. However, HDL-C decreased by less than 1 mg/dL, and triglycerides increased by 10%. Importantly, for all of these measures – systolic and diastolic blood pressure, triglycerides, HDL-C, fasting glucose, and uric acid – there was no difference between the 3 levels of sugars consumption (interaction P for all measures >0.05). As already noted, an increase in weight (slightly less than 1 kg) occurred across the entire cohort of 352 individuals, resulting in an increase in waist circumference over the course of the 10-week intervention. In this study, energy intake increased by approximately 300 kcal from baseline to the end of the intervention. These increases in calories were driven largely by increases in the groups that consumed 30% of their calories from sugars. Thus, the Lowndes et al.41 study represents a hypercaloric condition. These data support the concept that if sugars consumption increases risk factors for metabolic syndrome, the changes are small and mixed and may be the result of increased calories and weight gain rather than the added sugars per se. Furthermore, there is no difference between 8%, 18%, and 30% of calories with regard to risk factors for metabolic syndrome.
Systematic reviews and meta-analyses of controlled feeding trials have failed to show an adverse effect of fructose on metabolic syndrome criteria (weight, fasting glucose, fasting triglycerides, HDL-C, and blood pressure) in isocaloric substitution trials39,60,67,68,77,103. Although there may be a dose threshold for fasting triglycerides in earlier subgroup analyses of isocaloric substitution trials (a finding that has not been replicated in updated analyses69), a consistent adverse effect of fructose on some metabolic syndrome criteria – weight, fasting glucose, and fasting triglycerides, but not HDL-C or blood pressure – is restricted to hypercaloric addition trials.39,60,77,103 The same pattern is seen for the effect of total sugars on body weight in the WHO-commissioned systematic review and meta-analysis.31 The results for 3 metabolic syndrome criteria (fasting triglycerides, HDL-C, and blood pressure) from another systematic review and meta-analysis of the effect of total sugars in randomized controlled trials remain small, inconsistent, and difficult to explain.70
Thus, findings related to risk factors for metabolic syndrome appear to be mixed. This may be due to differences in the type or amount of sugars studied (eg, sucrose or HFCS vs fructose or glucose) and the duration of trials.
NONALCOHOLIC FATTY LIVER DISEASE
The prevalence of nonalcoholic fatty liver disease (NAFLD) has increased dramatically over the past 20 years.105,106 Nonalcoholic fatty liver disease now represents the leading cause of liver failure and the main reason for liver transplantation around the world. Concern about fatty infiltration of the liver and the potential effect of fructose has been evaluated by a number of investigators. Some investigators have suggested the basic underlying mechanism of fructose metabolism in the liver as a potential reason for concern about fat accumulation in the liver. As already indicated, while fructose and glucose are metabolized by different pathways in the liver, the pathways are interactive, and only 1% to 5% of consumed fructose is converted to free fatty acids and, ultimately, to triglycerides in humans.107,108 In some early investigations, it was noted that certain high-carbohydrate diets can dramatically enhance this process.109 In animals such as rodents, as much as 60% to 70% of consumed fructose may be converted into triglycerides through the process of de novo lipogenesis.110
Prospective cohort studies
There do not seem to be any prospective cohort studies that have investigated the relation of sugars or SSBs to NAFLD. It should be noted that a recently published cross-sectional analysis from the Framingham Study reported that regular SSB consumption was associated with greater likelihood of fatty liver disease, particularly in overweight and obese individuals. Diet soda intake was not associated with NAFLD.111
Randomized controlled trials
Stanhope et al.63 gave individuals 25% of energy as fructose or glucose and found increased de novo lipogenesis in the fructose-consuming group. Lé et al.85 gave offspring of diabetics doses of fructose at 3.5 mg/kg of lean body mass (35% of energy requirement) and found some increased accumulation of liver fat. In a 6-month trial, Maersk et al.66 compared 1 L of SSB (≈25% of energy requirement as sugars), low-fat milk, aspartame-sweetened diet cola, and water. They reported increases in liver fat in the SSB-consuming group. In contrast, Silbernagel et al.101 compared 4 weeks of a very high-fructose diet with 4 weeks of a very high-glucose diet (≈30% of energy requirement as either fructose or glucose) and found no differences in liver fat accumulation over a 10-week trial.
Johnston et al.112 compared high-fructose diets with high-glucose diets in 20 healthy men with central adiposity. The trial had two separate 2-week periods, an isocaloric period and a hypercaloric period, separated by 6 weeks. During the isocaloric period, individuals on either the high-fructose diet or the high-glucose diet did not develop any significant change in hepatic triglycerides or serum levels of liver enzymes. However, in the hypercaloric period, both the high-fructose diet and the high-glucose diet produced significant increases in these parameters, without significant differences between the groups. The investigators concluded that these changes are mediated by energy rather than by a specific macronutrient. Bravo et al.113 compared 3 different levels of added sugars, 8%, 18%, and 30% of calories from either HFCS or sucrose, in a 10-week free-living trial of 68 men and women between the ages of 20 and 60 years of age and found no increases liver fat and no differences between the 3 different doses of sugars.
Two separate systematic reviews and meta-analyses have been conducted to synthesize the effects of fructose on markers of NAFLD in the available controlled feeding trials.114,115 There was no evidence of adverse effects on alanine aminotransferase or intraheptocellular lipids in substitution trials, in which fructose-containing added sugars were compared with starch or other sugars under energy-matched conditions. Fructose, however, did increase alanine aminotransferase and intraheptocellular lipids in hypercaloric addition trials. In the absence of an adverse effect of fructose on markers of NAFLD in isocaloric substitution trials, the adverse effects seen in the hypercaloric addition trials appear to be mediated by excess calories.
