Abstract
Phenotypically diverse memory CD8+ T cells are present in the lungs that either re-circulate or reside within the tissue. Understanding the key cellular interactions that regulate the generation and then persistence of these different subsets is of great interest. Recently, DNGR-1+ dendritic cell (DC) mediated priming was reported to control the generation of lung resident but not circulating memory cells following respiratory viral infection. Here, we report an important role for Ly6C+ inflammatory monocytes (IMs) in contributing to the persistence of memory CD8+ T cells but not their generation. Effector CD8+ T cells expanded and contracted normally in the absence of IMs, but the memory compartment declined significantly over time. Quite unexpectedly, this defect was confined to tissue resident and circulating CXCR3hiCX3CR1lo memory cells but not CXCR3hiCX3CR1int and CXCR3loCX3CR1hi subsets. Thus, two developmentally distinct innate cells orchestrate the generation and persistence of memory T cell subsets following a respiratory virus infection.
Keywords: CD8+ T cells, vaccinia, lung, monocytes, resident memory T cells
BLURB FOR ETOC
The cellular networks that orchestrate the generation and maintenance of different CD8 T cell memory subsets are poorly defined. We show that in CCR2−/− mice, both circulating and resident memory cells bearing CXCR3hiCX3CR1lo phenotype were significantly reduced but other subsets remained intact. Thus, we describe a new understanding about how distinct innate immune cells impact phenotypic specialization and persistence of memory T cell subsets – findings that we believe will lead to new ways of thinking about how immunity against respiratory viruses works.
INTRODUCTION
CD8+ memory T cells populate the lung mucosae after clearance of acute respiratory viral infection and contribute greatly to immune defense against secondary encounters with the same virus1. Mounting studies have reported existence of remarkably diverse phenotypic subsets within the memory CD8+ T cell compartment2, 3. Initial ‘programming’ events that occur during the priming phase in local draining lymph nodes (LNs) and the environmental cues received when CD8+ T cells infiltrate inflamed tissues greatly influence the phenotypic specialization, tissue localization, and longevity of effector and memory pools4. In the respiratory tract, central-like CXCR3hiKLRG1lo cells exhibit optimal recall proliferative and self-renewal capacity and function as frontline defenses by mobilizing to the bronchiolar epithelial foci and airway lumen, where virus infection and replication are commonly localized5, 6. In sharp contrast, highly differentiated effector-like CXCR3loKLRG1hi cells fail to undergo significant recall proliferation but efficiently localize to the peribronchoarterial areas and in interalveolar septa in order to prevent systemic viral dissemination5. Using CXCR3 versus CX3CR1 division scheme, similar phenotypic diversity was reported to exist in the memory compartment reflecting the degree of differentiation and tissue surveillance properties of different memory subsets7. A subset of these memory cells differentiates further in response to local stimuli, acquires tissue residence, and are referred as Tissue Resident Memory (TRM) cells8. It is becoming increasingly clear that this highly sophisticated partitioning of memory subsets in the respiratory tract is necessary to enhance the efficiency of antigen-specific host defense. However, precise factors that influence the generation and maintenance of specific memory T cell subsets are poorly defined.
Multiple immune cells are known to interact with CD8+ T cells in the LNs and the lung in a highly orchestrated manner to ensure their full activation, differentiation and maintenance. In this regard, much work over the years has been focused on understanding the role of dendritic cells (DCs) and CD4+ T cells in priming and maintenance of CD8+ T cells respectively9, 10. Interestingly, a recent study demonstrated that CD8+ T cell trafficking and effector functions in virus infected lung required guidance from a preceding wave of infiltrating innate immune cells such as neutrophils11. Whether recruitment of other innate immune cells to the inflamed lung also affect subsequent CD8+ T cell immune response is not fully understood.
Inflammatory Monocytes (IM) are derived from hematopoietic stem cell progenitors in the bone marrow, and high expression of the chemoattractant receptor CCR2 is required for their egress from the bone marrow into circulation and entry into peripheral tissues in response to inflammatory stimuli12. Within the inflamed tissue, IMs can mediate either direct antimicrobial activity or differentiate into macrophages and DC subsets13. Although IMs have been shown to play a protective role in some bacterial14–16, fungal17, 18 and protozoan infections19, 20, they are also known to drive inflammatory diseases such as atherosclerosis21. IM derived DCs have also been shown to present antigens in local draining LNs during systemic bacterial and fungal infections and are suggested to contribute to T cell responses16–18, 22–24. However, the role of IMs during viral infections, especially the ones that infect mucosal surfaces such as the lungs, remains controversial since both beneficial25 and pathologic26 responses have been reported. Moreover, whether IMs are involved in regulating specific subsets of virus-specific effector or memory CD8+ T cells within the specialized niches of the lung remains unknown.
