Abstract
Ultraviolet radiation induces DNA damage products, largely in the form of pyrimidine dimers, that are both toxic and mutagenic. In most organisms, including Arabidopsis, these lesions are repaired both through a dimer-specific photoreactivation mechanism and through a less efficient light-independent mechanism. Several mutants defective in this “dark repair” pathway have been previously described. The mechanism of this repair has not been elucidated, but is thought to be homologous to the nucleotide excision repair mechanisms found in other eukaryotes. Here we report the complementation of the Arabidopsis uvh1 dark repair mutant with the Arabidopsis homolog of the yeast nucleotide excision repair gene RAD1, which encodes one of the subunits of the 5′-repair endonuclease. The uvh1-2 mutant allele carries a glycine→aspartate amino acid change that has been previously identified to produce a null allele of RAD1 in yeast. Although Arabidopsis homologs of genes involved in nucleotide excision repair are readily identified by searching the genomic database, it has not been established that these homologs are actually required for dark repair in plants. The complementation of the Arabidopsis uvh1 mutation with the Arabidopsis RAD1 homolog clearly demonstrates that the mechanism of nucleotide excision repair is conserved among the plant, animal, and fungal kingdoms.
UV radiation induces two major DNA damage products: cyclobutane pyrimidine dimers (CPDs) and pyrimidine[6-4]pyrimidinone dimers (6-4 products), each making up approximately 75% and 25% of UV-induced damage, respectively (Mitchell and Nairn, 1989). These lesions block the progress of both DNA and RNA polymerases and therefore have a toxic effect even in tissues that are not actively dividing. Because plants are obligatorily exposed to solar radiation it is especially important that they be able to efficiently remove UV-induced DNA damage. Plants are known to produce two distinct photolyases that efficiently remove both CPDs and 6-4 products in the presence of blue light (Britt, 1999). Plants also repair 6-4 products in the absence of light, although this “dark” repair mechanism is less efficient than the photolyase-dependent pathway. In contrast, in Arabidopsis seedlings the light-independent repair of CPDs is too slow to be detected by conventional assays (Britt et al., 1993). The biochemical basis of this “dark repair” mechanism has not yet been elucidated in plants.
In other eukaryotes, the repair of UV-induced dimers is known to proceed through both the light-independent nucleotide excision repair (NER) pathway and the photolyase dependent photoreactivation pathway. Placental mammals are generally thought to lack functional photolyases (although this is still subject to debate) and appear to repair UV-induced damage strictly via NER. This pathway, extensively characterized in yeast and mammals, is not specific to the repair of dimers but is an all-purpose pathway with a remarkably broad substrate “specificity” (Sancar, 1996). At least 11 genes are required for the recognition of lesions, the dual incision of the damaged strand, and the removal of the damaged oligonucleotide. In Saccharomyces cerevisiae one of these genes, RAD1, encodes one of the two subunits of the endonuclease complex that incises the DNA 5′ of the damage (Davies et al., 1995). In bacteria, fungi, and mammals the efficiency of this pathway is greatly enhanced when the damage is present in the transcribed strand of DNA (Friedberg, 1996); whether or not this transcription-coupled repair occurs in plants remains to be determined.
Several UV-sensitive mutants of Arabidopsis have been identified (Britt et al., 1993; Jenkins et al., 1995; Jiang et al., 1997). Some of these, including the uvh1-1 mutant originally isolated by the Mount laboratory (Harlow et al., 1994), are known to be defective in the light-independent repair of 6-4 photoproducts. In this paper we demonstrate that the ethyl methanesulfonate (EMS)-induced uvh1-2 allele (Jiang et al., 1997) carries a mutation in an Arabidopsis homolog of the yeast gene RAD1 and that the wild-type Arabidopsis RAD1 homolog complements the UV-sensitive and repair-defective phenotype of the uvh1-1 mutant.