Thus, it would appear that normal levels of fructose-containing sugars do not increase liver fat in humans, and that there are no differences in a wide range of consumption levels varying from 8% of calories (25th percentile population consumption level of fructose) to 30% of calories (95th percentile population consumption level of fructose) when fructose is consumed under isocaloric conditions. In contrast, it appears quite clear from addition trials that short-term overfeeding of fructose increases liver fat content dose dependently.116 It should be noted, however, that many of these trials were 10 weeks in duration or shorter; thus, longer-term trials will be needed to determine whether sugars consumption increases the risk of NAFLD.
PUBLIC POLICY ISSUES
The classic determination of upper limits involves the establishment of either a maximum dose at which there is no adverse event or a concentration at which there is an absence of cytotoxic perturbation.117 On the basis of these kinds of data, a threshold level above which a negative outcome is documented, or an upper limit threshold, can be established. The Institute of Medicine has taken this approach for many vitamins and minerals.5
In the absence of an identified threshold, the no observable adverse effect level (NOAEL) often serves as a surrogate. A typical safety or uncertainty factor of 100 is applied to a NOAEL to allow for interspecies differences among animal models and for intraspecies variations among humans. An acceptable daily intake (ADI) is typically based on NOAEL ÷ 100. When total intake (exposure) from all possible sources does not pose a hazard (remember, risk = hazard × exposure), the risk becomes negligible and a numerical value for an ADI is not assigned. Thus, in the absence of a defined ADI, one could expect a lifetime exposure to a substance would not result in a health risk. This includes a lifetime exposure to sweeteners.118
Oral toxicity studies are germane to the understanding of sucrose safety and the potential development of an upper limit. When rats were provided a 100% sucrose-plus-vitamins diet over a 4-week period, the animals lost 30% body weight,119 as would be expected following consumption of a nutrient-deficient yet energy-adequate diet. A study of adult rats fed 80% sucrose diets for 26 weeks indicated enlarged livers, kidneys, and hearts as well as hypercholesterolemia.120 Adult rats fed by gavage, a solution that provided sucrose doses ranging from 5 to 80 g/kg of body weight for 30 days, demonstrated mortality threshold at 24 g/kg.121 The calculated median lethal dose (LD50) of sucrose from this study was 34.5 ± 7.0 g/kg. Loss of body weight, depressed food intake, and decreased water consumption were noted within the first 24 hours after administration of the sucrose solution at doses up to 20 g/kg. A similar study of young male rats indicated an acute oral LD50 (100 d) of 28.5 ± 1.3 g/kg.122 Interestingly, previous animal studies indicated no signs of toxicity at 2 to 3 times the LD50 when animals were fed intragastrically on an empty stomach.123,124 Recent data indicate the 50th percentile of added caloric sweeteners consumption for Americans is approximately 1.1 g/kg.125
Within the United States, sucrose from sugar cane or sugar beets is considered generally recognized as safe (GRAS) (21CFR184.1854) and may be used without limitation other than good manufacturing practice (CFR; updated May 5, 2016).126 This GRAS status suggests sucrose is safe to consume at typical usage levels and meets typical toxicological assessment principles and criteria outlined under guidelines developed by the Organization for Economic Co-operation and Development and the International Conference for Harmonisation. Importantly, the US statute also suggests an undeclared ADI value for sucrose, in the absence of a formal statement. However, several recent publications maintain dietary sucrose is toxic, citing decreased survival among female mice fed sucrose at 25% of energy for 40 weeks127 or the contribution of sucrose to noncommunicable disease.128 Even the WHO commented that excess sucrose consumption contributes to pandemic obesity.1 The recent WHO guidelines on sugars intake for adults and children state that excess sugars consumption is associated with excess body weight gain,1 which is consistent with the “excess” comments noted in the report of the 2010 DGAC.7 These outcomes of excess consumption are not components of classical toxicological assessments.
Despite the absence of any classical toxicological concerns of sucrose at typical consumption levels, there are many dietary guidelines, emerging statutes, and consumer litigations that continue to call for a reduction in sugars intake. Since the 1977 McGovern report Dietary Goals for the United States,129 which called for sugars consumption not to exceed 15% of total energy intake, all subsequent dietary guidelines within the United States have recommended a reduction in sugars intake, particularly for consumers with the highest intakes. The Institute of Medicine130 advised added sugars should not comprise more than 25% of total energy intake. The 2010 DGAC supported this position and noted that sugars are like any other macronutrient that contributes to weight gain when consumed in excess.7 However, the 2015 DGAC recommended that sugars intake constitute a maximum of 10% of total energy to reduce excess body weight, reduce the risk of type 2 diabetes, and decrease the risk of hypertension, stroke, and CHD in adults.8 This recommendation is consistent with the 2015 WHO report, which advises a maximum free sugars intake of 10% of total energy for adults and children for a lifetime.1
At least 65 countries have implemented dietary guidelines or public health policies to curb sugars consumption in an effort to encourage maintenance of healthy body weight.131 Within the United States, a 2014 ballot initiative to place a $0.01-per-ounce tax on SSBs was approved by voters in Berkeley, California. This is the first voter-approved taxation on SSBs. In January 2015, the Alliance for Healthier Vermont advocated a $0.02-per-ounce excise tax on SSBs, an initiative that appears to be favored by Governor Peter Shumlin but opposed by several state senators. The state legislature is considering two bills, one that supports this excise tax (H.235) and another that supports the application of collected funds to subsidize healthcare costs (H.24). In addition, Vermont’s House Committee on Human Services is considering a bill (H.89) that proposes to require SSBs sold or distributed in the state to bear a health and safety warning label. In February 2015, the California State Senate introduced SB 203 (Sugar-Sweetened Beverage Safety Warning Act), intended to warn consumers of the potentially harmful effects of beverages with added sugars.132 Justifications for this bill were based on a 2005 report by the Center for Safety in the Public Interest and a university survey133 that state those who consume at least one SSB daily are nearly 30% more likely to be obese. Not mentioned by the bill’s proponents is a 2014 study that indicates the prevalence of obesity and overweight status is about 30% in each category among nonconsumers of SSBs.134 Following the latest public hearing (April 2015), SB 203 failed to survive a vote by the Senate Committee on Health.