Using a respiratory poxvirus infection in mice and adoptive transfer of naïve TCR transgenic T cells in CCR2-deficient mice, we asked how CCR2-dependent mobilization of IMs influences the phenotype and differentiation potential of effector and memory CD8+ T cells. We found that IMs were dispensable for activation, expansion, and early memory generation of circulating and lung resident CD8+ T cells. Unexpectedly, however, CXCR3hiCX3CR1lo memory cells did not persist long term in the absence of IMs as did CXCR3hiCX3CRint or CXCR3loCX3CR1hi cells. These data highlight a previously unappreciated role for IMs in regulating the phenotypic diversity and abundance of memory CD8+ T cell subsets after an acute infection with a DNA virus.
RESULTS
Expansion of inflammatory monocytes in response to intranasal vaccinia virus infection and the impact of CCR2 deficiency on innate immune cells
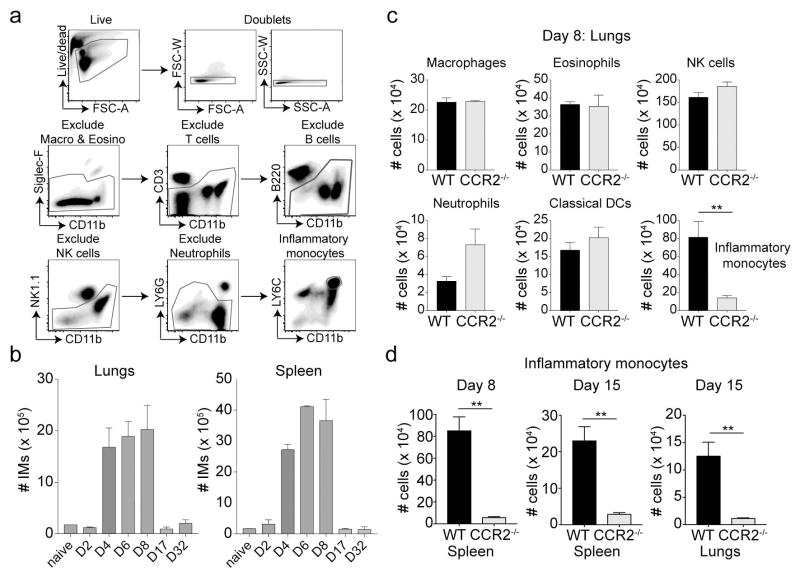
First, we assessed the kinetics of IM recruitment to the lungs and the spleen following intra-nasal (i.n.) infection of naïve wild type (WT) C57BL/6 mice with a mouse-adapted vaccinia virus Western Reserve (VacV-WR) strain. In mice, IMs are identified by high expression of Ly6C and CD11b12. Our gating strategy for identifying IMs (Figure 1a) included exclusion of dead cells and doublets through acquisition of live/dead violet dye and forward and side scatter characteristics followed by exclusion of macrophages and eosinophils (Siglec F+CD11bint), T cells (CD3+CD11blo), B cells (B220+CD11blo), NK cells (NK1.1+CD11bint) and neutrophils (LY6G+CD11bhi). In uninfected (naïve) mice, very few IMs were present in the lung or the spleen, as expected (Figure 1b). After infection, the absolute number of IMs recovered from the lung and spleen increased between days 2 and 4, and peaked between days 4 and 8 (Figure 1b). However, by day 17 post-infection, IM numbers contracted and were maintained at steady levels through day 32 post-infection (Figure 1b). Thus, a nonlethal respiratory VacV-WR infection triggers a rapid and sustained IM response in the infected tissues.
Figure 1. Expansion of inflammatory monocytes in response to respiratory vaccinia virus infection.
WT BL/6 mice were infected with VACV-WR (i.n) and lung and spleen cells were harvested. (a) Gating strategy for identifying IMs includes exclusion of other prominent cell types. Cells were stained with live/dead dye followed by CD3, B220, CD11b, NK1.1, Ly6G and Ly6C. (b) Total number of LY6C+ cells were calculated at days 0, 2, 4, 6 and 8 post-infection. (c & d) Lungs were harvested from BL/6 mice and CCR2−/− recipient mice at day 8 post-infection with rVacV-WR-OVA. Cells were stained as described in (a) and total cell numbers of each innate cell type was determined. *, P < 0.05; **, P < 0.01 and results are a representative of one of the three repeats with mean ± SEM (n = 3 mice/group).
