Abstract
Objective
Little is known about long-term metabolic (energy expenditure) adaptation after bariatric surgery.
Methods
Resting metabolic rate under basal conditions (RMR), total daily energy expenditure (TDEE) and body composition were measured in 25 participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2).
Results
Six months after surgery, BMI (±SD) decreased (47 ± 6 to 37 ± 5 kg/m2), body fat from 48 ± 6 to 40 ± 6% fat and fat-free mass from 67 ± 9 to 60 ± 9 kg. In absolute terms RMR and TDEE both decreased significantly (1730 ± 278 vs. 1430 ± 200 and 2879 ± 544 vs. 2369 ± 304 kcal/day) and the achieved energy balance was −1293 ± 355 kcal/day. Sixteen of these participants underwent repeated measures at ~24 months; TDEE decreased 6 months postoperative (2957 ± 540 kcal/day to 2423 ± 324, P=0.0003), but at ~24 months, TDEE (2602 ± 471 kcal/day) was not significantly different compared to Month 6. The average negative energy balance from Baseline to Month 24 was −379 ± 131 kcal/day.
Conclusions
RMR and TDEE fall precipitously in the first six months after bariatric surgery but these adaptive changes were no longer significant after two years.
Keywords: Energy expenditure, bariatric surgery, obesity
Introduction
Bariatric surgery has been shown to be effective for accomplishing durable weight loss and improved survival (1–6). There is wide variation in the amount of weight loss and regain after bariatric surgery. Pories (2) reported that the percent excess weight loss 10 years following gastric bypass was a mean of 55% with a range from 0.9% to 103%. In addition, the LABS (Longitudinal Assessment of Bariatric Surgery) consortium recently identified five weight loss trajectories following both gastric bypass and adjustable gastric banding also demonstrating a wide variability of post-operative weight loss (7–9). Variable regain of weight occurs following all bariatric surgical procedures (7–9).
Detailed studies of the mechanism(s) of action following gastric bypass are needed in order to devise strategies for improving the outcomes of bariatric surgery. Here we wanted to assess whether total daily energy expenditure (TDEE) and resting metabolic rate under basal conditions (RMR) change in patients undergoing bariatric surgery post-operatively compared to that measured pre-operatively. Our primary hypothesis was that TDEE and RMR would decrease in patients undergoing bariatric surgery at six months and 24 months post-operatively compared to baseline.
Methods
Subjects
We studied patients who were enrolled in the LABS clinical trial (7) including 88% Roux-en-Y gastric bypass (none “long limb”), 8% adjustable gastric banding and 4% biliopancreatic bypass with duodenal switch. Participants were recruited at Oregon Health & Science University (OHSU). All inclusion/exclusion criteria matched those of LABS (7). In addition, eligible subjects were excluded if they were unable to walk at 2 mph for 15 minutes, or weighed greater than 227 kg at the screening visit because this was the upper limit of the Dual X-ray Absorptiometry scanner table. Oregon Health & Science University and Mayo Clinic Institutional Review Boards approved the study, and written informed consent was obtained.
Study design
Free-living LABS bariatric surgery patients were studied for 18-day periods on three occasions, at baseline before surgery, at six months and at approximately 24 months after bariatric surgery. LABS patients followed best practice guideless for post-bariatric surgery care (7) that included education regarding the composition and energy content of their diets, calcium, multivitamin and B12 supplementation. Subjects were not given an exercise prescription.
Measurements of RMR
At each time point (pre-operatively and at six and 24 months after surgery), RMR was measured on three consecutive mornings (days 16, 17, 18) between 06:00 and 08:00 h in the subjects who had slept uninterrupted the previous nights in the OHSU GCRC (subjects were admitted to the GCRC on the evening of day 11). Subjects were not moved prior to measurements and had not eaten since 2100 the night before. For each measurement, the facemask equipped indirect calorimeter (Columbus Instruments, Columbus, OH) was calibrated using gases of known composition. Subjects were awake, semirecumbent (10° head bed tilt), lightly clothed, and in thermal comfort (68–74°F) in a dimly lit, quiet room. Measurements were performed for 30 min, during which time subjects were not allowed to talk or move. RMR was the average of the final 25 min for the three consecutive days of data (days 16–18).
Measurements of TDEE
Total daily energy expenditure (TDEE) was measured using doubly labeled water (DLW) at these same three time points during the study. A baseline urine was collected and then subjects drank approximately 10 atom percent (AP) 18O water mixed with 99.8 AP 2H water at a dose of approximately 1.8 g of the 10 AP 18O water and 0.12 g of the 99.8 AP 2H water per kg of estimated total body water. The cup was then washed with 50 mL of tap water and that drunk by the subjects. Subjects voided at one hour after the dose and then voids were collected at four and six hours after the dose and then at 14 days after dose. The urine specimens were frozen (−10°C) until analyzed. Urine were decolorized with dry carbon black and the 18O and 2H abundances analyzed by equilibration with CO2 and chromium reduction, respectively (10). TDEE was calculated using equation A6 and the revised dilution space ratio, with an assumed RQ of 0.86 (11, 12). The laboratory has demonstrated accuracy of 1–2% and a coefficient of variation of 4–7% (12).
