Abstract
Background
Intrapartum electronic fetal monitoring (EFM) is the most commonly used tool in obstetrics in the United States, however, which EFM patterns predict acidemia remains unclear.
Objective
This study was designed to describe the frequency of patterns seen in labor using modern nomenclature, and to test the hypothesis that visually interpreted patterns are associated with acidemia and morbidities in term infants. We further identified patterns prior to delivery, alone or in combination, predictive of acidemia and neonatal morbidity.
Design
This was a prospective cohort study of 8,580 women from 2010 to 2015. Patients were all consecutive women laboring at ≥ 37 weeks gestation with a singleton cephalic fetus. EFM patterns during the 120 minutes prior to delivery were interpreted in 10-minute epochs. Interpretation included the Category system and individual EFM patterns per the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) criteria as well as novel patterns. The primary outcome was fetal acidemia (umbilical artery pH ≤ 7.10); neonatal morbidities were also assessed. Final regression models for acidemia adjusted for nulliparity, pregestational diabetes, and advanced maternal age. Area under the receiver-operating characteristic curves (AUC) were used to assess the test characteristics of individual models for acidemia and neonatal morbidity.
Results
Of 8,580 women, 149 (1.7%) delivered acidemic infants. Composite neonatal morbidity was diagnosed in 757 (8.8%) neonates within the total cohort. Persistent Category I, and 10-minute period of Category III, were significantly associated with normal pH and acidemia, respectively. Total deceleration area was most discriminative of acidemia (AUC=0.76; 95% CI: 0.72–0.80), and deceleration area with any 10 minutes of tachycardia had the greatest discriminative ability for neonatal morbidity (AUC=0.77; 95% CI: 0.75–0.79). Once the threshold of deceleration area is reached the number of cesareans needed-to-be performed to potentially prevent one case of acidemia and morbidity is 5 and 6, respectively.
Conclusions
Deceleration area is the most predictive EFM pattern for acidemia, and combined with tachycardia for significant risk of morbidity, from the EFM patterns studied. It is important to acknowledge that this study was performed in patients delivering at or beyond 37 weeks which may limit the generalizability to preterm populations. We also did not use computerized analysis of the EFM patterns because human visual interpretation was the basis for the NICHD categories, and importantly, it is how EFM is used clinically.
Keywords: electronic fetal monitoring, acidemia, pregnancy, term infants, deceleration area, neonatal morbidity
Introduction
Intrapartum electronic fetal monitoring (EFM) is the most commonly used tool in obstetrics in the United States (U.S.), with 85% of the over 4 million deliveries each year having EFM.1 Over the last 60 years, EFM has become the standard of care in U.S. hospitals to monitor the fetus during labor, despite the lack of evidence to support its ability to reduce neonatal morbidity and mortality.2,3 Acidemia at the time of birth is a risk factor for neonatal morbidity, including neurologic injury and mortality,4,5 and EFM promised to be a non-invasive tool to reduce adverse outcomes by identifying fetuses developing acidemia.6,7 It gained widespread use without supportive scientific evidence. Obstetric care providers use EFM patterns to identify fetuses at risk for acidemia and to make clinical decisions regarding delivery, often by cesarean. This has contributed, at least in part, to the dramatic rise in the cesarean rate.8,9
Data regarding EFM patterns and their association with acidemia are limited.10 After an early description, in 1969, associating groups of EFM patterns with progressively worse umbilical artery pH,11 randomized controlled trials failed to demonstrate improved outcomes with the use of EFM during labor.12–17 A recent meta-analysis of over 37,000 women, evaluated the effectiveness of EFM during labor and concluded the use of continuous EFM was not associated with improved Apgar scores, reduced hypoxic ischemic encephalopathy or neonatal mortality.13 Despite widespread adoption of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Category system in the U.S.,18 which EFM patterns predict acidemia remains unclear. Moreover, obstetric care providers need guidance to help them use the patterns seen at the bedside to understand the likelihood of normal pH or acidemia.
We aimed to describe the frequency of EFM patterns seen in labor using modern nomenclature, and assess their association with acidemia and neonatal morbidity. We hypothesized that some visually interpreted EFM patterns are associated with acidemia and morbidities in term infants, while others can help identify infants with a normal pH. We further aimed to identify EFM patterns, which alone or in combination, are predictive of acidemia and neonatal morbidity.
Materials and Methods
This was a single-center prospective cohort study of all consecutive women in labor at ≥ 37 0/7 weeks gestation with a singleton, non-anomalous infants from 2010 through 2015. The Washington University School of Medicine Human Research Protection Office approved this study prior to enrollment (ID# 201102438). Universal continuous EFM and umbilical artery pH are standard of care at our institution. Patient were included if they had umbilical artery pH availability and sufficient EFM, defined as at least 30 minutes of EFM data in the 120 minutes prior to delivery. This definition prevented exclusion of cases with clinical events precluding optimization of continuous monitoring, as well as to enable generalizability. Participants who did not experience labor, did not have continuous EFM, or did not have an umbilical artery pH were excluded.
The primary outcome was fetal acidemia, defined as umbilical artery pH ≤ 7.10. A pH ≤7.10 was chosen to test the possibility that EFM patterns could assist in identifying term fetuses who have developed an abnormal pH but not severe enough to cause morbidity. To obtain the umbilical artery pH, a segment of the umbilical artery cord was clamped immediately after delivery. Whole blood was analyzed centrally using an automated benchtop analyzer (GEM Premier 4000 Analyzer, Bedford, MA, USA) to measure the umbilical artery pH and associated components. Secondary outcomes included neonatal composite morbidity, which included any one or more of the following: mechanical ventilation, seizures, treatment for sepsis, respiratory distress, meconium aspiration, therapeutic hypothermia and death.