Deficiency of CCR2 markedly reduces the ability of IMs to egress from bone marrow and impairs their trafficking to inflamed sites during infection27, 28, but after VacV-WR infection, we found little or no effect on the accumulation of other innate immune cells such as macrophages, eosinophils, NK cells, neutrophils, and DCs in the lungs at day 8 post-infection (Figure 1c). Similar results were observed in the spleen (not shown). In sharp contrast, we found that the absolute number of IMs in the lung and spleen were markedly lower in infected CCR2−/− mice than the infected WT controls (Figure 1d). Similar results were observed at later time-points (day 32 post-infection) (Supplementary figure 1). These results indicate that although CCR2 deficiency severely impairs recruitment of IMs to infected tissue, accumulation of other innate immune cells remains unaffected.
Expansion and differentiation of antigen-specific CD8+ T cells in CCR2 deficient mice following intranasal infection with vaccinia virus
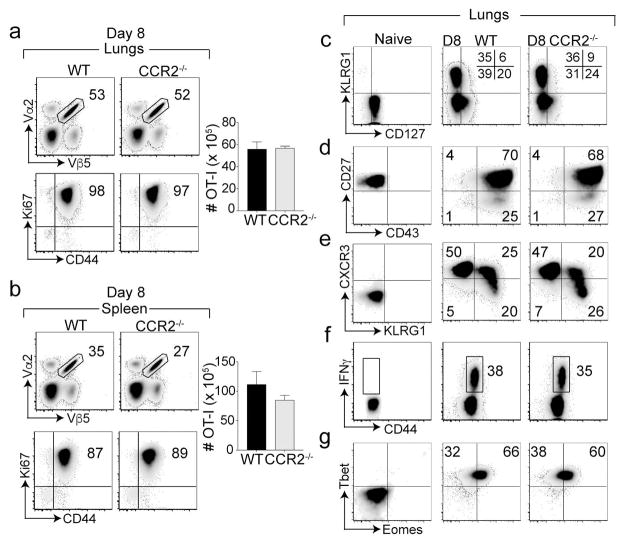
We next used CCR2−/− mice to evaluate the role of IMs in CD8+ T cell response to respiratory VacV-WR infection. To facilitate functional characterization on a per-cell basis, we adoptively transferred equal numbers of FACS sorted naïve (CD8+CD44lo) T cell receptor (TCR) Vα2Vβ5 transgenic OT-I cells specific for H-2Kb/OVA257–264 into naïve WT and CCR2−/− mice and one day later, infected them i.n. with recombinant VacV-WR expressing the full-length ovalbumin protein (rVacV-WR-OVA). Recombinant VacV-WR-OVA was cleared from the infected lungs before day 3 post-infection with no overt signs of disease or effect on weight loss (not shown). Consistent with this, at the peak of the antiviral CD8+ T cell response29, 30, similar frequencies and cell numbers of OT-I cells accumulated in the lungs in both recipient groups (Figure 2a). Although modestly reduced frequencies of OT-I cells were observed in spleens of infected CCR2−/− mice compared with WT recipients, the absolute numbers were not significantly reduced (Figure 2b). Also, no changes were observed in the frequencies of the endogenous immunodominant B8R20–27/kb-reactive population in the lungs and spleens (Supplementary figure 1a, b). Notably, almost all transferred OT-I cells were proliferating irrespective of CCR2 deficiency as noted by similar percentages of Ki67+ cells in both recipient groups (Figure 2a, b). Collectively, these data indicate that IMs are dispensable for optimal clonal expansion and accumulation of antigen-specific CD8+ effector T cells.
Figure 2. Inflammatory monocytes do not affec expansion and accumulation of antigen specific CD8 T cells.
