Abstract
Background
Previous clinical studies of autosomal dominant polycystic kidney disease (ADPKD) reported that loss of kidney function usually follows a steep and relentless course. A detailed examination of individual patterns of decline in estimated glomerular filtration rate (eGFR) has not been performed.
Study Design
Longitudinal post hoc analysis of data collected during the Halt Progression of Polycystic Kidney Disease (HALT-PKD) trials.
Setting & Participants
494 HALT PKD Study A (younger study participants, preserved eGFR) and 435 Study B (older study participants, reduced eGFR) who had > 3 years of follow-up and ≥ 7 eGFR assessments.
Measurements
Longitudinal eGFR assessments using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation.
Predictors
Demographic, clinical, laboratory and imaging features of participants.
Outcomes
Probability of linear and nonlinear decline patterns or of stable eGFR calculated for each participant from a Bayesian model of individual eGFR trajectories.
Results
The majority of participants (62.5% in Study A and 81% in Study B) had a linear decline in eGFR during up to 8 years of follow-up. A proportion (22% in Study A and 13% in Study B) of progressors had a nonlinear pattern. 15.5% of participants in Study A and 6% in Study B had a prolonged (≥ 4.5 years) period of stable eGFR. These individuals (Study A) had significantly smaller total kidney volumes, higher renal blood flow, lower urinary albumin excretion, and lower body mass index at baseline and at study end. In Study B participants with reduced but stable eGFR were older than the progressors. Two thirds of nonprogressors in both studies had PKD1 mutations, with enrichment for weak nontruncating mutations.
Limitations
Relatively short follow-up of a clinical trial population.
Conclusions
Although many individuals with ADPKD have a linear decline in eGFR, prolonged intervals of stable GFR occur in a substantial fraction. Lower body mass index was associated with more stable kidney function in early ADPKD.
Keywords: Autosomal dominant polycystic kidney disease (ADPKD), Bayesian models, end-stage renal disease (ESRD), estimated glomerular filtration rate (eGFR), eGFR trajectory, eGFR slope, total kidney volume, mutation analysis, kidney disease progression
Autosomal dominant polycystic kidney disease (ADPKD) is estimated to affect one in 400–1000 people in the United States and over 12 million worldwide, leading to kidney failure in the majority of afflicted patients 1–3. The predominant causative genes are PKD1 on chromosome 16 and PKD2 on chromosome 4. Mean age at onset of end-stage renal disease (ESRD) is 56 years for patients with the more common PKD1 genotype and 73 years for those with a PKD2 mutation 2–5.
Although renal cysts start to develop in utero and grow throughout life, glomerular filtration rate (GFR) usually remains normal until the 4th or 5th decade, likely due to compensatory hyperfiltration by noncystic nephrons 6,7. Previous natural history studies concluded that once GFR begins to fall, there is a relentless downhill course to ESRD within 5–10 years 7–10. In the MDRD (Modification of Diet in Renal Disease) Study, ADPKD patients had a very fast decline in measured GFR of 5.8 mL/min per year 11.
Several recent publications have described nonlinear trajectories of kidney function decline and prolonged periods of nonprogression in a significant number of patients with chronic kidney disease (CKD) of different etiologies 12–14. Long-term follow-up of individual trajectories may identify factors associated with periods of stable kidney function and periods of faster decline, thus providing opportunities for intervention 15.
Individual patterns of kidney function decline over time have not been studied in large cohorts of patients with ADPKD. We used data from the recently completed Halt Progression of Polycystic Kidney Disease (HALT-PKD) trials 16,17 to test the hypothesis that GFR decline in ADPKD is not always linear and rapid, and that periods of stability can occur even in advanced disease. Significant deviations from a linear pattern of estimated GFR (eGFR) decline complicate patient care because optimal timing of dialysis access creation or preemptive kidney transplantation will be less predictable. Clinical trials for testing interventions would need to be larger and of longer duration if there is a substantial proportion of individuals with ADPKD who have spontaneous nonlinear patterns of eGFR decline.
METHODS
Study Population
The design of the HALT PKD trials (ClinicalTrials.gov study numbers NCT00283686 and NCT01885559) and primary results have been published 16–18. The trials adhered to the Declaration of Helsinki and were approved by the Institutional Review Boards of each center. All study participants gave informed consent. Study A randomized 558 subjects (aged 15–49 years, eGFR >60 mL/min/1.73 m2) in a 2×2 factorial design to either a low (95/60–110/75 mmHg) or standard (120/70–130/80 mm Hg) blood pressure (BP) goal using either lisinopril and placebo or lisinopril and telmisartan, with other medications added as needed to achieve the BP goal (based on home BP measurements). Study B randomized 486 patients (aged 18–64 years, eGFR 25–60 mL/min/1.73 m2) to either lisinopril and placebo or lisinopril and telmisartan to achieve a BP goal of 120–130/70–80 mmHg. Subjects were treated for 5–8 years in both studies. Sufficient (> 3 years) follow-up time to calculate eGFR trajectories was available for 494 Study A and 435 Study B participants who are the study population for this analysis.
Cardiac and renal magnetic resonance imaging (MRI) was obtained at baseline and after 2, 4 and 5 years in Study A but not in Study B. Mutation analysis was performed as described by Heyer et al 19.
The primary outcome for Study A was percent annual increase in total kidney volume (TKV); for Study B the composite of time to ESRD, death, or 50% reduction in eGFR. We used the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation to calculate eGFR 20, based on centralized serum creatinine measurements by isotope-dilution mass spectrometry (IDMS) at baseline, 4 and 12 months, then every 6 months until either the trial ended (Study A and B) or a subject reached an end point (Study B only).
Clinical events, particularly hospitalizations and episodes of acute kidney injury (AKI), were documented at each patient visit and reviewed by an adjudication committee. AKI was defined as an elevation of serum creatinine by ≥ 0.3 mg/dL from the most recent baseline within a fitting clinical context. Data about gross hematuria were obtained from symptoms questionnaires.
Statistical Analysis
We categorized 929 individual eGFR trajectories into 3 mutually exclusive groups: 1) progressive and linear, 2) progressive and nonlinear, and 3) nonprogessive (linear and nonlinear combined due to small numbers). The term nonprogressive applies to the time frame of the HALT PKD trials and does not imply that ADPKD is a nonprogressive disorder in some patients. All analyses were run separately for Study A and Study B.