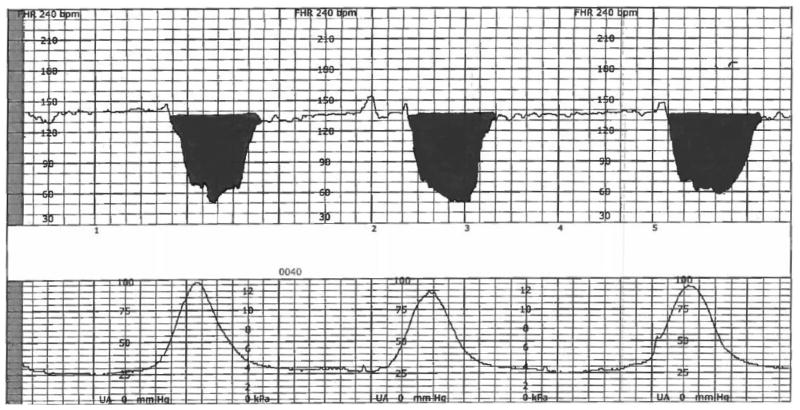
EFM patterns in the final 120 minutes prior to delivery were interpreted in 10-minute epochs by trained obstetric research nurses with high inter- and intraobserver reliability. The obstetric research nurses underwent blind re-reading of 30 EFM tracings after every 500 patients during the course of the study, with a range of Spearman’s correlation coefficients of 0.83 – 0.95, mean differences in deceleration area of 108 ± 248 (intra-) and 154 ± 424 (inter-), and were blind to all outcome and clinical data as well as group assignment.19 EFM recordings were obtained with external and internal monitors, as clinically indicated. Five elements of the EFM recording were extracted using the strict and unambiguous definitions from the NICHD criteria (Table 1)20 and then used to categorize the EFM recordings into one of the three accepted categories: Category I, Category II, or Category III, as well as quantitatively and semi-quantitatively (Table 2).20,21 We estimated deceleration area as the sum of the areas within the deceleration, and each area was estimated as ½ x duration x depth in the final 120 minutes of EFM as a measure of both quantity and severity (Figure 1).22–24
Table 1.
NICHD definitions of fetal heart rate parameters
| Uterine activity: presence of contractions |
Baseline fetal heart rate: approximation of the mean fetal heart rate of increments of 5 beats per minute (bpm), using a 10-minute window, and excluding periodic changes; at least 2 of 10 minutes must be spent at the baseline, or the baseline for that period is indeterminate
|
Baseline fetal heart rate variability: the visual quantification of amplitude of the fluctuations of the fetal heart rate at the baseline; thought to be a physiologic result of the interplay between fetal sympathetic and parasympathetic nervous systems
|
| Accelerations: visually apparent abrupt increase in fetal heart rate, moving from baseline to peak in <20 seconds; must be at least 15 bpm above baseline and last at least 15 seconds |
Decelerations: a visually apparent decrease in fetal heart rate at least 15 bpm below the baseline, and further classified by type
|
Table 2.
EFM Pattern Definitions
| Semi-quantitative Measures of Fetal Heart Rate Patterns in the Final 120 Minutes |
|---|
| Always: Present for all 120 minutes |
| Mostly: Present for ≥ 80 minutes |
| Ever: Present for ≥ 10 minutes |
| Total number of decelerations: the number of each type of deceleration (early, variable, late, prolonged) and their timing within the 120 minute period was extracted |
| Normal (Category I) |
Category I EFM recordings include all of the following:
|
| Indeterminate (Category II) |
| Category II EFM recordings include all not categorized as Category I or Category III. Category II EFM recordings may represent an appreciable fraction of those encountered in clinical care. Examples of Category II EFM recordings include any of the following:
|
| Abnormal (Category III) |
| Category III EFM recordings include either: Absent variability and any of the following:
|
Figure 1.
Illustration of deceleration area*
*Deceleration area was estimated by width of widest aspect of deceleration (below the baseline) measured in seconds, multiplied by the maximum depth below the baseline, divided by two
Statistical Analyses
Descriptive and bivariate analyses compared baseline characteristics between those with acidemia and those without; Chi-square or Fisher exact test, for categorical variables, and the Mann-Whitney U test, for continuous variables. Log-binomial regressions were used to estimate the risk ratios for acidemia in the presence of each EFM pattern in the final 120 minutes prior to delivery. Initial regressions contained the following covariates: advanced maternal age (≥ 35 years old), body mass index (BMI), pregestational diabetes, parity, prostaglandin, Foley bulb, infant birthweight, and maternal fever. Additionally, delivery year was tested as a possible covariate but was found to be insignificant and thus did not remain in the final model. Covariates were retained in the model if their impact on the effect size of the primary covariate was more than 10%. The final regression models adjusted for nulliparity, pregestational diabetes and advanced maternal age. The association and predictive ability of EFM patterns and normal pH was also estimated.
Area under the receiver-operating characteristic curves (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive values (NPV) estimated the discriminative efficiency of EFM patterns, alone or in combination, to predict acidemia and morbidity. AUCs were compared using the method of Delong.25 The optimal cut points of total deceleration for acidemia or neonatal morbidity were identified using the Youden index, which maximizes the sum of sensitivity and specificity.26 The predictive models for acidemia and neonatal morbidity were internally validated using bootstrap analysis of 10,000 samples with replacement to obtain more stable and robust estimates.
Historically, clinical reliance has been placed on the presence of moderate variability as a reliable feature for normal pH even in the setting of other concerning patterns; therefore, various periods of moderate variability were forced into the final predictive models for acidemia to test whether those models would have diminished predictive ability. We estimated the number of cesareans that need to be performed, also considered the number needed to treat (NNT), to prevent one case of acidemia or neonatal morbidity based on the most predictive models and the Youden cut point. All analyses were performed using SAS software, Version 9.4 for Windows (Cary, North Carolina).
Results
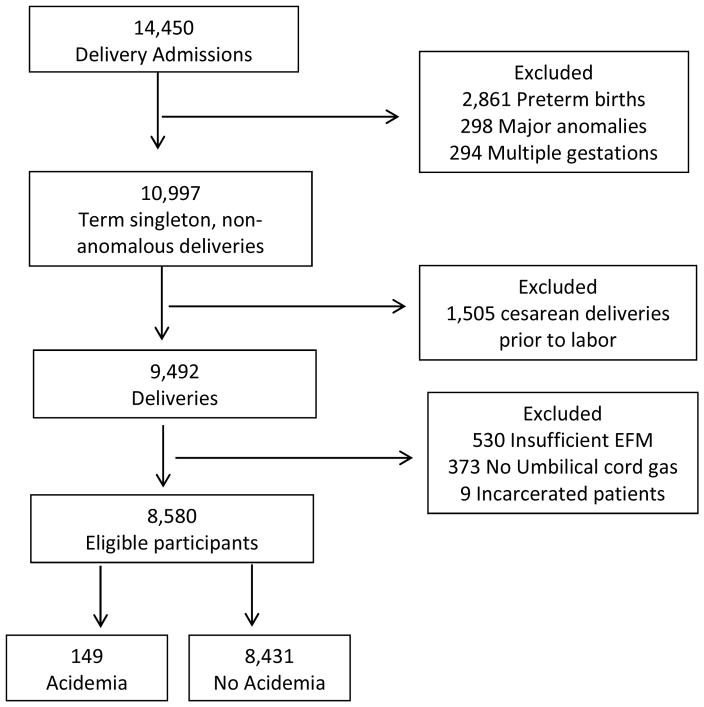
A total of 14,450 women admitted for delivery during the study period, and 10,997 were ≥ 37 0/7 weeks’ gestation, singleton and without a diagnosis of a major anomaly. In all, 912 did not meet inclusion criteria and were excluded: 530 for insufficient EFM, 373 lacking an umbilical artery pH, and 9 who were incarcerated, leaving 8,580 for analysis (Figure 2). Of the 8,580 women meeting inclusion criteria, 149 (1.7%) delivered infants with acidemia and 8,431 (98.3%) without acidemia. Women who delivered infants with acidemia were more likely to be nulliparous, older, have pregestational diabetes and a higher median BMI In addition to higher rates of cesarean delivery, cesarean for indication of non-reassuring fetal status, and prostaglandin and Foley bulb use (Table 3).