Measurements of body composition
Body fat was measured at the start and end of each DLW period using Dual X-ray Absorptiometry (DXA) (13) (Discovery A (S/N 80132) Hologic, Lunar, Madison, WI). The vast majority of DXA scans were completed with the entire body captured in the field of the X-ray. In a small number of scans, subjects were unable to be captured in the field; here the subject was scanned with the results multiplied by 2 for the full body estimate. Fat mass was the average of the % DXA fat mass multiplied by the scale weight on Days 0 and 14 of the TDEE protocol. Average FFM at each time point was calculated as the difference between the subject’s body weight and fat mass as determined by the two DXA.
Data and statistical analyses
Sample sizes for race other than white or surgical procedure other than Roux-en-Y were small (1 or 2 per group) and thus analyses were performed without subcategorization. Results are reported as mean ± SD.
Testing for Metabolic Adaptation at Month Six and Month 24
To test for the presence of change at both six months and 24 months, we performed ANOVA and post hoc paired two-sample t-tests comparing resting metabolic rate and total energy expenditure at baseline to month 6 resting metabolic rate and baseline to month 24 resting metabolic rate using SPSS v 21 (Armonk, NY, 2012).
The t-test alone cannot isolate the presence of metabolic adaptation since the difference between baseline and six month energy expenditures may be different due to lower metabolic rates associated with reduced weight or a change beyond the effects of reduced fat-free mass (adaptation). To determine whether metabolic rates are lower due to metabolic adaptation, we regressed a line with baseline resting metabolic rate as the dependent variable and baseline fat free mass as the independent variable in Microsoft Excel (Seattle, WA 2011). We then calculated the residuals between measured energy expenditure and that predicted from current FFM using the baseline linear relationship described above. Finally, a one-sample t-test was performed to determine whether the mean difference (bias) was different from 0. The one sample t-test was conducted in SPSS v 21 (Armonk, NY, 2012)
Estimation of the Energy Deficit and Energy Intake
Weight change results from energy imbalance and can result from uncompensated changes in energy intake, total daily energy expenditure (TDEE) or both. To understand the rates of weight change over time we applied the first law of thermodynamics by the use of energy balance models (14) to calculate the magnitude of energy deficit from body composition changes. To isolate the roles of energy intake and TDEE, we also used these models to calculate metabolizable energy intake (Ei).
Change in energy stores (ES) was calculated applying changes in body composition (kg) as measured by DXA using the formula:
From this formula we can also calculate energy balance (the difference between metabolizable energy intake and energy expended) at six months and 24 months, which is equal to ES. These calculations were performed in Microsoft Excel (Seattle, WA 2011). Gender was not examined as a variable due to small sample size.
Results
Subjects were weight stable or lost small amounts of weight during each of the 10 day DLW periods indicating they were in or close to energy balance. Among those for whom two measures were available at each DLW period, body masses were: baseline (n= 20) Day 0, 130 ± 19 kg and Day 10, 129 ± 19 kg (R2 = 0.99), 6 Months (n= 24) Day 0, 101 ± 16 kg and Day 10, 99 ± 16 kg (R2 = 0.99) and 24 months (n=9) Day 0, 94 ± 13 and Day 10, 94 ± 13 Kg (R2 = 0.99)
Baseline and six month measures
Subjects included and subjects excluded from analysis
At baseline 34 people signed consent forms; 30 were women. Nine subjects were excluded from analysis because they did not complete TDEE or RMR measurements; the principal reason for this was that subjects did not want to return for 6 month follow-up measurements. Thus the data presented here are derived from 25 people; 22 women, age 45 ± 11 years; BMI 47 ± 6 kg/m2 (Table 1). The three men were aged 61, 49 and 49 years with BMI values of 42, 41 and 47 kg/m2 respectively.
Table 1.