Following the approach of Li et al. 12, 21, we used Bayesian smoothing models to estimate the individual eGFR trajectories for each of the 494 Study A and 435 Study B participants, using R statistical software, version 3.1.3 (R Foundation for Statistical Computing). Bayesian smoothing models allow individual eGFR trajectories to be estimated using information from the raw data, but without bias from the analyst implementing the models. Similar to Li et al.,12 we classified individual trajectories as being nonlinear if the difference in mean slopes between the first and second halves of the follow-up period was > 3 ml/min/1.73 m2 per year. We also classified trajectories as having a significant nonprogression period if all of the following were satisfied: 1) the length of the period was at least 4.5 years or the entire follow-up period for the participant; 2) the slope was no steeper than −2 ml/min/1.73 m2 per year during every month of the period; and 3) the average decline in eGFR during the entire period was no more than 1 ml/min/1.73 m2 per year, which equals the generally accepted age-related decline 12. Posterior probabilities of nonlinearity and nonprogression were calculated as the proportion of 3,000 Monte Carlo simulated trajectories that satisfied the criteria 12. If the probability of nonlinearity was > 0.5, we classified the trajectory as nonlinear; similarly, if the probability of nonprogression was > 0.5, the trajectory was classified as nonprogressive.
For each study, baseline demographic and clinical characteristics were summarized across the 3 trajectory groups using sample means and standard deviations or sample proportions. We also examined the distribution of genotype (both studies) and MRI class (Study A), an index of kidney disease severity based on TKV at a given age 22, across the trajectory groups (imaging not performed in Study B). Between-group comparisons were made using analysis of variance (ANOVA) and Chi Square tests, or their non-parametric counterparts. Associations between trajectory groups and outcomes (annual rate of TKV increase and slopes of eGFR decline) were assessed using linear mixed models. Predictors included month, separate interactions between month and each of the study arms (BP goal and drug), trajectory group, the month-by-trajectory group interaction, and random effects for the intercept and slope. Finally, the rates of clinical events (AKI, hematuria, and hospitalizations) were calculated for each trajectory group and further split between participants based on whether they experienced steeper eGFR declines in the first half of study follow-up. Statistical analyses were performed using SAS 9.4 and R version 3.1.3.
RESULTS
Study A
Mean follow-up time of the 494 Study A participants was 6.1±1.2 years; 309 (62.5%) were classified as linear progressors (examples in Fig. 1); 108 (21.9%), as nonlinear progressors (Fig. 2); and 77 (15.6%), as nonprogressors (Fig. 3).
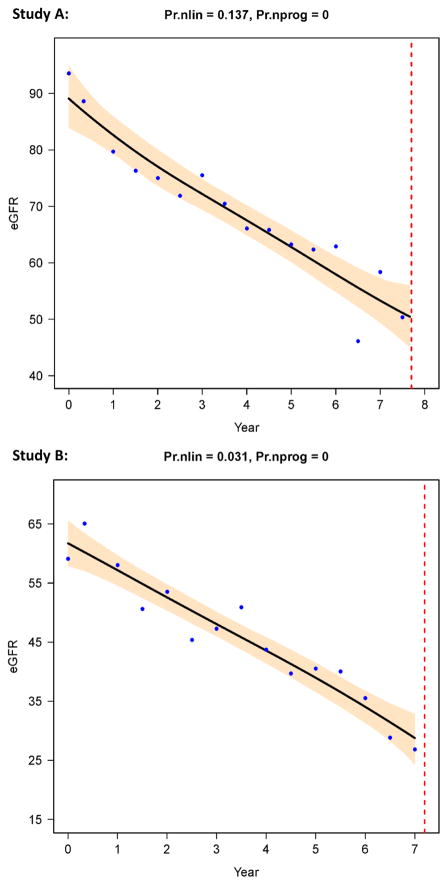
Figure 1. Progressive linear patterns in Study A (top) and B (bottom).
Examples of individual eGFR trajectories with low probabilities of nonlinearity (Pr.nlin) and zero probability of nonprogression (Pr.nprog). The X-axis shows years from baseline visit until the end of the HALT PKD trial, and the Y-axis shows eGFR in mL/min/1.73 m2. The dots are actual eGFR data derived from centralized serum creatinine measurements, the black line is the estimated trajectory (the average of 3,000 Monte Carlo simulated curves), with 95% Bayesian confidence intervals in grey. The vertical dotted line signifies the end of study participation of that individual. The top figure is from a Study A participant with a truncating PKD1 mutation, the bottom figure from a Study B participant, also with a truncating PKD1 mutation. Figure courtesy of L. Lee.
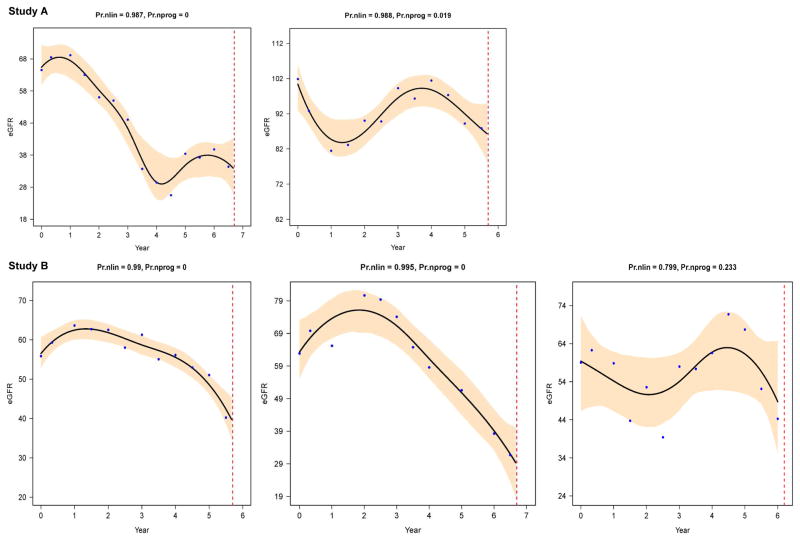
Figure 2. Progressive nonlinear patterns in Study A (top) and B (bottom).
Shown are 5 individual eGFR trajectories as examples of a nonlinear pattern, with high probabilities of nonlinearity (Pr.nlin) and very low probabilities of nonprogression (Pr.nprog). The setup of the 5 trajectories is the same as in figure 1. Figure 2 demonstrates the high variability in the shapes of nonlinear progression. The top left figure is from a Study A participant with a nontruncating PKD1 mutation, the top right figure from a Study A participant with a truncating PKD2 mutation. The bottom left figure is from a Study B participant with a truncating PKD2 mutation, the middle figure from a Study B participant with a truncating PKD1 mutation, and the bottom right figure from a Study B participant with no mutation detected. Figure courtesy of L. Lee.
