Abstract
ALT-803 is a fusion protein complex consisting of an interleukin (IL)-15 superagonist and a dimeric IL-15 receptor alpha sushi domain IgG1 Fc fusion protein. When administered to mice, ALT-803 is capable of inducing natural killer (NK) and CD8+ T cell proliferation and activation, and effectively promoting potent anti-tumor responses. Currently, ALT-803 is in clinical trials for treatment of various solid tumors and hematological malignancies. In the initial phase of these clinical studies, intravenous (iv) injection was used according to the route used in pre-clinical efficacy studies. In order to evaluate the possible advantage of subcutaneous (sc) injection versus iv injection, this study compared the biological activity of the two treatment regimens of ALT-803 in pre-clinical in vivo models. The pharmacokinetics, immune stimulation, and anti-tumor efficacy of iv and sc injection routes of ALT-803 in C57BL/6 mice were compared. The half-life of ALT-803 was 7.5 h for iv versus 7.7 h for sc with the maximal detected serum concentration of ALT-803 to be 3926 ng/ml at 0.5 h time-point following iv injection versus 495 ng/ml at 16 h post sc injection. Biodistribution studies indicated that sc ALT-803, similarly to iv ALT-803 as previously reported, has a greater tissue distribution and longer residence time in lymphoid tissues compared to recombinant IL-15. Notably, ALT-803 when administered either iv or sc induced comparable proliferation and activation of CD8+ T and NK cells and resulted in similar reductions of tumor burden. A toxicity study of mice receiving multiple injections of ALT-803 for 4 weeks by iv or sc routes revealed equivalent immune-related changes. The gradual absorbance into the blood stream and lower maximal blood levels of ALT-803 in sc-injected mice, along with similar anti-tumor efficacy support the administration of ALT-803 by sc injection in patients with various malignancies and infectious diseases.
Keywords: Interleukin-15, Interleukin-15Rα, ALT-803, CD8 T cells, NK cells, Immunotherapy
1. Introduction
Cytokines have a pivotal role in the regulation of the immune system and have been approved in therapeutic applications to treat cancer. The common γ-chain cytokine interleukin (IL)-15, is a growth factor for activation and proliferation of natural killer (NK) cells and CD8+ T cells [1,2]. In contrast to other common γ-chain cytokines such as IL-2, IL-15 is not associated with activation induced cell death (AICD) and does not induce capillary leak syndrome in mice and non-human primates [3,4]. IL-15 has a distinct role in prolonged maintenance of memory T-cells against invading pathogens, supporting development and activation of NK cells, and is a desirable immunotherapy candidate against cancer [4–8]. We developed an IL-15 superagonist complex, ALT-803, to circumvent the shortcomings of recombinant IL-15 and advance an IL-15-based immunotherapy into clinical evaluation for cancer and infectious diseases. As previously reported, ALT-803 is a fusion protein complex consisting of an IL-15N72D superagonist and a dimeric IL-15 receptor alpha sushi domain IgG1 Fc fusion protein (IL-15RαSu/Fc) [9,10]. ALT-803 was shown to exhibit superior immunostimulatory activity, prolonged serum half-life, and more potent anti-tumor activity compared to recombinant IL-15 in various mouse models [11,12].
Clinical trials evaluating ALT-803 for treatment of solid tumors and hematologic malignancies are underway. In initial clinical trials, intravenous (iv) administration of ALT-803 showed a dose-dependent immune cell activation, which correlated with increased serum IFNγ and IL-6 levels and constitutional side effects [13]. Thus, we hypothesized that subcutaneous (sc) administration of ALT-803 may lessen the constitutional adverse events (AEs) by lowering peak serum level of ALT-803. In order to demonstrate the possible advantage of sc injection, we compared the immunologic effects and anti-tumor activity of ALT-803 using the different dosing routes in pre-clinical models. The two treatment regimens of ALT-803 were evaluated and pharmacokinetics, immune cell activity, anti-tumor activity, and biodistribution were assessed in pre-clinical in vivo studies. The results reported herein strongly support a treatment regimen of sc administration of ALT-803 in clinical applications.
2. Materials and methods
2.1. Mice and cell lines
Six- to eight-week old female C57BL/6NHsd mice (B6) were purchased from Envigo Corporation (Indianapolis, IN) and were housed in the animal facility at Altor BioScience (Miramar, FL). All animal studies were performed according to NIH animal care guidelines under Institutional Animal Care and Use Committee approved protocols.
The murine 5T33 multiple myeloma cell line [14] was kindly provided by Dr. Ulrich van Andrian (Harvard Medical School, Cambridge, MA). Cells were routinely cultured in RPMI-1640 (Invitrogen, Grand Island, NY) supplemented with 10% fetal calf serum (HyClone, Waltham, MA) at 37 °C with 5% CO2.
2.2. Pharmacokinetic analysis
ALT-803 (IL-15N72D:IL-15RαSu/Fc) was generated as previously described [10]. ALT-803 (0.2 mg/kg) was administered to B6 mice (6 weeks old) by iv (tail vein) or sc (right flank) injections. A dose equivalent volume of PBS was injected into a group of B6 mice as vehicle control. Serum was prepared from blood from the tail vein at varying time-points. ALT-803 serum levels were assessed by ELISA (anti-IL-15 antibody (Ab) capture and HRP-conjugated donkey anti-human IgG Ab), which detects the intact ALT-803 complex of IL-15N72D:IL-15RαSu/Fc fusion protein [10]. The pharmacokinetics of ALT-803 were analyzed with PK Solution 2.0 software (Summit Research Services, Montrose, CO).