Meanwhile, on June 1, 2015, the San Francisco Board of Supervisors advanced three city ordinances that will require warning labels on advertising for SSBs, ban SSB ads on city property, and prohibit city departments from purchasing or selling SSBs. Specifically, the warning label would read “WARNING: Drinking beverages with added sugar(s) contributes to obesity, diabetes, and tooth decay. This is a message from the City and County of San Francisco.”135
Another argument of the bill’s supporters (California Center for Public Health Advocacy, 2015) is that liquid sugars are digested and metabolized differently than solid forms and thus pose a greater public health concern. This controversy was addressed by the 2005 DGAC and 2010 DGAC. These two advisory committees reviewed 74 studies pertinent to this point. The 2005 DGAC found conflicting evidence that liquid and solid foods differ in their effect on calorie compensation, and the 2010 DGAC concluded that then-current (2005–2009) evidence from randomized controlled trials of sufficient size and duration was insufficient to support a possible difference between liquid and solid sugars intake in body weight control. A subsequent publication noted, “despite public perception that SSB are linked to increased body weight compared with whole foods, evidence-based reviews of this topic do not support that liquid calories are processed differently in the body.”136
There is a global movement to levy taxes on SSBs in an effort to fund medical and public health initiatives and interventions directed to reduce obesity.137,138 In Canada, the Childhood Obesity Foundation called for restricted marketing of SSBs and SSB “smart taxation” to fund efforts to curb unhealthy weights and reduce childhood obesity.139 The Mexican government levied an SSB tax of 1 peso (about $0.07) per liter (≈$0.02 per ounce) in January 2014. According to Mexico’s National Institute of Public Health, preliminary data indicate a 10% reduction in purchases of SSBs during the first 3 months after implementation and a 4.7% to 6.4% reduction in sales of soft drinks, while sales of plain water have increased 13%.140 Interestingly, sales of soft drinks with sugars and non-nutritive sweetener blends are on the rise.140
Banning SSBs in public schools, reducing their availability across university campuses, and eliminating the ability to purchase them with food stamps under the Supplemental Nutrition Assistance Program reflect efforts within the National School Lunch Program141 and other initiatives to limit soda consumption and intake of excess calories. In California, SB 12 (2007) required competitive foods be delivered at all grade levels and required the implementation of several nutrition standards in 2009. A recent study indicated students in participating schools consumed about 17 g less added sugars than cohorts from outside of California.142 These results were based on 24-recall data and did not include body weight data or assess off-campus consumption of sugary beverages. A similar study among high school students under a soda-restriction policy in Boston indicated a slight yet insignificant decline in the total consumption of SSBs between 2004 and 2006.143 A longitudinal study among 6300 5th- and 8th-grade students in 40 states suggested more strict regulations on food nutrition content may contribute to decreased body mass index.144 A similar conclusion was suggested from a study among about 9100 middle-school students attending 64 schools with different SSB policies.145
CONCLUSION
In light of differing recommendations for upper limits of consumption of fructose-containing sugars, considerable controversy and confusion exists both in the nutrition community and among public policy makers. Data from higher-quality scientific studies, including systematic reviews and meta-analyses of randomized controlled trials and individual controlled trials, have yielded contrasting results. It appears that sugars (including fructose) do not have any adverse effects when tested in isocaloric exchange for other carbohydrates; most adverse effects, it seems, appear under conditions of hypercaloric exchange. As recently concluded by the European Food Safety Authority, the scientific evidence on the effects of sugars on health is insufficient to set an upper limit for consumption. Indeed, effects are largely dependent on the overall diet and energy balance. As such, it is unknown whether restrictive guidelines on sugars intakes, when promulgated in isolation and dissociated from global recommendations on overall diet quality, can represent an effective strategy to curb the prevalence of obesity and metabolic disorders. Any recommendations must be dependent on energy balance and nutrient adequacy. These considerations should inject a note of caution into restrictive guidelines such as those promulgated by respected scientific organizations such as the WHO, the American Heart Association, and the Scientific Advisory Committee on Nutrition. For now, it would appear prudent to avoid excessive calories from sugars, although the scientific basis for restrictive guidelines is far from settled.