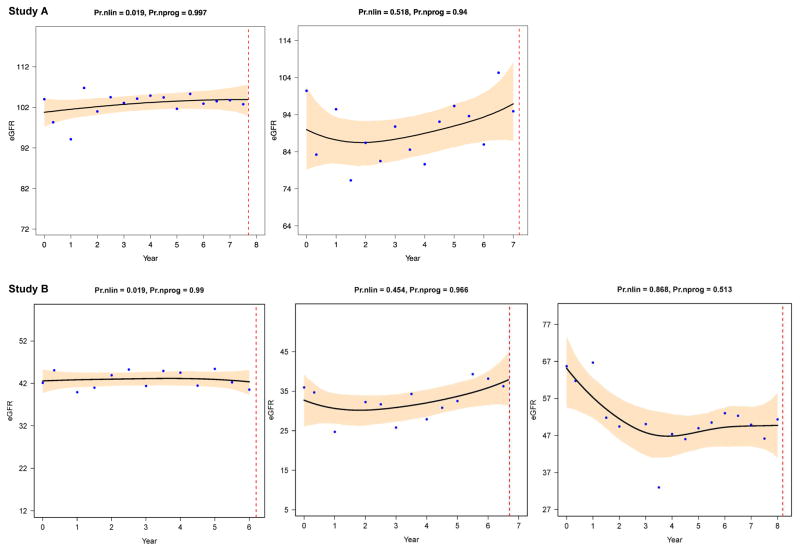
Figure 3. Nonprogressive patterns in Study A (top) and B (bottom).
These 5 examples of individual eGFR trajectories have high probability of nonprogression (Pr.nprog > 0.5, often > 0.9). The upper row left figure shows an example of a linear (probability of nonlinearity very low) nonprogressive eGFR trajectory from a Study A participant with a truncating PKD2 mutation; the upper right figure shows a nonlinear (probability of nonlinearity > 0.5) nonprogressive pattern from a Study A participant with a truncating PKD1 mutation. The bottom row left and middle figures show 2 examples of stable (Pr.nprog > 0.9) eGFR in Study B participants with low eGFR at baseline, the left figure from a participant with a truncating PKD1 mutation and the middle figure from a subject with no mutation detected. The bottom right figure is an example of nonlinear (Pr.nlin > 0.5) nonprogression (Pr.nprog > 0.5): an initial rapid eGFR decline is followed by an extended (> 4.5 years) period of nonprogression; the trajectory is from a Study B participant with a truncating PKD1 mutation. Setup is the same as in figure 1. Figure courtesy of L. Lee.
The three groups were similar in many baseline characteristics, including age, gender, baseline eGFR and left ventricular mass index (Table 1). However, the nonprogressors (as defined for this study) had significantly smaller TKV and height-adjusted TKV, higher renal blood flow, and lower urinary albumin excretion than the 2 progressor groups. The nonprogressors contained a higher proportion of low-risk (class 1A and 2A of the Irazabal imaging classification 22) and a lower proportion of high-risk (class 1D and 1E) patients than the 2 progressor groups (Table 1). Mean slopes for annual TKV increase were 6.4% (95% confidence interval [CI], 6.0–6.8) for linear progressors, 6.3% (95% CI, 5.6–7.0) for nonlinear progressors, and 4.8% (95% CI, 4.0–5.7) for nonprogressors (p = 0.006 between groups).
Table 1.
Study A, baseline characteristics of the three groups
| Measure and category | Progressor & Linear (n=309) | Progressor & Nonlinear (n=108) | Nonprogressor (n=77) | P |
|---|---|---|---|---|
| Sex | 0.9 | |||
| Male | 163 (52.8%) | 56 (51.9%) | 38 (49%) | |
| Female | 146 (47.2%) | 52 (48.1%) | 39 (51%) | |
| PKD genotype | 0.1 | |||
| NMD | 23 (7.6%) | 9 (8.7%) | 9 (12%) | |
| PKD1 | 231 (76.7%) | 79 (76.0%) | 46 (62%) | |
| PKD2 | 47 (15.6%) | 16 (15.4%) | 19 (26%) | |
| Previous use of any ARB at screening | 52 (17.9%) | 18 (18.6%) | 13 (18%) | 0.9 |
| Previous use of any ACEi at screening | 151 (52.1%) | 43 (44.3%) | 36 (49%) | 0.4 |
| Drug group | 0.8 | |||
| Lisinopril/Telmisartan | 152 (49.2%) | 52 (48.1%) | 35 (46%) | |
| Lisinopril/Placebo | 157 (50.8%) | 56 (51.9%) | 42 (55%) | |
| BP group | 0.6 | |||
| Low BP | 156 (50.5%) | 54 (50.0%) | 34 (44%) | |
| Standard BP | 153 (49.5%) | 54 (50.0%) | 43 (55%) | |
| Family history of ADPKD | 272 (88.0%) | 92 (85.2%) | 65 (84%) | 0.6 |
| Hematuria in past 6 mo | 8 (2.6%) | 8 (7.4%) | 3 (4%) | 0.07 |
| Smoking history | 0.9 | |||
| Never | 184 (59.7%) | 64 (61.0%) | 43 (57%) | |
| Former | 92 (29.9%) | 32 (30.5%) | 27 (36%) | |
| Current | 32 (10.4%) | 9 (8.6%) | 6 (8%) | |
| MRI Class ╪ | < 0.001 | |||
| 1A + 2A | 26 (8.5%) | 10 (9.4%) | 22 (29%) | |
| 1B + 1C | 173 (56.4%) | 52 (49.1%) | 44 (57%) | |
| 1D + 1E | 108 (35.2%) | 44 (41.5%) | 11 (14%) | |
| Age at baseline (years) | 37.3 ± 8.0 | 36.7 ± 8.5 | 36.6 ± 8.7 | 0.7 |
| Age at diagnosis of ADPKD | 27.8 ± 10.1 | 26.3 ± 10.0 | 29.9 ± 9.0 | 0.05 |
| Age at diagnosis of HTN | 30.6 ± 8.9 | 30.6 ± 9.2 | 31.7 ± 8.7 | 0.6 |
| Home average SBP (mmHg) | 124.4 ± 9.5 | 124.7 ± 9.7 | 121.7 ± 8.1 | 0.1 |
| Home average DBP (mmHg) | 82.8 ± 7.7 | 83.3 ± 7.9 | 80.7 ± 7.5 | 0.1 |
| LVMI (g/m2) | 64.1 ± 12.9 | 64.0 ± 13.1 | 63.4 ± 13.2 | 0.9 |
| TKV (mL) | 1287.2 ± 759.3 | 1379.7 ± 750.5 | 800.5 ± 458.2 | < 0.001 |
| Height-adjusted TKV (mL/m) | 732.3 ± 421.9 | 793.7 ± 411.1 | 463.6 ± 257.3 | < 0.001 |
| Renal blood flow (ml/min/1.73m2) | 587.6 ± 201.0 | 602.6 ± 193.6 | 664.9 ± 205.3 | 0.03 |
| eGFR (ml/min/1.73m2) | 89.9 ± 17.5 | 89.4 ± 16.7 | 94.9 ± 15.6 | 0.05 |
| Urine aldosterone (μg/24 | h) 12.1 ± 9.7 | 11.4 ± 8.3 | 13.0 ± 10.5 | 0.3* |
| Urine albumin (mg/24 h) | 19.1 [13.0–33.3] | 20.1 [11.7–37.4] | 15.1 [10.2–22.7]) | 0.01* |
| BMI (kg/m2) | 27.5 ± 5.3 | 27.1 ± 4.5 | 25.6 ± 4.2 | 0.01 |
| Weight (kg) | 84.1 ± 18.3 | 81.6 ± 16.1 | 76.1 ± 15.9 | < 0.01 |
| Urinary creatinine excretion (mg/24 h) | 1528.9 ± 735.5 | 1470.1 ± 549.1 | 1529.5 ± 606.1 | 0.7 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range].