Anthropometric and energetic data at baseline and 6 months post bariatric surgery
| Baseline | 6 months post-surgery | P | |||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | ||
| Age (22 women: 3 men) years | 44.5 | ± 10.6 | |||
| Body Mass Index (Kg/m2) | 47.4 | ± 6.1 | 36.5 | ± 5.1 | <0.001 |
| Weight (kg) | 130.5 | ± 20.4 | 99.8 | ± 16.1 | <0.0001 |
| Body fat (%) | 47.9 | ± 5.6 | 39.6 | ± 6.3 | <0.001 |
| Body fat (kg) | 63.0 | ± 15.3 | 40.0 | ± 10.9 | <0.001 |
| Fat free mass (kg) | 67.4 | ± 9.3 | 59.8 | ± 8.9 | <0.001 |
| Total body water (kg) | 44.4 | ± 6.4 | 40.1 | ± 6.0 | <0.001 |
| RMR (kcal/day) | 1730 | ± 278 | 1430 | ± 200 | <0.0001 |
| RMR Residual (kcal/day) | 0 | ± 186 | 131 | ± 163 | = 0.002 |
| TDEE (kcal/day) | 2879 | ± 544 | 2369 | ± 304 | <0.001 |
| TDEE Residual (kcal/day) | 0 | ± 420 | 227 | ± 339 | =0.008 |
Anthropometric and energetic data for 25 subjects (22 women, 3 men) at baseline and 6 months after bariatric surgery. Body composition was determined using dual-X-ray absorptiometry (DXA), and total body water was derived from deuterium dilution. RMR, basal metabolic rate; FFM, fat-free mass; TDEE, total daily energy expenditure. RMR was determined by indirect calorimeters and TDEE using doubly labeled water. Data are shown as mean ± standard deviation (SD). P values are for paired analyses. Residuals were calculated by predicting RMR and TDEE based upon the respective baseline relationships with FFM before surgery. The residuals were calculated as Predicted – Actual. The equation that described the baseline relationship between FFM and RMR was: RMR = 22.1 * FFM + 243 (R2 = 0.55, P<0.001). The equation that described the baseline relationship between FFM and TDEE was: TDEE = 37.0 * FFM + 382 (R2 = 0.40, P<0.001).
The 25 bariatric surgeries included 22 cases of Roux-en-Y gastric bypass, two cases of adjustable gastric banding (34 and 49 years old, 50 and 47 kg/m2, weight loss 29 and 21 kg respectively) and one case of biliary pancreatic diversion with a duodenal switch (33 years, 51 kg/m2, weight loss 43 kg) Subjects continued their usual occupations and activities throughout the measurement periods. Of the 25 subjects, 21 identified themselves as white, one African American, one white/Native American, one as white/African American/Native American and one as white/African American/’other’.
Six month repeated measures of body weight and body composition
As expected, weight and fat loss six months after bariatric surgery were significant. Mean body weight decreased from 131 ± 20 kg to 100 ± 16 kg (P<0.001), representing, 24 ± 5% mean decrease. Body fat decreased from 63 ± 15 kg to 40 ± 11 kg (P<0.001), a 37 ± 8% decrease (Table 1). Fat loss was highly variable ranging from 7.3 to 42.9 kg (15.6 – 50.8% reduction). Fat-free mass (FFM) decreased significantly from 67 ± 9 vs. 60 ± 9 kg (p<0.001), a modest fractional loss in fat-free mass (11 ± 4%). The ratio of fat mass (FM) loss/FFM loss was 2.6 (±2.4):1.
Baseline and six month repeated measures of TDEE and RMR
RMR was measured before and six months after bariatric surgery (Table 2). In absolute terms, RMR decreased with weight loss from 1730 ± 278 to 1430 ± 200 kcal/day (p<0.001). As expected RMR correlated with fat-free mass but not fat mass.
Table 2.
Anthropometric and energetic at baseline and 6 and 24 months post bariatric surgery.
| Baseline | 6 months post-surgery | 24 months post-surgery | P (0 vs 6 months) | P (6 vs 24 month) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | Mean | SD | Mean | SD | |||
| Age (14 women: 2 men) years | 46.3 | ± 11.2 | ||||||
| Body Mass Index (Kg/m2) | 47.8 | ± 5.2 | 37.3 | ± 4.4 | 31.7 | ± 9.2 | <0.0001 | 0.03 |
| Weight (kg) | 134.5 | ± 13.0 | 104.2 | ± 9.9 | 96.7 | ± 12.3 | <0.0001 | 0.005 |
| Body fat (%) | 47.0 | ± 6.4 | 39.7 | ± 7.5 | 37.6 | ± 6.1 | <0.0001 | 0.05 |
| Body fat (kg) | 63.7 | ± 13.4 | 41.7 | ± 10.4 | 36.7 | ± 9.2 | <0.0001 | 0.01 |
| Fat free mass (kg) | 70.8 | ± 7.0 | 62.5 | ± 6.9 | 60.0 | ± 7.2 | <0.0001 | 0.02 |
| Total body water (kg) | 46.2 | ± 4.9 | 41.7 | ± 5.0 | 40.6 | ± 4.8 | <0.0001 | ns |
| RMR (kcal/day) | 1792 | ± 267 | 1460 | ± 192 | 1563 | ± 216 | <0.0001 | ns |
| RMR Residual (kcal/day) | 13.3 | ± 196 | 161 | + 139 | 3.0 | ± 141 | =0.02 | ns |
| TDEE (kcal/day) | 2957 | ± 540 | 2423 | ± 324 | 2602 | ± 471 | 0.0003 | ns |
| TDEE Residual (kcal/day) | 47.3 | ± 410 | 273 | ± 377 | 94 | ± 550 | ns | ns |
Anthropometric and energetic data for 16 subjects (14 women, 2 men) at baseline and 6 months and 24 months after bariatric surgery. Body composition was determined using dual-X-ray absorptiometry (DXA), and total body water was derived from deuterium dilution. RMR, basal metabolic rate; FFM, fat-free mass; TDEE, total daily energy expenditure. RMR was determined by indirect calorimeters and TDEE using doubly labeled water. Data are shown as mean ± standard deviation (SD). P values are for paired analyses. Residuals were calculated by predicting RMR and TDEE based upon the respective baseline relationships with FFM. The residuals were calculated as Predicted – Actual. The equation that described the baseline relationship between FFM and RMR was: RMR = 22.1 * FFM + 243 (R2 = 0.55, P<0.001). The equation that described the baseline relationship between FFM and TDEE was: TDEE = 37.0 * FFM + 382 (R2 = 0.40, P<0.001).