Acknowledgments
Declarations of interest. J.M.R.’s research laboratory has received unrestricted grants, and J.M.R. has received consulting fees from ConAgra Foods, Kraft Foods, the Florida Department of Citrus, PepsiCo International, The Coca Cola Company, the Corn Refiners Association, Weight Watchers International, and various publishers. J.M.R.’s research laboratory has received unrestricted grant funding to conduct research trials, and J.M.R. has received consulting fees from a variety of companies, organizations, publishers, or trade associations that utilize, market, or publish information about fructose, HFCS, or sucrose and, hence, have an ongoing interest in the metabolism and health effects of these sugars.
J.S.W. is a consultant and advisor to the food and beverage industry in the area of nutritive sweeteners. He receives compensation from scientific societies, research institutes, food industry councils, trade organizations, and individual companies. Clients have a commitment to nutritive sweetener research, development, production, applications, safety, nutrition, and education.
J.L.S. is funded by a PSI Graham Farquharson Knowledge Translation Fellowship, a Canadian Diabetes Association Clinician Scientist award, a Canadian Institutes of Health Research (CIHR) Institute of Nutrition, Metabolism and Disease/Canadian Nutrition Society New Investigator Partnership Prize and a Banting and Best Diabetes Centre Sun Life Financial New Investigator Award. He has received research support from the Canadian Institutes of Health Research, the American Society of Nutrition, the Canadian Diabetes Association, the Banting and Best Diabetes Centre, the Calorie Control Council, The Coca-Cola Company (investigator initiated, unrestricted), the Dr Pepper Snapple Group (investigator initiated, unrestricted), Pulse Canada, and the International Tree Nut Council Nutrition Research and Education Foundation. He has received reimbursement of travel expenses, speaker fees, and/or honoraria from the American Society of Nutrition, the Canadian Diabetes Association, the Canadian Nutrition Society, the University of Alabama at Birmingham, the Oldways Preservation Trust, the Nutrition Foundation of Italy, the Calorie Control Council, the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes, the International Life Sciences Institute North America, Abbott Laboratories, the Canadian Sugar Institute, the Dr Pepper Snapple Group, The Coca-Cola Company, the Corn Refiners Association, the World Sugar Research Organization, the Dairy Farmers of Canada, the Società Italiana di Nutrizione Umana, the III World Congress of Public Health Nutrition, C3 Collaborating for Health, White Wave Foods, Rippe Lifestyle, mdBriefcase, the Federation of European Nutrition Societies, the New York Academy of Sciences, the International Diabetes Federation, and Food Minds. He has ad hoc consulting arrangements with Winston and Strawn LLP, Perkins Coie LLP, and Tate and Lyle. He is on the Clinical Practice Guidelines Expert Committees of the Canadian Diabetes Association, the European Association for the Study of Diabetes, and the Canadian Cardiovascular Society, as well as an expert writing panel of the American Society of Nutrition for a scientific statement on sugars. He is a member of the International Carbohydrate Quality Consortium, an Executive Board Member of the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials Foundation. He serves as an unpaid scientific advisor for the International Life Sciences Institute North America, Food, Nutrition, and Safety Program, and the Committee on Carbohydrates. His wife is an employee of Unilever Canada.
K.-A.L. is employed by the Nestlé Research Center, which is a subsidiary of Nestlé Ltd. The submitted work was done independently of Nestlé’s interests and represents the author’s personal views.
R.C. is Executive Vice President of Polyscience Consulting, which he cofounded in 1999. During the period of 2010 through the first quarter of 2016, he provided consultative services and/or served on an advisory council to the following: Abbott Nutrition; the Almond Board of California; the American Society for Quality; Aspire Food Group; Assure Water; Authen Technologies; Barilla; Bayer; the California Walnut Commission; The Coca-Cola Company (honorarium directly given to charity); the Corn Refiners Association; the Danish Agriculture and Food Council; the Dairy Council of California; Dentons LLP; E.T. Horn; the FMC Corporation (honorarium directly given to charity); Food Minds; HyCite; Jenner and Block LLP; Kellogg; Kerry Ingredients; the Malaysian Palm Oil Council; McDonalds; Mead Johnson; the Mushroom Council; the National Fisheries Institute; the National Restaurant Association; Nestlé SA; Obi “Probiotic” Soda; Petcurean; Quaker Oats; Schwann Foods; Senomyx (honorarium directly given to charity); Spherix; the US Department of Agriculture; Whitewave; and Yakult.
T.J.A. has no relevant interests to declare.