p value from log transformed variable
Imaging classification (based on TKV growth rate) by Irazabal et al.22, where MRI class 1A and 2A represent the lowest risk for GFR decline, class 1B and 1C an intermediate risk, and class 1D and 1E the highest risk for rapid decline in GFR.
ACEi: Angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BMI: body mass index; BP: blood pressure; DBP, diastolic BP; eGFR: estimated glomerular filtration rate (using Chronic Kidney Disease Epidemiology Collaboration creatinine equation); HTN: hypertension; LVMI, left ventricular mass index; NMD: no mutation detected; PKD, polycystic kidney disease; SBP, systolic BP; TKV: total kidney volume.
The BPs during the trial were similar in the three groups, but the number of medications needed to achieve the BP goal was slightly lower in the nonprogressor group (Table 2). By definition, eGFR decline was significantly greater in the progressors than the nonprogressors who had no significant change in eGFR (mean slope, 0.09 [95% CI, −0.4 to 0.5] mL/min/1.73 m2 per year; p = 0.7). Mean slopes for linear and nonlinear progressors were −3.5 (95% CI, −3.2 to −3.7) and −3.7 (95% CI, −3.3 to −4.0) mL/min/1.73 m2 per year (p < 0.001 between groups). We found no relationship between baseline age and rate of eGFR decline in linear progressors (Table S1).
Table 2.
Study A, characteristics of the 3 groups during and at end of trial
| Measure | Progressor & Linear (n=309) | Progressor & Nonlinear (n=108) | Non progressor (n=77) | P |
|---|---|---|---|---|
| 50% reduction in eGFR | 0 | 0 | 0 | --- |
| Average home SBP during study (mmHg) | 114.9 ± 7.6 | 114.7 ± 7.7 | 113.9 ± 6.8 | 0.6 |
| Average home DBP during study (mmHg) | 76.3 ± 6.5 | 76.5 ± 6.8 | 74.8 ± 6.9 | 0.2 |
| Average no. of medications needed to control BP (step) | 2.9 ± 1.7 | 2.8 ± 1.8 | 2.3 ± 1.6 | 0.03 |
| TKV at 5 years (mL) | 1782.0 ± 1083.9 | 2020.7 ± 1324.0 | 1056.9 ± 608.2 | < 0.001 |
| htTKV at 5 years (mL/m) | 1013.0 ± 596.6 | 1160.6 ± 727.6 | 613.2 ± 344.4 | < 0.001 |
| RBF at 5 years (ml/min/1.73 m2) | 508.6 ± 197.3 | 470.5 ± 202.1 | 627.3 ± 163.3 | < 0.001 |
| Follow-up duration for eGFR (years) | 6.1 ± 1.2 | 5.8 ± 1.3 | 6.2 ± 1.2 | 0.04 |
| eGFR at study end (ml/min/1.73 m2) | 66.5 ± 23.1 | 66.2 ± 23.2 | 91.5 ± 16.4 | < 0.001 |
| Urine aldosterone at study end (μg/24 h) | 6.2 ± 4.8 | 6.8 ± 5.0 | 7.2 ± 4.8 | 0.2 |
| Weight at study end (kg) | 87.9 ± 20.3 | 84.4 ± 17.6 | 79.9 ± 17.0 | < 0.01 |
| Urinary creatinine excretion at study end (mg/24 h) | 1442.0 ± 531.2 | 1420.4 ± 624.2 | 1436.0 ± 545.5 | 0.9 |
Note: Values for categorical variables are given as number; values for continuous variables, as mean ± standard deviation.
BP: blood pressure; DBP, diastolic BP; eGFR: estimated glomerular filtration rate (using Chronic Kidney Disease Epidemiology Collaboration creatinine equation); htTKV: height-adjusted TKV; RBF: renal blood flow; SBP, systolic BP; TKV, total kidney volume.
Nonprogressors had a lower body mass index (BMI) that was not explained by their smaller TKV (only 400–500 ml) nor due to lower muscle mass, as daily creatinine excretion was not different from progressors either at baseline or at the end of the trial (Tables 1 and 2). While all subjects gained weight, nonprogressors were significantly slimmer both at the beginning and end of the trial.
The majority (62%) of nonprogressors had the PKD1 genotype and 84% had a family history of ADPKD, therefore typical disease. Weak nontruncating PKD1 mutations as described by Heyer et al.19 were enriched among the nonprogressors but were not exclusive, as 44% had a truncating PKD1 mutation (table a of Item S1).
Episodes of AKI were slightly more frequent in the progressor groups, whereas gross hematuria and hospitalizations were equally common in the 3 groups (Table S2), with no obvious temporal relationship between these events and steeper eGFR slope (Item S2).
Study B
Mean follow-up time for the 435 Study B participants was 5.4±1.4 years; 352 (81%) were classified as linear progressor (Fig. 1); 58 (13%), as nonlinear progressor (Fig. 2); and 25 (6%), as nonprogressor (Fig. 3).