Equal numbers (5 × 104) of WT naïve (CD44lo) OT-I (Vα2+Vβ5+) transgenic CD8 T cells were adoptively transferred into BL/6 mice and CCR2−/− mice, and infected with rVacV-WR-OVA (2 × 104 PFU i.n) the following day. (a) Lungs, (b) spleen were harvested at days 8 post-infection and stained for CD8, CD44, Vα2, Vβ5 extracellularly and Ki67 intra-nuclearly and frequencies and cell numbers of OT-I CD8 T cells determined. Lung cells pre-gated on CD8, CD44, Vα2, and Vβ5 were stained for (c) KLRG1 & CD127, (d) CD27 & CD43 (e) KLRG1 & CXCR3 (g) Tbet & Eomes. (f) Lungs cells were re-stimulated in vitro with OVA and OT-I cells were stained for IFN-γ. *, P < 0.05; **, P < 0.01 and results are the mean ± SEM (n = 3 mice/group). Similar results were obtained in two independent experiments.
Canonical CD8+ T cell differentiation can be analyzed using two main division schemes: KLRG1 versus CD127 and CD27 versus CD43. The former divides effector CD8+ T cells into alternate effector subsets with potential to die [(KLRG1+CD127−) short-lived effector cells or (SLECs)] during contraction phase or long-lived memory cells [KLRG1−CD127+] memory precursor effector cells (MPECs) 31. The frequencies of SLECs and MPECs were similar between infected WT and CCR2−/− recipients (Figure 2c). Moreover, the proportion of effector CD8+ T cell subsets divided based on the CD27 versus CD43 division scheme also remained intact in the lungs of infected CCR2−/− mice compared with WT controls (Figure 2d). By using CXCR3 and KLRG1, we recently showed that SLECs can be further divided into intermediate (CXCR3hiKLRG1hi) and terminally differentiated (CXCR3loKLRG1hi) cells5. Again, generation of these subsets were not affected by the loss of CCR2 (Figure 2e). Similar results were also observed for splenic OT-I cells (not shown). Furthermore, OT-I cells adoptively transferred into CCR2−/− and WT control mice exhibited a similar capacity to produce IFN-γ in response to ex vivo stimulation with OVA-derived SIINFEKL peptide (Figure 2f). This is consistent with the observation that T-bet and Eomes expression by lung-infiltrating effector CD8+ T cells were also similar in infected CCR2−/− and WT recipients (Figure 2g), suggesting that IMs are dispensable for functional maturation of effector CD8+ T cells. Overall, these data indicate that IMs do not regulate CD8+ T cell expansion, accumulation, or differentiation at the peak of antiviral CD8+ T cell response.
Inflammatory monocytes contribute to the persistence of memory CD8+ T cells
The majority of effector CD8+ T cells present at the peak of the response undergo drastic contraction between days 8 and 15, and few cells persist long-term to seed the memory pool. Although IMs have been shown to play important roles in memory CD8+ T cell recall responses32, whether IMs affect lymphoid and non-lymphoid memory cell development after a respiratory virus infection is not known. Following the peak response to VacV-WR-OVA infection, OT-I cells transferred into CCR2 and WT recipients contracted similarly at day 15, both in the lungs as well as in the spleen (Supplementary figure 2a, b). Interestingly, at day 50 post-infection, we found lower frequencies of memory OT-I cells in CCR2−/− recipients compared to WT controls (Supplementary figure 2c, d). These observations indicate that, loss of IMs does not affect the contraction of effector CD8+ T cells, but severely depletes the pool of long-lived memory CD8+ T cells.
Inflammatory monocytes are dispensable for the generation of early lung-resident TRM cells but required for their long-term maintenance
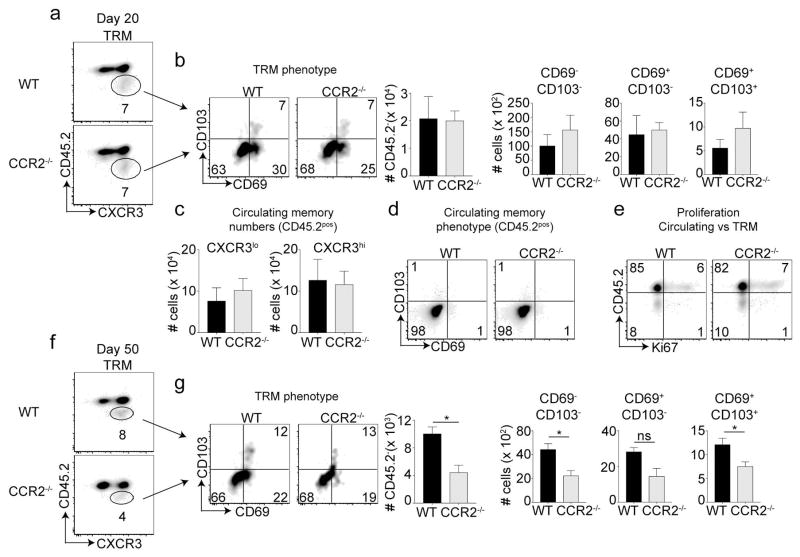