Figure 2.
Participants
Table 3.
Baseline Characteristics
| Acidemia (N=149) | No Acidemia (N=8431) | P value | |
|---|---|---|---|
|
| |||
| Maternal age, median (IQR), years | 28 (22, 32) | 25 (21, 30) | .005 |
|
| |||
| Advance maternal age, No. (%), years | 24 (16.1) | 740 (8.8) | .003 |
|
| |||
| Gestational age at delivery median (IQR), weeks | 39 w 4 d (38 w 5 d, 40 w 2 d) | 39 w 3 d (38 wks 3 d, 40 w 2 d) | .15 |
|
| |||
| Maternal race, No. (%) | .67 | ||
| African-American | 94 (63.1) | 5471 (64.9) | |
| Caucasian | 39 (26.2) | 1902 (22.6) | |
| Latina | 7 (4.7) | 606 (7.2) | |
| Asian | 6 (4.0) | 311 (3.7) | |
| Other or Unknown | 3 (2.0) | 141 (1.7) | |
|
| |||
| BMI (median, IQR) | 32.4 (27.5, 38.8) | 30.9 (27.1, 35.8) | .02 |
|
| |||
| Preeclampsia/Hypertension, No. (%) | 30 (20.1) | 1514 (18.0) | .56 |
|
| |||
| Gestational diabetes, No. (%) | 6 (4.0) | 255 (3.0) | .46 |
|
| |||
| Pregestational diabetes, No. (%) | 6 (4.0) | 117 (1.4) | .02 |
|
| |||
| Nulliparous, No. (%) | 80 (53.7) | 3525 (41.8) | .005 |
|
| |||
| Prior low transverse cesarean, No. (%) | 18 (12.1) | 647 (7.7) | .07 |
|
| |||
| Labor type, No. (%) | .54 | ||
| Spontaneous | 43 (28.9) | 2498 (29.6) | |
| Augmented | 35 (23.5) | 2217 (26.3) | |
| Induction | 71 (47.7) | 3716 (44.1) | |
|
| |||
| Regional anesthesia, No. (%) | 137 (92.0) | 7603 (90.2) | .56 |
|
| |||
| Prostaglandin, No. (%) | 37 (24.8) | 1491 (17.7) | .03 |
|
| |||
| Foley bulb, No. (%) | 30 (20.1) | 949 (11.3) | .001 |
|
| |||
| Oxytocin, No. (%) | 97 (65.1) | 5649 (67.0) | .69 |
|
| |||
| Birth weight (median, IQR) | 3285 (2948, 3628) | 3235 (2937, 3545) | .55 |
|
| |||
| Birth weight > 4000, No. (%), grams | 10 (6.7) | 453 (5.4) | .59 |
|
| |||
| Birth weight < 1800, No. (%), grams | 1 (0.67) | 1 (0.01) | .03 |
|
| |||
| Mode of delivery, No. (%) | <.001 | ||
| Vaginal delivery | 44 (29.5) | 6663 (79.0) | |
| Operative vaginal | 16 (10.7) | 396 (4.7) | |
| Cesarean | 89 (59.7) | 1372 (16.3) | |
|
| |||
| Cesarean indications, No. (%) | <.001 | ||
| Arrest | 8 (9.0) | 443 (32.3) | |
| Exhaust | 0 (0.0) | 2 (0.2) | |
| Expulsive | 0 (0.0) | 1 (0.1) | |
| Nonreassuring fetal status | 48 (53.9) | 478 (34.8) | |
| Other | 4 (4.5) | 106 (7.7) | |
| Multiple indications* | 29 (32.6) | 341 (24.9) | |
| Unknown | 0 (0.0) | 1 (0.1) | |
|
| |||
| Maternal fever, No. (%) | 12 (8.1) | 370 (4.4) | .05 |
BMI, body mass index
Combination of all the cesarean indications
Associated EFM Patterns and Predictors of normal umbilical artery pH
Category I throughout the final 120 minutes was rare, occurring in 0.4% (n=37/8580) of patients, but all had a normal pH. In the last 120 minutes prior to delivery infants who had mostly Category I tracings (adjusted risk ratio [aRR], 1.01; 95% confidence interval [CI], 1.01–1.02) or ever had Category I tracings (aRR 1.02; 95% CI 1.01–1.03) were significantly more likely to have normal pH. The specificity of these EFM parameters for normal pH (always, mostly, and ever Category I) were 64.4%, 57.7% and 65.8%, respectively. Moderate variability alone, in any number of epochs (always, mostly, ever), was not a significant independent factor to predict normal pH. Presence of any acceleration in the 120 minutes prior to delivery, whether spontaneous or induced by scalp stimulation, was independently associated with a normal pH (aRR 1.02; 95% 1.01–1.03) (Table 4).
Table 4.
