Abstract
Background
In our companion paper, we reported that the phosphodiesterase type 4 inhibitor apremilast reduced ethanol intake and preference in different drinking models in male and female C57BL/6J mice. In the current study, we measured the effects of apremilast on other behaviors that are correlated with ethanol consumption.
Methods
The effects of apremilast (20 mg/kg) on the following behaviors were studied in male and female C57BL/6J mice: locomotor response to a novel situation; ethanol- or LiCl-induced conditioned taste aversion (CTA) to saccharin; conditioned place preference (CPP) and conditioned place avoidance (CPA) to ethanol; severity of handling-induced convulsions after ethanol administration; ethanol-induced anxiolytic-like behavior in the elevated plus maze; duration of ethanol-induced loss of the righting reflex (LORR); recovery from ethanol-induced motor impairment on the rotarod; and acute functional tolerance (AFT) to ethanol.
Results
Apremilast did not change the acquisition of ethanol-induced CPP, severity of acute withdrawal from ethanol, or ethanol's anxiolytic-like effect. Apremilast did not alter the extinction of ethanol- or LiCl-induced CTA, but may interfere with acquisition of CTA to ethanol. Apremilast increased the acquisition of CPA to ethanol, reduced locomotor responses to a novel situation, and prolonged the duration of LORR and the recovery from acute motor incoordination induced by ethanol. Longer recovery from the ataxic effect of ethanol may be attributed to the reduced development of AFT to ethanol after apremilast pretreatment.
Conclusions
Our results suggest that apremilast increases the duration of ethanol intoxication by reducing AFT. Apremilast also reduces some aspects of general reward and increases ethanol's aversive properties, which might further contribute to its ability to reduce ethanol drinking.
Keywords: PDE4 inhibitor, loss of righting reflex, acute ethanol withdrawal, acute functional tolerance, C57BL/6J mice
Introduction
Phosphodiesterase type 4 (PDE4) inhibitors decrease ethanol seeking and consumption in rodent models (Blednov et al., 2014, Hu et al., 2011, Wen et al., 2012) and have been studied as potential treatments for neuroinflammatory diseases. However, clinical use of these drugs is limited by their gastrointestinal side effects. In our companion manuscript, we showed that apremilast, a PDE4 inhibitor with lower pro-emetic activity, produced stable decreases in ethanol intake in male and female C57BL/6J mice in different drinking tests (Blednov et al.). Ethanol drinking may be positively or negatively correlated with other behaviors (Blednov et al., 2012, Green and Grahame, 2008), and it is important to study the relevant behaviors to understand how apremilast decreases drinking and to further evaluate its potential as a repurposed drug for treating alcohol use disorder (AUD).
There is a strong positive correlation between consumption of ethanol and sweet solutions (Bachmanov et al., 1996, Blednov et al., 2008, Yoneyama et al., 2008). Ethanol drinking is also positively correlated with response to novelty (Blednov et al., 2012) and the rewarding properties of ethanol, as measured in the conditioned place preference (CPP) test (Green and Grahame, 2008). Conditioned taste aversion (CTA) (Blednov et al., 2012, Green and Grahame, 2008, Risinger and Cunningham, 1998) and acute ethanol withdrawal severity are used as indices of the aversive effects of ethanol and are negatively correlated with voluntary ethanol consumption (Belknap et al., 2008, Hitzemann et al., 2009, Metten et al., 1998). Duration of the loss of righting reflex (LORR) measures ethanol's hypnotic or sedative effects and is negatively correlated with the severity of acute ethanol withdrawal in mice (Blednov et al., 2012). Increased sensitivity to sedation, ataxia, or other adverse effects would be expected to decrease the amount of ethanol consumed, although this is not always observed in rodent models.
In humans, AUD is also associated with negative reinforcing states. For example, individuals may drink alcohol to reduce anxiety or symptoms related to alcohol withdrawal. A large international study found a strong association between anxiety and alcohol and other substance use disorders, and the onset of anxiety disorders typically preceded that of drug abuse (Merikangas et al., 1998), suggesting that alcohol's anxiolytic effects may be important in the initiation of drinking. Furthermore, a low level of response to alcohol was shown to be a predictor of future alcohol-related problems (Schuckit, 1994). In addition to a subject's innate or initial sensitivity to ethanol, a major determinant of the level of response to ethanol is the ability to develop acute functional tolerance (AFT) during a single ethanol exposure. AFT is characterized by greater intoxication during the ascending phase of the blood ethanol concentration curve than at equivalent concentrations on the descending phase. AFT has a genetic component and occurs in humans as well as in animal models (Erwin and Deitrich, 1996, Bennett et al., 1993, Radcliffe et al., 2006). Decreased acute sensitivity to ethanol may be an important risk factor for the development of AUD.
Considering the ethanol-related behaviors discussed above and their contribution to drinking, it is relevant to examine the effects of potential drugs to treat AUD on these behaviors. In our companion article, we showed that apremilast reduced preference for ethanol but did not change preference for sweet solutions (Blednov et al., 2018). In this study, we measured the effects of apremilast pretreatment on other relevant behaviors in both male and female mice. Apremiliast increased the acute hypnotic and intoxicating effects of ethanol and reduced AFT. An increase in the aversive properties of ethanol could contribute to the ability of apremilast to reduce drinking.
Materials and Methods
Mice
Male and female C57BL/6J mice were from a colony maintained in the Animal Resources Center at The University of Texas at Austin (UT Austin). Original breeders were purchased and replenished every 6 months from The Jackson Laboratory (Bar Harbor, ME). Mice were group-housed (4 or 5 per cage) in temperature- and humidity-controlled rooms with free access to food and water using a 12-h light/dark cycle (lights on at 7:00 a.m.). Experiments began when the mice were 2-3 months old. Mice were allowed to adapt to the testing rooms for about one week before behavioral testing. Separate groups of mice were used for the different behavioral tests in this study and that of our companion paper (Blednov et al., 2018), with the exception of the locomotor activity tests in which the same mice were used but different time periods were analyzed in the two studies. Experiments were approved by the Institutional Animal Care and Use Committee at UT Austin.
Drug Administration
Apremilast was purchased from Toronto Research Chemicals Inc. (Toronto, ON, Canada). It was freshly prepared as a suspension in saline with 3-4 drops of Tween-80 and administered daily in a volume of 0.05 ml/10 g of body weight 1h or 3h before experiments. Control mice received the same volume of saline containing 3-4 drops of Tween-80. Apremilast (20 mg/kg, p.o.) was given once daily for up to 6 days. This dose was chosen based on its ability to significantly reduce ethanol drinking in both the continuous and intermittent two-bottle choice tests without reducing total fluid intake (Blednov et al., 2018). Ethanol (Sigma-Aldrich, St. Louis, MO) was freshly prepared in saline and injected i.p. in a volume of 0.1 ml/10 g of body weight. Control mice received the same injection volume of saline.
Conditioned Taste Aversion
A negative correlation was observed between CTA and ethanol drinking, suggesting that ethanol's aversive properties may limit voluntary ethanol consumption (Risinger and Cunningham, 1998). In CTA procedures, an association is established between an environmental, conditioned stimulus (e.g., sweet solution) and the unconditioned stimulus (e.g., ethanol). Mice were adapted to a water-restricted schedule of 2h per day for 7 days. Saline or apremilast was given daily 3h before experimental or control (during intermittent days with limited access to water) sessions. At 48-h intervals on days 1, 3, 5, 7, 9, and 11, mice received 1-h access to saccharin (Sigma-Aldrich; 0.15% w/v sodium saccharin in tap water). For the first 4 days, mice received saline injections, and saccharin intake on day 3 (trial 0 in Figs. 1 and 2) was used as the control measure (100%). Beginning on day 5 (trial 2 in Figs. 1 and 2), half of the mice received daily injections of saline and half received daily injections of apremilast. Immediately after 1-h access to saccharin on day 5, mice were injected with saline, ethanol (2.5 g/kg), or lithium chloride (LiCl, 10 mEq/kg; Sigma-Aldrich). Mice had 30-min access to tap water 5h after each saccharin-access period to prevent dehydration. On intervening days, mice had 2-h access to water at standard times in the morning. Reduced consumption of saccharin was used to measure CTA. In a separate experiment, apremilast was used as the unconditioned stimulus and was injected instead of ethanol, after the saccharin sessions.