RESULTS
Plants are known to possess some mechanism for the light-independent removal of pyrimidine dimers, but the mechanism underlying this process is poorly understood. In contrast, the process of NER has been extensively characterized in bacteria, yeasts, and mammals. Yeasts and mammals share approximately a dozen genes that are required for the NER of UV-induced damage (Sancar, 1996). Bacteria possess a similar mechanism, although there are fewer proteins involved and little homology between the bacterial and eukaryotic proteins.
In contrast, a search of GenBank, including the nearly complete Arabidopsis genomic sequence, reveals that higher plants possess obvious homologs of many of the eukaryotic genes required for NER (Britt, 1999). To determine whether these homologs actually are required for dark repair of UV-induced lesions in Arabidopsis, we sought to determine whether the cloned sequence of the Arabidopsis homolog of the yeast repair endonuclease RAD1 would restore UV-resistance to a previously isolated repair-defective mutant, uvh1, that maps to the same location on chromosome 5 (Jiang et al., 1997).
Identification of an Arabidopsis RAD1 Homolog and Transformation into uvh1
Using the human XPF amino acid sequence (a homolog of RAD1 in yeast) we searched GenBank via tBLASTn and found an Arabidopsis homolog: the P1 clone MEE6 in the Kazusa chromosome 5 database. This clone has a amino acid sequence (based on the Kazusa's predicted splicing pattern) with 50.6% homology and 38.6% identity to the human XPF protein throughout the length of the sequence (the homology was actually a little higher when a different predicted splice pattern was used for the comparisons; this will be discussed later in this paper). MEE6 is located at the 14- to 16-Mb region on chromosome V. An Arabidopsis mutant defective in dark repair, uvh1, maps at roughly the same location (Jiang et al., 1997). In yeast, RAD1 forms part of the heterodimer responsible for incision of the DNA 5′ of the damage (Siede et al., 1993), and defects in this activity result in a UV-sensitive phenotype. To determine whether the Arabidopsis RAD1 homolog corresponds to the UVH1 gene, we transformed the wild-type AtRAD1 gene into the uvh1-1 mutant line.
The AtRAD1 genomic sequence was amplified by PCR using the clone MEE6 as a template. The resulting product was subcloned into pBART, a binary Agrobacterium tumefaciens transformation vector carrying the BART (Basta resistance) herbicide resistance gene as a selectable marker (K. Richardson, unpublished data). Constructs containing AtRAD1 in the sense orientation were transformed into uvh1-1 via the in planta procedure (Clough and Bent, 1998). Both sense and antisense constructs were transformed into the parent line Columbia. Transformed T1 progeny from three T0 plants were selected on soil using the herbicide Basta. Two transformed plants did not produce any progeny resistant to the herbicide. One T0 plant (plant 3) produced two T1 progeny resistant to the herbicide (lines 3-1 and 3-2). These two lines were self-pollinated to produce T2 populations. Line 3-1 was completely characterized.
The T2 progeny of line 3-1 expressed herbicide resistance to sensitivity in a ratio of 285 to 118. This ratio is consistent with the presence of one Basta-resistance locus (χ2 = 3.93; P > 0.05). The presence of the appropriate transformed DNA was confirmed by PCR (data not shown).
Characterization of the uvh1/AtRAD1 Line
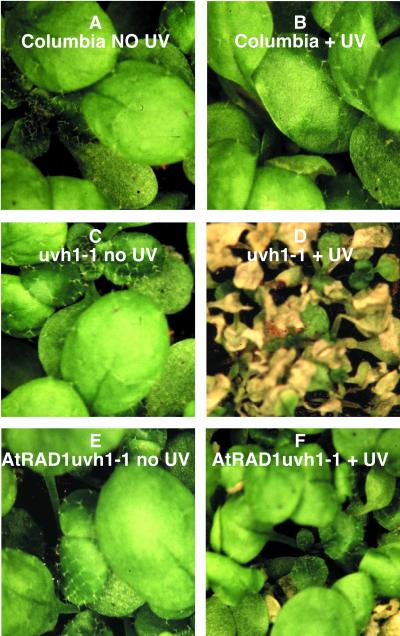
Functional complementation of uvh1-1 by AtRAD1 was tested by irradiation of the T2 progeny of line 3-1 with UV-C at 200 J m−2, a dose that produces a UV-sensitive phenotype in the uvh1-1 mutant but does not affect the wild type. Figure 1 shows the AtRAD1 transformed uvh1-1 mutant line that displayed a largely UV-resistant phenotype, although occasional individuals displayed the UV-sensitive phenotype of the progenitor uvh1 line. Given the fact that this line is segregating approximately 3:1 for the AtRad1 transgene, this phenotype is consistent with complementation of the uvh1deficiency by the AtRAD1 gene.