2.3. Immune cell activation and proliferation analysis
B6 mice were injected iv or sc with ALT-803 or PBS as described above. Mice were humanely sacrificed and spleens were harvested at 0, 16, 24, 48, and 72 h following injection. For splenic immune cell phenotyping, splenocytes were isolated and stained with the fluorochrome-conjugated monoclonal antibodies (mAbs) against mouse CD4, CD8, NK1.1, NKp46, KLRG1, CD25, and intracellular Foxp3 and granzyme B molecules (Biolegend, San Diego, CA). The stained cells were analyzed on a FACSverse with FACSuite software (BD Biosciences, San Jose, CA). Serum cytokine levels of ALT-803-treated mice were also assessed using a Cytometric Bead Array, Mouse Inflammation Kit (BD Biosciences, San Jose, CA) according to manufacturer’s instructions. The data were analyzed using Flow Cytometric Analysis Program (FCAP) Array Software (BD Biosciences, San Jose, CA).
2.4. ALT-803 tissue biodistribution studies
B6 mice were injected iv or sc with 3–7 MBq of 64Cu-labeled ALT-803 [12,15]. Static PET scans were performed on anesthetized animals at various time-points after injection using an Inveon microPET/microCT hybrid scanner (Siemens). Data acquisition, image reconstruction, and region-of-interest analysis to calculate the percentage injected dose per gram of tissue (%ID/g) for major organs were conducted as previously described [12,15].
2.5. Tumor model
Six-week old B6 mice were injected iv with 1 × 107 5T33 myeloma cells on day 0. On study days (SD) 10 and 14, ALT-803 (0.2 mg/kg) or PBS were administered into B6 mice by iv or sc injection. On SD17, mice were humanely sacrificed and spleen and bone marrow (BM) cells from hind leg femurs were harvested. The levels of BM myeloma cells were assessed by intracellular staining with FITC-conjugated anti-mouse IgG2b which is expressed on 5T33 tumor cells. To assess the effect of ALT-803 on immune cell stimulation in 5T33 tumor-bearing mice, NK and CD8+ T cells were also evaluated. BM cells and splenocytes of tumor-bearing mice were stained with fluorochrome-conjugated mAbs against mouse CD8, KLRG1, NK1.1 or NKp46. The stained cells were analyzed as described above.
2.6. Toxicity of repeated sc injections of ALT-803
Eight-week old female B6 mice (5 mice/group) were injected sc with a high dose of ALT-803 (1.0 mg/kg) or a PBS once a week for 4 weeks (SD1, 8, 15, and 22). Cage side observations including mortality, morbidity, and signs of study drug toxicity were conducted at least three times per week. Animals were weighed prior to each dosing and on the day of sacrifice (SD26). Blood samples were collected into heparinized tubes on SD26, 4 days after administration of the final dose of ALT-803. Mice were sacrificed on SD26, necropsied, organs (spleen, peripheral lymph nodes, liver, lungs, heart, thymus, kidneys) were removed for weight determination. Hematologic and chemical analysis of the serum was performed by the Comparative Pathology Laboratory/Department of Pathology at the University of Miami, Miller School of Medicine, Miami, FL.
3. Results and discussion
3.1. Pharmacokinetics evaluation of ALT-803 following iv or sc administration
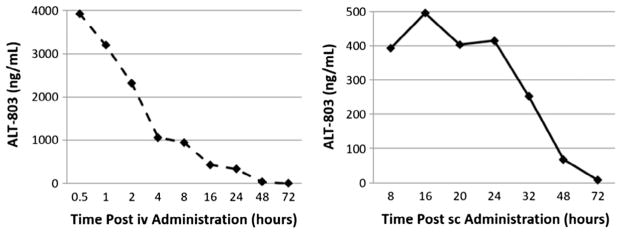
We have previously reported that in ICR (CD-1) mice receiving 1.0 mg/kg ALT-803 by iv injection, the estimated serum half-life values of ALT-803 using anti-IL-15 Ab-based or anti-human IgG Fc Ab-based ELISAs were about 25 h and 18 h, respectively [10]. To compare the pharmacokinetics of ALT-803 following iv or sc administration, groups of C57BL/6 mice (3 mice/time-point) were injected iv or sc with 0.2 mg/kg of ALT-803. Serum levels of ALT-803 were assessed at varying time-points by ELISA (anti-IL-15 Ab capture and HRP-conjugated donkey anti-human IgG Ab detection), which detected the intact complex of ALT-803. The pharmacokinetic profile of ALT-803 in these mice was analyzed using PK Solution 2.0 software and serum concentrations of ALT-803 are shown in Fig. 1. The estimated elimination half-life values of ALT-803 following iv or sc administration was 7.50 h and 7.71 h, respectively. The maximal detected serum concentrations (Cmax) of ALT-803 were 3926 ng/ml at 0.5 h time-point following iv injection and 495 ng/ml at 16 h time-point following sc injection.
Fig. 1.
Pharmacokinetics of ALT-803 following iv and sc administration in B6 mice. ALT-803 was iv or sc injected into B6 mice at 0.2 mg/kg. At various time-points after dosing, blood was collected, and serum samples were prepared. Serum levels of ALT-803 were assessed by ELISA and ALT-803 concentrations (ng/ml) in mouse serum samples were plotted.
This finding indicates that the serum half-life of ALT-803 following sc administration is comparable with half-life following iv administration. Due to the slower absorbance time of sc injection, we used the Cmax of ALT-803 to determine the blood concentration of ALT-803 achieved by the different routes of administration. Based on these concentrations, the Cmax of ALT-803 by iv administration was 7-fold higher than that by sc administration. Since the higher serum levels of ALT-803 may induce more frequent and severe systematic side effects related to inflammatory cytokines [13], this finding suggests that ALT-803 administration via sc injection may result in less toxicity in patients.