The 3 groups were similar in all examined baseline characteristics except for age; interestingly, the nonprogressors (14 male, 11 female) were older than the progressors (Table 3). Muscle mass, estimated by daily creatinine excretion, was not significantly different between the 3 groups. The BPs during the trial and number of medications needed to achieve the goal were not different between groups (Table 4). Nonprogressors remained in the trial significantly longer because none of them reached an end point, whereas over 50% of progressors did (Table 4). Nonprogressors had a nonsignificant decline in eGFR (mean slope, −0.55 [95% CI, −1.16 to 0.07] mL/min/1.73 m2 per year; p = 0.08), whereas mean slopes for linear and nonlinear progressors were −3.9 (95% CI, −3.8 to −4.1) and −3.8 (95% CI, −3.3 to −4.2) mL/min/1.73 m2 per year (p < 0.001 between groups). Among linear progressors, younger age was associated with faster progression; a 10-year decrease in baseline age resulted in a 0.86 mL/min/year steeper eGFR slope (p < 0.001) (Table S1).
Table 3.
Study B, baseline characteristics of the 3 groups
| Measure and category | Progressor & Linear (n=352) | Progressor & Nonlinear (n=58) | Non progressor (n=25) | P |
|---|---|---|---|---|
| Sex | 0.3 | |||
| Male | 168 (47.7%) | 34 (58.6%) | 14 (56.0%) | |
| Female | 184 (52.3%) | 24 (41.4%) | 11 (44.0%) | |
| PKD genotype | 0.06 | |||
| NMD | 15 (4.4%) | 4 (7.0%) | 4 (16.7%) | |
| PKD1 | 279 (82.5%) | 42 (73.7%) | 16 (66.7%) | |
| PKD2 | 44 (13.0%) | 11 (19.3%) | 4 (16.7%) | |
| Previous use of any ARB at screening | 86 (24.8%) | 14 (24.1%) | 4 (16.0%) | 0.7 |
| Previous use of any ACEi at screening | 200 (57.6%) | 28 (48.3%) | 15 (60.0%) | 0.4 |
| Drug group | 0.3 | |||
| Lisinopril/Telmisartan | 166 (47.2%) | 32 (55.2%) | 15 (60.0%) | |
| Lisinopril/Placebo | 186 (52.8%) | 26 (44.8%) | 10 (40.0%) | |
| Family history of ADPKD | 314 (89.2%) | 51 (87.9%) | 19 (76.0%) | 0.1 |
| Hematuria in past 6 mo | 10 (2.8%) | 1 (1.8%) | 1 (4.0%) | 0.7 |
| Smoking history | 0.3 | |||
| Never | 218 (63.2%) | 33 (57.9%) | 12 (48.0%) | |
| Former | 101 (29.3%) | 18 (31.6%) | 12 (48.0%) | |
| Current | 26 (7.5%) | 6 (10.5%) | 1 (4.0%) | |
| Age at baseline (years) | 49.0 ± 7.9 | 47.4 ± 9.3 | 53.3 ± 7.9 | 0.01 |
| Age at diagnosis of ADPKD, y | 33.0 ± 12.3 | 33.3 ± 11.7 | 38.4 ± 13.5 | 0.1 |
| Age at diagnosis of HTN, y | 36.7 ± 10.0 | 35.7 ± 9.9 | 40.0 ± 11.9 | 0.2 |
| Home average SBP (mmHg) | 124.4 ± 10.6 | 125.3 ± 11.0 | 124.7 ± 14.2 | 0.9 |
| Home average DBP (mmHg) | 81.4 ± 8.0 | 83.3 ± 9.4 | 81.0 ± 8.7 | 0.4 |
| eGFR (ml/min/1.73 m2) | 48.1 ± 11.3 | 49.6 ± 13.4 | 53.4 ± 10.6 | 0.07 |
| Urine aldosterone (μg/24 h) | 9.5 ± 6.7 | 9.3 ± 5.8 | 10.3 ± 6.5 | 0.7* |
| Urine albumin (mg/24 h) | 30.0 [17.6–78.0] | 32.9 [15.6–85.2] | 20.0 [12.3–42.3] | 0.08* |
| BMI (kg/m2) | 27.8 ± 5.3 | 28.8 ± 5.2 | 27.5 ± 5.3 | 0.4 |
| Weight (kg) | 83.7 ± 19.8 | 87.5 ± 17.5 | 82.8 ± 18.4 | 0.4 |
| urinary creatinine excretion (mg/24 h) | 1425.4 ± 624.2 | 1510.2 ± 666.8 | 1702.3 ε ± 725.9 | 0.08 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range].
p value from log transformed variable
2 subjects had initial 24-hour creatinine excretion of 3.5 and 3.8 g/24 h with sodium excretion of 309 and 520 mmol/24 h, therefore these were likely significant over-collections; the 2 subjects did not report taking any dietary creatine-containing supplements. Excluding those 2 subjects from the nonprogressor analysis shows an initial creatinine excretion of 1535 ± 452 mg/24 h.
ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BMI: body mass index; BP: blood pressure; DBP, diastolic BP; eGFR: estimated glomerular filtration rate (using Chronic Kidney Disease Epidemiology Collaboration creatinine equation); HTN: hypertension; NMD: no mutation detected; PKD, polycystic kidney disease; SBP, systolic BP; TKV: total kidney volume.
Table 4.
Study B, characteristics of the 3 groups during and at end of trial
| Measure | Progressor & Linear (n=352) | Progressor & Nonlinear (n=58) | Non progressor (n=25) | P |
|---|---|---|---|---|
| 50% reduction in eGFR | 155 (44.0%) | 23 (39.7%) | 0 (0%) | < 0.001 |
| Reached any end point * | 184 (52.3%) | 31 (53.4%) | 0 (0%) | < 0.001 |
| Average home SBP during trial (mm Hg) | 118.9 ± 4.9 | 119.9 ± 6.1 | 118.5 ± 8.2 | 0.4 |
| Average home DBP during trial (mm Hg) | 79.1 ± 4.8 | 79.5 ± 5.3 | 78.2 ± 5.9 | 0.6 |
| Average no. of medications needed to control BP (step) | 2.8 ± 2.1 | 3.3 ± 2.5 | 3.1 ± 2.2 | 0.2 |
| Follow-up duration for eGFR (years) | 5.4 ± 1.4 | 4.9 ± 1.6 | 6.6 ± 0.8 | < 0.001 |
| eGFR at study end (ml/min/1.73 m2) or end point | 26.6 ± 10.7 | 28.7 ± 13.3 | 45.4 ± 11.4 | < 0.001 |
| Urine aldosterone at study end (μg/24 h) or end point | 5.2 ± 3.6 | 6.2 ± 5.4 | 5.9 ± 3.6 | 0.1 |
| Time to end point (months) | 67.4 ± 15.9 | 62.1 ± 18.5 | 79.0 ± 10.1 | < 0.001 |
| Weight at study end (kg) | 85.1 ± 19.9 | 85.7 ± 17.9 | 80.9 ± 18.1 | 0.6 |
| Urinary creatinine excretion at study end (mg/24 h) | 1296.4 ± 638.1 | 1372.5 ± 561.8 | 1242.8 ε ± 414.9 | 0.6 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation.
