Abstract
T-cell lymphoma is a rare hematologic malignancy with an incidence rate between 10% and 20% of that of non-Hodgkin lymphomas. Patients with peripheral T-cell lymphoma (PTCL) generally have a poor prognosis when treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP)/CHOP-like chemotherapy; once relapse occurs, it is mostly regarded as an incurable disease. To overcome the chemorefractoriness of PTCL, several novel agents have been developed. Since the first approval of pralatrexate, a dihydrofolate reductase inhibitor, for relapsed/refractory PTCL by the US Food and Drug Administration, several new agents, such as romidepsin (histone deacetylase inhibitor), brentuximab vedotin (antibody–drug conjugate targeting CD30), chidamide (histone deacetylase inhibitor), and mogamulizumab (anti-CC chemokine receptor 4 monoclonal antibody), have been approved as a therapeutic option for relapsed/refractory PTCL in several countries, including the US, Europe, China, and Japan. Forodesine is a novel, potent purine nucleoside phosphorylase inhibitor that is effective against T-cell malignancies. Although the clinical development of forodesine was discontinued in the US and Europe, a multicenter Phase I/II study of oral forodesine for relapsed PTCL was recently completed in Japan. The overall response rate was 24% (10 of 41 patients), which included four patients with complete response. In general, the toxicity of forodesine is manageable. As the study met the primary end point, forodesine was approved for the treatment of relapsed/refractory PTCL in Japan in March 2017, which was the first approval of forodesine in the world. As forodesine is an oral formulation, it is more convenient than other novel intravenous agents approved for PTCL. However, it is necessary to appropriately manage opportunistic infections and secondary lymphomas possibly associated with long-lasting lymphocytopenia caused by forodesine. In this manuscript, we have summarized the currently available evidence for forodesine and discussed the clinical implications for PTCL treatment.
Keywords: forodesine, PTCL, T-cell lymphoma, new agents, lymphoma, non-Hodgkin lymphoma, PNP, purine nucleoside phosphorylase
Introduction
T-cell/natural killer (NK)-cell lymphoma is a rare lymphoid neoplasm and its incidence rate is 10%–20% of that of non-Hodgkin lymphomas (NHLs).1,2 T/NK-cell lymphoma is divided into several histologic subtypes based on the WHO classification,1 including peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS); angioimmunoblastic T-cell lymphoma (AITL); nodal peripheral T-cell lymphoma with T follicular helper (TFH) phenotype; anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL); ALK-negative ALCL, adult T-cell leukemia-lymphoma (ATL); and extranodal NK/T-cell lymphoma, nasal type (ENKL). Among these, ATL and ENKL are considered distinct disease entities associated with specific viral infections, and the recommended first-line treatment strategies are different from those of other T-cell lymphomas.3,4 Therefore, this review focuses on T-cell lymphomas except for ATL and ENKL.
Systemic T-cell lymphomas (so-called, peripheral T-cell lymphomas [PTCLs]) are difficult to treat and have unfavorable prognoses, mainly because most PTCLs are refractory to conventional cytotoxic chemotherapies. Except for ALK-positive ALCL with lower risk by International Prognostic Index, which has favorable prognosis,2 patients with PTCLs generally have poor prognosis when treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP)/CHOP-like chemotherapy; the 5-year progression-free survival (PFS) rate is 31%–36% and the 5-year overall survival (OS) rate is 48%–55%.5,6 Once relapse occurs, the disease is regarded as mostly incurable.7
To overcome the chemorefractoriness of PTCLs, several agents have been developed. Since the approval of pralatrexate, a dihydrofolate reductase inhibitor, for relapsed/refractory PTCL by the US Food and Drug Administration (FDA),8 several new agents, such as romidepsin (histone deacetylase inhibitor),9–12 brentuximab vedotin (antibody–drug conjugate targeting CD30),13–15 chidamide (histone deacetylase inhibitor),16 and mogamulizumab (anti-CC chemokine receptor 4 monoclonal antibody),17–20 have been approved as a therapeutic option for relapsed/refractory PTCL in several countries, including the US, Europe, China, and Japan. The results of the key trial of these agents are summarized in Table 1, and their schematic mechanisms of actions are shown in Figure 1.8–11,13–16,18–22
Table 1.