Conditioned Place Preference
The CPP protocol was carried out as described previously (Blednov et al., 2003). Six acrylic boxes were individually enclosed in light- and sound-attenuating, ventilated chambers (Med Associates, St. Albans, VT). Each box consisted of two compartments containing a different floor type (either bars set in a grid or small round holes). Infrared light sources and photodetectors were used to measure general activity and location of the mouse in the left or right compartment. Total activity counts and location were computer recorded. Saline or apremilast was given daily 3h before the experimental session. Before training, naïve mice were first habituated to chambers with covered floors; the next day mice received saline i.p. and were given 30-min access to both chambers. The next day, half of the mice were designated for pretreatment with saline and the other half for pretreatment with apremilast. Half of the mice from each group were given ethanol (2 g/kg, i.p.) and placed in a conditioning chamber containing a bar floor for 5 min, and the other half were placed in a chamber containing a floor with round holes. The next day, mice were given an equivalent volume of saline i.p. and placed in the opposite chamber for 5 min. This pattern was repeated for 8 days (four saline- and four ethanol-conditioning sessions). The other half of the mice received saline on conditioning days 1, 3, 5, and 7 and ethanol (2 g/kg, i.p.) on conditioning days 2, 4, 6, and 8. Twenty-four hours after the last conditioning session, mice were given saline i.p. and then had access to both chambers for 30 min. The time spent in the ethanol-paired side during the habituation session was subtracted from the time spent in that side during the test session to determine a CPP score, which was compared with a theoretical mean of 0 (no CPP) and then compared between treatment groups. We also compared the time spent on the bar floor when it was paired with ethanol [Bar+] and when it was paired with saline [Bar-].
Conditioned Place Avoidance
The conditioned place avoidance (CPA) test was performed as described (Cunningham et al., 1998). Saline or apremilast was given daily 3h before the experimental session. The protocol was identical to the CPP test, except ethanol (2 g/kg, i.p.) was given immediately after the conditioned stimulus. Half of the mice from each group (pretreated with saline or apremilast) received ethanol after sessions on the floor with round holes, while the other half received ethanol after sessions on the bar floor. On alternative days, mice received injections of saline after placement on the opposite type of floor.
Acute Withdrawal from Ethanol
Mice were scored for handling-induced convulsion (HIC) severity 30 min before and immediately before ethanol injection, and these pre-drug baseline scores were averaged. After injecting a single dose of ethanol in saline (4 g/kg, i.p.), the HIC score was measured every hour until the HIC level reached baseline. Acute withdrawal was measured as the area under the curve, above the pre-drug level (Crabbe et al., 1991). To evoke HIC, an investigator picked up the mouse by the tail and, if necessary, gently rotated it 180°. The HIC was scored as follows: 5, tonic-clonic convulsion when lifted; 4, tonic convulsion when lifted; 3, tonic-clonic convulsion after a gentle spin; 2, no convulsion when lifted, but tonic convulsion elicited by a gentle spin; 1, facial grimace only after a gentle spin; 0, no convulsion or grimace when spun. Saline or apremilast (20 mg/kg) was given 1h before ethanol injection.
Elevated Plus Maze
The elevated plus maze was used to measure basal anxiety-like behavior and the effect of apremilast on ethanol-mediated anxiolytic-like behavior (Blednov et al., 2001). Mice were taken to the testing room one day before the experiment and were tested between 10:00 and 12:00 a.m. under ambient room light. Mice were weighed and injected with saline or ethanol (1.25 g/kg, i.p.) 5 min before the test. Each mouse was placed on the central platform of the maze facing an open arm and allowed to freely explore for 5 min, during which time the following measurements were recorded manually: number of open arm entries, number of closed arm entries, total number of entries, time spent in open arms, and time spent in closed arms. The subject was considered to be on the central platform or any arm when all four paws were within its perimeter. Pretreatment with saline or apremilast (20 mg/kg) was carried out 1h before saline or ethanol injections.
Locomotor Response to Novelty
The Opto-microvarimex animal activity meter (Columbus Instruments, Columbus, OH) was used to measure locomotor activity in standard cages covered by a plastic lid with holes for ventilation. On the day of the experiment, mice were placed in individual cages, and activity was monitored every 10 min for 22h. The first 80 min represents the drug effect on response to a novel situation (novelty). Saline or apremilast (20 mg/kg) was administered 1h before monitoring of motor activity. For chronic drug treatment, apremilast (20 mg/kg) was given daily for 6 days, and locomotor activity was measured on day 6, 1h after the last drug injection.
Loss of Righting Reflex
Sensitivity to the sedative effects of ethanol (3.6 g/kg, i.p.) was measured by the duration of LORR (Marley et al., 1986). When mice became ataxic, they were placed in the supine position in V-shaped plastic troughs until they were able to right themselves three times within 30s. Sleep time was defined as the time from being placed in the supine position until the righting reflex was regained. Saline or apremilast (20 mg/kg) was either injected once 1h before ethanol or daily for 6 days for chronic treatment.
Recovery from Ethanol-Induced Motor Incoordination
Ethanol impairs rotarod performance at doses above 1 g/kg (Johnston et al., 1986, Stromberg, 1988). Mice were trained on a fixed-speed rotarod (Economex; Columbus Instruments, Columbus, OH) at 10 rpm until they were able to remain on the rotarod for 60s. Every 15 min after ethanol injection (2 g/kg, i.p.), each mouse was placed on the rotarod and latency to fall was measured until the mouse was able to remain on the rotarod for 60s. Saline or apremilast (20 mg/kg) was either injected once 1h before ethanol or daily for 6 days for chronic treatment.
Acute Functional Tolerance
AFT to the ataxic effects of ethanol was determined using the two-dose procedure (Erwin and Deitrich, 1996). Ethanol-naïve mice were trained to balance on the rotarod (10 rpm) for a 60-s period. After training, half of the mice received saline and the other half received apremilast injections. One hour later, mice were given ethanol (1.75 g/kg, i.p.) and placed on the rotarod until they fell off. They were tested in 5-min intervals until they could balance on the rotarod for 60s. At this time (t1), a retro-orbital blood sample was collected to measure blood ethanol concentration (BEC1) as described below. Mice then received a second ethanol injection (2 g/kg, i.p.) and were tested in 5-min intervals until they regained the ability to balance on the rotarod for 60s (t2). Then a second blood sample was collected for BEC determination (BEC2). AFT was defined as the difference in BEC at t2 versus t1 (BEC2 - BEC1).
Blood Ethanol Concentration
Retro-orbital blood samples (∼20 μl) were collected in capillary tubes and centrifuged for 6 min at 3,100g using a Haematospin 1400 centrifuge (Analox Instruments, London, UK). Plasma samples were stored at −20°C until BECs were determined in 5-μl aliquots using an AM1 Alcohol Analyzer (Analox Instruments). The machine was calibrated every 15 samples using an industry standard, and BECs were determined using commercially available reagents according to the manufacturer's instructions. Samples were averaged from duplicate runs and expressed as mg/dl.
Statistical Analysis
Data are reported as mean ± S.E.M. values. Prism (GraphPad Software, Inc., La Jolla, CA) was used to perform one-way or two-way repeated measures ANOVA, Bonferroni post-hoc tests, and Student's t-tests.