3.2. Proliferation and stimulation of splenocytes following ALT-803 iv or sc administration
Due to the higher Cmax of ALT-803 following iv administration, we hypothesized that ALT-803 would have increased immunostimulatory activity when administered iv than that by sc administration. To investigate this, splenocytes were collected for phenotyping from ALT-803-treated mice at 0 (control mice), 16, 24, 48 and 72 h post-treatment. Splenocytes were stained with fluorochrome-labeled mAbs specific to mouse CD4, CD8, NK1.1 and KLRG1 (as an activation marker) and analyzed on a FACSverse with FACSuite software.
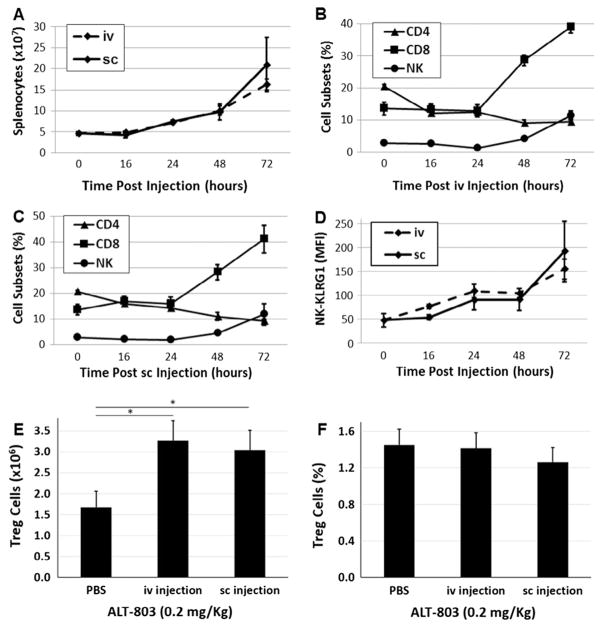
Surprisingly, as shown in Fig. 2A, ALT-803 administered either by iv or sc injection induced an equivalent increase in total splenocyte number by 48–72 h post-treatment. It was also demonstrated that ALT-803, administered either by iv or sc injection, promoted an equivalent level of CD8+ T and NK cell proliferation at 48 h and 72 h post-treatment (Fig. 2B and C). Notably, the percentage of CD4+ T cells in spleens decreased slightly in both injection groups at 48 and 72 h post-ALT-803 treatment. These results were anticipated since in previous studies [11,12], ALT-803 was shown to promote the proliferation of NK and CD8+ T cells at a significantly higher degree than CD4+ T cells and B cells. In addition to examining the lymphocyte subset changes, we also investigated NK cell activation using KLRG1 as an activation marker. As shown in Fig. 2D, KLRG1 expression on NK cells was significantly upregulated to comparable levels in both iv and sc treated mice. In contrast to lymphocyte subset changes which occurred at 48 h post-treatment, KLRG1 upregulation occurred 24 h following iv or sc administration of ALT-803, suggesting that both iv and sc administration at the mg/kg dose level provide equivalent short-term immune stimulation. Additionally, there was no significant difference in the percentage or number of mouse splenic CD4+CD25+Foxp3+ T regulatory (Treg) cells in the iv or sc treatment groups at 3 days post-ALT-803 administration (0.2 mg/kg) (Fig. 2E and F).
Fig. 2.
Proliferation and activation of splenocytes induced following iv and sc administration of ALT-803. Splenocytes were prepared from the ALT-803-treated mice from Fig. 1 at the 0, 16, 24, 48 and 72 h time-points. Splenocytes were stained with fluorochrome-labeled mAbs specific to mouse CD4, CD8, NK1.1, KLRG1, CD25, and Foxp3 and analyzed on a FACS-verse with FACSuite software. Splenocyte numbers (A), the percentages (B: iv C: sc) of CD4+ T cells, CD8+ T cells and NK1.1+ NK cells, and mean fluorescent intensity (MFI, D) of KLRG1 on NK cells were plotted. CD4+CD25+Foxp3+ Treg cells numbers (E) and percentages (F) at 72 h post iv and sc ALT-803 treatment are plotted; (*) indicates a p-value < 0.05.
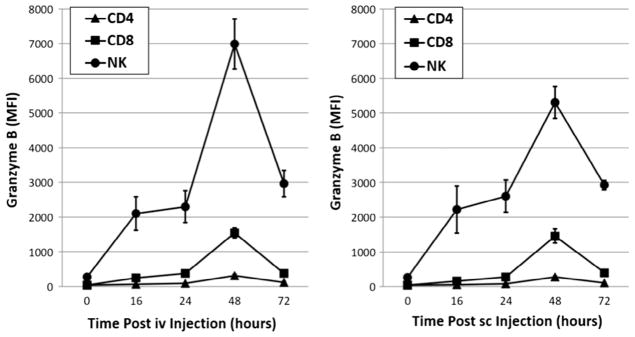
We also investigated the impact of iv versus sc ALT-803 administration on a key effector protein, granzyme B, which mediates NK cell cytotoxicity. ALT-803 iv dosing has been previously shown to up-regulate granzyme B expression by NK cells [11,12]. Our data show that iv and sc administration of ALT-803 equally induced expression of granzyme B by NK cells (Fig. 3). In summary, these data demonstrate that iv and sc administration of ALT-803 can activate CD8+ T and NK cells in an equivalent manner. These results also indicate that sc administration of ALT-803 is likely to enhance the cytotoxic potential of human NK cells to the same extent as iv administration.
Fig. 3.
Up-regulation of granzyme B expression in CD8+ T and NK cells following iv or sc administration of ALT-803. Splenocytes were prepared from ALT-803-treated mice from Fig. 1 at the 16, 24, 48 and 72 h time-points. Splenocytes were stained with fluorochrome-labeled mAbs specific to mouse CD8 and NK1.1 followed by intracellular granzyme B staining. Expression levels of granzyme B by activated CD8+ T and NK cells were determined by flow cytometry.