Clinical efficacy of selected novel agents for relapsed/refractory T-cell lymphoma
| Agents | Disease subtype | No of Pts | ORR (%) | CR rate (%) | Median PFS (months) | Median DoR (months) | References |
|---|---|---|---|---|---|---|---|
| Brentuximab vedotin | ALCL | 58 | 86 | 57 | 20 | 12.6 | Pro et al (2012)13 |
| CD30-positive PTCL | 34 | 41 | 24 | 2.6 | 6.7 | Horowitz et al (2014)14 | |
| CD30-positive CTCL | 64 | 67 | 16 | 16.7 | 16.7 | Prince et al (2017)15 | |
| Vorinostat | CTCL | 74 | 30 | NA | 4.9 | 5.6 | Mann et al (2007)21 |
| Romidepsin | CTCL | 71 | 34 | 5 | NA | 13.7 | Piekarz et al (2009)9 |
| CTCL | 96 | 38 | 1 | 8 | 15 | Whittaker et al (2010)10 | |
| PTCL | 130 | 25 | 15 | 4 | 28 | Coiffier et al (2014)11 | |
| Belinostat | PTCL | 120 | 26 | 11 | 1.6 | 13.6 | O’Connor et al (2015)22 |
| Chidamide | PTCL | 79 | 28 | 14 | 2.1 | 9.9 | Shi et al (2015)16 |
| Pralatrexate | PTCL | 111 | 29 | 11 | 3.5 | 10.1 | O’Connor et al (2011)8 |
| Mogamulizumab | CCR4-positive PTCL/CTCLa | 37 | 35 | 14 | 3 | NA | Ogura et al (2014)18 |
| CCR4-positive PTCL | 35 | 11 | 3 | 2 | 2.8 | Zinzani et al (2016)19 | |
| CTCL | 38 | 37 | 7.9 | 11.4 | 10.4 | Duvic et al (2015)20 | |
| Forodesine | PTCLa | 41 | 24 | 10 | 1.9 | 10.4 | Shibayama et al (2017)42 |
| CTCL | 101 | 11 | 0 | NA | 6.4 | Dummer et al (2014)39 |
Note:
Refractory patients were not included in these studies.
Abbreviations: ALCL, anaplastic large cell lymphoma; CCR4, CC chemokine receptor 4; CR, complete response; CTCL, cutaneous T-cell lymphoma; DoR, duration of response; NA, not available; ORR, overall response rate; PTCL, peripheral T-cell lymphoma; Pts, patients; PFS, progression-free survival.
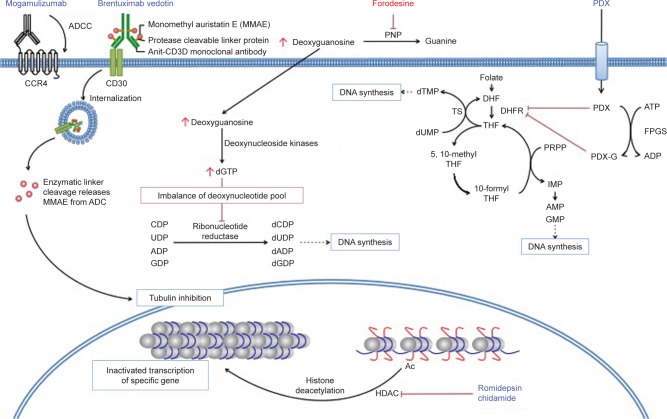
Figure 1.
Selected novel agents for PTCL.
Abbreviations: ADC, antibody–drug conjugate; ADCC, antibody-dependent cell-mediated cytotoxicity; CCR4, CC chemokine receptor 4; DHF, dihydrofolate; DHFR, dehydrofolate reductase; FPGS, folylpolyglutamate synthase; HDAC, histone deacetylase; IMP, inosine monophosphate; PDX, pralatrexate; PDX-G, polyglutamylated pralatrexate; PNP, purine nucleoside phosphorylase; PRPP, 5-phosphoribosyl-1-pyrophosphate; PTCL, peripheral T-cell lymphoma; THF, tetrahydrofolate; TS, thymidylate synthase.