Results
Conditioned Taste Aversion
Intake was calculated as a percentage of trial 0 consumption for each mouse by dividing the amount of saccharin solution consumed in subsequent conditioning trials by the amount consumed in trial 0 (before conditioning). Compared with saline pretreatment (Fig. 1A, trial 2), apremilast (20 mg/kg) pretreatment (Fig. 1B, trial 2) reduced saccharin (0.15% w/v) consumption (p < 0.001, Student's t-test). In saline-pretreated mice, ethanol (2.5 g/kg)-saccharin pairings significantly reduced saccharin consumption across trials (3-6), indicating development of CTA (F1,74 = 44.2, p < 0.0001, effect of pairing; F3,222 = 20.7, p < 0.0001, effect of trial; F3,222 = 9.1, p < 0.0001, pairing × trial interaction; Fig. 1A). In contrast, in apremilast-treated mice, ethanol-saccharin pairings did not reduce saccharin intake compared with the saline-paired group, suggesting lack of development of CTA (F1,73 = 0.11, p > 0.05, effect of pairing; F3,219 = 6.4, p < 0.001, effect of trial; Fig. 1B). Comparison of control mice (saline-saline, Fig. 1A) with apremilast-treated mice (saline-apremilast, Fig. 1B) showed significant reduction of saccharin intake in the apremilast-pretreated group (F1,71 = 30, p < 0.001, effect of pretreatment). During extinction of ethanol-induced CTA, both saline- (F1,39 = 33.3, p < 0.0001, effect of pairing; F7,273 = 14.4, p < 0.0001, effect of time; F7,273 = 11.1, p < 0.0001, pairing × time interaction; Fig. 1C) and apremilast-pretreated (F1,39 = 5.4, p < 0.05, effect of pairing; F7,273 = 16, p < 0.0001, effect of time; F7,273 = 6.8, p < 0.0001, pairing × time interaction; Fig. 1D) mice gradually increased preference for saccharin. Although apremilast-pretreated mice appeared to show faster extinction compared with the saline-paired group, this may be explained by their higher preference for saccharin from the beginning of the extinction period compared with saline-paired mice. We also note that the slopes of the extinction curves were similar for both groups, suggesting that apremilast did not affect extinction of CTA. Because apremilast-pretreated mice demonstrated extinction, we suggest that these mice also developed CTA to ethanol (albeit weaker than the saline-paired group), but this effect was masked by decreased saccharin consumption in the apremilast-saline group. There were no sex-dependent differences in these and the following CTA experiments, and data from male and female mice were combined.
Figure 1. Apremilast does not alter the extinction of conditioned taste aversion developed in response to ethanol.
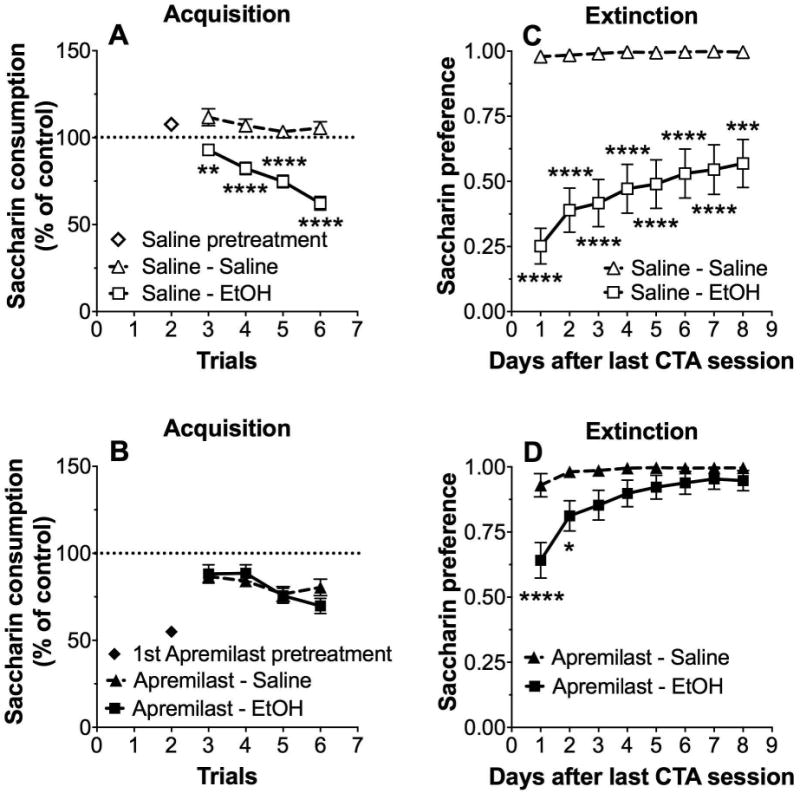
Changes in saccharin (0.15% w/v) consumption produced by injection of saline or ethanol (EtOH, 2 g/kg) expressed as a percent of the control trial (trial 0) in (A) saline-pretreated and (B) apremilast (20 mg/kg)-pretreated male and female mice (n= 35-40 per group). Preference for saccharin measured during extinction in (C) saline-pretreated and (D) apremilast-pretreated male and female mice (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 compared with saline control by Bonferroni post-hoc tests; n= 19-22 per group).
We then tested the effects of LiCl (10 mEq/kg) on saccharin consumption. Similar to the results shown in Fig. 1, compared with saline, pretreatment with apremilast (20 mg/kg) reduced saccharin consumption (p < 0.001, Student's t-test; Fig. 2A vs. 2B, trial 2). In saline-treated mice, LiCl-saccharin pairings significantly reduced saccharin consumption across trials (3-6), indicating development of CTA (F1,46 = 43.4, p < 0.0001, effect of pairing; F3,138 = 31.4, p < 0.0001, effect of trial; F3,138 = 23.7, p < 0.0001, pairing × trial interaction; Fig. 2A). However in apremilast-treated mice, LiCl-saccharin pairings did not significantly reduce saccharin intake compared with saline-saccharin pairings, indicating lack of development of CTA (F1,44 = 3.19, p > 0.05, effect of pairing; F3,132 = 23.4, p < 0.0001, effect of trial; F3,132 = 13.27, p < 0.0001, pairing × trial interaction; Fig. 2B). Comparison of control mice (saline-saline; Fig. 2A) with apremilast-treated mice (saline-apremilast; Fig. 2B) showed significant reduction of saccharin intake in the apremilast-pretreated group (F1,54 = 15.6, p < 0.001, effect of pretreatment). However, both saline- and apremilast-treated mice demonstrated strong initial aversion to saccharin during the extinction phase of LiCl-induced CTA, which was followed by a gradual increase in saccharin consumption over subsequent extinction sessions (F1,46 = 347; p < 0.0001, effect of pairing; F7,322 = 6.4, p < 0.0001, effect of time; F7,322 = 2.6, p < 0.05, pairing × time interaction for saline-pretreated groups; Fig. 2C; and F1,43 = 292, p < 0.0001, effect of pairing; F7,301 = 12.3, p < 0.0001, effect of time; F7,301 = 7.4, p < 0.0001, pairing × time interaction for apremilast- pretreated groups; Fig. 2D).
Figure 2. Apremilast does not change the extinction of conditioned taste aversion developed in response to LiCl.
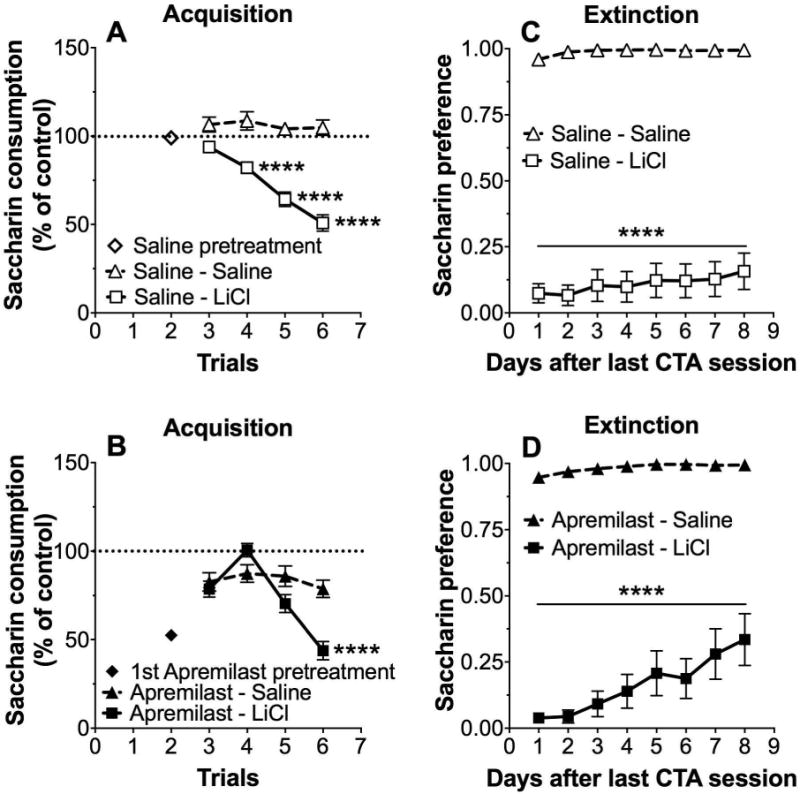
Changes in saccharin (0.15% w/v) consumption produced by injection of saline or LiCl (10 mEq/kg) expressed as a percent of the control trial (trial 0) in (A) saline-pretreated and (B) apremilast (20 mg/kg)-pretreated male and female mice (n= 18-28 per for saline and apremilast groups). Preference for saccharin during extinction in (C) saline-pretreated and (D) apremilast-pretreated male and female mice (****p < 0.0001 compared with saline control by Bonferroni post-hoc tests; n= 18-28 per group).