Association and prediction of EFM patterns for normal and abnormal pH
| pH > 7.10 (n= 8431) | pH ≤ 7.10 (n= 149) | Adjusted RR (95% CI)* | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|---|
| Normal pH | |||||
| Category I | |||||
| Always, No. (%) | 37 (0.4) | 0 (0.0) | -- | -- | -- |
| Mostly, No. (%) | 1038 (12.3) | 6 (4.0) | 1.01 (1.01, 1.02) | 55.2 (54.2, 56.4) | 64.4 (56.2, 72.1) |
| Ever, No. (%) | 6000 (71.2) | 78 (52.3) | 1.02 (1.01, 1.03) | 37.1 (36.0, 38.1) | 82.6 (75.5, 88.3) |
| Variability | |||||
| Always moderate, No. (%) | 2082 (24.7) | 27 (18.1) | 1.00 (0.99, 1.02) | 50.2 (49.1, 51.2) | 67.8 (59.6, 75.2) |
| Mostly moderate, No. (%) | 4856 (57.6) | 79 (53.0) | 1.00 (0.98, 1.01) | 50.1 (49.0, 51.1) | 67.7 (59.5, 75.1) |
| Accelerations | |||||
| Ever, No. (%) | 5790 (68.7) | 60 (40.3) | 1.02 (1.01, 1.03) | 66.6 (65.6, 67.6) | 61.7 (53.4, 69.6) |
| Abnormal pH | |||||
| Category III | |||||
| Always, No. (%) | -- | -- | -- | -- | -- |
| Mostly, No. (%) | -- | -- | -- | -- | -- |
| Ever, No. (%) | 18 (0.2) | 3 (2.0) | 8.72 (2.92, 26.04) | 69.1 (61.0, 76.4) | 50.1 (49.0, 51.2) |
| Baseline | |||||
| Ever bradycardic, No. (%) | 231 (2.7) | 5 (3.4) | 1.30 (0.54, 3.15) | 67.8 (59.6, 75.2) | 50.2 (49.1, 51.2) |
Adjusted for nulliparity, pregestational diabetes, and advanced maternal age
Associated EFM Patterns and Predictors of Acidemia
No patients had all or mostly periods of Category III tracings. Any 10-minute period of Category III tracing was rare, but these women were significantly more likely to have infant acidemia at birth (aRR 8.72; 95% 2.92–26.04), with sensitivity of 69.1% and specificity of 50.0% (Table 4 and Table 5).
Table 5.
EFM features discriminative of acidemia in the last 120 minutes prior to delivery*
| AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|
| Always Category II | 0.61 (0.56, 0.65) | 67.8 (59.6, 75.2) | 50.2 (49.1, 51.2) | 2.8 (1.9, 2.9) | 98.9 (98.5, 99.2) |
| Ever Category III | 0.62 (0.57, 0.66) | 69.1 (61.0, 76.4) | 50.0 (49.0, 51.2) | 2.4 (2.0, 2.9) | 98.9 (96.6, 99.2) |
| Ever moderate variability | 0.62 (0.57, 0.66) | 73.8 (66.0, 80.7) | 44.3 (43.2, 45.4) | 2.4 (1.9, 2.8) | 98.7 (98.6, 99.3) |
| Total number of decelerations | 0.66 (0.62, 0.71) | 68.5 (61.7, 77.0) | 58.8 (57.5, 59.6) | 2.9 (2.4, 3.5) | 99.1 (98.8, 99.3) |
| Number of decelerations & ever tachycardic | 0.67 (0.63, 0.72) | 73.2 (63.2, 78.3) | 53.9 (53.6, 56.8) | 2.8 (2.3, 3.3) | 99.1 (98.8, 99.3) |
| Total deceleration area | 0.76 (0.72, 0.80) | 73.5 (64.8, 79.8) | 67.2 (66.6, 68.6) | 4.0 (3.3, 4.8) | 99.3 (99.0, 99.5) |
| Total deceleration area & ever tachycardic | 0.77 (0.73, 0.80) | 66.0 (57.7, 73.6) | 76.2 (75.4, 77.3) | 5.0 (4.0, 6.0) | 99.2 (98.9, 99.4) |
| Total deceleration area, ever tachycardic & always moderate variability | 0.77 (0.73, 0.81) | 71.4 (68.5, 82.8) | 68.5 (66.3, 69.4) | 4.0 (3.4, 5.0) | 99.2 (99.1, 99.5) |
| Total deceleration area, ever tachycardic & mostly moderate variability | 0.77 (0.73, 0.81) | 64.6 (56.3, 72.3) | 78.5 (77.9, 79.8) | 5.3 (4.4, 6.5) | 99.2 (98.9, 99.4) |
| Total deceleration area, ever tachycardic & ever moderate variability | 0.77 (0.74, 0.81) | 63.4 (61.3, 76.7) | 72.6 (71.6, 73.6 | 4.3 (3.7, 5.4) | 99.2 (99.0, 99.4) |
Prevalence of acidemia 1.7% (n= 149/8580)
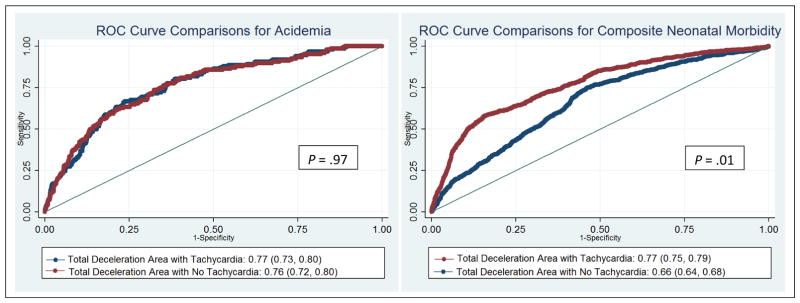
Of all the EFM patterns and features, total deceleration area was most discriminative of acidemia with AUC of 0.76 (95% CI: 0.72, 0.80). The Youden maximal cut point for this model was 42,152, with sensitivity of 63.4% and specificity of 67.2%. The NPV was high (99.2%), with a low PPV (4.0%). A model adding ever baseline tachycardia to total deceleration area did not significantly improve the discriminative ability for acidemia (AUC: 0.77 versus 0.76, P = .97) (Figure 3A). Importantly, always, mostly, or ever moderate variability did not dampen the ability to predict acidemia in the final models (Table 5). In the presence of deceleration area over 42,152 within 120 minutes, the NNT was suggested at 5 cesareans to prevent one case of acidemia.
Figure 3.
Comparisons for acidemia (A) and composite neonatal morbidity (B)
EFM Predictors of Neonatal Morbidity
Composite neonatal morbidity was diagnosed in 8.8% (n= 757/8580) of neonates within the total cohort. Definitions and frequencies of the components of the composite neonatal morbidity are described in Table 6. Some neonates were diagnosed with more than one complication, with the most prevalent morbidities being respiratory distress (43.9%, n=332/757) and requiring treatment for suspected sepsis (82.8%, n=627/757).
Table 6.