Figure 1.
Phenotype of Columbia, uvh1-1, and the complemented line AtRAD1 uvh1-1 before and after UV irradiation. Two-week-old seedlings were irradiated with 200 J m−2 UV-C and kept in the dark for 3 d to prevent photoreactivation. Seedlings were removed from the dark and put under white light for 1 d when the pictures were taken. A, Columbia seedlings before irradiation. B, Columbia seedlings after irradiation show characteristic UV-resistant phenotype. C, uvh1-1 seedlings before irradiation. D, uvh1-1 seedlings show the characteristic UV-sensitive phenotype with yellow, wrinkled leaves. E, AtRAD1 uvh1-1 before irradiation. F, AtRAD1 uvh1-1 displays a UV-resistant phenotype with one UV-sensitive seedling; this may be a segregant that lacks the AtRAD1 transgene.
The T1 progeny of wild-type plants transformed with the AtRAD1 antisense construct were also characterized. Of 20 T0 plants, six produced one transformant each, and two produced two transformants each, as determined by resistance to the herbicide. Presence of the antisense construct in each T1 line was confirmed by PCR of DNA extracted from pooled T2 seeds produced by individual T1 plants (data not shown). The T2 progeny of these eight antisense lines (approximately 25 T2 plants from each T1 antisense plant) were UV-C irradiated, and all displayed a UV-C-resistant phenotype (data not shown). This suggests that the expression of the AtRAD1 antisense RNA has little or no effect on DNA repair in wild-type plants. Transformation with the empty vector similarly had no effect on the UV sensitivity of either the wild-type line or the uvh1-1 mutant.
To further characterize the UV resistance of the complemented line we performed the root-bending assay for UV sensitivity (Jiang et al., 1997). Seedlings of the mutant uvh1-1, its wild-type progenitor Columbia, and the complemented line AtRAD1 uvh1-1 (line 3-1) were UV-B irradiated at 0, 1, 2, 3, and 4 kJ m−2, doses that have been previously shown to produce the UV-sensitive phenotype in uvh1-2 mutant. Table I shows the relative root growth of the three strains compared with the average of root growth in the respective unirradiated strain (100% root growth). The complemented line was not statistically different at the 95% confidence level from relative root growth in the wild-type seedlings at all doses based on a Student's t-test analysis (ts = 0.5, 0.6, 1.07, and 1.4, at 1, 2, 3, and 4 kJ m−2 respectively). The relative root growth in uvh1-1 was statistically different at the 95% confidence level from the relative root growth of both Columbia (ts = 6.17 and 11.88) and the complemented line (ts = 3.5 and 8.23) at 3 and 4 kJ m−2, respectively.
Table I.
Root-bending assay
| UV-B Dose | Root Growth after Irradiationa
|
||
|---|---|---|---|
| Columbia | uvh1-1 | AtRAD1uvh1-1 | |
| kJ m−2 | % | ||
| 0 | 100.0 ± 7.6 | 100.0 ± 17.8 | 100.0 ± 19.1 |
| 1 | 98.7 ± 59.3 | 116.0 ± 55.8 | 92.0 ± 42.5 |
| 2 | 65.6 ± 49.4 | 72.4 ± 46.1 | 73.4 ± 51.3 |
| 3 | 78.2 ± 44.0 | 12.7 ± 22.8* | 61.4 ± 49.5 |
| 4 | 80.5 ± 41.0 | 5.9 ± 15.2** | 67.8 ± 45.1 |
New root growth was measured 2 d after irradiation and compared with root growth in an unirradiated line of the same genotype (= 100% root growth). Data statistically different at the 95% confidence level from the relative root growth of both Columbia (ts = 6.17* and 11.88**) and the complemented line (ts = 3.50* and 8.23**).