References
- 1. World Health Organization. World Health Organization. Guidelines: sugars intake for adults and children.http://www.who.int/nutrition/publications/guidelines/sugars_intake/en/. Published 2015. Accessed October 7, 2016. [Google Scholar]
- 2. Scientific Advisory Committee on Nutrition. Carbohydrates and Health Report. London, England: The Stationery Office; 2015. [Google Scholar]
- 3. Marriott BP, Olsho L, Hadden L, et al. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003–2006. Crit Rev Food Sci Nutr. 2010;50:228–258. [DOI] [PubMed] [Google Scholar]
- 4. Johnson RK, Appel LJ, Brands M, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–1020. [DOI] [PubMed] [Google Scholar]
- 5. Institute of Medicine, Panel on Macronutrients, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary carbohydrates: sugars and starches. In: Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 6. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed.Washington, DC: US Government Printing Office; 2010. [Google Scholar]
- 7. US Department of Agriculture, US Department of Health and Human Services. Report of the Advisory Committee on the Dietary Guidelines for Americans, 2010. http://www.nutriwatch.org/05Guidelines/dga_advisory_2010.pdf. Published May 28, 2010. Accessed October 7, 2016. [Google Scholar]
- 8. US Department of Agriculture, US Department of Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Department of Agriculture, Center for Nutrition Policy and Promotion; 2015. [Google Scholar]
- 9. US Department of Agriculture, US Department of Health and Human Services. Dietary guidelines for Americans, 2015–2020. https://health.gov/dietaryguidelines/2015/guidelines/. Published January 2016. Accessed October 5, 2016. [Google Scholar]
- 10. European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition, and Allergies. Scientific opinion on the substantiation of health claims related to fructose and reduction of post-prandial glycaemic responses (ID 558) pursuant to Article 13(1) of Regulation (EC) no 1924/2006. EFSA J. 2011;9:2223–2238. [Google Scholar]
- 11. Le MT, Frye RF, Rivard CJ, et al. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism. 2012;61:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanover LM, White JS. Manufacturing, composition, and applications of fructose. Am J Clin Nutr. 1993;58(5 suppl):724S–732S. [DOI] [PubMed] [Google Scholar]
- 13. Sigman-Grant M, Morita J. Defining and interpreting intakes of sugars. Am J Clin Nutr. 2003;78:815S–826S. [DOI] [PubMed] [Google Scholar]
- 14. Thorens B, Mueckler M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab. 2010;298:E141–E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58(5 suppl):754S–765S. [DOI] [PubMed] [Google Scholar]
- 16. Tappy L, Le K. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. [DOI] [PubMed] [Google Scholar]
- 17. Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity (Silver Spring). 2011;19:868–874. [DOI] [PubMed] [Google Scholar]
- 18. Walker RW, Dumke KA, Goran MI. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition. 2014;30:928–935. [DOI] [PubMed] [Google Scholar]
- 19. White JS, Hobbs LJ, Fernandez S. Fructose content in popular beverages made with and without high fructose corn syrup. Nutrition. 2015;31:417–418. [DOI] [PubMed] [Google Scholar]
- 20. White JS, Hobbs LJ, Fernandez S. Fructose content and composition of commercial HFCS-sweetened carbonated beverages. Int J Obes (London). 2015;39:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welsh JA, Sharma AJ, Grellinger L, et al. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr. 2011;94:726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang Q, Zhang Z, Gregg EW, et al. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marriott BP, Fink CJ, Krakower T. Worldwide consumption of sweeteners and recent trends. In: Rippe JM, ed. Fructose, High Fructose Corn Syrup, Sucrose and Health. New York, NY: Springer Science; 2014. [Google Scholar]
- 24. White JS. Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Adv Nutr. 2013;4:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Department of Agriculture, Economic Research Service. Calories average daily per capita calories from the US food supply, adjusted for spoilage and other waste. Loss-adjusted food availability documentation. http://www.ers.usda.gov/data-products/food-availability-(per-capita)-data-system/loss-adjusted-food-availability-documentation.aspx. Updated August 24, 2016. Accessed October 5, 2016. [Google Scholar]
- 26. Carden TJ, Carr TP. Food availability of glucose and fat, but not fructose, increased in the US between 1970 and 2009: analysis of the USDA food availability data system. Nutr J. 2013;12:130 doi:10.1186/1475-2891-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. Obesity and overweight. Fact sheet no. 311. http://www.mclveganway.org.uk/Publications/WHO_Obesity_and_overweight.pdf. Published September 2006. Accessed October 7, 2016. [Google Scholar]
- 29. Organisation for Economic Cooperation and Development. Overweight and obesity among adults. In: Health at a Glance: Europe 2012. Paris, France: OECD Publishing; 2012: 62–64. [Google Scholar]
- 30. Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–1437. [DOI] [PubMed] [Google Scholar]
- 31. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2013;346:e7492 doi:10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 32. Malik VS, Pan A, Willett WC, et al. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forshee R, Anderson P, Storey M. Sugar-sweetened beverages and body mass index in children and adolescents: a meta-analysis. Am J Clin Nutr. 2008;87:1662–1671. [DOI] [PubMed] [Google Scholar]
- 34. Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr. 2009;89:438–439; author reply 439–440. [DOI] [PubMed] [Google Scholar]
- 35. Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:1228S–1235S. [DOI] [PubMed] [Google Scholar]