In the next experiment, we tested the ability of apremilast itself to serve as an unconditioned stimulus and induce CTA. Compared with saline, pairings with apremilast (20 mg/kg) reduced saccharin consumption across trials, indicating development of CTA (F1,22 = 17.3, p < 0.001, effect of treatment; Fig. 3A). Apremilast-induced CTA led to a prolonged reduction in the preference for saccharin during extinction (F1,22 = 39.8, p < 0.0001, effect of treatment; F7,154 = 6.2, p < 0.0001, effect of time; F7,154 = 5.2, p < 0.0001, treatment × time interaction; Fig. 3B).
Figure 3. Apremilast induces the development of conditioned taste aversion.
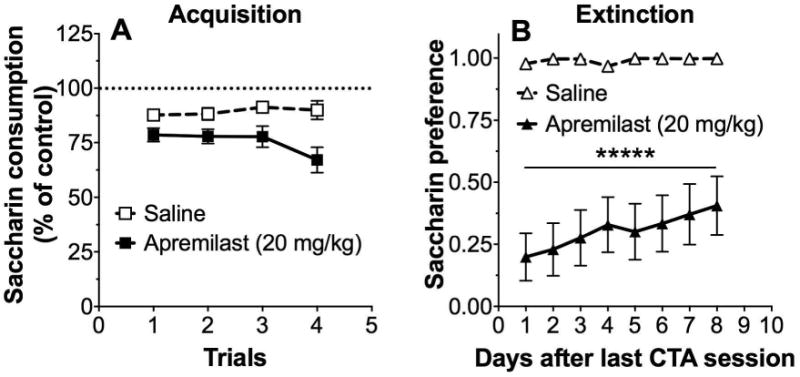
(A) Changes in saccharin (0.15% w/v) consumption produced by injection of saline or apremilast (20 mg/kg) expressed as a percent of the control trial (trial 0). (B) Preference for saccharin during extinction in saline-pretreated and apremilast-pretreated male and female mice (****p < 0.0001 compared with saline control by Bonferroni post-hoc tests; n= 12 per group).
Conditioned Place Preference
CPP is often used to examine the rewarding properties of ethanol and other drugs of abuse. Both saline- and apremilast (20 mg/kg)-pretreated groups showed CPP for ethanol as measured by the CPP score (p < 0.001; t-test for comparison of each group with a theoretical mean of 0; Fig. 4A). Both groups of mice spent more time on the bar floor when it was paired with ethanol (Bar+) than when it was paired with saline (Bar-) (F1,62 = 128, effect of pairing, p < 0.0001; Fig. 4B). There was no main effect of apremilast or interaction between apremilast and ethanol treatment on these measures. Locomotor activity during each 5-min ethanol [CS+] and saline [CS-] conditioning trial is shown in Fig. 4C and D. Ethanol increased locomotor activity in mice pretreated with saline (F1,64 = 21.6, p < 0.0001, effect of ethanol; F3,192 = 4.2, p < 0.01, effect of trial; F3,192 = 28.3, p < 0.0001, ethanol × trial interaction; Fig. 4C). In mice pretreated with apremilast (20 mg/kg), there was a trial-dependent effect on locomotor activity (F3,192 = 7.6, p < 0.001, effect of trial; F3,192 = 14.1, p < 0.001, ethanol × trial interaction), but no effect of ethanol (Fig. 4D). In both saline- and apremilast-pretreated mice, the [CS-] group showed a trial-dependent reduction in motor activity, as expected when animals habituate to the chamber (one-way ANOVA: F3,96 = 33.8, p < 0.0001, saline-treated mice, Fig. 4C; F3,96 = 29.3, p < 0.0001, apremilast-pretreated mice, Fig. 4D). Trial-dependent increases in motor activity were observed in the [CS+] saline-pretreated group, indicating locomotor sensitization to ethanol (one-way ANOVA: F3,96 = 9.3, p < 0.0001), but this increase did not occur in the [CS+] apremilast-pretreated group (one-way ANOVA: F3,96 = 2.6, p > 0.05). There were no sex-dependent differences, and data from male and female mice were combined.
Figure 4. Apremilast does not alter acquisition of conditioned place preference for ethanol (2 g/kg).
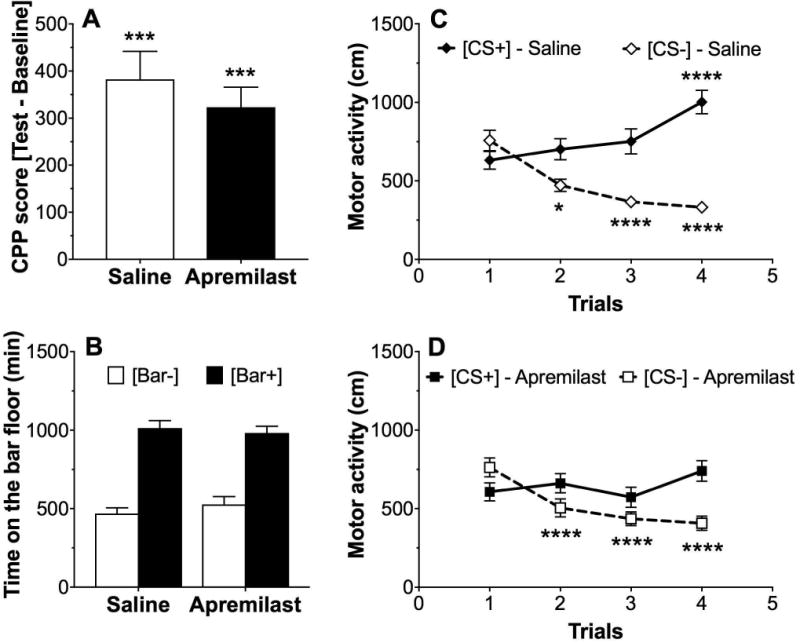
(A) CPP scores were significantly greater than 0 (theoretical mean) in both apremilast (20 mg/kg)-pretreated and saline-pretreated male and female mice (***p < 0.001 compared with no CPP; one-sample t-test), but were not different from each other. (B) Time spent on the bar floor when paired with ethanol [Bar+] was greater than time spent on the bar floor when paired with saline [Bar-] in both groups. (C, D) Locomotor activity when paired with saline [CS-] vs. ethanol [CS+] in (C) saline-pretreated and (D) apremilast (20 mg/kg)-pretreated male and female mice (*p < 0.05 and ****p < 0.0001compared with corresponding trial 1 by Bonferroni post-hoc tests; n= 16-17 per group).
Conditioned Place Avoidance
In the conditioned place avoidance (CPA) procedure, there was an effect of ethanol in mice pretreated with apremilast as measured by the CPA score (p < 0.05, t-test for comparison with theoretical mean of 0) but not in mice pretreated with saline (Fig. 5A). However, when we compared the time spent on the bar floor when it was paired with ethanol vs. saline, there were significant effects of ethanol pairing (F1,66 = 13.7, p < 0.001) and apremilast pretreatment (F1,66 = 7.5, p < 0.01), but there was no interaction between them (Fig. 5B). There were no sex-dependent differences, and data from male and female mice were combined.
Figure 5. Effect of apremilast on conditioned place avoidance to ethanol (2 g/kg).