Recently, forodesine (formerly known as BCX-1777), a novel purine nucleoside phosphorylase (PNP) inhibitor, was approved for the treatment of relapsed/refractory PTCL in Japan; this was the first approval of forodesine in the world. In this manuscript, we have summarized the currently available evidence for forodesine and discussed its clinical implications for the current and future treatment of PTCLs.
Role of PNP and effects of PNP inhibition
The enzyme PNP participates in the purine salvage pathway and is present at high levels in lymphoid tissues. It catalyzes the phosphorolysis of 2′-deoxyguanosine (dGuo) to a guanine base and 2′-deoxyribose-1-phosphate.23 PNP has been proposed as one of the most promising therapeutic targets for T-cell malignancies, because children born with PNP deficiency have severely reduced T-cell counts, with relatively normal B-cell immunity.24 Preclinical studies revealed that PNP deficiency increased the dGuo concentration in plasma and in T cells.25 An increased concentration of dGuo results in deoxyguanosine triphosphate (dGTP) accumulation (Figure 1). This causes an imbalance of the deoxynucleotide pool, which reduces the activity of ribonucleotide reductase and leads to T-cell apoptosis.26 Based on this hypothesis, the intravenous infusion therapy of dGuo was evaluated; however, this strategy was unable to achieve higher dGuo plasma levels because the half-life (t1/2) of plasma dGuo is only 12 s. Therefore, the pharmacological inhibition of PNP was thought to be a more suitable method to achieve T-cell suppression.
Several compounds that inhibit PNP have been developed, but most did not reach clinical trials because of low efficacy associated with a low inhibitory potency against PNP.27,28 Peldesine (BCX-34) is a relatively potent PNP inhibitor (IC50 [50% inhibitory concentration] = 30 nM).29 The compound was tested in patients with cutaneous T-cell lymphoma (CTCL) and psoriasis, which is a T-cell-mediated skin disorder. However, limited efficacy was observed, partly because of the rapid release of peldesine from PNP and the inability of peldesine to sufficiently inhibit PNP and elevate plasma dGuo to the level necessary for T-cell suppression. Furthermore, the pharmacokinetic (PK)/pharmacodynamic (PD) properties revealed that the oral dosing of peldesine could not achieve a sufficient increase in dGuo to suppress T cells.30,31
Forodesine is a novel, potent PNP inhibitor (IC50 = 0.48–1.57 nM).32,33 It is a transition state analog of PNP and is between 100- and 1,000-fold more potent than previous PNP inhibitors.34,35 Although forodesine is also a member of purine analogs, it contains nitrogen in the ring of D-ribofuranose and C-glycosidic bond (Figure 2). In contrast to other purine nucleoside analogs, forodesine does not act via direct incorporation into DNA but displays a highly selective PNP inhibition because of such a unique structure.
Figure 2.
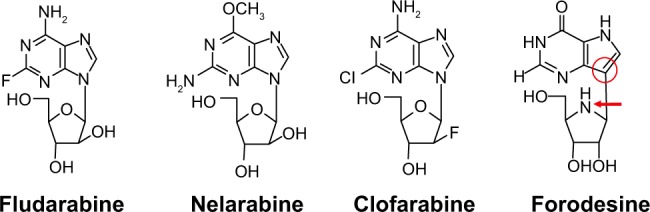
Structures of purine nucleoside analogs.
Notes: Forodesine is a member of purine nucleoside analogs. Unlike the other purine nucleoside analogs, forodesine contains nitrogen in the ring of D-ribofuranose (red arrow) and C-glycosidic bond (red ring).
Based on the promising preclinical results, clinical trials of forodesine for PTCL, CTCL, and T-cell leukemia have been conducted.
Clinical trials of forodesine
Early clinical trials for T-cell leukemia
Gandhi et al conducted the first-in-human Phase I study of forodesine in patients with relapsed/refractory T-cell malignancies.36 In this study, forodesine was administered intravenously via a 30-minute infusion, at a dose level of 40 mg/m2. The dosing pattern comprised a single administration on day 1, and then two administrations at 12-hour intervals on days 2–5; a 21- to 28-day cycle was used. The starting dose and schedule of forodesine were determined based on data from preclinical studies in primates. Based on the activity and toxicity, the suggested maximum daily exposure in primates was 20 mg/kg/day, which was considered to be the equivalent of 800 mg/m2/day.