Distribution of components of composite neonatal morbidity (n= 757)
| Component | N (% of total cohort)* | % Among Neonates with Composite Morbidity† | Definition |
|---|---|---|---|
| Neonatal death, No. (%) | 4 (0.04) | 0.5 | Death of the neonate |
| Hypothermic therapy, No. (%) | 42 (0.49) | 5.5 | Body temperature of the neonate is reduced at birth in order to reduce the possibilities of brain damage |
| Mechanical ventilation, No. (%) | 50 (0.58) | 6.6 | Assistive equipment for inhalation of oxygen and exhalation of carbon dioxide from the lungs |
| Respiratory distress, No. (%) | 332 (3.87) | 43.9 | The lungs of the neonate have not fully developed, thus posing difficulties in breathing |
| Meconium aspiration syndrome, No. (%) | 22 (0.26) | 2.9 | The presence of meconium in the lungs of the neonate |
| Seizure, No. (%) | 18 (0.21) | 2.4 | A neurologic dysfunction in the neonate |
| Treatment for Sepsis, No. (%) | 627 (7.3) | 82.8 | Requiring treatment for a presumed or proven blood infection in the neonate |
508 neonates were diagnosed with one complication, 190 with two complications, 36 with three complications, 16 with four complications, and 7 with five complications
Percentage of specific morbidity out of all neonates with composite morbidity (n = 757)
The best discriminative model for neonatal morbidity included total deceleration area (AUC: 0.66; 95% CI 0.64, 0.68), but the addition of ever baseline tachycardia (any 10-minute period) significantly improved the discriminative ability (AUC=0.77 versus 0.66, P = .01) (Figure 3B). The Youden maximal cut point was 50,761 for the total deceleration area alone. The model combining total deceleration areas and ever tachycardia had sensitivity of 57.5% and specificity was 84.6%. The NPV was high (95.4%), with a modest PPV (26.2%). As with the acidemia models, always, mostly, or ever moderate variability did not diminish the ability to discriminate morbidity. Any 10-minute period of Category III (AUC = 0.63) alone had the lowest discriminative ability (Table 7). In the presence of deceleration area at or above the Youden cut point and any 10 minutes of tachycardia in the final 120, the NNT was suggested at 6 cesareans to prevent one case of neonatal morbidity. Finally, 65 infants had both acidemia and morbidity, and deceleration area performed similarly in its ability to discriminate those with both (AUC 0.75, 95% CI 0.69 – 0.81).
Table 7.
EFM features discriminative of neonatal morbidity in last 120 minutes prior to delivery*
| AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|
| Always Category II | 0.64 (0.62, 0.66) | 69.5 (64.4, 71.2) | 57.4 (57.0, 59.9) | 13.6 (12.7, 14.9) | 95.1 (94.3, 95.6) |
| Ever Category III | 0.63 (0.61, 0.65) | 68.2 (64.7, 71.5) | 58.7 (57.6, 59.8) | 13.8 (12.7, 14.9) | 95.0 (94.4, 95.6) |
| Ever moderate variability | 0.65 (0.63, 0.67) | 67.9 (62.8, 69.7) | 58.8 (58.2, 60.4) | 13.7 (12.5, 14.8) | 95.0 (94.1, 95.4) |
| Number of decelerations & ever tachycardic | 0.75 (0.73, 0.77) | 57.5 (51.9, 59.1) | 84.4 (83.8, 86.3) | 26.3 (24.9, 29.4) | 95.4 (94.7, 95.7) |
| Number of severe decelerations & ever tachycardic | 0.76 (0.74, 0.78) | 53.4 (48.6, 55.8) | 88.0 (87.2, 88.7) | 28.5 (27.1, 32.1) | 95.1 (94.5, 95.5) |
| Total deceleration area | 0.66 (0.64, 0.68) | 74.8 (71.4, 77.9) | 54.0 (52.9, 55.2) | 13.4 (12.4, 14.5) | 95.7 (95.1, 96.3) |
| Total deceleration area & ever tachycardic | 0.77 (0.75, 0.79) | 57.5 (54.3, 61.7) | 84.6 (83.4, 85.1) | 26.2 (23.8, 28.2) | 95.4 (94.9, 96.0) |
| Total deceleration area, ever tachycardic & always moderate variability | 0.77 (0.75, 0.79) | 55.0 (51.8, 59.3) | 85.7 (84.6, 86.2) | 26.9 (24.5, 29.1) | 95.2 (94.7, 95.8) |
| Total deceleration area, ever tachycardic & mostly moderate variability | 0.77 (0.75, 0.79) | 68.7 (65.2, 73.2) | 72.1 (71.0, 73.1) | 19.0 (17.5, 20.6) | 96.0 (95.5, 96.5) |
| Total deceleration area, ever tachycardic & ever moderate variability | 0.77 (0.74, 0.79) | 60.6 (57.6, 64.9) | 80.9 (79.6, 81.4) | 23.2 (21.2, 25.1) | 95.6 (95.1, 96.1) |
Prevalence of composite neonatal morbidity 8.8% (n= 757/8580)
Comment
EFM patterns in the final 120 minutes prior to delivery identified acidemia and morbidity among infants born at or after 37 0/7 weeks’ gestation better than any previously published work. The most predictive EFM feature was total deceleration area for acidemia and a combination of total deceleration area and any 10-minute period of baseline tachycardia for neonatal morbidity. Any 10-minute period of Category III made acidemia 10-times more likely, though it occurs too rarely to be an efficient predictor. Category I throughout the entire final 120 minutes occurred only among those born with a normal pH, and this was predominantly driven by the presence of accelerations. All, mostly, or any moderate variability the final 120 minutes was not sufficient to independently predict a normal pH, and did not dampen the ability to predict acidemia with deceleration area, with or without tachycardia. Sensitivity of patterns, alone or in combination, was better than previously published but PPV were low, demonstrating that EFM is a poor screening tool for acidemia and morbidity.