Whether IMs play a role in the generation of lung resident TRM cells is not known. To examine this, CCR2−/− mice and WT mice infected i.n. with rVacV-WR-OVA were injected (i.v.) with fluorophore conjugated anti-CD45.2 antibody 3 minutes before euthanizing. This method labels all circulating cells expressing CD45.2, and the CD45.2 negative cells are generally classified as TRM cells33. Twenty days post-infection, which is considered early memory, we observed similar frequencies and absolute numbers of CD45.2-CXCR3hi TRM cells in CCR2−/− mice compared to their WT counterparts in the lungs (Figure 3a). The percentages and absolute cell numbers of TRM subsets identified based on CD69 versus CD103 were also similar between the two recipient groups (Figure 3b). No change was observed in the absolute cell numbers of either CXCR3hi or CXCR3lo circulating cells (Figure 3c). In contrast to CD45.2− resident memory cells, the CD45.2+ circulating cells were negative for both CD69 and CD103 (Figure 3d). Interestingly, Ki67 expression was confined to circulating cells of the lungs, and no differences were noted in the percentage of proliferating cells between CCR2−/− or WT recipients. In contrast, TRM cells of both recipients failed to express Ki67 indicating that these cells undergo negligible proliferation once they seed specific lung niche (Figure 3e). These results suggest that IMs are dispensable for the generation of early memory cells of both the circulating and tissue resident type.
Figure 3. Generation of tissue resident memory cells in CCR2 deficient mice.
(a–e) At day 20, recipient mice were injected with anti-CD45.2 antibody intravenously, three minutes before euthanizing and lungs were harvested. Frequencies and total number of TRM (CD45.2−) cells were determined after pre-gating on CD8+CD44+ OT-I (Vα2+Vβ5+) cells followed by staining with CXCR3. (b) The gated CD45.2 negative cells were analyzed for the expression of CD103 and CD69 using flow cytometry at respective days and absolute numbers of cells calculated. (c) Cells were stained with CXCR3 and absolute numbers of CD45.2+ cells (non-TRM cells) both CXCR3lo and CXCR3hi was quantified. (d) The gated CD45.2 negative cells were analyzed for the expression of CD103 and CD69 using flow cytometry at respective days and absolute numbers of cells calculated. (e) After extracellular staining, cells were stained with Ki-67 intra-nuclearly without peptide stimulation. (f–g) At day 50, recipient mice were injected with anti-CD45.2 antibody intravenously, three minutes before euthanizing and lungs were harvested. Frequencies and total number of TRM (CD45.2−) cells were determined after pre-gating on CD8+CD44+OT-I (Vα2+Vβ5+) cells followed by staining with CXCR3. (g) Cells were stained with CD69 and CD103 and total cell numbers in each gate were calculated. These experiments were performed twice with (n = 3 or 4 mice/group) and results are the mean ± SEM; with significance value determined by *, P < 0.05.
To determine if CCR2 deficiency affects TRM persistence, we analyzed TRM response at day 50 post-infection. Strikingly, there was approximately 50% reduction in the percentage and absolute number of CD45.2 negative cells in CCR2−/− recipients in the lungs (Figure 3f). Furthermore, analyzing these subsets based on CD69 and CD103 co-expression showed that the defect was specifically associated with the CD69−CD103− [double negative (DN)] and CD69+CD103+ [double positive (DP)] subsets but not the single positive CD69+CD103− subset (Figure 3g). These data indicate that IMs are required for long-term persistence of a subset of TRM (CD45.2 negative) cells in the lungs.