Five patients with relapsed/refractory T-cell malignancies (T-acute lymphoblastic leukemia [T-ALL], n=2; T-prolymphocytic leukemia, n=3) received an intravenous infusion of forodesine. In all patients, a sufficient increase of plasma dGuo was observed. From a cellular pharmacology perspective, four out of five patients achieved a 10- to 20-fold increase in the intracellular dGTP level within 24 h of the first infusion. Although no objective responses were observed, the four patients with increased intracellular dGTP level had stable disease, whereas the remaining patients had progressive disease. Based on these results, the antitumor activity of forodesine was thought to be correlated with intracellular accumulation of dGTP. After the first five patients were enrolled, a review of the clinical and pharmacological data suggested that an alternative dosing schedule of forodesine should be considered; therefore, enrollment in this study was stopped. The PK investigations of forodesine in plasma showed that 4–8 μM concentration was achieved from a 40 mg/m2 starting dose, which was therefore sufficient to achieve effective inhibition. However, the peak level of forodesine (median 5.4 μM) and the long t1/2 (median 10 h) suggested that once-daily dosing of 40 mg/m2 might be sufficient to provide and maintain adequate drug exposure for the inhibition of PNP.
Subsequently, Furman et al conducted a single-arm Phase II study of intravenous forodesine in patients with relapsed/refractory T-ALL.37 At the time of the interim analysis of this study, 34 patients were enrolled. The patients were intravenously administered forodesine 40 mg/m2/day for 5 days each week (one cycle) for a total of six cycles. The dose was escalated to 90 mg/m2 in patients who did not respond after cycle 2. The overall response rate (ORR) was 32% (11/34) with seven complete responses (CRs) and four partial responses (PRs). One patient who showed CR had continued to receive treatment for almost 1 year. Two patients with CR proceeded to hematopoietic stem cell transplantation after remission. In total, 18 patients received dose escalation to 90 mg/m2 after cycle 2. However, none of these patients demonstrated an improved response after dose escalation.
Overall, intravenous forodesine was reasonably effective as a single agent for relapsed/refractory T-ALL, with manageable toxicity. However, the subsequent clinical data for forodesine in T-ALL have not been published.
Clinical trials for CTCL
Duvic et al conducted a Phase I/II trial of oral forodesine in patients with relapsed/refractory CTCL.38 This study enrolled patients with stage IB disease or higher, who were orally administered forodesine between 40 and 320 mg/m2, once daily for 4 weeks, to determine the maximum tolerated dose (MTD) and/or the optimal biological dose based on the PK and PNP inhibition, as evidenced by the elevation of plasma dGuo levels. However, MTD was not reached at any dose level. Based on the plateau of the area under the curve (AUC) versus dose plot at 80 mg/m2 and above, and the same observations for plasma dGuo versus dose, the once daily administration of 80 mg/m2 was judged as the optimal biological dose. In the 36 patients treated at this dose, the ORR with modified severity-weighted assessment tool was 39% (14/36), with a median duration of response of 127 days. The grade 3 or higher subjective adverse events related to the study treatment were vertigo, diarrhea, generalized edema, and pneumonia (one each). With regard to laboratory events, the grade 3 elevations of AST, ALT, bilirubin, and alkaline phosphatase was observed in one patient. Grade 3 or higher lymphocytopenia and low CD4 counts were observed in 71% and 31% of patients, respectively.
Subsequently, a multicenter Phase II study of forodesine for relapsed/refractory CTCL at a fixed dose of 200 mg/day until disease progression was conducted.39 In total, 144 patients who had received three or more prior lines of systemic therapies were enrolled. Unlike the previous promising results, no CRs occurred and only 11% (11 of 101 modified intent-to-treat population) of patients achieved PRs; 50% (51/101) had stable disease and 35% (35/101) had progressive disease. Despite some durable responses (median of 261 days until progression), further clinical development for CTCL was halted.