3.3. Serum cytokine/chemokine induction in mice treated with iv or sc ALT-803
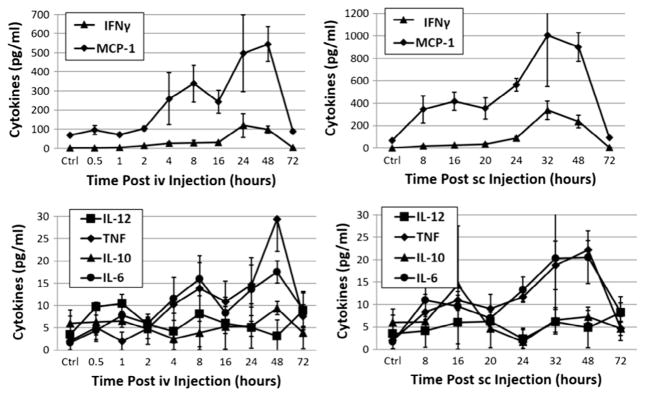
To further compare immune stimulation following iv and sc administration of ALT-803, we measured the levels of serum cytokines in peripheral blood of C57BL/6 mice treated with 0.2 mg/kg ALT-803 via iv or sc injections using Cytometric Bead Array Mouse Inflammation Kit (BD Biosciences). As shown in Fig. 4, IL-10 and IL-12 were not changed following ALT-803 treatment as shown in previous studies. IL-6 and TNF levels slightly increased at 48 h following ALT-803 treatment, regardless of the route of administration. It has been previously reported that IFNγ production by NK and CD8+ T cells played an important role in anti-tumor activity of iv treatment with ALT-803 and is induced by ALT-803 without requiring antigen-specific stimulation [11,12,16,17]. Thus, we also assessed whether sc administration of ALT-803 can induce IFNγ production in non-tumor-bearing mice. As illustrated in Fig. 4, levels of IFNγ production were dramatically induced by ALT-803 and reached the highest level at 24 h after iv treatment and 32 h after sc treatment. Interestingly, serum level of monocyte chemoattractant protein-1 (MCP-1/CCL2), a chemokine which plays a key role in regulating migration and infiltration of monocytes, memory T and NK cells [18], was also significantly increased in blood of mice treated with ALT-803 via iv or sc administration. This is the first report of MCP-1 upregulation by ALT-803 and it remains to be determined which cells produce MCP-1 following ALT-803 treatment and whether MCP-1 plays a role in the potent anti-tumor activity of ALT-803 by promoting infiltration of macrophages, memory T and NK cells to the tumor micro-environment.
Fig. 4.
Cytokine/chemokine induction by iv or sc administration of ALT-803. Serum samples were prepared from blood of ALT-803-treated mice at various time-points after ALT-803 iv or sc administration. Cytokine/chemokine levels in murine serum were determined by flow cytometry with Cytometric Bead Array Mouse Inflammation Kit.
3.4. Comparison of ALT-803 biodistribution following iv and sc administration in mice
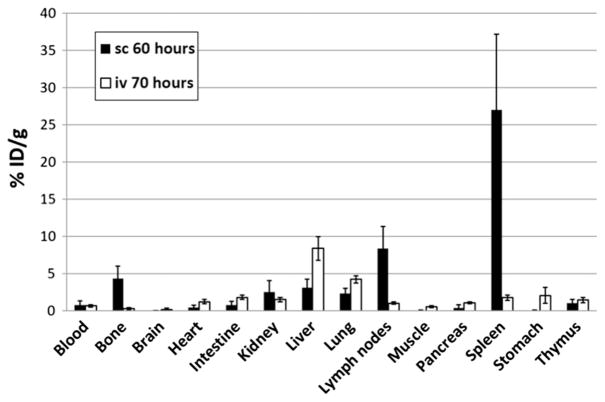
Previous tissue biodistribution studies in mice have shown much greater distribution and longer retention of iv-administered ALT-803 in the lymphoid organs when compared to iv-administered IL-15 [12]. In the current study, 64Cu-ALT-803 was administrated by sc injection in the same strain of C57BL/6 mice and the biodistribution of ALT-803 was assessed as shown in Fig. 5. Surprisingly, ALT-803 also exhibited greater biodistribution to the lymphoid organs, e.g. spleen and lymph nodes, when administered by sc administration (27.0 ± 10.2%ID/g and 8.4 ± 2.9%ID/g at 60 h post-injection respectively; n = 4), which was much higher than that for iv administration (1.8 ± 0.4%ID/g and 4.2 ± 0.5%ID/g at 70 h post-injection respectively; n = 4). This finding may help explain the observation that although ALT-803 sc administration resulted in a lower Cmax, it still exhibits similar immune stimulation as the iv administration, which showed a higher Cmax.
Fig. 5.
Biodistribution of ALT-803 by iv or sc administration. Tissue biodistribution of 64Cu-ALT-803 in B6 mice at 70 h (iv) and 60 h (sc) post-injection. Data are expressed as mean percentages + SD of %ID/g (n = 4/group).
3.5. Comparison of anti-tumor activity of iv and sc administration of ALT-803 in 5T33 myeloma-bearing mice
To investigate the anti-tumor efficacy of sc administration of ALT-803 in vivo, we employed the 5T33 myeloma mouse model which was previously used to demonstrate the anti-tumor activity of ALT-803 administered by iv injection [11]. In the current study, female B6 mice (6 mice/group) were injected iv with 5T33 myeloma cells (1 × 107/mouse) on day 0. ALT-803 (0.05 mg/kg or 0.2 mg/kg) was iv or sc injected on SD10 and SD14. To assess growth inhibition of 5T33 myeloma cells following iv and sc ALT-803 treatment, bone marrow (BM) cells were collected on SD17 and stained with fluorochrome-labeled mAbs specific to mouse CD8 and NK1.1 and intracellularly stained with FITC-conjugated anti-mouse IgG2b. The percentages of BM CD8+ T, NK and IgG2b+ 5T33 cells were evaluated by flow cytometry. As shown in the upper panel of Fig. 6, BM 5T33 myeloma cells were significantly reduced in mice treated with either iv or sc ALT-803 at the 0.2 mg/kg dose (p < 0.05) and 0.05 mg/kg dose (p = 0.03 for iv and p = 0.06 for sc) when compared to the PBS control group. No significant difference (p > 0.05) in efficacy was observed between iv and sc administration of ALT-803 in terms of reduction of tumor burden at both drug dose levels.