- 36. Almiron-Roig E, Palla L, Guest K, et al. Factors that determine energy compensation: a systematic review of preload studies. Nutr Rev. 2013;71:458–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sievenpiper JL, de Souza RJ. Are sugar-sweetened beverages the whole story? Am J Clin Nutr. 2013;98:261–263. [DOI] [PubMed] [Google Scholar]
- 38. Kaiser KA, Shikany JM, Keating KD, et al. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes Rev. 2013;14:620–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sievenpiper JL, de Souza RJ, Mirrahimi A, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012;156:291–304. [DOI] [PubMed] [Google Scholar]
- 40. Lowndes J, Sinnett S, Pardo S, et al. The effect of normally consumed amounts of sucrose or high fructose corn syrup on lipid profiles, body composition and related parameters in overweight/obese subjects. Nutrients. 2014;6:1128–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lowndes J, Sinnett S, Yu Z, et al. The effects of fructose-containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients. 2014;6:3153–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stanhope KL, Medici V, Bremer AA, et al. A dose-response study of consuming high-fructose corn syrup–sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saris WH, Astrup A, Prentice AM, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. The Carbohydrate Ratio Management in European National diets. Int J Obes Relat Metabol Disord. 2000;24:1310–1318. [DOI] [PubMed] [Google Scholar]
- 44. Poppitt SD, Keogh GF, Prentice AM, et al. Long-term effects of ad libitum low-fat, high-carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. Am J Clin Nutr. 2002;75:11–20. [DOI] [PubMed] [Google Scholar]
- 45. Teff KL, Grudziak J, Townsend RR, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Melanson KJ, Zukley L, Lowndes J, et al. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition. 2007;23:103–112. [DOI] [PubMed] [Google Scholar]
- 47. Yu Z, Lowndes J, Rippe J. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr Res. 2013;33:1043–1052. [DOI] [PubMed] [Google Scholar]
- 48. Jayalath VH, Sievenpiper JL, de Souza RJ, et al. Total fructose intake and risk of hypertension: a systematic review and meta-analysis of prospective cohorts. J Am Coll Nutr. 2014;33:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. [DOI] [PubMed] [Google Scholar]
- 50. Beulens JW, de Bruijne LM, Stolk RP, et al. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007;50:14–21. [DOI] [PubMed] [Google Scholar]
- 51. Sieri S, Krogh V, Berrino F, et al. Dietary glycemic load and index and risk of coronary heart disease in a large Italian cohort: the EPICOR study. Arch Intern Med. 2010;170:640–647. [DOI] [PubMed] [Google Scholar]
- 52. Burger KN, Beulens JW, Boer JM, et al. Dietary glycemic load and glycemic index and risk of coronary heart disease and stroke in Dutch men and women: the EPIC-MORGEN study. PLoS One. 2011;6:e25955 doi:10.1371/journal.pone.0025955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tasevska N, Park Y, Jiao L, et al. Sugars and risk of mortality in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2014;99:1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jayalath VH, de Souza RJ, Ha V, et al. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr. 2015;102:914–921. [DOI] [PubMed] [Google Scholar]
- 55. Xi B, Huang Y, Reilly KH, et al. Sugar-sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr. 2015;113:709–717. [DOI] [PubMed] [Google Scholar]
- 56. Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–1520. [DOI] [PubMed] [Google Scholar]
- 57. Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr. 2003;78:873S–880S. [DOI] [PubMed] [Google Scholar]
- 58. Bantle JP, Raatz SK, Thomas W, et al. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000;72:1128–1134. [DOI] [PubMed] [Google Scholar]
- 59. Miller M, Stone N, Ballantye C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. [DOI] [PubMed] [Google Scholar]
- 60. Chiavaroli L, Mirrahimi A, De Souza RJ, et al. Does fructose consumption elicit a dose–response effect on fasting triglycerides? A systematic review and meta-regression of controlled feeding trials. Can J Diab. 2012;36:S37. [Google Scholar]
- 61. Wang DD, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014;232:125–133. [DOI] [PubMed] [Google Scholar]
- 62. Rippe JM, Angelopoulos TJ. Fructose-containing sugars and cardiovascular disease. Adv Nutr. 2015;6:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marckmann P. Dietary treatment of thrombogenic disorders related to the metabolic syndrome. Br J Nutr. 2000;83(suppl 1):S121–S126. [DOI] [PubMed] [Google Scholar]
- 65. Raben A, Vasilaras T, Møller A, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–729. [DOI] [PubMed] [Google Scholar]
- 66. Maersk M, Belza A, Stødkilde-Jørgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283–289. [DOI] [PubMed] [Google Scholar]
- 67. Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr. 2008;88:1419–1437. [DOI] [PubMed] [Google Scholar]
- 68. Sievenpiper JL, Carleton AJ, Chatha S, et al. Heterogeneous effects of fructose on blood lipids in individuals with type 2 diabetes: systematic review and meta-analysis of experimental trials in humans. Diabetes Care. 2009;32:1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chiavaroli L, de Souza RJ, Ha V, et al. Effect of fructose on established lipid targets: a systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc. 2015;4:e001700 doi:10.1161/JAHA.114.001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Te Morenga LA, Howatson AJ, Jones RM, et al. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100:65–79. [DOI] [PubMed] [Google Scholar]
- 71. Feig D, Soletsky B, Johnson R. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension. J Am Med Assoc. 2008;300:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nguyen S, Choi HK, Lustig RH, et al. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bremer AA, Auinger P, Byrd RS. Relationship between insulin resistance–associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: findings from the 1999–2004 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2009;163:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Johnson R, Segal M, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. [DOI] [PubMed] [Google Scholar]