Clinical trials for PTCL
In Japan, a Phase I study of oral forodesine in patients with relapsed/refractory mature T/NK-cell malignancy was conducted.40 In this study, three dose levels (100, 200, and 300 mg, once daily) have been evaluated; 13 patients with relapsed/refractory PTCL and CTCL (five in the 100 mg cohort; three in the 200 mg cohort; five in the 300 mg cohort) were enrolled. One patient with ALK-negative ALCL treated at 100 mg/day achieved CR, and PR was observed in two patients with CTCL treated at 200 mg/day. With respect to the safety profile, the major adverse events of grade 3 or higher were lymphocytopenia (8/13, 62%), anemia (2/13, 15%), leukocytopenia (1/13, 8%), and pyrexia (1/13, 8%). No dose-limiting toxicity (DLT) was observed, even at the highest dose, and MTD was not determined. These data suggested that the MTD of forodesine might be much higher than that in this Phase I study. Based on the PK/PD data obtained from healthy volunteers (not published) in the Western countries, twice-daily (BID) administration of oral forodesine 300 mg was equivalent to intravenous forodesine 40 mg/m2 once daily, which was the dose tested in T-ALL and CTCL. Furthermore, the PK profiles (AUC, Cmax, and Cmin) of 300 mg BID were significantly higher than that of 200 mg BID, whereas the profiles of 300 mg BID and 400 mg BID were not. Therefore, the optimal dose of oral forodesine was expected to be 300 mg BID.
Subsequently, a pivotal single-arm Phase I/II study of oral forodesine at a higher dose was conducted in patients with relapsed PTCL in Japan (NCT01776411).41,42 The primary end point of the Phase II portion was the ORR; the sample size of the Phase II portion was calculated to require at least 43 patients based on Simon’s Minimax method. The threshold response rate was 10%, and the expected response rate was 25%. The response was assessed by using computed tomography scanning and positron emission tomography scanning by an independent imaging review using IWC 2007 criteria.43
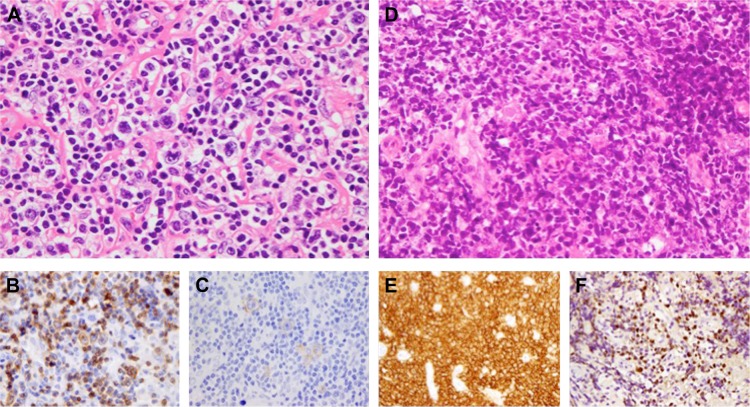
In the Phase I portion, 3+3 design was used and four patients received 300 mg BID (600 mg/day). No DLTs were observed in the three evaluable patients. Therefore, the recommended Phase II dose was determined to be 300 mg BID. In the Phase II portion, 44 patients received oral forodesine at 300 mg BID. The results presented at the American Society of Hematology annual meeting in 2017 reported the ORR in 41 evaluable patients as 24% (10/41), which included four patients (10%) with CRs.42 The median PFS and OS were 1.9 and 15.6 months, respectively, and the 2-year OS was 39%. From a toxicity perspective, serious adverse events were reported in 46% of patients (22/48). The most common serious adverse events were pneumonia (8%, 4/48), pyrexia (6%, 3/48), Pneumocystis jirovecii pneumonia (4%, 2/48), and anemia (4%, 2/48). Grade 4 lymphocytopenia, observed in 81% of patients, was thought to be a major cause of opportunistic infections during the study treatment. Furthermore, five patients (three patients with AITL and two patients with PTCL-NOS) developed diffuse large B-cell lymphoma (DLBCL). Four of the five patients had Epstein–Barr virus (EBV)-positive DLBCL, which suggested that durable severe lymphocytopenia might be associated with the development of these secondary lymphomas. For example, the histopathological findings of EBV-positive DLBCL that developed during the administration of forodesine (not in this trial, but in our institution) are shown in Figure 3.