Fig. 6.
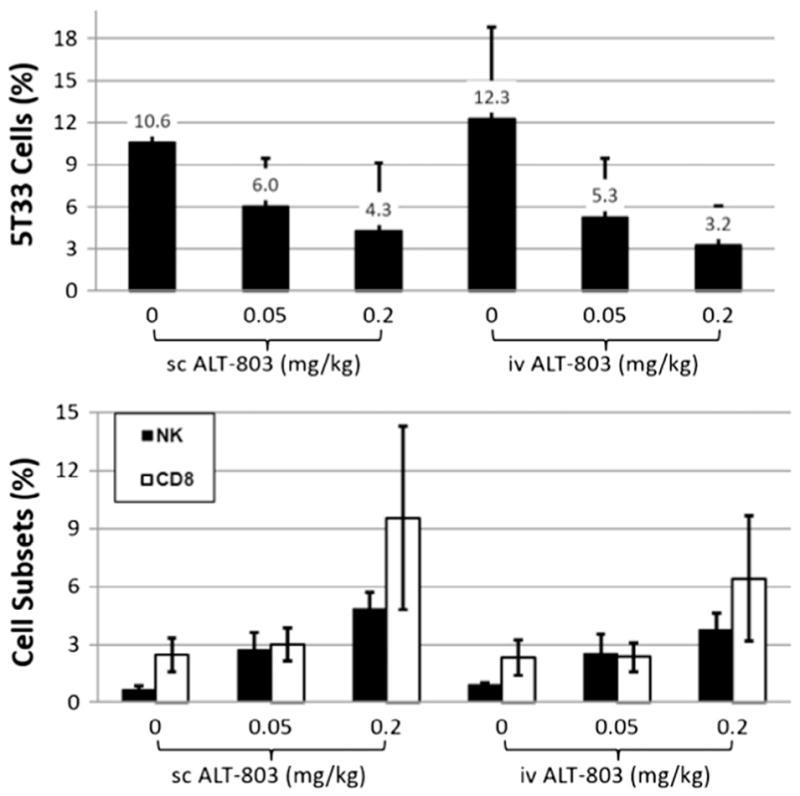
ALT-803 treatment via iv or sc results in similar reduction in tumor burden and stimulation of NK and CD8+ T cell proliferation in bone marrow of 5T33 myeloma-bearing mice. Female B6 mice (6 mice/group) were injected iv with 5T33 myeloma cells (1 × 107/mouse) on day 0. ALT-803 (0.05 mg/kg or 0.2 mg/kg) was injected iv or sc on SD10 and SD14. On SD17 the BM cells were prepared and stained with fluorochrome-labeled mAbs specific to mouse CD8 and NK1.1 and then intracellularly stained with FITC-conjugated anti-mouse IgG2b. The percentages of BM CD8+ T, NK cells and IgG2b+ 5T33 cells were evaluated by flow cytometry. The percentages of 5T33 cells (upper panel) or CD8+ T and NK cells (lower panel) in BM were plotted.
It was also observed that the percentage of NK cells in BM significantly increased in 5T33 tumor-bearing mice treated with iv and sc ALT-803 at 0.05 mg/kg and 0.2 mg/kg (Fig. 6 lower panel, p < 0.05). In addition, the percentage of CD8+ T cells in BM significantly increased in 5T33 tumor-bearing mice treated with iv and sc ALT-803 at 0.2 mg/kg (p < 0.05) but not at 0.05 mg/kg (p > 0.05). However, there were no significant differences in BM NK or CD8+ T cells percentages between the mice treated with iv or sc ALT-803.
To further evaluate immune stimulation by ALT-803 in 5T33 tumor-bearing mice, CD8+ T and NK cells in spleens were evaluated. Splenocytes were prepared from the 5T33-tumor bearing mice as described above, stained with fluorochrome-labeled mAbs specific against mouse CD8 and NKp46, and analyzed by flow cytometry. As shown in Fig. 7, the percentages of CD8+ T cells in spleens were significantly increased in 5T33 tumor-bearing mice treated with iv or sc ALT-803 at 0.05 mg/kg and 0.2 mg/kg. The percentage of NK cells in spleens also increased in 5T33 tumor-bearing mice treated with ALT-803 at 0.05 mg/kg but not at 0.2 mg/kg. This response is different from that observed for NK cells in BM. There were no significant differences in splenic NK or CD8+ T cells between mice treated with iv or sc ALT-803. For both control and tumor-bearing C57BL/6 mice, iv or sc administration of ALT-803 up to 0.2 mg/kg did not induce any signs of toxicity. In particular, there was no indication of injection site reaction or inflammatory reactions in mice administered ALT-803 by sc injection. These findings indicate that ALT-803 exhibits a comparable anti-tumor and immunostimulatory activity with both iv and sc administration.
Fig. 7.