IMs impact the persistence of specific memory CD8+ T cell subsets
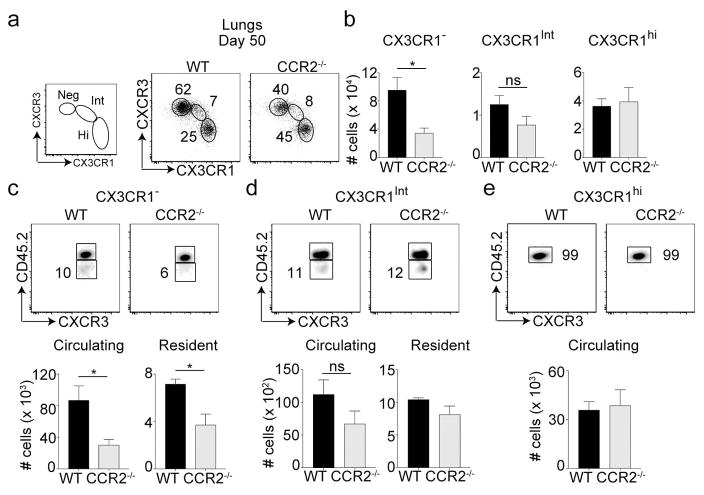
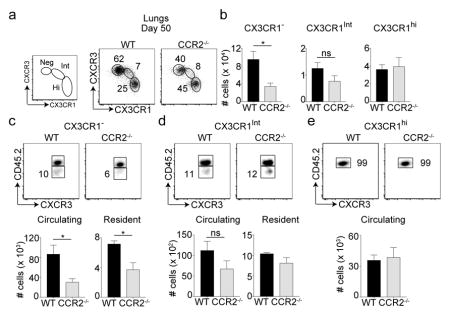
We recently showed that both CXCR3hi and CXCR3lo memory cells are required for providing immunity against respiratory VacV infection5. Subsequently, fractalkine receptor CX3CR1 was also shown to further distinguish these memory subsets (CX3CR1: negative, intermediate and high)7. At day 50 post-infection, we found that only the CXCR3hiCX3CR1neg subset was reduced in CCR2−/− recipients compared to WT recipients (Figure 4a, b). Both the frequencies and numbers of CXCR3hiCX3CR1int and CXCR3loCX3CR1hi subsets were similar between the two recipient groups. Similar results were also observed in the spleen tissue (Supplementary figure 4). TRM cells are thought to be derivatives of or replenished by the CX3CR1neg or CX3CR1int subsets7. In line with this, both CX3CR1neg and CX3CR1int subsets seemed to harbor tissue resident memory cells along with circulating cells (Figure 4c, d). In contrast, CX3CR1hi cells were mostly vascular as previously reported7 and remained unchanged in CCR2−/− recipients (Figure 4e). Strikingly, tissue resident and circulating cells present only within the CX3CR1neg subset were significantly reduced in CCR2−/− mice compared to WT controls (Figure 4c). No differences were observed in resident and circulating cells of CX3CR1int (peripheral memory subset) between the two groups (Figure 4d). These results provide evidence that IMs contribute to the persistence of CXCR3hiCX3CR1neg circulating and tissue resident memory cells in the lung.
Figure 4. Long-term maintenance of tissue resident memory cell subset segregated based on CXCR3 and CX3CR1 expression.
(a–e) Cells were also stained with CX3CR1 and CXCR3 and (a) frequencies and (b) total cell numbers of were calculated. (c) CX3CR1neg (d) CX3CR1int (e) CX3CR1hi were also analyzed for TRM cells after recipient mice were injected with anti-CD45.2 antibody intravenously. Total number of circulating/vascular (CD45.2+) and resident (CD45.2−) cells were calculated for each of the CX3CR1 subsets. These experiments were performed twice with (n = 3 or 4 mice/group) and results are the mean ± SEM; with significance value determined by *, P < 0.05;
DISCUSSION
Recently, DNGR-1+ DCs were reported to be required for the generation of lung TRM cells following respiratory VacV and influenza virus infections34. In the present study, we found that CD8+ T cell differentiation at the peak of effector phase in the lungs and initial TRM formation is intact in VacV infected CCR2−/− recipient mice, implying that IMs are likely not involved in the initial programming and generation of CD8+ T cell memory. However, long-term persistence of specific memory CD8+ T cells, including TRM cells, is impaired in the absence of IMs.