Figure 3.
Epstein–Barr virus (EBV)-positive diffuse large B-cell lymphoma (DLBCL) developed during forodesine therapy.
Notes: A 68-year-old female was diagnosed with anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma. Lymph node biopsy at the initial diagnosis revealed the disseminated presentation of large atypical lymphocytes (A, hematoxylin and eosin, ×600). Immunohistochemical staining revealed that the tumor cells were positive for CD3 (B, ×600) and CD30 (C, ×600). The patient received eight cycles of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) and achieved complete remission. Six months after the completion of CHOP chemotherapy, the first relapse was confirmed and the patient joined the Phase I study of oral forodesine.35 After 4 years of forodesine administration (100 mg, once daily), the patient experienced epigastric pain. Endoscopic examination revealed gastric ulcer lesions and a biopsy demonstrated the diffuse growth of large atypical lymphocytes (D, hematoxylin and eosin, ×400). Immunohistochemical staining revealed that the tumor cells were positive for CD20 (E, ×400). EBV early RNA 1 (EBER1) expression was detected in tumor cells by in situ hybridization (F, ×400). The patient was diagnosed with EBV-positive DLBCL. The patient received cyclophosphamide, vincristine, procarbazine, and prednisolone (C-MOPP) in combination with rituximab, and subsequently achieved complete remission.
Based on the results of this pivotal study, oral forodesine was approved for the treatment of relapsed PTCLs in Japan in March 2017. Although this study met the primary end point, careful consideration is required in the application of this evidence into daily clinical practice, because: 1) the threshold response rate of 10% appears too low considering the efficacy of recently approved agents for T-cell lymphomas; 2) the study did not include refractory patients; and 3) the incidence of secondary EBV-positive DLBCL (10%, 5 out of 48 patients) is relatively high.
Conclusion and future perspectives
PNP inhibition has been expected to be a novel treatment option for the treatment of T-cell lymphomas for many years; forodesine finally achieved its first approval for relapsed/refractory PTCL in Japan. Oral forodesine (300 mg BID) appeared to show better efficacy than that observed in lower dose previous trials of forodesine for PTCL and CTCL. In general, the toxicity of forodesine is manageable, and the oral formulation is more convenient than other novel intravenous agents approved for T-cell lymphomas. However, it is necessary to carefully manage opportunistic infections and secondary lymphomas possibly associated with durable lymphocytopenia.
Forodesine may contribute as one of the reasonable options for the treatment of relapsed T-cell lymphomas that have unmet medical needs. Although the ORR was not very high, some patients with T-cell lymphomas who received forodesine displayed favorable responses with long response durations. Further investigations to find the predictive markers of forodesine are warranted, as they may identify the patients who will receive maximum benefit from this novel oral agent.
Acknowledgments
This work was partially supported by the National Cancer Center Research and Development Fund (26-A-4, 29-A-3).