The results represent average of approximately 30 measurements ± sd. The actual values for each line for the 0 kJ m−2 UV-B dose are as follows: Columbia, 1.2 mm; uvh1-1, 2.1 mm; and AtRAD1uvh1-1, 3.8 mm.
uvh1 mutants are sensitive to the inhibitory effects of γ radiation on cell division. Irradiation of uvh1 seeds at relatively low doses (10 kilorads) results in the production of a “γ-plantlet,” a miniature plant in which the cells expand but do not divide. Arabidopsis γ-plantlets produce cotyledons (as this organ is largely preformed in the seed) but are delayed in the production of postembryonic true leaves (as the production of these organs requires cell division). In other words, irradiated uvh1 seeds germinate as quickly as unirradiated seeds, expand their cotyledons, and respond to light by turning green like their unirradiated sibs, but they are delayed in the production of true leaves for approximately 2 weeks. This effect is illustrated in Figure 2. To determine whether AtRAD1 uvh1-1 displays a wild-type level of γ resistance, seeds of AtRAD1 uvh1-1, Columbia, and uvh1-1 were treated with 10 kilorads of γ radiation and screened for leaf formation following irradiation. Thirteen days after irradiation, all of the Columbia seedlings (n = 25) had produced leaves. In contrast, none of the uvh1-1 seedlings (n = 25) had formed leaves. The T2 progeny of the transformed line presented an intermediate phenotype after γ-irradiation: 24 of 28 seedlings produced leaves. This restoration of resistance in a fraction of the seedlings is consistent with the fact that the transgene is segregating 3:1 in this line (χ2 = 1.715; P > 0.05). Figure 2 shows the AtRAD1-transformed uvh1-1 mutant line. Most of the transformants display a γ-resistant phenotype; a few γ-sensitive individuals are also observed.
Figure 2.
Phenotype of Columbia, uvh1-1, and the complemented line AtRAD1 uvh1-1 before and after γ-irradiation. Seeds in water were treated with 10 kilorads of γ radiation and screened for leaf formation 14 d after irradiation. A, Columbia seedlings before irradiation. B, Columbia seedlings after γ-radiation show leaf formation, characteristic of a γ-resistant phenotype. C, uvh1-1 seedlings before irradiation. D, uvh1-1 seedlings show the characteristic “γ-plantlet” phenotype where cotyledons are visible but there is a delay in the production of postembryonic true leaves. E, AtRAD1 uvh1-1 before irradiation. F, AtRAD1 uvh1-1 displays a γ-resistant phenotype with a few individuals displaying the “γ-plantlet” phenotype, probably due to segregation of the AtRAD1 transgene.
Arabidopsis seedlings repair 6-4 products in the dark, although this light-independent pathway for repair is not as efficient as the photolyase-dependent pathway. uvh1 mutants are defective in the dark repair of 6-4 products (Jiang et al., 1997). To determine whether the AtRAD1 transgene restored wild-type levels of dark repair to the uvh1 mutant we irradiated T4 seedlings from AtRAD1 uvh1-1 line 3-1, as well as uvh1 and wild-type controls with UV-B at 1.32 kJ m−2 and measured the concentration of 6-4 products at t = 0 and t = 24 h. As is shown in Table II, the uvh1-1 mutant did not repair 6-4 products induced in a period of 24 h (98% of the induced 6-4 products still remained). Both the wild-type Columbia and the transgenic AtRAD1 uvh1-1 demonstrated about the same amount of repair with 47% and 41% of the initial 6-4 products removed in a 24-h period, respectively, indicating that the AtRAD1 homolog also complemented the repair deficiency of uvh1-1. The value for the induction of dimers was significantly lower in the AtRAD1 complemented line; we believe this is because the percent germination in our seeds stock was low (approximately 60%). Although our procedure extracts DNA from intact seeds, the seeds coats are highly UV absorbent, and the initial values of dimer/gram DNA are expected to be lower.