Precisely how IMs regulate the maintenance of memory CD8+ T cells in the lungs, especially those with CX3CR1neg phenotype needs further examination. One possibility is that IMs co-localize with only the CX3CR1neg subset providing pro-survival signals for their maintenance. Recently, infiltrating IMs were reported to localize near sites of intestinal TRM cells, thereby driving their differentiation and persistence35. IMs were also shown to regulate not all but specific subsets of TRM cells designated as CD103−CD69+, and IM derived IL-18 was shown to be important for TRM generation. In our study, the nature of signals (cytokines, co-stimulatory molecules, or residual antigen) provided by IMs is not currently known. In addition to IL-18, IM derived IL-15 has been implicated in reactivation of memory CD8+ T cells32, 35. Moreover, maintenance of TRM cells was reported to be dependent on IL-1536, 37, although IL-15 independent mechanisms have also been reported38, 39. Interestingly, in the context of VacV infection, IMs are a rich source of IL-15 in the lungs (Abboud and Salek-Ardakani, unpublished results) and thus, could possibly provide IL-15 mediated survival signals for long-term TRM persistence. Furthermore, presentation of local residual antigen in the specific lung niche by IMs to enable prolonged survival of highly stable CX3CR1neg cells cannot be ruled out40. Indeed, IMs infected with VacV in vitro can process and present both soluble and peptide antigen via MHC-I complex (Abboud and Salek-Ardakani, unpublished results). This raises the possibility that IMs could also act as residual antigen depots and sustain the survival of specific memory CD8+ T cell subsets by providing low level TCR stimuli. In line with this, non-proliferating TRM cells were reported to receive TCR stimulus in the skin tissue following VacV infection, although the cell type providing the signal to TRM cells was not determined40. Further in-depth studies elucidating these factors will inform the mechanistic basis for IM involvement in maintenance of memory CD8+ T cell subsets and allow for manipulation of these processes to enhance immunological memory of the desirable T cell phenotype.
METHODS
Mice
Eight- to twelve week-old female C57BL/6 (CD45.2) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6129S4-CCR2tm1lfc/J (Cat# 004999) or CCR2−/− mice were also obtained from The Jackson Laboratory (Bar Harbor, ME) and bred and maintained at University of Florida animal facility. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Florida (OLAW Assurance # A3377-01).
Viruses and infections
Recombinant vaccinia virus (VacV-WR) and virus expressing full-length ovalbumin (rVacV-WR-OVA) were grown in HeLa cells and subsequently tittered on VeroE6 cells as described previously41. Mice were intra-nasally infected with either 1.5 × 104 plaque-forming units (PFU) of VacV-WR or 2 × 104 PFU of VacV-WR-OVA in a volume of 10μL.
CD8+ T cell adoptive transfer
For adoptive transfer experiments, 5 × 104 naive WT OT-I CD8+ T cells were purified from spleens with MACS Technology (Miltenyi Biotec), FACS sorted, and transferred into WT C57BL/6 mice via the intra-venous route as described previously42, 43. One day later, mice were infected with rVacV-WR-OVA as above.
Flow cytometry
Preparation of cells, extracellular/intracellular staining, data acquisition, and data analysis were performed as described previously44–47.
Statistical analysis
Data were analyzed using GraphPad Prism version 5.0 software (GraphPad, San Diego, CA). Statistical analyses were performed using a two-tailed, unpaired Student’s t-test with 95% confidence intervals unless otherwise indicated. Two-way ANOVA was used to determine differences in weight loss profiles, and the Mantel-Cox test was utilized for survival analysis. Unless otherwise indicated, data represent the mean ± SEM; P <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This study was supported by NIH grants AI77079 and AI087734 to S.S.-A. V.T. was supported by NIH grant T32 AR007603-15. P.D. was supported through The American Association of Immunologists Careers in Immunology Fellowship Program.
We thank Dr Amanda Posgai for her assistance with technical editing of the manuscript.
Footnotes
AUTHOR CONTRIBUTION
PD designed and performed experiments, analyzed and interpreted data, and wrote the manuscript, VT, JS and GA performed experiments, and reviewed the manuscript, and SS-A conceived and designed experiments and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare that no conflicts of interest exist related to the work presented herein.