Footnotes
Disclosure
DM had received honoraria from Takeda, Janssen, and Eisai and research funding from Dai-ichi Sankyo, Chugai, Kyowa Hakko Kirin, Ono, Celgene, Janssen, GSK, Eisai, Mundipharma, Takeda, AbbVie, MSD, Sanofi, Pfizer, Otsuka, Novartis, Solasia, and Zenyaku. KI has received research funding from Celgene, Daiichi Sankyo, Eisai, HUYA Bioscience International, Mundipharma, Solasia, and Takeda; consultancy from Celgene; and honararia from Eisai, Kyowa Hakko Kirin, and Takeda. KT has received research funding from Eisai, Mundipharma, Celgene, Solasia Pharma, Kyowa Hakko Kirin, and HUYA Bioscience International. The authors report no other conflicts of interest in this work.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D, International T-Cell Lymphoma Project International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 3.Makita S, Tobinai K. Disease oriented treatment of T-cell lymphoma. Hematol Oncol. 2017;35:54–59. doi: 10.1002/hon.2401. [DOI] [PubMed] [Google Scholar]
- 4.Makita S, Tobinai K. Clinical features and current optimal management of natural killer/T-cell lymphoma. Hematol Oncol Clin North Am. 2017;31:239–253. doi: 10.1016/j.hoc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Reimer P, Rüdiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 6.Kitahara H, Maruyama D, Maeshima AM, et al. Prognosis of patients with peripheral T cell lymphoma who achieve complete response after CHOP/CHOP-like chemotherapy without autologous stem cell transplantation as an initial treatment. Ann Hematol. 2017;96:411–420. doi: 10.1007/s00277-016-2891-8. [DOI] [PubMed] [Google Scholar]
- 7.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 11.Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11. doi: 10.1186/1756-8722-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maruyama D, Tobinai K, Ogura M, et al. Romidepsin in Japanese patients with relapsed or refractory peripheral T-cell lymphoma: a phase I/II and pharmacokinetics study. Int J Hematol. 2017;106:655–665. doi: 10.1007/s12185-017-2286-1. [DOI] [PubMed] [Google Scholar]
- 13.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123:3095–3100. doi: 10.1182/blood-2013-12-542142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390:555–566. doi: 10.1016/S0140-6736(17)31266-7. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y, Dong M, Hong X, et al. Results from a multicenter, openlabel, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766–1771. doi: 10.1093/annonc/mdv237. [DOI] [PubMed] [Google Scholar]
- 17.Makita S, Tobinai K. Mogamulizumab for the treatment of T-cell lymphoma. Expert Opin Biol Ther. 2017;17:1145–1153. doi: 10.1080/14712598.2017.1347634. [DOI] [PubMed] [Google Scholar]
- 18.Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32:1157–1163. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 19.Zinzani PL, Karlin L, Radford J, et al. European phase II study of mogamulizumab, an anti-CCR4 monoclonal antibody, in relapsed/refractory peripheral T-cell lymphoma. Haematologica. 2016;101:e407–e410. doi: 10.3324/haematol.2016.146977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duvic M, Pinter-Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125:1883–1889. doi: 10.1182/blood-2014-09-600924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13:2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33:2492–2499. doi: 10.1200/JCO.2014.59.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BK, Cha S, Parks RE., Jr Purine nucleoside phosphorylase from human erythrocytes. I. Purification and properties. J Biol Chem. 1968;243:1763–1770. [PubMed] [Google Scholar]
- 24.Giblett ER, Ammann AJ, Wara DW, Sandman R, Diamond LK. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975;1:1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- 25.Carson DA, Kaye J, Seegmiller JE. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s) Proc Natl Acad Sci U S A. 1977;74:5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carson DA, Kaye J, Matsumoto S, Seegmiller JE, Thompson L. Biochemical basis for the enhanced toxicity of deoxyribonucleosides toward malignant human T cell lines. Proc Natl Acad Sci U S A. 1979;76:2430–2433. doi: 10.1073/pnas.76.5.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazmers IS, Mitchell BS, Dadonna PE, et al. Inhibition of purine nucleoside phosphorylase by 8-aminoguanosine: selective toxicity for T lymphoblasts. Science. 1981;214:1137–1139. doi: 10.1126/science.6795718. [DOI] [PubMed] [Google Scholar]