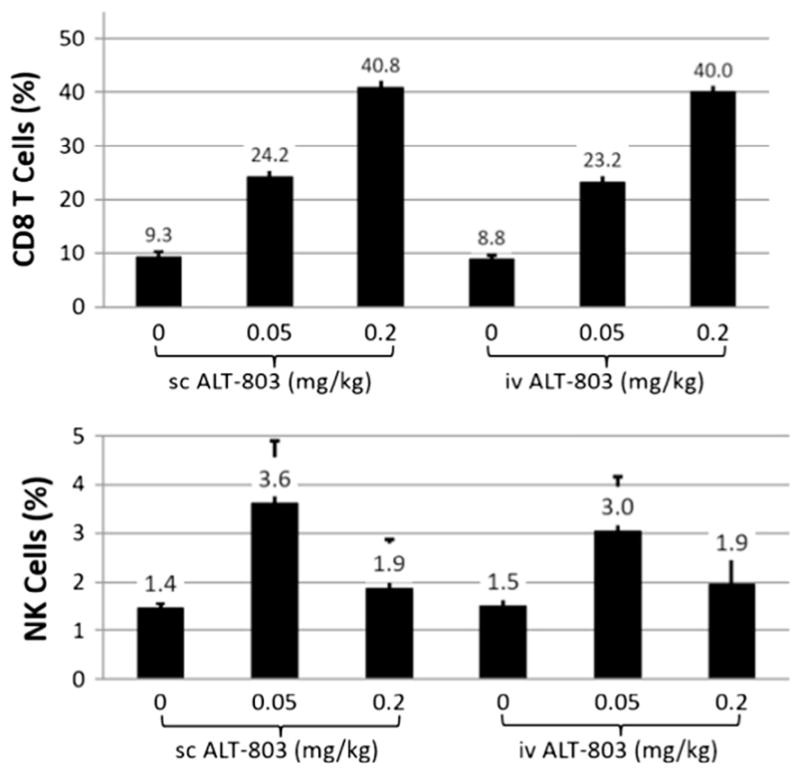
Comparison of the effects of iv and sc ALT-803 treatment on CD8+ T and NK cells in the spleens of 5T33 myeloma-bearing mice. B6 female mice (6 mice/group) were injected iv with 5T33 myeloma cells (1 × 107/mouse) on day 0. ALT-803 (0.05 mg/kg or 0.2 mg/kg) was injected iv or sc on SD10 and SD14. On SD17, splenocytes were prepared, stained with fluorochrome-labeled mAbs specific to mouse CD8 and NKp46, and analyzed by flow cytometry. The percentages of CD8+ T (upper panel) and NK cells (lower panel) in spleens were plotted.
3.6. Toxicological effects of repeated sc administration of ALT-803
To assess possible toxicities associated with repeated sc injections, female B6 mice (n = 5/group) were injected sc with high dose ALT-803 (1.0 mg/kg, 4-fold higher than the optimal dose of ALT-803 in previous anti-tumor and immunostimulatory studies) or PBS (dose equivalent volume) once a week for 4 weeks. Animals receiving ALT-803 (1.0 mg/kg) by sc injection exhibited a slight decrease in body weight as compared to PBS control mice (Fig. 8). However, this difference was not statistically significant (p = 0.5795) and body weight was recovered to control levels by week 4. Animals were monitored throughout the study period for signs of study drug-related toxicities, such as mortality, morbidity, ruffed fur, hunched posture, diarrhea, and lethargy. All mice received the complete treatment course and survived to study termination on SD26. Hunched posture was observed in the sc ALT-803 treatment group 4 days after the first injection (SD5), however, the animal’s posture returned to normal by SD7. No other signs of toxicity were observed during the study period. Following the fourth and final sc dose of ALT-803, peripheral blood was collected in heparinized micro-tubes and used for analysis of hematologic (Table 1) and chemical (Table 2) parameters. As was shown in a previous toxicity study examining repeated iv injection of ALT-803 [12], mice receiving multiple sc injections of 1.0 mg/kg ALT-803 exhibited significant differences in hematologic parameters as compared to PBS controls. Most notably, there was a 9-fold increase in the absolute white blood cells (WBC) and lymphocyte counts. Significant increases in neutrophil (8-fold), monocyte (7-fold), eosinophil (6-fold) and basophil (4-fold) counts were also observed in the sc ALT-803-treated mice. There was also a change in red blood cell parameters, consistent with a treatment-related shift from erythropoiesis to leukocyte production observed and described previously with iv administered ALT-803 [12]. For example, an increase in absolute cell counts for WBCs (15-fold), lymphocytes (18-fold), neutrophils (10-fold), monocytes (11-fold), eosinophils (7-fold) and basophils (6-fold) was previously observed in female C57BL/6 mice following 4 weekly iv doses of 1 mg/kg ALT-803 [12], [unpublished data].
Fig. 8.
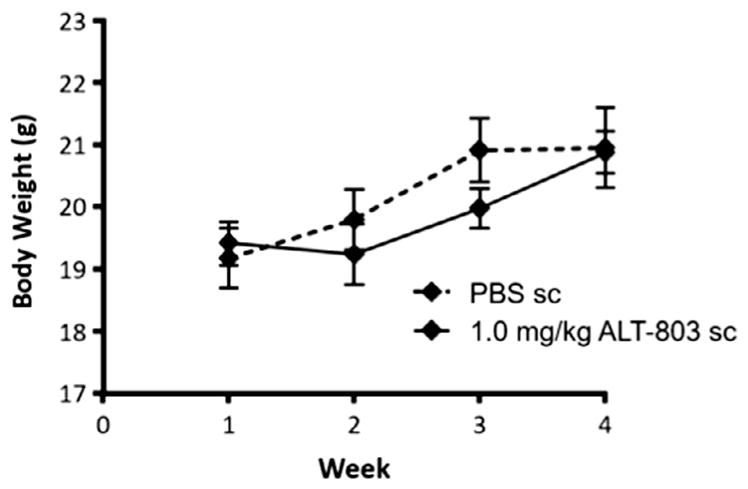
Body weight of C57BL/6 female mice during sc treatment with ALT-803. C57BL/6 female mice (n = 5/group) were injected with 1.0 mg/kg ALT-803 or PBS (dose equivalent volume) sc once weekly for 4 weeks. Mice were weighed prior to injection and at the termination of the study (SD25).