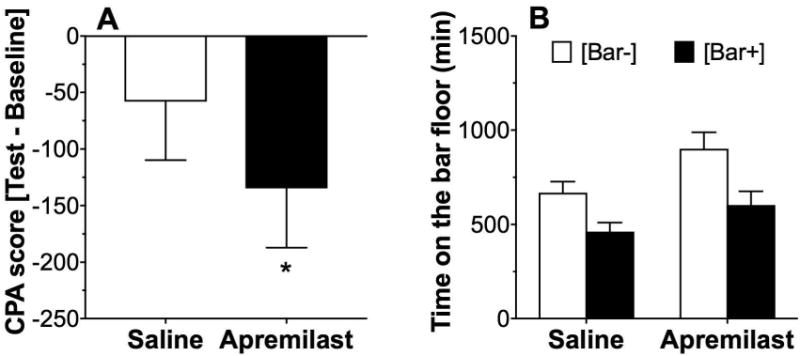
(A) The CPA score was significantly less than 0 (theoretical mean) only in the apremilast (20 mg/kg)-pretreated group (*p < 0.05 compared with no CPA by one-sample t-test), and the CPA score in the apremilast-pretreated group was lower compared with the score in the saline-pretreated group (p < 0.05, two-tailed t-test). (B) Time spent on the bar floor when paired with saline [Bar-] was greater than time spent on the bar floor when paired with ethanol [Bar+] after pretreatment with saline or apremilast (n= 17-18 per group).
Acute Ethanol Withdrawal
A single ethanol dose (4 g/kg) suppressed basal HIC in all mice for ∼5-7 h (Fig. 6A). Recovery of HIC to the basal level was similar for saline- and apremilast (20 mg/kg)-pretreated mice (Fig. 6A). No group differences were found for areas below or above the basal HIC level (Fig. 6B and C). There were no sex-dependent differences, and data from male and female mice were combined.
Figure 6. Apremilast does not alter acute withdrawal from ethanol.
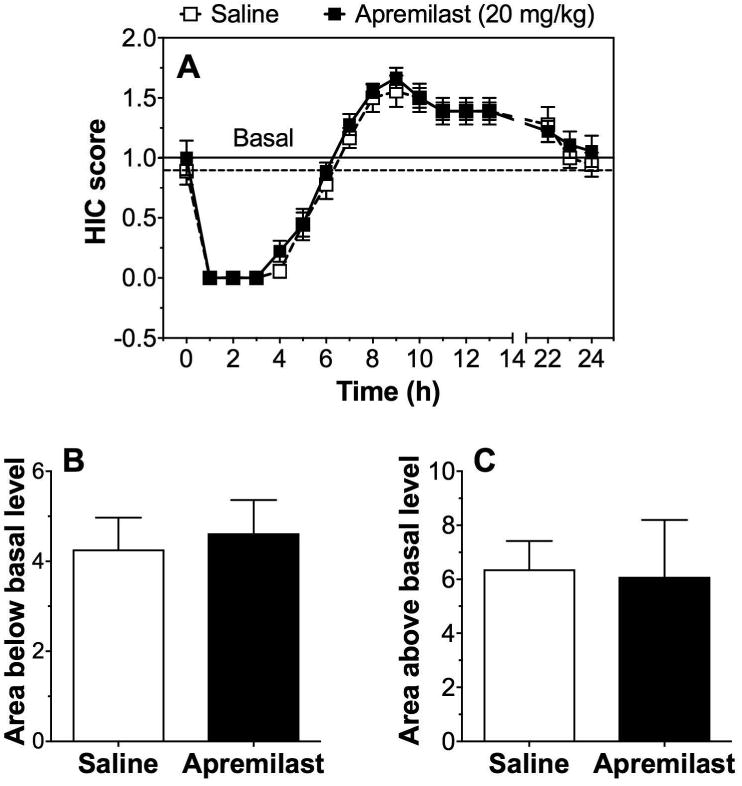
(A) Handling-induced convulsion (HIC) scores were measured every hour after injection of ethanol (4 g/kg) in male and female mice pretreated with saline or 20 mg/kg of apremilast. Horizontal lines represent the basal HIC level for control (dashed line) and apremilast-pretreated (solid line) groups. (B) Area under the curve (below basal) and (C) area above the curve (above basal; n= 9 per group).
Elevated Plus Maze
In the elevated plus maze, locomotor activity was measured by the number of entries into the closed arms, and anxiety-like behavior was measured by the percentage of time spent in the open arms after saline or ethanol. There were no differences between the sexes, and data from male and female mice were combined. Ethanol (1.25 g/kg, i.p.) increased the percentage of time spent in open arms (F1,60 = 34.2, p < 0.0001, effect of treatment; Fig. 7A) and the percentage of open arm entries (F1,60 = 47.3, p < 0.0001, effect of treatment; Fig. 7B) but did not alter the number of entries into the closed arms (Fig. 7C). There was no effect of apremilast (20 mg/kg) on these responses.
Figure 7. Apremilast does not alter open or closed arm entries in the elevated plus maze.
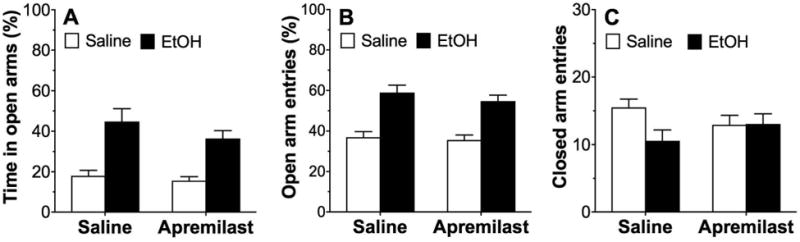
Ethanol (1.25 g/kg) increased (A) the percentage of time spent in the open arms and (B) the percentage of entries into the open arms in both saline-pretreated and apremilast (20 mg/kg)-pretreated male and female mice but did not alter (C) the number of entries into the closed arms (n= 16 per group).
Locomotor Response to Novelty
The initial 80 minutes of locomotor activity represents the drug effect on response to a novel situation (novelty). Analysis of this time period showed that acute administration of apremilast (20 mg/kg) significantly reduced the response to novelty in both male (F7,210 = 23.5, p < 0.0001, effect of time; F1,30 = 5.9, p < 0.05, effect of treatment; F7,210 = 3.1, p < 0.01, time × treatment interaction; Fig. 8A) and female (F7,210 = 26, p < 0.0001, effect of time; F1,30 = 7.2, p < 0.05, effect of treatment; F7,210 = 4.5, p < 0.0001, time x treatment interaction; Fig. 8B) mice. Because ethanol drinking was measured after chronic drug administration, we also studied motor activity after chronic (6 daily injections) administration of apremilast (20 mg/kg). Repeated treatment with apremilast significantly reduced the response to novelty in male mice (F1,30 = 7.9, p < 0.01, effect of treatment; F7,210 = 39.6, p < 0.0001, effect of time; F7,210 = 2.8, p < 0.01, time × treatment interaction; Fig. 8C) but did not change the novelty response in female mice (Fig. 8D).
Figure 8. Apremilast decreases the locomotor response to novelty.