Total Daily Energy Expenditure was measured before and six months after bariatric surgery (Table 2). In absolute terms, TDEE decreased with weight loss from 2879 ± 544 to 2369 ± 304 kcal/day (p<0.001) as did total body water (Table 2). The amount of fat loss was not predicted by initial body weight or by baseline RMR or TDEE using linear models.
The mean measured change in FFM was 7.66 ± 3.0 Kg; TDEE, 510 ± 433 kcal/day, and RMR, 300 ± 203 kcal/day. The change in TDEE correlated with the change in FFM (R = 0.49, p=0.01). There was no correlation between the change in TDEE and the change in RMR or between the change in TDEE and the change in body fat. There was no association between the change in RMR and the change in body fat or fat free mass after surgery.
Baseline, 6 and 24 month repeated measures
Subjects included and subjects excluded from analysis
The data presented here are derived from 16 people; 14 women, age 46 ± 11 years. Fourteen of the patients underwent Roux-en-Y gastric bypass, one completed adjustable gastric banding and one, biliary pancreatic diversion with a duodenal switch. Of the 16 subjects, 12 identified themselves as white, 1 African American, 1 white/Native American, 1 as white/African American/Native American and 1 as white/African American/’other’. The reasons that 9 of the 25 subjects did not complete the 24 month follow-up included that they chose to not repeat the measurements, or could not be located for follow-up.
Baseline, 6 and 24 month repeated measures of body weight and body composition
As expected in these 16 patients who underwent repeated measures of body composition at 24 months post-operatively, the patients’ weight, body fat and fat free mass continued to decrease from postoperative months 6 through 24 (Table 2)(Figure 1). After two years, fat loss was highly variable 27.0 ± 10.2 (11.3 – 47.2) kg). The rate, at which body composition changed was less between months six and 24 compared to baseline and month 6 (Table 2). This slowing or plateauing of rate of weight loss was expected (7, 8).
Figure 1.
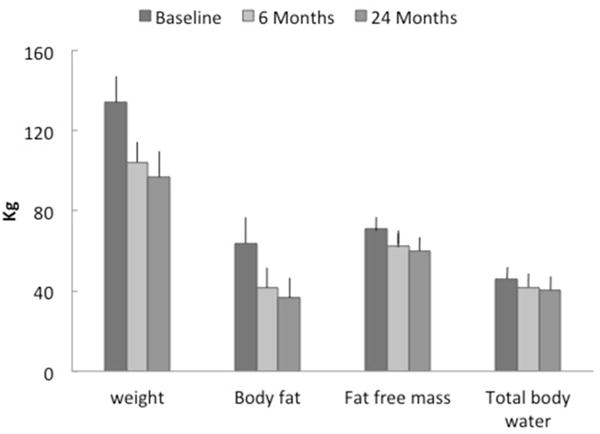
Body composition in 16 subjects (14 women, 2 men) at Baseline, 6 months and 24 months after bariatric surgery. Body fat and fat free mass were measured using dual-X-ray absorptiometry and total body water by deuterium dilution.
Baseline, 6 and 24 month repeated measures of RMR
Similar to the above, for the 16 subjects who completed the assessments through 24 months, measured RMR decreased in the first six months after surgery, but the change between months six and 24 was not significant (Table 2). FFM contributed most to the variance of RMR (Figure 2).
Figure 2.
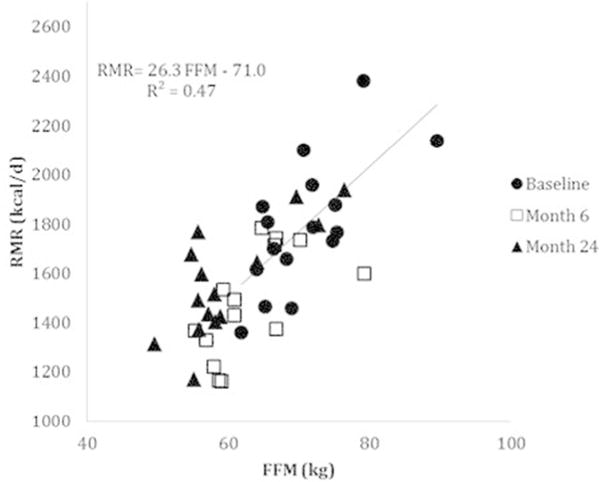
Resting metabolic rate (RMR) versus fat-free mass (FFM) in 16 subjects (14 women, 2 men) at Baseline, 6 months and 24 months after bariatric surgery. RMR was determined by indirect calorimetry in the basal state and body composition was determined using dual-X-ray absorptiometry.