Table II.
6-4 Repair assay in Arabidopsis seedlings UV-B irradiated at 0 and 24 h after irradiation
| Time Point | 6-4 Products
|
||
|---|---|---|---|
| Columbia | uvh1-1 | AtRAD1uvh1-1 | |
| h | |||
| 0 | 6.05 ± 1.20 | 4.65 ± 0.07 | 9.45 ± 2.19 |
| 24 | 3.20 ± 0.99 | 4.55 ± 0.35 | 5.55 ± 0.21 |
The results represent an average of two measurements ± sd.
Characterization of the EMS-Induced Mutation of uvh1-2
The uvh1-1 and uvh1-2 alleles were generated with the mutagen EMS in different genetic backgrounds (Columbia and Landsberg erecta [Ler]), respectively. The mutants were independently isolated and characterized (uvh1-1 in David Mount's laboratory at the University of Arizona [Harlow et al., 1994]). As uvh1-2 had been characterized in our laboratory we proceeded to sequence this allele. Because the AtRAD1 wild-type sequence available in the database was derived from ecotype Columbia, we also sequenced the wild-type allele of AtRAD1/UVH1 from the Ler line. During the completion of our sequencing project the cDNA sequence of the Arabidopsis uvh1-1 allele was deposited in GenBank by the Mount laboratory (accession no. AF160500). Although this mutant allele has a defect at a splice junction (Liu et al., 2000), the non-mutant splice joints of this cDNA sequence provide a better match to the human and yeast amino acid sequences than the cDNA structure predicted by Kazusa (for the human homolog, the cDNA provides 51.3% and 39% similarity and identity, respectively, whereas the computer-predicted intron/exon structure suggests a 50.6% and 38.6% for similarity and identity). Therefore, we used the cDNA provided by the Mount laboratory to predict the location of introns and exons in our genomic sequence. Our Ler UVH1 sequence is available in the database (accession no. AF277377).
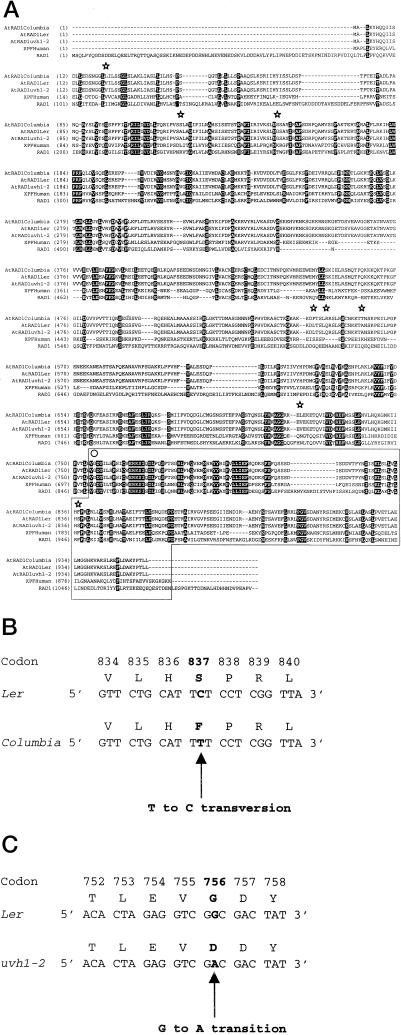
Forty-six nucleotide differences were observed in the coding regions of the wild-type AtRAD1 gene between the Ler and Columbia alleles. This degree of variation is often observed when alleles from the two ecotypes are compared (i.e. Kuittinen and Aguade, 2000). Thirty-eight of these mutations correspond to silent mutations, and eight corresponded to missense mutations; these are diagrammed in Figure 3A. A single interesting missense mutation was a TA→GC transversion in Ler, which resulted in a substitution of a Phe by a Ser in Ler in position 837 in the C-terminal region of the protein (Fig. 3B). This region (marked within a box, Fig. 3A) of the protein appears to be highly conserved in humans and yeast and is thought to be directly involved in forming the RAD1/RAD10 complex (Bardwell et al., 1993). However, the importance of each of these conserved residues has not been tested by mutagenesis. A Phe is found at this position in yeast, humans, and the Columbia ecotype of Arabidopsis. One might expect that a substitution of a Phe, a very hydrophobic amino acid, by a Ser, a polar amino acid, might disrupt this interaction with the RAD10 homolog with RAD1 in this region. However, this substitution obviously does not abolish the action of the Arabidopsis RAD1 protein, since it appears in one of the wild-type backgrounds (Ler, Ser) but not in the other wild-type background (Columbia, Phe).