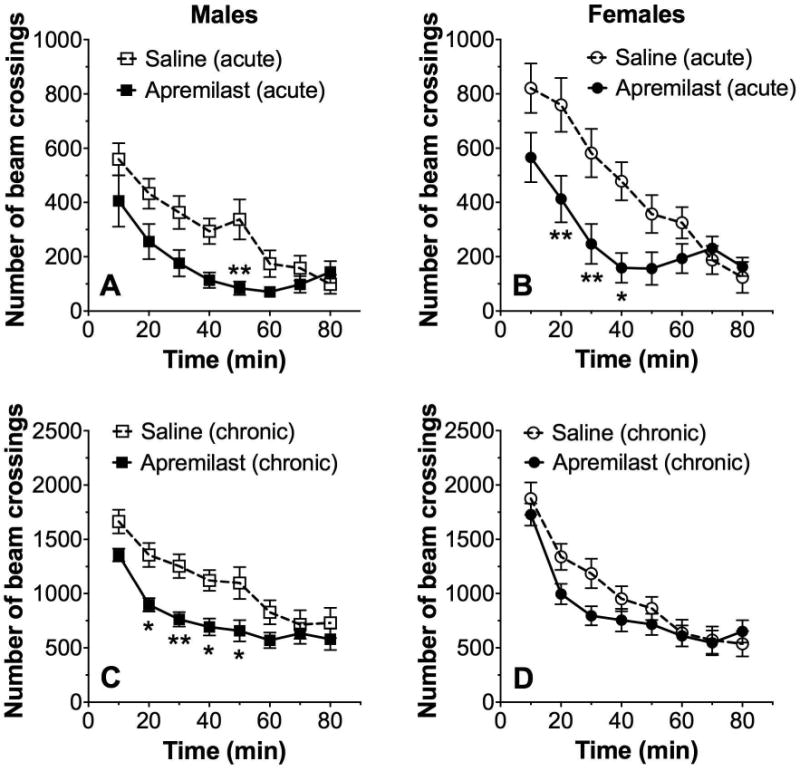
Acute administration of apremilast (20 mg/kg) reduced the novelty response in male (A, n= 16) and female (B, n= 16) mice. Repeated administration of apremilast (20 mg/kg) for 6 days reduced the novelty response in male (C, n= 16) and female (D, n= 16) mice (*p < 0.05 and **p < 0.01 compared with saline control by Bonferroni post-hoc tests).
Loss of Righting Reflex
Since there is a positive correlation between the negative part of the HIC curve (protection from HIC) and duration of LORR (Blednov et al., 2012), we next studied the effect of apremilast (20 mg/kg) on the duration of ethanol (3.6 g/kg)-induced LORR. Acute administration of apremilast increased the LORR duration (p < 0.001, Student's t-test; Fig. 9A). Repeated administration of apremilast for 6 days also prolonged the LORR duration (p < 0.001, Student's t-test; Fig. 9B). There were no sex-dependent differences, and data from male and female mice were combined.
Figure 9. Apremilast increases the duration of ethanol-induced loss of righting reflex.
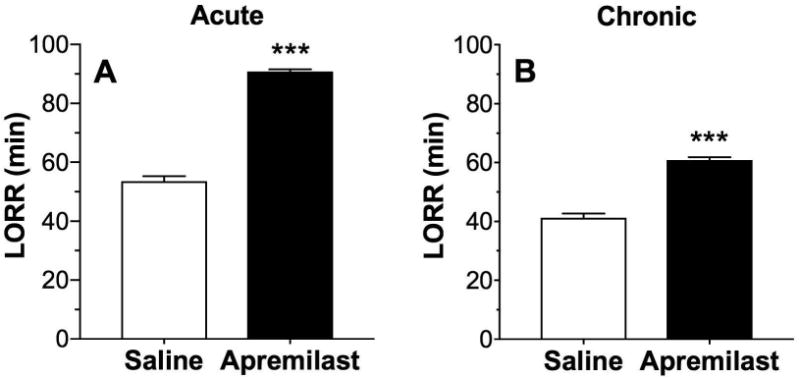
Duration of ethanol (3.6 g/kg)-induced LORR (min) in saline-pretreated and apremilast (20 mg/kg)-pretreated male and female mice after acute (A, n= 11) and chronic (B, 6 daily injections, n= 6) exposure (***p < 0.001 compared with saline control by t-tests).
Recovery from Ethanol-Induced Motor Impairment
The rotarod test was used to measure the effects of apremilast (20 mg/kg) on ethanol (2 g/kg)-induced motor impairment. Both acute and chronic (6 daily injections) administration of apremilast prolonged the recovery from ethanol-induced motor impairment (Fig. 10A, acute administration: F1,30 = 71.7, p < 0.0001, effect of treatment; F10,300 = 302.2, p < 0.0001, effect of time; F10,300 = 20.7, p < 0.0001, time × treatment interaction; Fig. 10B, chronic administration: F1,30 = 21.7, p < 0.0001, effect of treatment; F7,210 = 281.8, p < 0.0001, effect of time; F7,210 = 7.9, p < 0.0001, time × treatment interaction). There were no sex-dependent differences, and data from male and female mice were combined.
Figure 10. Apremilast increases the duration of ethanol-induced motor impairment.
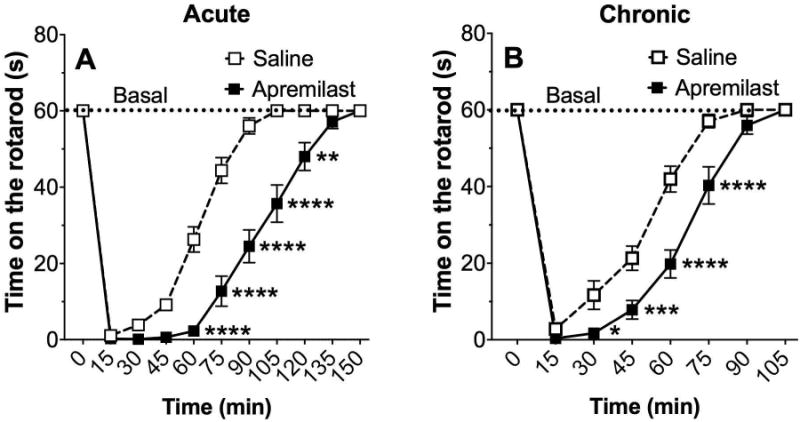
Time on the rotarod after ethanol (2 g/kg, i.p.) following acute (A) and chronic (B, 6 daily injections) pretreatment with saline or 20 mg/kg of apremilast (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 compared with saline control by Bonferroni post-hoc tests; n= 16).
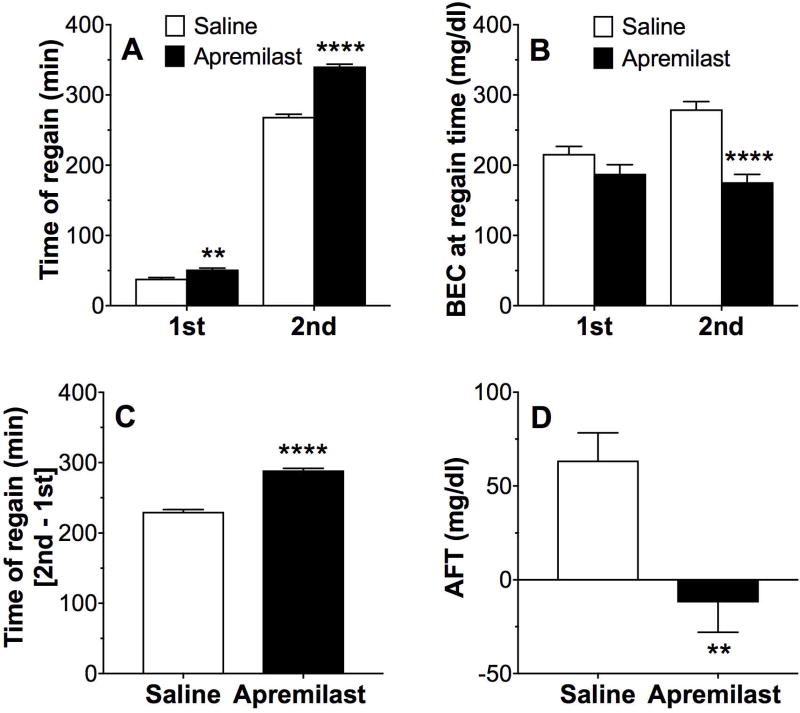
Acute Functional Tolerance to Ethanol-Induced Motor Impairment
We also used the rotarod to measure acute tolerance to the ataxic effects of ethanol. The time to recover from motor impairment after the first and second ethanol injections was significantly longer in apremilast (20 mg/kg)-pretreated vs. saline-pretreated mice (F1,32 = 165, p < 0.0001, effect of pretreatment; F1,32 = 14968, p < 0.0001, effect of recovery; F1,32 = 192, p < 0.0001, pretreatment × recovery interaction; Fig. 11A). The BECs measured after the first ethanol exposure (BEC1 after 1.75 g/kg ethanol) did not differ between groups, whereas BEC2 (after 2 g/kg ethanol) was lower in apremilast- vs. saline-pretreated mice (F1,32 = 29.2, p < 0.001, effect of pretreatment; F1,32 = 5.6, p < 0.05, effect of recovery; F1,32 = 12.1, p < 0.01, pretreatment × recovery interaction; Fig. 11B). The difference in recovery time between the second and first ethanol injections was longer in apremilast-pretreated mice (p < 0.0001; Fig. 11C). AFT (defined as BEC2 - BEC1) to the intoxicating effects of ethanol was decreased in apremilast- compared with saline-pretreated mice (63.5 ± 14.8 vs. -12.03 ± 15.9, p < 0.01; Fig. 11D). There were no sex-dependent differences, and data from male and female mice were combined.