Baseline, 6 and 24 month repeated measures of TDEE
In these 16 patients, measured TDEE decreased between baseline and 24 months (2957 ± 540 kcal/day vs. 2602 ± 471 kcal/day) (Table 2). Measured TDEE, however, did not change significantly between six and 24 months despite patients continuing to lose weight. TDEE/RMR did not change significantly between baseline (1.68 ± 0.31) and six months (1.70 ± 0.33) and 24 months post-operatively (1.70 ± 0.35).
Evidence for Metabolic Adaptation
To test whether RMR or TDEE displayed metabolic adaptation after surgery, EE prediction equations were developed using baseline data. Before surgery, the relationship between RMR (kcal/d) and FFM ((kg) was:
The equation that described the relationship between FFM and TDEE at baseline was:
The paired t-test revealed that the six month RMR was significantly different from baseline, (p=0.02). The bias (average of the residuals) between the predicted RMR from baseline data and the six month RMR was −161 ± 139 kcal/d indicating that on average RMR decreased beyond that accounted by weight loss at 6 months which was evidence of metabolic adaptation. However, at 24 months, the bias was small −3 ± 141 kcal/d and not significantly different from 0, (p=0.12).
TDEE also changed more than was expected for change in body composition, but only at the six month assessment. The average residual between measured and predicted TDEE based on FFM was significant (−227 ± 340 kcal/d) at six months, but not 24 months (0 ± 555 kcal/d).
We also tested the influence of including FM in the TDEE prediction equation. In doing so it was found that FM was a significant predictor when included along with FFM, but that the coefficient for FM in these subjects with class III obesity was negative −i.e. TDEE decreased with increasing FM – and that it actually increased the estimate of metabolic adaptation at six months (−454 ± 365 kcal/d, p<0.001). For the evaluation of the TDEE data at 24 months, we also tested whether using data from the 16 subjects at baseline that were present for the 24 month measurement period would change the estimate of metabolic adaptation and it did influence the sign of the estimate, but it remained insignificant (218 ± 480 kcal/d, NS).
Calculated Energy Deficit and Energy Intake
The achieved energy deficit between Baseline to Month six was −1293 ± 355 kcal/d in 25 subjects while the achieved energy deficit from Month six to Month 24 was –93 ± 126 kcal/d in 16 subjects, which was a 93% reduction in magnitude comparing to the estimate between Baseline and Month Six. The calculated Ei was 1111 ± 435 kcal/d between Baseline and Month six and 2420 ± 409 kcal/d between Month six and Month 24.
Conclusion
It is not well understood how weight loss is sustained after bariatric surgery (15–17). Our results indicate that TDEE decreases following bariatric surgery and that the decrease at six months is due to both a reduction in FFM (accounting for 56% of the change in TDEE), a major predictor of TDEE, and a metabolic adaptation (44%) that decreases TDEE more than the reduction in FFM predicts. The decrease in TDEE is maintained at 24 months, but at this point in time after surgery there was no longer evidence indicating metabolic adaptation.
Previous data suggest that patients undergoing bariatric surgery are physically inactive (18–25) and it is often assumed the after bariatric surgery, physical activity increases. Our data and others (19, 22) does not support this. Rather our results indicate that weight loss occurs not because of an increase in TDEE, but rather that weight occurs despite a reduction in TDEE. Our data supports prior findings of large reductions in caloric intake despite these studies utilizing food records and dietary recall (23) which are often inaccurate (26). More accurate methods of quantitating nutrient intake such as our energy deficit calculations combined with TDEE, following gastric bypass demonstrate evidence of a large reduction in metabolizable energy intake. It should be noted that the energy deficit/balance approach cannot isolate the influences between reduced dietary consumption and increased malabsorption, which identified 62% and 18% reductions in Ei the first six months and next 18 months after surgery, respectively. Despite the widespread popularity of gastric bypass for weight loss, the mechanisms of action remain incompletely defined.
We had a comparatively large data set to look at the short and intermediate-term impact of bariatric surgery on human energetics (Table 1). We showed substantial variance in fat loss in our subjects as others have reported. In the first six months after surgery, RMR and TDEE fell precipitously by 17% and 18%, respectively. The reductions in RMR and TDEE at 6 and 24 Months were due to roughly equal reductions in metabolic body size as defined by FFM and metabolic adaptation during the period of rapid weight loss, which is similar to results reported by others (27). Eighteen months later (two years post-operatively), this metabolic adaption to short-term term weight loss had started to dissipate as both RMR and TDEE where no longer significantly less than predicted based on FFM at 24 months. Due to participant dropout between 6 and 24 months, however, the power of our study was reduced.