End points were initiation of dialysis, kidney transplantation, death, or 50% reduction in eGFR.
Excluding the 2 subjects with likely over-collections of urine from the nonprogressor analysis results in ending creatinine excretion of 1280 ± 405 mg/24 h.
BP: blood pressure; DBP, diastolic BP; eGFR: estimated glomerular filtration rate (using Chronic Kidney Disease Epidemiology Collaboration creatinine equation); SBP, systolic BP.
Two thirds of the nonprogressors in Study B had the PKD1 genotype; one third of those had a truncating PKD1 mutation, but weak nontruncating mutations were more common among the PKD1 nonprogressors (table b of Item S1). Most (76%) nonprogressors in Study B had a family history of ADPKD, suggesting typical disease.
Episodes of AKI occurred more frequently in the nonlinear progressor group, hematuria was most common among nonprogressors, whereas hospitalizations occurred with similar frequency in the 3 groups (Table S3). There was no obvious temporal relationship between these events and steeper eGFR slopes (Item S2).
Because over 90% of families in both studies had only one member participating and contributing data, assessment for family clustering of eGFR trajectory patterns was limited (Table S4).
DISCUSSION
Previous studies of the clinical course of ADPKD suggested that once GFR begins to fall, the course is relentlessly downhill, leading to ESRD within 5–10 years 7–10. A linear progressive decline in GFR was thought to be a general feature of progressive chronic kidney diseases 12,23, but recently data from large longitudinal studies have shown that nonlinear trajectories of progression are seen in up to 40% of patients 12–15. Here we explore the individual progression patterns of 929 participants in the HALT PKD trials using similar statistical techniques as described previously 12. We are unaware of other large-scale examinations of actual eGFR trajectories in ADPKD.
The majority of participants in the HALT PKD trials did have a linear progressive loss of kidney function, even if they started with a normal baseline eGFR of ≥ 90 mL/min/1.73 m2. However, a significant proportion of progressors (26% in Study A and 14% in Study B) had periods of variable slopes resulting in nonlinear patterns as defined in this study. Nonlinear patterns had many different shapes; periods of slow or no eGFR decline could be followed by periods of faster decline, and vice versa. Duration of these periods was 2–4 years. Because the categorization of (non)linearity is based on the average of 3000 simulations of eGFR trajectories, it is unlikely that nonlinearity was simply caused by outlying data points or physiological GFR variability, particularly in progressors who already had declining kidney function. Mild to moderate changes in body weight are also not a major factor, as creatinine excretion was not different between pattern categories, and in AASK (African American Study of Kidney Disease and Hypertension) there was no association between moderate weight change and eGFR 24. Intake of nonsteroidal or other medications that could change GFR was discouraged for all HALT PKD participants.
We could not identify any predictors for nonlinear trajectories either at baseline or during the trials. Events such as AKI, gross hematuria and hospitalizations occurred with similar frequencies during periods of steep and less steep or stable eGFR, but numbers were low. Study A participants were generally healthy and had infrequent hospitalizations or AKI 16,18. In Study B there was a slightly higher incidence of AKI and hospitalization in nonlinear progressors but no clear temporal relationship with steeper GFR decline. It is likely that eGFR trajectories reflect a complex interplay between genetic, environmental and life event factors, coupled with varying capability of remaining nephrons to compensate. In addition, although cysts appear to grow at a constant rate, the effect on GFR may not be linear, because cysts located in the medulla can suddenly obstruct a much larger number of collecting ducts when they reach a critical size than peripheral cysts, leading to an abrupt decline in GFR, with subsequent stabilization due to adaptation of remaining nephrons 7.
The observation that patients with ADPKD have periods of varying eGFR decline may not be new to clinicians, but here we have quantified the frequency with which this occurs by an unbiased method. The finding that eGFR can stabilize or improve for a few years, even at a very reduced level, is novel, and is in contrast to other recent studies which generally describe an accelerated loss of kidney function in the last 2 years before initiation of dialysis 13,14. Our findings have implications for clinical care (e.g. kidney transplantation may be safely postponed) as well as for design of interventional trials, which needs to account for spontaneous changes in eGFR slopes that cannot be predicted by clinical variables 25.
Almost 16% of Study A participants had no significant decline in eGFR during HALT PKD, although they had the same age and similar baseline eGFR as the progressors. However, they had significantly smaller TKV and a slower growth rate, consistent with previous reports from the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP), where baseline height-adjusted TKV ≥ 600 mL/m predicted progression to stage 3 CKD within 8 years (sensitivity, 74%; specificity, 75%) 26,27. Although the mean increase in TKV among nonprogressors was 4.8% per year, corresponding to MRI class 1D of the Irazabal classification 22, these nonprogressors had a significantly higher percentage of subjects with mild disease (MRI classes 1A/2A) and a lower proportion with severe disease (MRI classes 1D/E) than the progressors. Nonprogressors with higher MRI classes were younger than progressors and still at an early stage of ADPKD when GFR declines are not yet apparent (Table S5). Nonprogressors had higher renal blood flow than progressors at baseline and even more pronounced at 5 years, consistent with an earlier disease stage, as impairment of renal blood flow precedes the decline in eGFR 28–30.
Nonprogressors in Study A had significantly lower BMI than progressors, not explained by their slightly smaller kidneys. This is intriguing in light of recent animal studies demonstrating that mild-to-moderate food restriction can ameliorate PKD without malnutrition 31,32. Employing food restriction is based on the observation that PKD1 mutant cells use aerobic glycolysis for energy production, and that glucose deprivation results in lower cell proliferation 33. Maintaining a lower BMI in young adulthood may slow the progression of ADPKD.
The nonprogressor group in Study B, although small, is perhaps the most remarkable. These subjects were older than the progressors but their change in eGFR during 6.6 years of observation was less than the age-related decline, in the absence of significant weight or muscle mass loss. These subjects may represent the mildest end of the relationship between older age at onset of kidney function loss and slower rate of decline that we describe for Study B. Nonprogressors had a slightly lower incidence of AKI than progressors, but similar frequencies of hematuria and hospitalizations.