Original publications described the association between EFM patterns and fetal pH, 6,27–29 but not the ability of EFM to predict and prevent injury. A recent meta-analysis summarized the findings from 13 randomized controlled trials, comparing the ability of EFM to identify infants with acidemia and morbidity to intermittent auscultation or intermittent EFM.13 While continuous EFM was associated with a decrease in neonatal seizures, there was no evidence that EFM reduced the risk of acidemia or any other important measure or marker of neonatal morbidity or mortality.13 Importantly, the evidence on association between EFM and acidemia was low quality and prone to selection bias.13 Our study was in response to the summary document from the 2008 consensus conference on EFM, which published the NICHD Category system for describing and defining EFM patterns, and specified the need for observation studies to describe specific patterns as well focus on indeterminate (Category II) patterns and association with varying outcomes.18
EFM is used as a screening test, applied to almost every woman presenting for labor and delivery in a hospital setting, but also as a diagnostic test, with the expectation that EFM can identify infants with acidemia and enable clinical intervention to improve outcomes. Prior research described poor test characteristics for using EFM either as a screening or diagnostic test for cerebral palsy.30 Our findings describe better test properties than previously published for the ability of EFM to differentiate between term infants with acidemia and risk of morbidity and those without. While sensitivity remains weak, we found reasonable predictive ability of specific patterns for acidemia and morbidity with the suggested NNT of cesareans to prevent one case being 5 and 6, respectively. These findings regarding deceleration area are also consistent with abnormal in The International Federation of Gynecology and Obstetrics (FIGO) classification system.31
We previously performed a retrospective cohort study of over 5000 women describing the frequency and estimating the relationship between EFM patterns in the 30 minutes prior to delivery and acidemia.32 Findings suggested prolonged, late and variable decelerations were associated with acidemia and, for non-NICHD features, deceleration area was most predictive of acidemia.32 However, this study was limited by its retrospective nature, with patients only in the second stage of labor and with EFM patterns in the last 30 minutes prior to delivery.32 A recently published case-control study of 204 infants also found that deceleration area was the superior feature to identify infants with acidemia.33 These led us to hypothesize that deceleration area might be an important predictor of acidemia and morbidity within a large, modern, and unselected cohort. Additionally, the predictive value of tachycardia within our study remains consistent with findings from animal studies, which suggests that with labor-like cord occlusions there is an increase in the magnitude and slope of the fetal heart rate. 34,35 The physiology of decelerations has been well-described36–40 and, regardless of type, they represent periods of compromised gas exchange for the fetus. Furthermore, the authors acknowledge the conflict between the uses of low pH as an outcome for the sensitivity and specificity of EFM tracings, given the primary purpose is to avoid fetal acidemia; however, this study provides evidence for providers regarding interpretation of Category II patterns and the potential to prospectively test future impact on acidemia and morbidity.34,35 The temporal relationships of EFM patterns are important because prior animal studies also suggest the possibility that rather than pH falling over time, it is the ability to restore normal pH with multiple and prolonged periods of impaired gas exchange that explains the acidemia. This explanation would also be supported by findings of the importance of deceleration area, such as in the present study.41–43
In this study, the policies of universal cord gas acquisition and continuous intrapartum EFM significantly reduced selection bias, inherent in previous studies where the measurement of umbilical artery pH was dependent on physician judgment and the clinical condition of the infant. Additionally, the large prospective design allowed collection of neonatal morbidities, which are rare at term, enabling estimation of the association of EFM patterns with neonatal morbidity. Formally trained obstetric research nurses interpreted the EFM recordings blind to all clinical data.
Some potential limitations include the inability to necessarily generalize these findings to intrapartum EFM patterns of pregnancies less than 37 weeks and those with known anomalies. We did not use computerized analysis of the EFM patterns because human visual interpretation was the basis for the NICHD categories, and importantly, it is how EFM is used clinically. Furthermore, the authors acknowledge the conflict between the uses of low pH as an outcome for the sensitivity and specificity of EFM tracings, given the primary purpose is to avoid fetal acidemia; however, this study provides the potential for physicians to gain additional knowledge to clinically intervene in future obstetric care. We chose a pH cut-off of 7.10 to explore the ability of EFM patterns to predict infants who have developed an abnormal pH but are not yet in a pH range where the morbidity risk is very high (i.e. below 7.00). However, in choosing this threshold, we did not explore the ability of patterns to predict milder pH abnormalities. Another possible limitation is that the inherent observational nature of this research design, although obligatory to complete this study, may have impacted prevalence estimates because of the latent impact of obstetric interventions. However, it is also important that the key predictive features of EFM in our findings are not currently used in clinical practice and thus, it should not have influenced the estimates of this study. For this reason, the findings are useful in the context of EFM, and the prospective use of these findings and features could assist in intervening and improving outcomes. Deceleration area was estimated, but potential inaccuracies would have been non-differential with respect to presence or absence of acidemia. We assessed EFM patterns and features only in the last 120 min prior to delivery. While features within this time period proximal to delivery would be most associated with outcomes, it does not take into account earlier events and EFM features. Despite these potential limitations, our findings contribute to the paucity of existing literature on intrapartum EFM patterns.
The clinical translation of our findings is an important consideration. Tachycardia is a visually identifiable EFM pattern, but deceleration is less so. We found the respective Youden cut points of deceleration area for optimal prediction to be 42,152 and 50,761, for acidemia and morbidity respectively. If a patient contracts every 3 minutes in the last 120 minutes prior to delivery, she will have about 40 contractions. If decelerations 60 beats below the baseline for 60 seconds occur with even 24 or 28 of those, respectively, she will reach that threshold regardless of the type of decelerations. Clinically, the current approach to EFM patterns can include position change, blood pressure management, oxygen supplementation, and amnioinfusion.44,45 However, there is no guidance regarding how long to observe these patterns before deciding to intervene with delivery. Our findings offer guidance as to objectively define thresholds at which the risk of acidemia and morbidity are such that the number of cesareans needed-to-be performed to prevent one case of acidemia or morbidity are relatively low.
In summary, we found that specific EFM patterns associated with and predictive of acidemia and morbidity at near-term and term. Persistent Category I, and any 10-minute period of Category III, were significantly associated with normal pH and acidemia, respectively. Deceleration area was most predictive of acidemia, and in combination with any 10-minute period of heart rate baseline tachycardia for neonatal morbidity, even when periods of moderate variability were present. While EFM remains a poor screening test, clinically, these patterns can help identify infants developing acidemia. At identifiable thresholds of deceleration area, the NNT of cesareans to potentially prevent one case of acidemia or morbidity was relatively low. These specific features of EFM patterns should be considered in the clinical interpretation and algorithms to improve the performance of EFM.
Implications and Contributions.
We aimed to measure EFM patterns in the two hours before delivery and assess their association with acidemia and neonatal morbidity among term infants.
Deceleration area, and deceleration area combined with tachycardia, were most discriminatory for acidemia and neonatal morbidity, respectively.