After bariatric surgery, it appears that the energetic changes in the short term may not be sustained for even two years. For example, absolute RMR falls precipitously and then returns upwards by two years. The idea of short-term energetic adaptation giving way to a longer-term change is supported by the RMR data adjusted for FFM, where short-term decrease in RMR reverses by two years. These changes are mirrored by the changes in TDEE explaining why TDEE/RMR is constant. It would be interesting to follow patients even longer, but we speculate it is most likely that the trend of metabolic adaptation reverting towards zero would not change. This is consistent with the results from human energy balance studies in which energy expenditure is reduced for body composition during periods of rapid weight loss induced by negative energy balance, but either not significantly reduced or only slightly reduced after weight regain (28). It should be noted that our subjects were nearly in energy balance at baseline and 24 months, but energy balance as evidenced by weight stability at 24 months. The insignificant metabolic adaptation we observed at 24 months contrasts with the report from the Biggest Loser where the metabolic adaptation was sustained for 6 years although others suggest that this was an overstatement (29, 30) and provides further evidence that weight loss induced metabolic adaptation is not permanent (37). The difference may reflect the different mechanisms of weight loss – i.e. intestinal surgery with its concurrent altered satiety feedback signaling (1), microbiome (31) or other changes versus the effect of extreme diet and exercise (32, 33).
There are several studies with which to compare our data (27, 34–36). Su et al followed 11 women who underwent ileogastrostomy for 6–8 weeks after surgery. Over this short period of time, RMR was not affected although TDEE significantly predicted weight loss (34). Das et al (27) studied 30 woman before and 14 months after bariatric surgery using DLW before and after gastric bypass. Das reported that TDEE decreased following gastric bypass in proportion to the reduction in LBM. However, these studies could not track both the short-term (six month) and longer-term effects of weight loss because the protocol included only one post-surgical time point. It should be noted that in our study, the TDEE and BMR values were smaller than of Das et al (27), despite similar body size, age and sex distributions. It is not known if this difference contributed to the unusual negative coefficient for fat mass at baseline and thus our finding that that use of TDEE prediction equation using both fat mass and fat free mass suggested a greater metabolic adaptation at 6 months than just fat mass alone. Because the sample sizes were small in both studies, we advise caution in trying to interpret the coefficients in such prediction equations.
Our studies contrast those performed in gastric bypass rats, which show increases in TDEE and RMR, along with a 17% decrease in ad libitum food intake (37). The difference appears to be a species effect. The rat studies expressed energy expenditure as mL of O2 consumed per h per kg body weight raised to the 0.75 power. Our findings were based on differences after linear adjustment for FFM. We did not use the ratio of expenditure per unit of FFM because the TDEE regression line did not pass through the origin, a requirement of expressing results as a ratio.
There are limitations to our study that we acknowledge. Our subjects went from a mean BMI of 48 kg/m2 to a BMI of 32 kg/m2. Even two years post-operatively, our subjects met criteria for obesity. If people transition to a healthy BMI, their energetic parameters might undergo further change. The studies we conducted were not large and so may have been underpowered to detect small changes. That said, the studies are arduous to conduct and represent the best available existing data to examine our stated hypothesis. A third important limitation was that most of our volunteers were women. It is impossible to say from these studies whether men would have responded differently; we respect that the impact of sex is unanswered in this work although it was not one of our hypotheses. Lastly, we lost a third of our subjects to follow-up between months 6 and 24 (as might be expected). However, there is no evidence that this biased the results as both cohorts at six months showed similar energetic changes and weight loss. A further question that arises that we failed to address, is whether the weight loss and adaptive changes we report result specifically because of bariatric surgery or because of weight loss alone. Several studies (38–41) have documented how energy expenditure decreases with non-surgical weight loss and that during weight regain adaptive metabolic reductions rapidly reverses even though all of the lost weight is not regained (42). Thus, the lack of metabolic adaptation we demonstrated at 24 months may be from physiological reversal of adaptive reductions in metabolism. We cannot be certain whether the changes we document are because of bariatric surgery and/or weight loss because we did not include a weight-matched nonsurgical weight loss group, because of the difficulty in achieving such large changes in weight through non-surgical clinical methods. This is important because mechanisms specific to surgery cannot, from our data, be separated out from those of physiological weight loss (43, 44).
In conclusion, RMR and TDEE fall precipitously in the first six months after surgery, even when data are adjusted for FFM. The changes can be explained by a combination of reductions in FFM and a metabolic adaptation. However, the metabolic adaptation, abated sometime between six and 24 months resulting in a partial absolute upward shift in energy expenditure between six and 24 months after surgery. If the underlying thermogenic mechanism were understood, interventions could be designed to prevent this.
Study importance questions.
What is already known about this subject?