Interestingly, approximately two thirds of nonprogressors in both studies had the PKD1 genotype. PKD1 families with late-onset or no ESRD have been reported previously 34–36, and approximately one third of PKD1 mutations are nontruncating, resulting in milder disease 37. Here we demonstrate enrichment of weak nontruncating PKD1 mutations among nonprogressors of both Study A and B. Subjects with no mutation detected are also enriched among nonprogressors; some might have the recently discovered and rare GANAB (glucosidase IIα subunit) mutation, which causes very mild ADPKD 38. Genetic mosaicism due to new mutations could account for a few nonprogressors, but most nonprogressors did have a family history of ADPKD.
HALT PKD had four treatment arms 16,17, but participants were similarly distributed among study arms across the 3 pattern groups (see Table 1 and Table 3). This indicates that treatments did not have a differential effect on patterns of eGFR decline.
Limitations of this analysis are the relatively short observation time of 5–8 years and limited information about individual clinical events. Other shortfalls are the absence of MRI imaging in Study B and the use of eGFR as opposed to measured GFR, which is more accurate but not practical to obtain in large trials. A recent report from the Chronic Renal Insufficiency Cohort (CRIC) study concluded that assessing declines in measured versus estimated GFR did not improve prediction of ESRD 39.
Study strengths are the large number of individuals with ADPKD; centralized IDMS-traceable serum creatinine determinations obtained every 6 months; rigorous analysis using Bayesian models; detailed genetic data; and meticulous BP control in all subjects, avoiding confounding by uncontrolled hypertension. Patients with diabetes, proteinuria, significant heart disease or systemic illness were excluded from enrollment, thus minimizing confounding by comorbidities with negative impact on kidney function.
In summary, this is to our knowledge the first large analysis of individual patterns of eGFR decline in ADPKD. Loss of kidney function is not always linear and rapid; prolonged stabilization of GFR can occur even in advanced disease. Future trials evaluating the effect of treatment on eGFR will need to recognize the fact that eGFR slopes in ADPKD can have varying configurations and can change in 13%–22% of patients without apparent cause.
Supplementary Material
Item S1: Distribution of mutation strength across pattern groups for PKD1 participants.
Item S2: Relationship between clinical events and changes of eGFR slopes in nonlinear progressors.
Table S1: Mean eGFR slopes of linear progressors by age and study.
Table S2: AKI, gross hematuria episodes, and hospitalizations during the trial, Study A.
Table S3: AKI, gross hematuria episodes, and hospitalizations during the trial, Study B.
Table S4: Count of family size.
Table S5: Distribution of Irazabal MRI classes and mean ages across pattern groups.
Acknowledgments
Support: Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK62402 to Dr Schrier, DK62411 to Dr Perrone, DK62410 to Dr Torres, DK082230 to Dr Moore-Patterson, DK62408 to Dr Chapman, and DK62401 to Washington University in St. Louis) and the National Center for Research Resources General Clinical Research Centers (RR000039 to Emory University, RR000585 to the Mayo Clinic, RR000054 to Tufts Medical Center, RR000051 to the University of Colorado, RR023940 to the University of Kansas Medical Center, and RR001032 to Beth Israel Deaconess Medical Center), National Center for Advancing Translational Sciences Clinical and Translational Science Awards (RR025008 and TR000454 to Emory University, RR024150 and TR00135 to the Mayo Clinic, RR025752 and TR001064 to Tufts University, RR025780 and TR001082 to the University of Colorado, RR025758 and TR001102 to Beth Israel Deaconess Medical Center, RR033179 and TR000001 to the University of Kansas Medical Center, and RR024989 and TR000439 to Cleveland Clinic), by funding from the Zell Family Foundation (to the University of Colorado), and by a grant from the PKD Foundation. Mutation analysis was supported by DK62410-S1 to Dr Harris and the Mayo Translational PKD Center (DK090728). Study drugs were donated by Boehringer Ingelheim Pharmaceuticals Inc (telmisartan and matched placebo) and Merck & Co Inc (lisinopril). For the primary report of the HALT PKD trials (published in NEJM in 2014), individuals at the National Institute of Health participated in the study design, interpretation of the data, and writing of the report. For this article, aside from Dr Flessner’s authorship role, the funders had no role in the design, data collection, interpretation, and writing of the current secondary analysis or decision to submit the manuscript for publication.
We thank Dr Liang Li, Department of Biostatistics, MD Anderson Cancer Center, for providing the programming code for trajectory models; all figures were created using programming codes provided by Dr. Li. We also thank Diane Comer for her extraordinary help with statistical analyses. Most of all we thank the hundreds of patients who took part in the HALT PKD trials and the dedicated study coordinators who guided them through the years of participation.