Table 1.
Hematology parameters of ALT-803-treated C57BL/6 female mice on SD26.
| PBS (n = 5) | ALT-803 1.0 mg/kg (n = 5) | |
|---|---|---|
| White Blood Cell Count (WBC) K/μL | 5.8 ± 1.4 | 52.3 ± 32.7* |
| Red blood cell count (RBC) M/μL | 10.7 ± 0.3 | 7.9 ± 1.0* |
| Hemoglobin concentration (HB) g/dL | 15.0 ± 0.4 | 12.0 ± 1.4* |
| Hematocrit (HCT)% | 47.4 ± 1.5 | 38.8 ± 4.2* |
| Mean Corp. Volume (MCV) fL | 44.4 ± 0.5 | 49.5 ± 1.3* |
| Mean Corp. Hemoglobin (MCH) pg | 14.2 ± 0.4 | 15 ± 0* |
| Mean Corp. Hemoglobin Conc. (MCHC) g/dL | 31.8 ± 0.4 | 30.8 ± 0.5* |
| Platelet Count (PLT) K/μL | 801 ± 132 | 588 ± 122* |
| Mean platelet volume (MPV) fL | 5.7 ± 0.24 | 6.1 ± 0.2 |
| Segmented neutrophils ×103/μL | 1.2 ± 0.2 | 10.1 ± 4.5* |
| Lymphocytes ×103/μL | 4.3 ± 0.8 | 39.8 ± 17.9* |
| Monocytes ×103/μL | 0.2 ± 0.05 | 1.4 ± 0.8* |
| Eosinophils ×103/μL | 0.13 ± 0.03 | 0.82 ± 0.4* |
| Basophils ×103/μL | 0.03 ± 0.01 | 0.12 ± 0.04* |
| RBC morphology | Normal | Mild to moderate anisocytosis (5 of 5) Mild to moderate poikilocytosis (4 of 5) |
| Platelet morphology | Normal | Normal |
| WBC morphology | Normal | Normal |
indicates a p-value < 0.05.
Table 2.
Blood chemistry parameters of ALT-803-treated C57BL/6 female mice on SD26.
| PBS (n = 5) | ALT-803 1.0 mg/kg (n = 5) | |
|---|---|---|
| Total protein (TP) g/dl | 6.4 ± 0.3 | 6.3 ± 0.5 |
| Aspartate aminotransferase (AST) U/L | 193.2 ± 83.7 | 261.8 ± 47.5 |
| Alanine aminotransferase (ALT) U/L | 74.6 ± 47.5 | 105.2 ± 41.1 |
| Alkaline phosphatase (ALP) U/L | 136.6 ± 9.9 | 87.8 ± 42.5* |
| Creatinine (CREA) mg/dL | 0.3 ± 0.07 | 0.2 ± 0.0* |
| Total bilirubin (TBIL) mg/dL | 1.3 ± 0.2 | 1.1 ± 0.3 |
| Blood urea nitrogen mg/dL | 19.4 ± 2.5 | 23.5 ± 5.1 |
indicates a p-value < 0.05.
Chemical analysis of the blood from sc ALT-803 treatment group showed a significant decrease in alkaline phosphatase and creatinine but no significant change in levels of aspartate or alanine amino-transferases or blood urea nitrogen. In summary, the hematological and chemical changes observed in sc ALT-803-treated mice in this study were similar (if not, possibly less in magnitude) to the changes seen in the blood of mice receiving repeated iv injections of the same dose (1.0 mg/kg) of ALT-803.
On SD25 mice were humanely sacrificed and necropsied. No signs of inflammatory skin reactions were noted in the ALT-803 treatment group. Specifically, no site injection reactions were seen in mice treated by sc ALT-803. Organs (spleen, lymph nodes, liver, kidneys, lung, heart, and thymus) were harvested, weighed, and analyzed and differences were recorded as shown in Fig. 9. The most significant changes were 5.5-fold increase in spleen weight and 3-fold increase in lymph node weight of mice treated with ALT-803 compared to PBS controls. A significant increase in liver weight was also observed in ALT-803 treated mice, whereas there was no significant difference in the weights of kidneys, lung, heart, and thymus between ALT-803 and PBS treatment groups. As previously reported [12] and shown in Fig. 5, ALT-803 has the greatest biodistribution to the spleen, liver, and lymph nodes. The increased liver weight in ALT-803-treated mice can be attributed in part to induced NK cell proliferation and activation. These cells normally reside at high frequency in this tissue. The AST levels in mice receiving ALT-803 though slightly higher compared to PBS were not significantly different (p = 0.147). Although there appears to be a slight increase in lung weight in mice treated with ALT-803 as compared to PBS, this difference was also not statistically significant (p = 0.07). Overall, these results are comparable to the results seen in animals given multiple iv injections of ALT-803 (i.e., 6.5-fold increase in spleen weight at 1.0 mg/kg [12]). Taken together, C57BL/6 mice could tolerate either iv or sc administration of ALT-803 at a dose level of 1 mg/kg, which is 4-fold higher than the optimal dose level of 0.2 mg/kg.
Fig. 9.