Bariatric surgery is an effective method for weight loss. The mechanism by which weight loss is achieved is little understood.
There are adaptive changes in energy expenditure associated with substantial weight loss although the longevity and time-course of these changes are unclear.
What does this study add?
Resting metabolic rate (measured under basal conditions) and total daily energy expenditure fall precipitously in the first six months after bariatric surgery, even when data are adjusted for fat free mass.
However these changes, which can be explained by a combination of reductions in fat free mass and a metabolic adaptation, are not sustained even after two years.
Acknowledgments
We thank the volunteers and the staff of the Oregon Health & Science University Clinical Research Center.
Funding: This work was supported by a grant from the National Institutes of Health, USA (U01-DK66555 Oregon Health & Science University and DK 72479 Mayo Clinic).
Footnotes
Author Contributions: BMW, DAS, and JAL conceived the study. CES carried out the clinical study. DAS, DT, MP, SKMS, CES and JAL analyzed data. JAL wrote the first draft of the manuscript and all the authors were then involved in writing the paper and approved the submitted version.
JAL is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
No potential conflicts of interest relevant to this article were reported.
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50. doi: 10.1097/00000658-199509000-00011. discussion 50-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston EH, Huerta S, Arthur D, Lee S, De Shields S, Heber D. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002;236(5):576–82. doi: 10.1097/00000658-200211000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald KG, Jr, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, et al. The Gastric Bypass Operation Reduces the Progression and Mortality of Non-Insulin-Dependent Diabetes Mellitus. J Gastrointest Surg. 1997;1(3):213–20. doi: 10.1016/s1091-255x(97)80112-6. [DOI] [PubMed] [Google Scholar]
- 5.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240(3):416–23. doi: 10.1097/01.sla.0000137343.63376.19. discussion 23-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugerman HJ, Londrey GL, Kellum JM, Wolf L, Liszka T, Engle KM, et al. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. American journal of surgery. 1989;157(1):93–102. doi: 10.1016/0002-9610(89)90427-3. [DOI] [PubMed] [Google Scholar]
- 7.Courcoulas AP, Christian NJ, O’Rourke RW, Dakin G, Patchen Dellinger E, Flum DR, et al. Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surg Obes Relat Dis. 2015 doi: 10.1016/j.soard.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfe BM, Belle SH. Long-term risks and benefits of bariatric surgery: a research challenge. Jama. 2014;312(17):1792–3. doi: 10.1001/jama.2014.12966. [DOI] [PubMed] [Google Scholar]
- 9.Gourash WF, Ebel F, Lancaster K, Adeniji A, Koozer Iacono L, Eagleton JK, et al. Longitudinal Assessment of Bariatric Surgery (LABS): retention strategy and results at 24 months. Surg Obes Relat Dis. 2013;9(4):514–9. doi: 10.1016/j.soard.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorsen T, Shriver T, Racine N, Richman BA, Schoeller DA. Doubly labeled water analysis using cavity ring-down spectroscopy. Rapid communications in mass spectrometry : RCM. 2011;25(1):3–8. doi: 10.1002/rcm.4795. [DOI] [PubMed] [Google Scholar]
- 11.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250(5 Pt 2):R823–30. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 12.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267(4 Pt 1):E585–90. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- 13.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307(5709):584–6. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 14.Thomas DM, Westerterp K. Energy balance, energy turnover, and risk of body fat gain. The American journal of clinical nutrition. 2017;105(2):540–1. doi: 10.3945/ajcn.116.141887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes. N Engl J Med. 2014;371(7):682. doi: 10.1056/NEJMc1407393. [DOI] [PubMed] [Google Scholar]
- 16.Burguera B, Agusti A, Arner P, Baltasar A, Barbe F, Barcelo A, et al. Critical assessment of the current guidelines for the management and treatment of morbidly obese patients. J Endocrinol Invest. 2007;30(10):844–52. doi: 10.1007/BF03349226. [DOI] [PubMed] [Google Scholar]
- 17.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ (Clinical research ed) 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid RE, Carver TE, Andersen KM, Court O, Andersen RE. Physical activity and sedentary behavior in bariatric patients long-term post-surgery. Obesity surgery. 2015;25(6):1073–7. doi: 10.1007/s11695-015-1624-8. [DOI] [PubMed] [Google Scholar]