- 75. Forman JP, Choi H, Curhan GC. Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol. 2009;20:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Waring WS, Adwani SH, Breukels O, et al. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart. 2004;90:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ha V, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on blood pressure a systematic review and meta-analysis of controlled feeding trials. Hypertension. 2012;59:787–795. [DOI] [PubMed] [Google Scholar]
- 78. Angelopoulos TJ, Lowndes J, Sinnett S, et al. Fructose containing sugars do not raise blood pressure or uric acid at normal levels of human consumption. J Clin Hyperten. 2014;17:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 81. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131–1141. [DOI] [PubMed] [Google Scholar]
- 83. Montonen J, Jarvinen R, Knekt P, et al. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137:1447–1454. [DOI] [PubMed] [Google Scholar]
- 84. Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–684; quiz 714–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lê KA, Ith M, Kreis R, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89:1760–1765. [DOI] [PubMed] [Google Scholar]
- 86. de Koning L, Malik VS, Kellogg MD, et al. Sweetened beverage consumption, incident coronary heart disease and biomarkers of risk in men. Circulation. 2012;125:1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Basu S, Yoffe P, Hills N, et al. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS One. 2013;8:e57873 doi:10.1371/journal.pone.0057873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: a global perspective. Glob Public Health. 2013;8:55–64. [DOI] [PubMed] [Google Scholar]
- 89. Mielke JG, Taghibiglou C, Liu L, et al. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem. 2005;93:1568–1578. [DOI] [PubMed] [Google Scholar]
- 90. Maiztegui B, Borelli MI, Raschia MA, et al. Islet adaptive changes to fructose-induced insulin resistance: β-cell mass, glucokinase, glucose metabolism, and insulin secretion. J Endocrinol. 2009;200:139–149. [DOI] [PubMed] [Google Scholar]
- 91. Rizkalla SW, Boillot J, Tricottet V, et al. Effects of chronic dietary fructose with and without copper supplementation on glycaemic control, adiposity, insulin binding to adipocytes and glomerular basement membrane thickness in normal rats. Br J Nutr. 1993;70:199–209. [DOI] [PubMed] [Google Scholar]
- 92. Thorburn AW, Storlien LH, Jenkins AB, et al. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr. 1989;49:1155–1163. [DOI] [PubMed] [Google Scholar]
- 93. Tsilas CS, de Souza RJ, Tawfik R, et al. No relation between total sugars intake and incident diabetes: a systematic review and meta-anaylsis of cohorts. In: Proceedings of the 32nd International Symposium on Diabetes and Nutrition. June 25–27, 2014. Reykjavik, Iceland. [Google Scholar]
- 94. Buijsse B, Boeing H, Drogan D, et al. Consumption of fatty foods and incident type 2 diabetes in populations from eight European countries. Eur J Clin Nutr. 2015;69:455–461. [DOI] [PubMed] [Google Scholar]
- 95. Xi B, Li S, Liu Z, et al. Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2014;9:e93471 doi:10.1371/journal.pone.0093471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Teff K, Elliott S, Tschop M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. [DOI] [PubMed] [Google Scholar]
- 98. Aeberli I, Gerber PA, Hochuli M, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr. 2011;94:479–485. [DOI] [PubMed] [Google Scholar]
- 99. Aeberli I, Hochuli M, Gerber PA, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care. 2013;36:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Moore MC, Davis SN, Mann SL, et al. Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes. Diabetes Care. 2001;24:1882–1887. [DOI] [PubMed] [Google Scholar]
- 101. Silbernagel G, Machann J, Unmuth S, et al. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr. 2011;106:79–86. [DOI] [PubMed] [Google Scholar]
- 102. Faeh D, Minehira K, Schwarz JM, et al. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54:1907–1913. [DOI] [PubMed] [Google Scholar]
- 103. Cozma AI, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care. 2012;35:1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. National Cholesterol Education Program Expert Panel (NCEP) on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 105. Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162:496.e1–500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524.e1–530.e1.; quiz e60. [DOI] [PubMed] [Google Scholar]
- 107. Tappy L, Le KA. Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin Res Hepatol Gastroenterol. 2012;36:554–560. [DOI] [PubMed] [Google Scholar]
- 108. Sun SZ, Empie MW. Fructose metabolism in humans – what isotopic tracer studies tell us. Nutr Metab (London). 2012;9:89 doi:10.1186/1743-7075-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Acheson KJ, Schutz Y, Bessard T, et al. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am J Clin Nutr. 1988;48:240–247. [DOI] [PubMed] [Google Scholar]
- 110. Sievenpiper JL, de Souza RJ, Kendall CW, et al. Is fructose a story of mice but not men? J Am Diet Assoc. 2011;111:219–220; author reply 220–212. [DOI] [PubMed] [Google Scholar]
- 111. Ma J, Fox CS, Jacques PF, et al. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol. 2015;63:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Johnston RD, Stephenson MC, Crossland H, et al. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145:1016–1025. [DOI] [PubMed] [Google Scholar]
- 113. Bravo S, Lowndes J, Sinnett S, et al. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab. 2013;38:681–688. [DOI] [PubMed] [Google Scholar]