Footnotes
Authors’ Contributions: Research idea and study design: GMB (for original HALT PKD trials: RWS, JJG, ABC, VET, RDP, WEB, TIS, MFF); data acquisition: GMB, RWS, ABC, KTB, MCH, DCM, VET, RDP, WEB, TIS, FFR-O; data analysis/interpretation: GMB, JJG, VET, RDP, WEB, PCH, KTB, FFR-O, MFF; statistical analysis: KZA, CGM. JJG died before the manuscript was submitted; GMB affirms that he contributed to data interpretation and vouches for his coauthorship status; all authors approved the final author list. Except as noted, each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: Dr Schrier reports having received fees for serving on advisory boards from Otsuka Pharmaceuticals, Janssen Pharmaceuticals, and Ikaria; Dr Perrone reports consulting fees from Sanofi–Genzyme and Vertex Pharmaceuticals and consulting fees and grant support through his institution from Otsuka Pharmaceuticals; Drs Torres and Harris report grant support from Otsuka Pharmaceuticals; Dr Steinman reports grant support from Kadmon, Fibrogen, and AMAG Pharmaceuticals and a consulting fee from Sanofi-Genzyme; Dr Rahbari-Oskoui reports fees for serving on advisory boards from Otsuka, Kadmon, and Astute medical and also research support from Otsuka; Dr Bae reports consulting fees from Kadmon Pharmaceuticals; Dr Chapman reports consulting fees from Kadmon, Otsuka Pharmaceuticals, and Pfizer and grant support from Otsuka Pharmaceuticals; and Dr Hogan reports receiving grant support from Novartis. The other authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iglesias CG, Torres VE, Offord KP, Holley KE, Beard CM, Kurland LT. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935–1980. Am J Kidney Dis. 1983;2(6):630–639. doi: 10.1016/s0272-6386(83)80044-4. [DOI] [PubMed] [Google Scholar]
- 2.Fick-Brosnahan GM, Ecder T, Schrier RW. Polycystic kidney disease. In: Schrier R, editor. Diseases of the Kidney. 7. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. pp. 547–588. [Google Scholar]
- 3.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 4.Torra R, Badenas C, Darnell A, et al. Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7(10):2142–2151. doi: 10.1681/ASN.V7102142. [DOI] [PubMed] [Google Scholar]
- 5.Hateboer N, v Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353(9147):103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 6.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: The major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1(1):148–157. doi: 10.2215/CJN.00330705. [DOI] [PubMed] [Google Scholar]
- 7.Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7(10):556–566. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- 8.Franz KA, Reubi FC. Rate of functional deterioration in polycystic kidney disease. Kidney Int. 1983;23(3):526–529. doi: 10.1038/ki.1983.51. [DOI] [PubMed] [Google Scholar]
- 9.Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41(5):1311–1319. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- 10.Choukroun G, Itakura Y, Albouze G, et al. Factors influencing progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;6(6):1634–1642. doi: 10.1681/ASN.V661634. [DOI] [PubMed] [Google Scholar]
- 11.Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. J Am Soc Nephrol. 1995;5(12):2037–2047. doi: 10.1681/ASN.V5122037. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012;59(4):504–512. doi: 10.1053/j.ajkd.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Hare AM, Batten A, Burrows NR, et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis. 2012;59(4):513–522. doi: 10.1053/j.ajkd.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Y, Muñoz A, Schwartz GJ, Warady BA, Furth SL, Abraham AG. Nonlinear trajectory of GFR in children before RRT. J Am Soc Nephrol. 2014;25(5):913–917. doi: 10.1681/ASN.2013050487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Chang A, Rostand SG, et al. A within-patient analysis for time-varying risk factors of CKD progression. J Am Soc Nephrol. 2014;25(3):606–613. doi: 10.1681/ASN.2013050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrier RW, Abebe KZ, Perrone RD, et al. Blood Pressure in Early Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2014;371(24):2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres VE, Abebe KZ, Chapman AB, et al. Angiotensin Blockade in Late Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2014;371(24):2267–2276. doi: 10.1056/NEJMoa1402686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman AB, Torres VE, Perrone RD, et al. The HALT polycystic kidney disease trials: Design and implementation. Clin J Am Soc Nephrol. 2010;5(1):102–109. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heyer CM, Sundsbak JL, Abebe KZ, et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(9):2872–2884. doi: 10.1681/ASN.2015050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. Erratum in: Ann Intern Med, 2011, 155 (6), 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crainiceanu CM, Ruppert D, Wand MP. Bayesian analysis for penalized spline regression using WinBUGS. J Stat Software. 2005;14:14. [Google Scholar]
- 22.Irazabal MV, Rangel LJ, Bergstralh EJ, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26(1):160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitch WE, Walser M, Buffington GA, Lemann J., Jr A simple method of estimating progression of chronic renal failure. Lancet. 1976;2(7999):1326–1328. doi: 10.1016/s0140-6736(76)91974-7. [DOI] [PubMed] [Google Scholar]
- 24.Chang A, Greene TH, Wang X, et al. The effects of weight change on glomerular filtration rate. Nephrol Dial Transplant. 2015;30(11):1870–1877. doi: 10.1093/ndt/gfv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badve SV, Palmer SC, Hawley CM, Pascoe EM, Strippoli GFM, Johnson DW. Glomerular filtration rate decline as a surrogate end point in kidney disease progression trials. Nephrol Dial Transplant. 2016;31(9):1425–1436. doi: 10.1093/ndt/gfv269. [DOI] [PubMed] [Google Scholar]
- 26.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354(20):2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 27.Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7(3):479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King BF, Torres VE, Brummer ME, et al. Magnetic resonance measurements of renal blood flow as a marker of disease severity in autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64(6):2214–2221. doi: 10.1046/j.1523-1755.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- 29.Torres VE, King BF, Chapman AB, et al. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2007;2(1):112–120. doi: 10.2215/CJN.00910306. [DOI] [PubMed] [Google Scholar]
- 30.Meijer E, Rook M, Tent H, et al. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5(6):1091–1098. doi: 10.2215/CJN.00360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warner G, Hein KZ, Nin V, et al. Food restriction ameliorates the development of polycystic kidney disease. J Am Soc Nephrol. 2016;27(5):1437–1447. doi: 10.1681/ASN.2015020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kipp KR, Rezaei M, Lin L, Dewey EC, Weimbs T. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol. 2016;310(8):F726–F731. doi: 10.1152/ajprenal.00551.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowe I, Chiaravalli M, Mannella V, et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med. 2013;19(4):488–493. doi: 10.1038/nm.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryynanen M, Dolata MM, Lampainen E, Reeders ST. Localisation of a mutation producing autosomal dominant polycystic kidney disease without renal failure. J Med Genet. 1987;24(8):462–465. doi: 10.1136/jmg.24.8.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hateboer N, Lazarou LP, Williams AJ, Holmans P, Ravine D. Familial phenotype differences in PKD1. Kidney Int. 1999;56(1):34–40. doi: 10.1046/j.1523-1755.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- 36.Pei Y, Lan Z, Wang K, et al. A missense mutation in PKD1 attenuates the severity of renal disease. Kidney Int. 2012;81(4):412–417. doi: 10.1038/ki.2011.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornec-Le Gall E, Audrézet MP, Chen JM, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24(6):1006–1013. doi: 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porath B, Gainullin VG, Cornec-Le Gall E, et al. Mutations in GANAB, encoding the glucosidase IIα subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet. 2016;98(6):1193–1207. doi: 10.1016/j.ajhg.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ku E, Xie D, Shlipak M, et al. Change in measured GFR versus eGFR and CKD outcomes. J Am Soc Nephrol. 2016;27(7):2196–2204. doi: 10.1681/ASN.2015040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1: Distribution of mutation strength across pattern groups for PKD1 participants.
Item S2: Relationship between clinical events and changes of eGFR slopes in nonlinear progressors.
Table S1: Mean eGFR slopes of linear progressors by age and study.
Table S2: AKI, gross hematuria episodes, and hospitalizations during the trial, Study A.
Table S3: AKI, gross hematuria episodes, and hospitalizations during the trial, Study B.
Table S4: Count of family size.
Table S5: Distribution of Irazabal MRI classes and mean ages across pattern groups.