Figure 3.
Amino acid sequence analyses of RAD1 genes. A, Alignment of residues 1 to 956 of AtRAD1Ler with AtRAD1Columbia, AtRAD1 uvh1-2, XPF (the human RAD1 homolog), and S. cerevisiae RAD1 amino acid sequences. The positions of amino acid changes observed between AtRAD1Ler and AtRAD1Columbia are marked with stars above the alignments. The Gly→Asp amino acid substitution is marked with a circle above the alignments. The conserved C-terminal region of the proteins is marked within a box. B and C, Sequence changes in Ler, Columbia, and uvh1-2. The positions of the mutations are in bold. B, The Phe→Ser amino acid substitution in Columbia that does not alter the UV-resistant phenotype of both wild-type strains (Columbia and Ler). C, The Gly→Asp amino acid substitution causing the UV-sensitive phenotype in uvh1-2.
One G→A transition was found when comparing the nucleotide sequences of AtRAD1 in the wild-type Ler background and the uvh1-2 mutant isolated from Ler. This mutation changed a GGC into a GAC codon resulting in the substitution of a Gly by an Asp residue at position 756 in the conserved C-terminal region of the RAD1 protein (Fig. 3C). The identical amino acid substitution occurs in the S. cerevisiae rad1-20 mutation and is thought to impair Rad1/Rad10 complex formation resulting in the UV-sensitive phenotype of this mutant (Siede et al., 1993).
DISCUSSION
The process of dark repair in plants is poorly understood. In mammals, pyrimidine dimers are excised via NER; this process is complex and requires the combined action of several genes. All of these genes have been cloned and sequenced (de Laat et al., 1999). However, UV-induced dimers are also excised in some fungi via a simpler and more lesion-specific base excision repair (BER) process (Yajima et al., 1995). BER differs substantially from the NER process; BER involves the action of a single fairly lesion-specific glycosylase that snips the damaged base from the sugar-phosphate backbone (Wood, 1996). In contrast, NER requires the combined actions of several proteins to recognize a broad range of damage products, which are removed as oligonucleotides. Thus it remains an open question as to whether plants use BER, NER, or perhaps some novel light-independent pathway for the repair of UV-induced dimers.
In Arabidopsis, dark repair mutants representing several complementation groups have been isolated (Britt, 1998). Only a handful of loci are represented by more than one allele, suggesting that more genes remain to be identified. The requirement for several genes suggests that NER might also be responsible for dark repair of dimers in plants, as it is in most other eukaryotes. Arabidopsis sequences homologous to NER genes can be easily identified by searching the database (Britt, 1999). The complementation of one of the Arabidopsis dark repair mutants with one of these NER homologs would unambiguously demonstrate that NER is involved in dark repair in plants.
In this paper we found that one of these mutants (uvh1) is complemented by the Arabidopsis homolog of the yeast NER gene RAD1. The RAD1 protein plays a direct role in NER by acting, as a heterodimer with RAD10, as the endonuclease that nicks the damaged strand 5′ of the lesion. Complementation of uvh1, a gene required for dark repair of pyrimidine dimers in plants, by the plant homolog of RAD1 indicates that the biochemical pathway for the light-independent repair of UV-induced dimers is highly conserved among plants, mammals, and fungi. Furthermore, the similar effects of the Gly→Asp substitution in the same location in uvh1-2 and the S. cerevisiae rad1-20 mutant indicates that the same interaction of RAD1 with a RAD10 homolog probably also exists in plants.