In the setting of high total deceleration area, the number of cesareans needed-to-be performed to prevent one case of acidemia or morbidity is relatively low. These specific features of EFM patterns should be incorporated into clinical interpretation and algorithms to improve the performance of EFM.
Acknowledgments
Sources of Support: Dr. Alison Cahill received was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01: HD 06161619-01A1).
The authors would like to acknowledge Elena Deych, MS and William D. Shannon, PhD, MBA for consultation and preliminary data analyses.
Footnotes
Conflict of Interest Statement: The authors report no conflicts of interest.
Authors’ contributions: Study concept and design: Drs. Cahill, Tuuli, Macones. Data collection: Drs. Cahill, Tuuli, Stout, Macones. Analysis and interpretation of data: Drs. Cahill, Tuuli, Stout, Macones, López. Drafting of manuscript: Drs. Cahill, Tuuli, Stout, Macones, López. Critical revision of the manuscript: Drs. Cahill, Tuuli, Stout, Macones, López. Obtain funding: Drs. Cahill, Tuuli, Macones.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey RE. Intrapartum fetal monitoring. American family physician. 2009;80(12):1388–1396. [PubMed] [Google Scholar]
- 2.McCusker J, Harris DR, Hosmer DW. Association of electronic fetal monitoring during labor with cesarean section rate and with neonatal morbidity and mortality. American Journal of Public Health. 1988;78(9):1170–1174. doi: 10.2105/ajph.78.9.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613–618. doi: 10.1056/NEJM199603073341001. [DOI] [PubMed] [Google Scholar]
- 4.Ruis KA, Ruis KA, Lehmann CU, Northington FJ, Lin DD, Graham EM. Neonatal brain imaging and the identification of metabolic acidemia and hypoxic-ischemic encephalopathy. J Matern Fetal Neonatal Med. 2009;22(10):823–828. doi: 10.1080/14767050902769990. [DOI] [PubMed] [Google Scholar]
- 5.Andres RL, Saade G, Gilstrap LC, et al. Association between umbilical blood gas parameters and neonatal morbidity and death in neonates with pathologic fetal acidemia. American journal of obstetrics and gynecology. 1999;181(4):867–871. doi: 10.1016/s0002-9378(99)70316-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Chauhan SP, Ananth CV, Vintzileos AM, Abuhamad AZ. Electronic fetal heart rate monitoring and its relationship to neonatal and infant mortality in the United States. American journal of obstetrics and gynecology. 2011;204(6):491 e491–410. doi: 10.1016/j.ajog.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Sholapurkar SL. Computerised interpretation of fetal heart rate patterns and correlation with fetal acidaemia. BJOG: An International Journal of Obstetrics & Gynaecology. 2014;121(13):1746–1747. doi: 10.1111/1471-0528.13039. [DOI] [PubMed] [Google Scholar]
- 8.Ananth CV, Chauhan SP, Chen H-Y, D’Alton ME, Vintzileos AM. Electronic Fetal Monitoring in the United States: Temporal Trends and Adverse Perinatal Outcomes. Obstetrics & Gynecology. 2013;121(5):927–933. doi: 10.1097/AOG.0b013e318289510d. [DOI] [PubMed] [Google Scholar]
- 9.Thacker SB, Stroup D, Chang M-h. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane database of systematic reviews. 2006;(3) doi: 10.1002/14651858.CD000063.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham EM, Petersen SM, Christo DK, Fox HE. Intrapartum Electronic Fetal Heart Rate Monitoring and the Prevention of Perinatal Brain Injury. Obstetrics & Gynecology. 2006;108(3 Part 1):656–666. doi: 10.1097/01.AOG.0000230533.62760.ef. [DOI] [PubMed] [Google Scholar]
- 11.Kubli FW, Hon EH, Khazin AF, Takemura H. Observations on heart rate and pH in the human fetus during labor. American journal of obstetrics and gynecology. 1969;104(8):1190–1206. doi: 10.1016/s0002-9378(16)34294-6. [DOI] [PubMed] [Google Scholar]
- 12.Impey L, Reynolds M, MacQuillan K, Gates S, Murphy J, Sheil O. Admission cardiotocography: a randomised controlled trial. The Lancet. 2003;361(9356):465–470. doi: 10.1016/S0140-6736(03)12464-6. [DOI] [PubMed] [Google Scholar]
- 13.Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;(5):Cd006066. doi: 10.1002/14651858.CD006066. [DOI] [PubMed] [Google Scholar]
- 14.Shy KK, Luthy DA, Bennett FC, et al. Effects of Electronic Fetal-Heart-Rate Monitoring, as Compared with Periodic Auscultation, on the Neurologic Development of Premature Infants. New England Journal of Medicine. 1990;322(9):588–593. doi: 10.1056/NEJM199003013220904. [DOI] [PubMed] [Google Scholar]
- 15.Vintzileos AM, Antsaklis A, Varvarigos I, Papas C, Sofatzis I, Montgomery JT. A randomized trial of intrapartum electronic fetal heart rate monitoring versus intermittent auscultation. Obstetrics and gynecology. 1993;81(6):899–907. [PubMed] [Google Scholar]
- 16.MacDonald D, Grant A, Sheridan-Pereira M, Boylan P, Chalmers I. The Dublin randomized controlled trial of intrapartum fetal heart rate monitoring. American journal of obstetrics and gynecology. 1985;152(5):524–539. doi: 10.1016/0002-9378(85)90619-2. [DOI] [PubMed] [Google Scholar]
- 17.Vintzileos AM, Smulian JC. Decelerations, tachycardia, and decreased variability: have we overlooked the significance of longitudinal fetal heart rate changes for detecting intrapartum fetal hypoxia? American journal of obstetrics and gynecology. 2016;215(3):261–264. doi: 10.1016/j.ajog.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 18.Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Journal of obstetric, gynecologic, and neonatal nursing: JOGNN/NAACOG. 2008;37(5):510–515. doi: 10.1111/j.1552-6909.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 19.Donker DK, van Geijn HP, Hasman A. Interobserver variation in the assessment of fetal heart rate recordings. Eur J Obstet Gynecol Reprod Biol. 1993;52(1):21–28. doi: 10.1016/0028-2243(93)90220-7. [DOI] [PubMed] [Google Scholar]
- 20.ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstetrics and gynecology. 2009;114(1):192–202. doi: 10.1097/AOG.0b013e3181aef106. [DOI] [PubMed] [Google Scholar]