References
- 1.Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. 2009;27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- 2.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 3.Rutishauser RL, Kaech SM. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol Rev. 2010;235:219–233. doi: 10.1111/j.0105-2896.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abboud G, Desai P, Dastmalchi F, et al. Tissue-specific programming of memory CD8 T cell subsets impacts protection against lethal respiratory virus infection. J Exp Med. 2016;213:2897–2911. doi: 10.1084/jem.20160167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hikono H, Kohlmeier JE, Takamura S, et al. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlach C, Moseman EA, Loughhead SM, et al. The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity. 2016;45:1270–1284. doi: 10.1016/j.immuni.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 9.Masson F, Mount AM, Wilson NS, et al. Dendritic cells: driving the differentiation programme of T cells in viral infections. Immunol Cell Biol. 2008;86:333–342. doi: 10.1038/icb.2008.15. [DOI] [PubMed] [Google Scholar]
- 10.Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol. 2016;16:102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim K, Hyun YM, Lambert-Emo K, et al. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015;349:aaa4352. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serbina NV, Jia T, Hohl TM, et al. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen H, Gordon S, North RJ. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serbina NV, Salazar-Mather TP, Biron CA, et al. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 16.Peters W, Scott HM, Chambers HF, et al. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohl TM, Rivera A, Lipuma L, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traynor TR, Herring AC, Dorf ME, et al. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J Immunol. 2002;168:4659–4666. doi: 10.4049/jimmunol.168.9.4659. [DOI] [PubMed] [Google Scholar]
- 19.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Robben PM, LaRegina M, Kuziel WA, et al. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters W, Cyster JG, Mack M, et al. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J Immunol. 2004;172:7647–7653. doi: 10.4049/jimmunol.172.12.7647. [DOI] [PubMed] [Google Scholar]
- 23.Flores-Langarica A, Marshall JL, Bobat S, et al. T-zone localized monocyte-derived dendritic cells promote Th1 priming to Salmonella. Eur J Immunol. 2011;41:2654–2665. doi: 10.1002/eji.201141440. [DOI] [PubMed] [Google Scholar]
- 24.Samstein M, Schreiber HA, Leiner IM, et al. Essential yet limited role for CCR2(+) inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. Elife. 2013;2:e01086. doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aldridge JR, Jr, Moseley CE, Boltz DA, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson TC, Beck MA, Kuziel WA, et al. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurihara T, Warr G, Loy J, et al. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuziel WA, Morgan SJ, Dawson TC, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goulding J, Bogue R, Tahiliani V, et al. CD8 T cells are essential for recovery from a respiratory vaccinia virus infection. J Immunol. 2012;189:2432–2440. doi: 10.4049/jimmunol.1200799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goulding J, Abboud G, Tahiliani V, et al. CD8 T cells use IFN-gamma to protect against the lethal effects of a respiratory poxvirus infection. J Immunol. 2014;192:5415–5425. doi: 10.4049/jimmunol.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soudja SM, Ruiz AL, Marie JC, et al. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galkina E, Thatte J, Dabak V, et al. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iborra S, Martinez-Lopez M, Khouili SC, et al. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1+ Dendritic Cells. Immunity. 2016;45:847–860. doi: 10.1016/j.immuni.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergsbaken T, Bevan MJ, Fink PJ. Local Inflammatory Cues Regulate Differentiation and Persistence of CD8+ Tissue-Resident Memory T Cells. Cell Rep. 2017;19:114–124. doi: 10.1016/j.celrep.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 37.Adachi T, Kobayashi T, Sugihara E, et al. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med. 2015;21:1272–1279. doi: 10.1038/nm.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbist KC, Field MB, Klonowski KD. Cutting edge: IL-15-independent maintenance of mucosally generated memory CD8 T cells. J Immunol. 2011;186:6667–6671. doi: 10.4049/jimmunol.1004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenkel JM, Fraser KA, Casey KA, et al. IL-15-Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells. J Immunol. 2016;196:3920–3926. doi: 10.4049/jimmunol.1502337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan TN, Mooster JL, Kilgore AM, et al. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J Exp Med. 2016;213:951–966. doi: 10.1084/jem.20151855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salek-Ardakani S, Moutaftsi M, Crotty S, et al. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181:7969–7976. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, De Trez C, Flynn R, et al. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol. 2009;182:6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salek-Ardakani S, Arens R, Flynn R, et al. Preferential use of B7.2 and not B7. 1 in priming of vaccinia virus-specific CD8 T cells. J Immunol. 2009;182:2909–2918. doi: 10.4049/jimmunol.0803545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salek-Ardakani S, Flynn R, Arens R, et al. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J Clin Invest. 2011;121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goulding J, Abboud G, Tahiliani V, et al. CD8 T Cells Use IFN-gamma To Protect against the Lethal Effects of a Respiratory Poxvirus Infection. J Immunol. 2014;192:5415–5425. doi: 10.4049/jimmunol.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abboud G, Stanfield J, Tahiliani V, et al. Transcription Factor Bcl11b Controls Effector and Memory CD8 T cell Fate Decision and Function during Poxvirus Infection. Front Immunol. 2016;7:425, 1–13. doi: 10.3389/fimmu.2016.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desai P, Abboud G, Stanfield J, et al. HVEM Imprints Memory Potential on Effector CD8 T Cells Required for Protective Mucosal Immunity. J Immunol. 2017;199:2968–2975. doi: 10.4049/jimmunol.1700959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.