Figure 11. Apremilast reduces acute functional tolerance to ethanol.
(A) Time to regain the ability to remain on the rotarod for 60s after the first (1.75 g/kg) and second (2 g/kg) ethanol injection in saline-pretreated and apremilast (20 mg/kg)-pretreated male and female mice. (B) BECs measured at the time of regaining motor function after the first and second ethanol injections in saline-pretreated and apremilast-pretreated male and female mice. (C) Difference in recovery time between the second and first ethanol injections. (D) Acute functional tolerance (AFT) to ethanol measured as the difference in BECs (mg/dl) after two ethanol injections (BEC2 - BEC1). (**p < 0.01 and ****p < 0.0001 compared with saline control by Bonferroni post-hoc (A, B) or t-tests (C, D); n =16-18 per group).
Discussion
The behavioral profile of apremilast has not previously been studied in detail. In this study, we tested the effects of apremilast on different behaviors that are correlated with ethanol consumption in mice and humans and that could potentially contribute to the reduced ethanol drinking reported in our companion manuscript (Blednov et al., 2018). Table 1 summarizes the effects of apremilast (20 mg/kg) observed in both of our studies.
Table 1. Effects of apremilast (20 mg/kg) on ethanol-related behaviors in male and female C57BL/6J mice.
| Test | Measurement | Apremilast vs. Saline | |
|---|---|---|---|
| Males | Females | ||
| 2BC - EtOH | Intake | ↓ | ↓ |
| Preference | ↓ | ↓ | |
| Total fluid intake | = | = | |
| 2BC-EOD - EtOH | Intake | ↓ | ↓ |
| Preference | ↓ | = | |
| Total fluid intake | ↓ | = | |
| 2BC - Saccharin | Preference | = | = |
| 2BC - Sucrose | Preference | = | = |
| CPP | Acquisition | = | = |
| CPA | Acquisition | ↑ | ↑ |
| Motor activity | Spontaneous | = | ↓ |
| Novelty response | ↓ | ↓ | |
| CTA - EtOH | Acquisition | ↓ | ↓ |
| Extinction | = | = | |
| CTA - LiCl | Acquisition | ↓ | ↓ |
| Extinction | = | = | |
| LORR | Sleep time | ↑ | ↑ |
| Motor impairment | Rotarod ataxia | ↑ | ↑ |
| AFT | BEC2-BEC1 | ↓ | ↓ |
| EtOH withdrawal | HIC | = | = |
| Anxiety-like behavior | EPM | = | = |
| Clearance | BEC | = | = |
AFT, acute functional tolerance; BEC, blood ethanol concentration; CPA, conditioned place avoidance; CPP, conditioned place preference; CTA, conditioned taste aversion; EPM, elevated plus maze; EtOH, ethanol; HIC, handling-induced convulsions; LiCl, lithium chloride; LORR, loss of righting reflex; 2BC, two-bottle choice; 2BC-EOD, two-bottle choice every-other-day; =, ↓, ↑ indicate no change, decreased, and increased responses in apremilast-pretreated compared with saline-pretreated (control) mice. Results for spontaneous motor activity and 2BC drinking experiments (EtOH, saccharin, sucrose) are from our companion manuscript (Blednov et al., 2018).
Apremilast did not alter development of ethanol-induced CPP, severity of acute ethanol withdrawal, or the anxiolytic-like effect of ethanol. Apremilast slightly increased the acquisition of CPA to ethanol. Locomotor responses to novelty were reduced in apremilast-treated mice. We note that rolipram, a prototype PDE4 inhibitor, reduced ethanol drinking in several rodent models (Blednov et al., 2014, Franklin et al., 2015, Wen et al., 2012) and also transiently reduced locomotor activity (Franklin et al., 2015, Wen et al., 2012). Apremilast showed a similar pattern of effects – decreased ethanol consumption and a transient decrease in spontaneous motor activity.
Apremilast prolonged the duration of LORR and the recovery from acute motor incoordination induced by ethanol but did not alter ethanol clearance. Longer recovery from the intoxicating effects of ethanol may be due to the ability of apremilast to decrease AFT, although its initial CNS depressant effects could also be a contributing factor. Increased sensitivity to ataxia, sedation, or other adverse effects would be expected to decrease the amount of ethanol consumed. In humans, decreased sensitivity to alcohol was shown to be a predictor of future alcohol-related problems (Schuckit, 1994).
There is a positive correlation between preference for sweet solutions and ethanol consumption (Bachmanov et al., 1996, Blednov et al., 2008, Yoneyama et al., 2008), but apremilast did not alter preference for saccharin or sucrose. Ethanol-induced CPP is another behavior that is positively correlated with ethanol consumption (Green and Grahame, 2008), but CPP was not affected by apremilast. However, locomotor activity recorded during conditioning trials of CPP suggested that apremilast limits the development of motor sensitization induced by ethanol, which may be correlated with ethanol consumption (Legastelois et al., 2014). Apremilast slightly increased the acquisition of CPA to ethanol, suggesting an increase in ethanol's aversive properties.
The severity of acute withdrawal from ethanol is negatively correlated with ethanol drinking (Metten et al., 1998), but pretreatment with apremilast did not change withdrawal severity in mice. CTA is also negatively correlated with voluntary ethanol consumption (Blednov et al., 2012, Green and Grahame, 2008, Risinger and Cunningham, 1998). We found that apremilast had a complex effect on CTA. Although apremilast reduced the acquisition of CTA to ethanol and LiCl, this was confounded by its ability to reduce saccharin intake on its own. This may also be explained by the initial CNS depressant effects of apremilast, which could interfere with learning and general reward-mediated behaviors. For example, apremilast did not alter preference for sucrose or saccharin in 24h 2BC drinking tests (Blednov et al., 2018), suggesting that the effects observed here are likely due to apremilast's acute CNS inhibitory actions. The apremilast-mediated reduction in saccharin intake was transient, because during extinction, control mice that received apremilast with paired injections of saline immediately showed high preference for saccharin. Overall, our results suggest that there was development of CTA to ethanol in apremilast-pretreated mice during the acquisition period; however, based on comparison of saline- and apremilast-pretreated mice during the extinction period, the CTA to ethanol was weaker in the apremilast group. In addition, when apremilast was used as the unconditioned stimulus, it induced CTA and may therefore evoke an aversive state independent of ethanol.
We also showed that apremilast significantly reduced the motor response to novelty. A positive relationship between the response to novelty and ethanol consumption in rodents has been observed in meta-analysis of behavioral phenotypes in several strains of mutant mice (Blednov et al., 2012). In addition, a GeneNetwork analysis of the “BXD Published Phenotypes” shows correlations between 2BC ethanol consumption and measures of response to novelty in the open field (data in Record ID 10153 for ethanol preference in males is correlated with measures of activity in a novel open field test in Record IDs 11349, 11414, and 11863; http://www.genenetwork.org/webqtl/main.py). Although this was a different test of novelty than was used in our study, it indicates the generality of the association between ethanol consumption and the novelty response. Furthermore, the clinical literature suggests a relationship between novelty seeking and alcohol craving and relapse in alcohol-dependent patients (Evren et al., 2012).
Voluntary ethanol consumption is correlated with different physiological and behavioral effects of ethanol (Green and Grahame, 2008), and the cumulative effects could be significant if several behaviors are affected simultaneously (Blednov et al., 2012). For example, apremilast increased ethanol's sedative and intoxicating effects, reduced AFT and response to novelty, potentially reduced some aspects of general reward, and increased the aversive properties of ethanol. Each of these actions would be expected to limit ethanol consumption on their own. Together, these changes may contribute to the robust decreases in ethanol drinking shown in our companion paper (Blednov et al., 2018).