- 28.Gilbertsen RB, Josyula U, Sircar JC, et al. Comparative in vitro and in vivo activities of two 9-deazaguanine analog inhibitors of purine nucleoside phosphorylase, CI-972 and PD 141955. Biochem Pharmacol. 1992;44:996–999. doi: 10.1016/0006-2952(92)90135-6. [DOI] [PubMed] [Google Scholar]
- 29.Viegas TX, Omura GA, Stoltz RR, Kisicki J. Pharmacokinetics and pharmacodynamics of peldesine (BCX-34), a purine nucleoside phosphorylase inhibitor, following single and multiple oral doses in healthy volunteers. J Clin Pharmacol. 2000;40:410–420. doi: 10.1177/00912700022008991. [DOI] [PubMed] [Google Scholar]
- 30.Morris PE, Jr, Elliott AJ, Walton SP, Williams CH, Montgomery JA. Synthesis and biological activity of a novel class of purine nucleoside phosphorylase inhibitors. Nucleosides Nucleotides Nucleic Acids. 2000;19:379–404. doi: 10.1080/15257770008033016. [DOI] [PubMed] [Google Scholar]
- 31.Conry RM, Bantia S, Turner HS, et al. Effects of a novel purine nucleoside phosphorylase inhibitor, BCX-34, on activation and proliferation of normal human lymphoid cells. Immunopharmacology. 1998;40:1–9. doi: 10.1016/s0162-3109(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 32.Bantia S, Miller PJ, Parker CD, et al. Purine nucleoside phosphorylase inhibitor BCX-1777 (Immucillin-H) – a novel potent and orally active immunosuppressive agent. Int Immunopharmacol. 2001;1:1199–1210. doi: 10.1016/s1567-5769(01)00056-x. [DOI] [PubMed] [Google Scholar]
- 33.Kilpatrick JM, Morris PE, Serota DG, Jr, et al. Intravenous and oral pharmacokinetic study of BCX-1777, a novel purine nucleoside phosphorylase transition-state inhibitor. In vivo effects on blood 2′-deoxyguanosine in primates. Int Immunopharmacol. 2003;3:541–548. doi: 10.1016/S1567-5769(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 34.Kicska GA, Long L, Hörig H, et al. Immucillin H, a powerful transition-state analog inhibitor of purine nucleoside phosphorylase, selectively inhibits human T lymphocytes. Proc Natl Acad Sci U S A. 2001;98:4593–4598. doi: 10.1073/pnas.071050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Kali A, Gandhi V, Ayoubi M, Keating M, Ravandi F. Forodesine: review of preclinical and clinical data. Future Oncol. 2010;6:1211–1217. doi: 10.2217/fon.10.83. [DOI] [PubMed] [Google Scholar]
- 36.Gandhi V, Kilpatrick JM, Plunkett W, et al. A proof-of-principle pharmacokinetic, pharmacodynamic, and clinical study with purine nucleoside phosphorylase inhibitor immucillin-H (BCX-1777, forode-sine) Blood. 2005;106:4253–4260. doi: 10.1182/blood-2005-03-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furman RR, Gore L, Ravandi F, Hoelzer D. Forodesine IV (Bcx-1777) is clinically active in relapsed/refractory T-cell leukemia: results of a phase II study (interim report) Blood. 2006;108:1851. Abstract. [Google Scholar]
- 38.Duvic M, Forero-Torres A, Foss F, Olsen E, Kim Y. Response to oral forodesine in refractory cutaneous T-cell lymphoma: interim results of a phase I/II study. Blood. 2007;110:122. Abstract. [Google Scholar]
- 39.Dummer R, Duvic M, Scarisbrick J, et al. Final results of a multicenter phase II study of the purine nucleoside phosphorylase (PNP) inhibitor forodesine in patients with advanced cutaneous T-cell lymphomas (CTCL) (Mycosis fungoides and Sézary syndrome) Ann Oncol. 2014;25:1807–1812. doi: 10.1093/annonc/mdu231. [DOI] [PubMed] [Google Scholar]
- 40.Ogura M, Tsukasaki K, Nagai H, et al. Phase I study of BCX1777 (forodesine) in patients with relapsed or refractory peripheral T/natural killer-cell malignancies. Cancer Sci. 2012;103:1290–1295. doi: 10.1111/j.1349-7006.2012.02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsukasaki K, Tobinai K, Uchida T, et al. Phase 1/2 study of forodesine in patients with relapsed peripheral T-cell lymphoma (PTCL) J Clin Oncol. 2016;34:7542. Abstract. [Google Scholar]
- 42.Shibayama H, Tobinai K, Tsukasaki K, et al. Updated report of a phase I/II multicenter study of forodesine, a purine nucleoside phosphorylase inhibitor, in Japanese patients with relapsed peripheral T-cell lymphoma. ASH 59th Annual Meeting and Exposition; December 9–12, 2017; Atlanta, GA. Abstract#4075. [Google Scholar]
- 43.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]