- 19.Berglind D, Willmer M, Eriksson U, Thorell A, Sundbom M, Udden J, et al. Longitudinal assessment of physical activity in women undergoing Roux-en-Y gastric bypass. Obesity surgery. 2015;25(1):119–25. doi: 10.1007/s11695-014-1331-x. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez-Marrero FA, Edens KL, Joyner MJ, Curry TB. Predicted vs. Actual Resting Energy Expenditure and Activity Coefficients: Post-Gastric Bypass, Lean and Obese Women. Obes Control Ther. 2014;1(2):1–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Bragge T, Lyytinen T, Hakkarainen M, Vartiainen P, Liikavainio T, Karjalainen PA, et al. Lower impulsive loadings following intensive weight loss after bariatric surgery in level and stair walking: a preliminary study. Knee. 2014;21(2):534–40. doi: 10.1016/j.knee.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Marrero FA, Miles J, Joyner MJ, Curry TB. Self-reported and objective physical activity in postgastric bypass surgery, obese and lean adults: association with body composition and cardiorespiratory fitness. Journal of physical activity & health. 2014;11(1):145–51. doi: 10.1123/jpah.2012-0048. [DOI] [PubMed] [Google Scholar]
- 23.Unick JL, Bond DS, Jakicic JM, Vithiananthan S, Ryder BA, Roye GD, et al. Comparison of two objective monitors for assessing physical activity and sedentary behaviors in bariatric surgery patients. Obesity surgery. 2012;22(3):347–52. doi: 10.1007/s11695-011-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bond DS, Jakicic JM, Unick JL, Vithiananthan S, Pohl D, Roye GD, et al. Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity (Silver Spring, Md) 2010;18(12):2395–7. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King WC, Belle SH, Eid GM, Dakin GF, Inabnet WB, Mitchell JE, et al. Physical activity levels of patients undergoing bariatric surgery in the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Relat Dis. 2008;4(6):721–8. doi: 10.1016/j.soard.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. doi: 10.3945/jn.115.219634. (1541-6100 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. The American journal of clinical nutrition. 2003;78(1):22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 28.Saltzman E, Roberts SB. The role of energy expenditure in energy regulation: findings from a decade of research. Nutr Rev. 1995;53(8):209–20. doi: 10.1111/j.1753-4887.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 29.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring, Md) 2016;24(8):1612–9. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuchnia A, Huizenga R, Frankenfield D, Matthie JR, Earthman CP. Overstated metabolic adaptation after “the biggest loser” intervention. Obesity (Silver Spring, Md) 2016;24(10):2025. doi: 10.1002/oby.21638. [DOI] [PubMed] [Google Scholar]
- 31.Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: a Systematic Review and Meta-analysis. Obesity surgery. 2017;27(5):1345–57. doi: 10.1007/s11695-017-2595-8. [DOI] [PubMed] [Google Scholar]
- 32.Matarese LE, Pories WJ. Adult weight loss diets: metabolic effects and outcomes. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2014;29(6):759–67. doi: 10.1177/0884533614550251. [DOI] [PubMed] [Google Scholar]
- 33.Seimon RV, Roekenes JA, Zibellini J, Zhu B, Gibson AA, Hills AP, et al. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol. 2015;418(Pt 2):153–72. doi: 10.1016/j.mce.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Su W, Jones PJ, Cleator IG, Phang PT, Birmingham CL. Determinants of weight loss following ileogastrostomy. Int J Obes Relat Metab Disord. 1996;20(5):481–7. [PubMed] [Google Scholar]
- 35.van Gemert WG, Westerterp KR, van Acker BA, Wagenmakers AJ, Halliday D, Greve JM, et al. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int J Obes Relat Metab Disord. 2000;24(6):711–8. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- 36.Westerterp KR, Saris WH, Soeters PB, ten Hoor F. Determinants of weight loss after vertical banded gastroplasty. Int J Obes. 1991;15(8):529–34. [PubMed] [Google Scholar]
- 37.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring, Md) 2009;17(10):1839–47. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 39.Sims EA, Horton ES. Endocrine and metabolic adaptation to obesity and starvation. The American journal of clinical nutrition. 1968;21(12):1455–70. doi: 10.1093/ajcn/21.12.1455. [DOI] [PubMed] [Google Scholar]
- 40.Astrup A, Gotzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, et al. Meta-analysis of resting metabolic rate in formerly obese subjects. The American journal of clinical nutrition. 1999;69(6):1117–22. doi: 10.1093/ajcn/69.6.1117. [DOI] [PubMed] [Google Scholar]
- 41.Toubro S, Sorensen TI, Ronn B, Christensen NJ, Astrup A. Twenty-four-hour energy expenditure: the role of body composition, thyroid status, sympathetic activity, and family membership. Journal of Clinical Endocrinology & Metabolism. 1996;81(7):2670–4. doi: 10.1210/jcem.81.7.8675595. [DOI] [PubMed] [Google Scholar]
- 42.Keys A, Brozek J, Henschel A, Mickelson O, Taylor HL. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 43.Ross R, Pedwell H, Rissanen J. Response of total and regional lean tissue and skeletal muscle to a program of energy restriction and resistance exercise. Int J Obes Relat Metab Disord. 1995;19(11):781–7. [PubMed] [Google Scholar]
- 44.Prentice AM, Goldberg GR, Jebb SA, Black AE, Murgatroyd PR, Diaz EO. Physiological responses to slimming. The Proceedings of the Nutrition Society. 1991;50(2):441–58. doi: 10.1079/pns19910055. [DOI] [PubMed] [Google Scholar]