To distinguish between the peripheral vs. central actions of apremilast following oral administration, we measured levels of apremilast in plasma, liver, and brain. Although plasma levels were expectedly higher, small amounts of apremilast did reach the brain 1-2h after a single oral dose (Blednov et al., 2018). Furthermore, the plasma levels that we measured were similar to levels obtained with an equivalent dose in humans, suggesting that apremilast is a viable drug candidate for clinical testing in alcoholic subjects. These findings also provide novel evidence that apremilast can act centrally to decrease drinking-related behaviors in mice. At the mechanistic level, rolipram and other PDE4 inhibitors inhibit neuroimmune activation and dysregulation of GABAA α4 receptors produced by ethanol (Avila et al., 2017, Carlson et al., 2016), and both neuroimmune and GABAergic signaling in brain have been implicated in the behavioral responses to ethanol (Blednov et al., 2017a, Blednov et al., 2017b, Robinson et al., 2014, Montesinos et al., 2016, Lobo and Harris, 2008, Roberto and Varodayan, 2017). The cumulative actions of apremilast to decrease ethanol consumption and other related behaviors in a preclinical model suggest that this novel PDE4 inhibitor should be investigated further as a repurposed drug to treat AUD. Moreover, PDE4B was recently identified as a novel gene associated with alcohol consumption in humans (Clarke et al., 2017). Subunit-selective PDE4 inhibitors, particularly those targeting PDE4A or PDE4B that are associated with anti-inflammatory activity (and not PDE4D which is associated with emetic effects), may reveal new drug treatments for AUD.
Acknowledgments
The authors thank Dr. Jody Mayfield for help writing, editing, and preparing figures. This research was supported by the National Institute on Alcohol Abuse and Alcoholism (grants AA013520/INIA Project and AA006399).
Footnotes
The authors declare no conflicts of interest.
References
- Avila DV, Myers SA, Zhang J, Kharebava G, McClain CJ, Kim HY, Whittemore SR, Gobejishvili L, Barve S. Phosphodiesterase 4b expression plays a major role in alcohol-induced neuro-inflammation. Neuropharmacology. 2017;125:376–385. doi: 10.1016/j.neuropharm.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Metten P, Beckley EH, Crabbe JC. Multivariate analyses reveal common and drug-specific genetic influences on responses to four drugs of abuse. Trends Pharmacol Sci. 2008;29:537–543. doi: 10.1016/j.tips.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RH, Cherek DR, Spiga R. Acute and chronic alcohol tolerance in humans: effects of dose and consecutive days of exposure. Alcohol Clin Exp Res. 1993;17:740–745. doi: 10.1111/j.1530-0277.1993.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Harris RA. Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci. 2014;8:129. doi: 10.3389/fnins.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Benavidez JM, Da Costa A, Mayfield J, Harris RA. Sedative and motor incoordination effects of ethanol in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res. 2017a;41:531–540. doi: 10.1111/acer.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Chernis J, Da Costa A, Mayfield J, Harris RA. Ethanol consumption in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res. 2017b;41:516–530. doi: 10.1111/acer.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Da Costa AJ, Tarbox T, Ponomareva O, Messing RM, Harris RA. Apremilast alters behavioral responses to ethanol in mice: I. Reduced consumption and preference. Alcohol Clin Exp Res. 2018 doi: 10.1111/acer.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Mayfield RD, Belknap J, Harris RA. Behavioral actions of alcohol: phenotypic relations from multivariate analysis of mutant mouse data. Genes Brain Behav. 2012;11:424–435. doi: 10.1111/j.1601-183X.2012.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. GIRK2 deficient mice. Evidence for hyperactivity and reduced anxiety. Physiol Behav. 2001;74:109–117. doi: 10.1016/s0031-9384(01)00555-8. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA. GABAA receptor alpha 1 and beta 2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther. 2003;305:854–863. doi: 10.1124/jpet.103.049478. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP, Morrow AL. Ethanol regulation of synaptic GABAA alpha4 receptors is prevented by protein kinase A activation. J Pharmacol Exp Ther. 2016;357:10–16. doi: 10.1124/jpet.115.230417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, Murray AD, Smith BH, Campbell A, Hayward C, Porteous DJ, Deary IJ, McIntosh AM. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117) Mol Psychiatry. 2017;22:1376–1384. doi: 10.1038/mp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK. Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res. 1991;550:1–6. doi: 10.1016/0006-8993(91)90397-e. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology (Berl) 1998;139:62–70. doi: 10.1007/s002130050690. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Deitrich RA. Genetic selection and characterization of mouse lines for acute functional tolerance to ethanol. J Pharmacol Exp Ther. 1996;279:1310–1317. [PubMed] [Google Scholar]
- Evren C, Durkaya M, Evren B, Dalbudak E, Cetin R. Relationship of relapse with impulsivity, novelty seeking and craving in male alcohol-dependent inpatients. Drug Alcohol Rev. 2012;31:81–90. doi: 10.1111/j.1465-3362.2011.00303.x. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, McClintick J, Ding ZM, McBride WJ, Bell RL. Reduction of alcohol drinking of alcohol-preferring (P) and high-alcohol drinking (HAD1) rats by targeting phosphodiesterase-4 (PDE4) Psychopharmacology (Berl) 2015;232:2251–2262. doi: 10.1007/s00213-014-3852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzemann R, Edmunds S, Wu W, Malmanger B, Walter N, Belknap J, Darakjian P, McWeeney S. Detection of reciprocal quantitative trait loci for acute ethanol withdrawal and ethanol consumption in heterogeneous stock mice. Psychopharmacology. 2009;203:713–722. doi: 10.1007/s00213-008-1418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang HT. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology (Berl) 2011;218:331–339. doi: 10.1007/s00213-011-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JE, Draski LJ, Molina JC, Burright RG, Reynoso G, Calendrillo BA, Isaacson RL. The effects of verapamil and ethanol on body temperature and motor coordination. Life Sci. 1986;39:2067–2072. doi: 10.1016/0024-3205(86)90357-7. [DOI] [PubMed] [Google Scholar]
- Legastelois R, Botia B, Coune F, Jeanblanc J, Naassila M. Deciphering the relationship between vulnerability to ethanol-induced behavioral sensitization and ethanol consumption in outbred mice. Addict Biol. 2014;19:210–224. doi: 10.1111/adb.12104. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Harris RA. GABA(A) receptors and alcohol. Pharmacol Biochem Behav. 2008;90:90–94. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley RJ, Miner LL, Wehner JM, Collins AC. Differential effects of central nervous system depressants in long-sleep and short-sleep mice. J Pharmacol Exp Ther. 1986;238:1028–1033. [PubMed] [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, Aguilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, DeWit DJ, Kolody B, Vega WA, Wittchen HU, Kessler RC. Comorbidity of substance use disorders with mood and anxiety disorders: results of the International Consortium in Psychiatric Epidemiology. Addict Behav. 1998;23:893–907. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S, Guerri C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res. 2016;40:2260–2270. doi: 10.1111/acer.13208. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Floyd KL, Lee MJ. Rapid ethanol tolerance mediated by adaptations in acute tolerance in inbred mouse strains. Pharmacol Biochem Behav. 2006;84:524–534. doi: 10.1016/j.pbb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcohol Clin Exp Res. 1998;22:1234–1244. [PubMed] [Google Scholar]
- Roberto M, Varodayan FP. Synaptic targets: Chronic alcohol actions. Neuropharmacology. 2017;122:85–99. doi: 10.1016/j.neuropharm.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Most D, Ferguson LB, Mayfield J, Harris RA, Blednov YA. Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans. Int Rev Neurobiol. 2014;118:13–39. doi: 10.1016/B978-0-12-801284-0.00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Stromberg C. Interactions of antidepressants and ethanol on spontaneous locomotor activity and rotarod performance in NMRI and C57BL/6 mice. J Psychopharmacol. 1988;2:61–66. doi: 10.1177/026988118800200201. [DOI] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, Liu Q, Wang WP, Lawrence AJ, Zhang HT, Liang JH. The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol Clin Exp Res. 2012;36:2157–2167. doi: 10.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]