- 21.Giannubilo SR, Buscicchio G, Gentilucci L, Palla GP, Tranquilli AL. Deceleration area of fetal heart rate trace and fetal acidemia at delivery: a case-control study. J Matern Fetal Neonatal Med. 2007;20(2):141–144. doi: 10.1080/14767050601134603. [DOI] [PubMed] [Google Scholar]
- 22.TOURNAIRE M, YEH S-Y, FORSYTHE A, HON EH. A Study of Fetal Heart Rate Deceleration Areas: I. Preliminary Exploration. Obstetrics & Gynecology. 1973;42(5):711–717. [PubMed] [Google Scholar]
- 23.Shelley T, Tipton RH. Dip area. A quantitative measure of fetal heart rate patterns. The Journal of obstetrics and gynaecology of the British Commonwealth. 1971;78(8):694–701. doi: 10.1111/j.1471-0528.1971.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 24.Tipton RH, Shelley TS. An index of fetal welfare in labour. The Journal of obstetrics and gynaecology of the British Commonwealth. 1971;78(8):702–706. doi: 10.1111/j.1471-0528.1971.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 26.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biometrical journal Biometrische Zeitschrift. 2008;50(3):419–430. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parer JT, King T, Flanders S, Fox M, Kilpatrick SJ. Fetal acidemia and electronic fetal heart rate patterns: is there evidence of an association? J Matern Fetal Neonatal Med. 2006;19(5):289–294. doi: 10.1080/14767050500526172. [DOI] [PubMed] [Google Scholar]
- 28.Clark SL, Hamilton EF, Garite TJ, Timmins A, Warrick PA, Smith S. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. American journal of obstetrics and gynecology. 2017;216(2):163.e161–163.e166. doi: 10.1016/j.ajog.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Michikata K, Sameshima H, Urabe H, Tokunaga S, Kodama Y, Ikenoue T. The Regional Centralization of Electronic Fetal Heart Rate Monitoring and Its Impact on Neonatal Acidemia and the Cesarean Birth Rate. Journal of Pregnancy. 2016;2016:7. doi: 10.1155/2016/3658527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. Multivariate analysis of risk. N Engl J Med. 1986;315(2):81–86. doi: 10.1056/NEJM198607103150202. [DOI] [PubMed] [Google Scholar]
- 31.Ayres-de-Campos D, Spong CY, Chandraharan E. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynaecol Obstet. 2015;131(1):13–24. doi: 10.1016/j.ijgo.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Cahill AG, Roehl KA, Odibo AO, Macones GA. Association and prediction of neonatal acidemia. American journal of obstetrics and gynecology. 2012;207(3):206 e201–208. doi: 10.1016/j.ajog.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 33.Martí Gamboa S, Lapresta Moros M, Pascual Mancho J, Lapresta Moros C, Castán Mateo S. Deceleration area and fetal acidemia. The Journal of Maternal-Fetal & Neonatal Medicine. 2016:1–7. doi: 10.1080/14767058.2016.1256993. [DOI] [PubMed] [Google Scholar]
- 34.Bennet L, Westgate JA, Liu YC, Wassink G, Gunn AJ. Fetal acidosis and hypotension during repeated umbilical cord occlusions are associated with enhanced chemoreflex responses in near-term fetal sheep. Journal of applied physiology (Bethesda, Md: 1985) 2005;99(4):1477–1482. doi: 10.1152/japplphysiol.00431.2005. [DOI] [PubMed] [Google Scholar]
- 35.Westgate JA, Bennet L, de Haan HH, Gunn AJ. Fetal heart rate overshoot during repeated umbilical cord occlusion in sheep. Obstetrics and gynecology. 2001;97(3):454–459. doi: 10.1016/s0029-7844(00)01123-6. [DOI] [PubMed] [Google Scholar]
- 36.Cahill AG, Roehl KA, Odibo AO, Macones GA. Association of atypical decelerations with acidemia. Obstetrics and gynecology. 2012;120(6):1387–1393. doi: 10.1097/aog.0b013e3182733b6e. [DOI] [PubMed] [Google Scholar]
- 37.Gaziano EP. A study of variable decelerations in association with other heart rate patterns during monitored labor. American journal of obstetrics and gynecology. 1979;135(3):360–363. doi: 10.1016/0002-9378(79)90705-1. [DOI] [PubMed] [Google Scholar]
- 38.Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. VIII. Atypical variable decelerations. American journal of obstetrics and gynecology. 1983;145(3):297–305. [PubMed] [Google Scholar]
- 39.Sameshima H, Ikenoue T. Predictive value of late decelerations for fetal acidemia in unselective low-risk pregnancies. Am J Perinatol. 2005;22(1):19–23. doi: 10.1055/s-2004-837272. [DOI] [PubMed] [Google Scholar]
- 40.Lear CA, Galinsky R, Wassink G, et al. Sympathetic neural activation does not mediate heart rate variability during repeated brief umbilical cord occlusions in near-term fetal sheep. The Journal of physiology. 2016;594(5):1265–1277. doi: 10.1113/JP270125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omo-Aghoja L. Maternal and fetal Acid-base chemistry: a major determinant of perinatal outcome. Annals of medical and health sciences research. 2014;4(1):8–17. doi: 10.4103/2141-9248.126602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Low JA, Lindsay BG, Derrick EJ. Threshold of metabolic acidosis associated with newborn complications. American journal of obstetrics and gynecology. 1997;177(6):1391–1394. doi: 10.1016/s0002-9378(97)70080-2. [DOI] [PubMed] [Google Scholar]
- 43.Derks JB, Oudijk MA, Torrance HL, et al. Allopurinol Reduces Oxidative Stress in the Ovine Fetal Cardiovascular System After Repeated Episodes of Ischemia-Reperfusion. Pediatr Res. 2010;68(5):374–380. doi: 10.1203/PDR.0b013e3181ef7780. [DOI] [PubMed] [Google Scholar]
- 44.Practice bulletin no. 116: Management of intrapartum fetal heart rate tracings. Obstetrics and gynecology. 2010;116(5):1232–1240. doi: 10.1097/AOG.0b013e3182004fa9. [DOI] [PubMed] [Google Scholar]
- 45.Intermittent Auscultation for Intrapartum Fetal Heart Rate Surveillance. ACNM Clinical Bulletin Number 11, March 2010 (Replaces Number 9, March 2007) The Journal of Midwifery & Women’s Health. 2010;55(4):397–403. doi: 10.1016/j.jmwh.2010.05.007. [DOI] [PubMed] [Google Scholar]