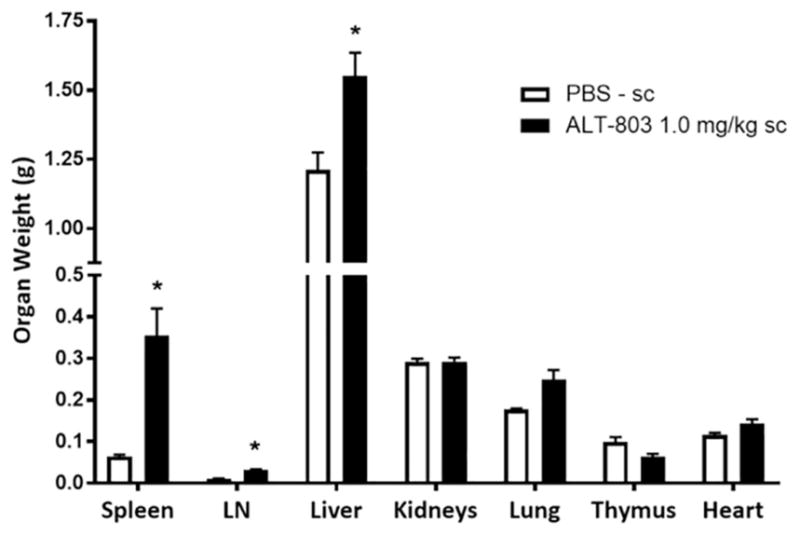
Organ weights of healthy C57BL/6 mice injected with sc ALT-803. Female B6 mice (5/group) were injected with 1.0 mg/kg ALT-803 or PBS (dose equivalent volume) sc once weekly for 4 weeks. Mice were humanely sacrificed on SD26, 4 days after the final injection. Necropsy was performed and organs were harvested for weight comparison as illustrated in this figure. LN = lymph nodes; (*) indicates a p value < 0.05.
4. Conclusions
It has been previously demonstrated that the IL-15N72D and IL-15RαSu/Fc genes can be co-expressed in recombinant CHO cells, form a functional protein complex, and be readily purified from cell culture supernatants as an IL-15 superagonist named ALT-803 [9,10]. ALT-803 is capable of inducing NK and CD8+ T cell proliferation and activation and effectively promoting potent anti-tumor responses. It was also reported that following iv ALT-803 administration, IFNγ production by host immune cells played an important role in the anti-tumor activity of ALT-803 [11]. ALT-803 was initially pursued in clinical trials using an iv route of administration. However, sc administration of ALT-803 may be more convenient as a clinical regimen and may be associated with fewer systemic toxicities due to lower ALT-803 peak serum concentration which is probably associated with immediate cytokine release. In the current study we compared the pharmacokinetics, immunostimulatory activities and anti-tumor efficacy of iv and sc administration of ALT-803 in C57BL/6 mice. We found the serum elimination half-life of ALT-803 to be 7.5 h for iv administration versus 7.7 h for sc administration. The maximal detected serum concentration of ALT-803 was 3926 ng/ml at 0.5 h time-point following iv administration and 495 ng/ml at 16 h time-point following sc administration. Despite the remarkable difference of peak serum level by iv and sc administration, we showed that ALT-803 administered iv or sc induced comparable proliferation of CD8+ T and NK cells and similarly activated immune cells which resulted in equivalent reduction of tumor burden in an experimental animal model. A toxicity study of mice receiving multiple iv or sc injections of ALT-803 for 4 weeks revealed comparable immune system-related changes. The gradual absorbance into the blood stream and lower maximal blood level of sc injected ALT-803, along with similar anti-tumor efficacy support the use of ALT-803 by sc administration in patients. Therefore, we have changed the dosing route of ALT-803 from iv to sc in all of our current clinical studies. Preliminary results of a clinical trial using ALT-803 in patients with relapsed/refractory hematological malignancies after allogenic stem cell transplantation (i.e., NCT01885897) indicated that sc administration induced constitutional AEs at a much lesser degree of severity and frequency than iv administration at the dose levels of 6 and 10 μg/kg [13]. At these dose levels, ALT-803 was reported to effectively promote the proliferation and activation of NK and T cells. Therefore, the results reported herein and the preliminary clinical results strongly support sc administration of ALT-803 in current and future clinical applications.
Acknowledgments
Funding
This work was supported, in part, by the University of Wisconsin -Madison, the National Institutes of Health [NIBIB/NCI 1R01CA169365 and P30CA014520]; and the American Cancer Society [125246-RSG-13-099-01-CCE].
Abbreviations
- IL-15N72D
human IL-15 aa 72N to D variant
- IL-15RαSu
human IL-15 receptor alpha sushi domain
References
- 1.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 2.Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res. 2004;10(18 Pt 2):6342s–6346s. doi: 10.1158/1078-0432.CCR-040029. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3(3):219–227. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldmann TA, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117(18):4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 6.Oh S, et al. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100(6):3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh S, et al. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci USA. 2004;101(42):15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munger W, et al. Studies evaluating the antitumor activity and toxicity of inter-leukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol. 1995;165(2):289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, et al. Novel human interleukin-15 agonists. J Immunol. 2009;183(6):3598–3607. doi: 10.4049/jimmunol.0901244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han KP, et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011;56(3):804–810. doi: 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013;73(10):3075–3086. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhode PR, et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol Res. 2016;4(1):49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JS, et al. ‘First-in-human’ phase I dose escalation trial of IL-15N72D/IL-15Rα-Fc superagonist complex (ALT-803) demonstrates immune activation with anti-tumor activity in patients with relapsed hematological malignancy. Blood. 2015;126(23):1957. [Google Scholar]
- 14.Radl J, et al. Animal model of human disease. Multiple myeloma. Am J Pathol. 1988;132(3):593–597. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, et al. A novel fusion of ALT-803 (Interleukin (IL)-15 Superagonist) with an antibody demonstrates antigen-specific antitumor responses. J Biol Chem. 2016;291(46):23869–23881. doi: 10.1074/jbc.M116.733600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong HC, Jeng EK, Rhode PR. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8 T cells into innate-like effector cells with antitumor activity. Oncoimmunology. 2013;2(11):e26442. doi: 10.4161/onci.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosario M, et al. The IL-15-based ALT-803 complex enhances FcgammaRIIIa-Triggered NK cell responses and in vivo clearance of B Cell lymphomas. Clin Cancer Res. 2016;22(3):596–608. doi: 10.1158/1078-0432.CCR-15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshmane SL, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]