- 114. Chung M, Ma J, Patel K, et al. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chiu S, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lecoultre V, Egli L, Carrel G, et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity. 2013;21:782–785. [DOI] [PubMed] [Google Scholar]
- 117. Hayes AW, Kruger CL. Hayes' Principles and Methods of Toxicology. 6th ed.Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 118. Mortensen A. Sweeteners permitted in the European Union: safety aspects. Scan J Food Nutr. 2006;50:104–116. [Google Scholar]
- 119. Shore V, Shore B. Effect of mercuric chloride on some kidney enzymes in chow-fed and sucrose-fed rats. Am J Physiol. 1960;198:187–190. [DOI] [PubMed] [Google Scholar]
- 120. Harper KH, Worden AN. Comparative toxicity studies on glucose, fructose, and sucrose [abstract]. Toxicol Appl Pharmacol. 1964;6:365. [Google Scholar]
- 121. Boyd EM, Godi I, Abel M. Acute oral toxicity of sucrose. Toxicol Appl Pharmacol. 1965;7:609–618. [DOI] [PubMed] [Google Scholar]
- 122. Constantopoulos G, Boyd E. Maximal tolerated amounts of sucrose given by daily intragastric administration to albino rats. Food Cosmet Toxicol. 1968;6:717–727. [Google Scholar]
- 123. Boyd EM. Predictive drug toxicity. Assessment of drug safety before human use. Can Med Assoc J. 1968;98:278–293. [PMC free article] [PubMed] [Google Scholar]
- 124. Richter CP. Self-regulatory Functions During Gestation and Lactation. Madison, NJ: Madison Printing; 1956. [Google Scholar]
- 125. Haley S. Sugar and sweeteners outlook. Washington, DC: US Department of Agriculture, Economic Research Service; Publication SSS-M-293, January 17, 2013. [Google Scholar]
- 126. Direct food substances affirmed as generally recognized as safe. Section 184.1854 Sucrose. Fed Regist. 2016;21CFR §184.1854. [Google Scholar]
- 127. Ruff JS, Hugentobler SA, Suchy AK, et al. Compared to sucrose, previous consumption of fructose and glucose monosaccharides reduces survival and fitness of female mice. J Nutr. 2015;145:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature. 2012;482:27–29. [DOI] [PubMed] [Google Scholar]
- 129. Staff of the Select Committee on Nutrition and Human Needs, US Senate. Dietary Goals for the United States. Washington, DC: US Government Printing Office; 1977. [Google Scholar]
- 130. Institute of Medicine of the National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 131. Clemens R, Papanikolaou Y. Crystalizing global sugar policy: public health promise or perception. In: Rippe JM, ed. Fructose, High Fructose Corn Syrup, Sucrose and Health. New York, NY: Springer; 2014:125–135. [Google Scholar]
- 132. Senate Bill 203. Sugar-sweetened beverages: safety warnings. 2015–2016 regular session. February 11, 2015 (Calif 2015). http://www.leginfo.ca.gov/pub/15-16/bill/sen/sb_0201-0250/sb_203_bill_20150211_introduced.htm. Accessed October 3, 2016. [Google Scholar]
- 133. Babey SH, Jones M, Yu H, et al. Bubbling over: soda consumption and its link to obesity in California. Policy Brief UCLA Cent Health Policy Res. 2009;PB2009-5:1–8. [PubMed] [Google Scholar]
- 134. Park S, Pan L, Sherry B, et al. Consumption of sugar-sweetened beverages among US adults in 6 states: Behavioral Risk Factor Surveillance System, 2011. Prev Chronic Dis. 2014;11:E65 doi:10.5888/pcd11.130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. City and County of San Francisco. Meeting agenda, Land Use and Transportation Committee, June 1, 2015. https://sfgov.legistar.com/View.ashx?M=A&ID=400743&GUID=E1C49D3B-459B-4448-8862-3F895C431852. Accessed October 7, 2016. [Google Scholar]
- 136. Slavin J. Beverages and body weight: challenges in the evidence-based review process of the Carbohydrate Subcommittee from the 2010 Dietary Guidelines Advisory Committee. Nutr Rev. 2012;70(suppl 2):S111–S120. [DOI] [PubMed] [Google Scholar]
- 137. Jou J, Techakehakij W. International application of sugar-sweetened beverage (SSB) taxation in obesity reduction: factors that may influence policy effectiveness in country-specific contexts. Health Policy. 2012;107:83–90. [DOI] [PubMed] [Google Scholar]
- 138. Andreyeva T, Chaloupka FJ, Brownell KD. Estimating the potential of taxes on sugar-sweetened beverages to reduce consumption and generate revenue. Prev Med. 2011;52:413–416. [DOI] [PubMed] [Google Scholar]
- 139. Childhood Obesity Foundation. Collaborative action on childhood obesity (CACO 1). Childhood Obesity Foundation website. http://childhoodobesityfoundation.ca/about/. Accessed October 5, 2016. [Google Scholar]
- 140. Guthrie A. Soda sales in Mexico rise despite tax. The Wall Street Journal. May 3, 2016. http://www.wsj.com/articles/soda-sales-in-mexico-rise-despite-tax-1462267808#:s3NptBM8z-HoCA, Accessed October 5, 2016. [Google Scholar]
- 141. Mello MM, Pomeranz J, Moran P. Policies Affecting Access to Sugar-Sweetened Beverages in Schools: a Legal and Regulatory Review. Report to the Robert Wood Johnson Foundation. Princeton, NJ: Robert Wood Johnson Foundation; 2006. [Google Scholar]
- 142. Taber DR, Chriqui JF, Chaloupka FJ. Differences in nutrient intake associated with state laws regarding fat, sugar, and caloric content of competitive foods. Arch Pediatr Adolesc Med. 2012;166:452–458. [DOI] [PubMed] [Google Scholar]
- 143. Cradock AL, McHugh A, Mont-Ferguson H, et al. Effect of school district policy change on consumption of sugar-sweetened beverages among high school students, Boston, Massachusetts, 2004–2006. Prev Chronic Dis. 2011;8:A74 https://www.cdc.gov/pcd/issues/2011/jul/10_0149.htm. Accessed October 3, 2016. [PMC free article] [PubMed] [Google Scholar]
- 144. Taber DR, Chriqui JF, Perna FM, et al. Weight status among adolescents in states that govern competitive food nutrition content. Pediatrics. 2012;130:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Johnson DB, Bruemmer B, Lund AE, et al. Impact of school district sugar-sweetened beverage policies on student beverage exposure and consumption in middle schools. J Adolesc Health. 2009;45(3 suppl):S30–S37. [DOI] [PubMed] [Google Scholar]