A comparison of the two wild-type alleles (in Ler versus Columbia) has also provided some insight into the interaction of the RAD1 and RAD10 proteins. The identification of a Phe→Ser substitution at residue 837 of two wild-type Arabidopsis alleles suggests that a residue known to be highly conserved in animals and fungi might not be absolutely essential for function, although it is also possible that the protein/protein contacts in plants are slightly different from those in animals and fungi.
Analyses of other available Arabidopsis mutants (and wild-type lines) and efforts at complementation of their UV-sensitive phenotypes with plant homologous of NER genes are currently in progress and should continue to shed light on the biochemical mechanism of dark repair in plants and other eukaryotes.
MATERIALS AND METHODS
Plant Material and Growth Conditions
We used Arabidopsis strains Columbia and Ler, and the UV-sensitive mutants UV hypersensitive 1 (uvh1) from both Columbia (uvh1-1; Harlow et al., 1994) and Ler (uvh1-2; Jiang et al., 1997) backgrounds. Plants were grown on either Sunshine mix number 2 (SunGro, Bellevue, WA) or nutritive agar (Kranz and Kirchheim, 1987) with a 24-h d. Plants were grown under cool-white lamps filtered through Mylar at an intensity of 100 to 150 μmol m−2 s−1. The temperature was set at 22°C and the humidity at 50%.
Sequence Analysis and Database Search
All database searches was done using the Genetics Computer Group (GCG, Madison, Wisconsin) package. DNA Strider (Commissariat à l'Energie Atomique, France) and Vector NTI Suite (InforMax, Bethesda, MD) were used for sequence analysis in conjunction with the GCG package. The program OLIGO (National Biosciences, Plymouth, MN) was used to help designing the primers that extended the RAD1 sequences as well as the primers used for sequencing. Sequencing was performed by the Davis Sequencing Group (Davis, CA) as well as the UCD Sequencing Facility (University of California, Davis).
Amplification of AtRAD1 from Arabidopsis and Subcloning into Expression Vectors
Using the human XPF sequence (accession no. HSU64315), a homolog of the yeast RAD1 gene, a BLAST search was performed to identify possible Arabidopsis homologs. The search identified a clone corresponding to an 83,698-bp fragment (accession no. AB010072, P1 clone MEE6) released by the Kazusa DNA Research Institute (Chiba, Japan). To amplify the AtRAD1, P1 clone MEE6 was requested from the Kazusa DNA Research Institute, and PCR was performed using primers designed to amplify the entire AtRAD1 genomic sequence. The following modified primers were used to introduce an XbaI site (T/CTAGA) approximately 6 bp upstream of the translation initiation codon ATG, and an XbaI site approximately 23 bp beyond the stop codon: 5′-GTCGTCTTCTAGAATGGCGCTGAAAT-3′(5′ primer) and 5′-ATGAGCTTCTAGATTAATAAGTGTTACCT-3′ (3′ primer). Bacteria containing the MEE6 clone provided the template for a reaction with the above primers. The 3,924-bp fragment was generated after using the Robocycler Gradient 96 (Stratagene, La Jolla, CA) with the following parameters: one cycle of denaturation at 95°C for 5 min followed by 45 cycles of denaturation at 95°C for 1 min 30 s, annealing at 50°C for 1 min 30 s, and an extension at 72°C for 4 min 30 s, followed by a final extension at 72°C for 10 min using Vent DNA Polymerase (New England Biolabs, Beverly, MA). The PCR product was then subcloned into the XbaI site of pZero (Invitrogen, Carlsbad, CA) and subsequently into the XbaI site of pART (Gleave, 1992) creating two expression cassettes, one with AtRAD1 in the sense orientation and the other with AtRAD1 in the antisense orientation in respect to the cauliflower mosaic virus 35S (CaMV35S) promoter and the ocs3′ terminator. NotI was subsequently used to excise the expression cassettes from pART7 and to subclone them into the NotI site of pBART, a T-DNA vector carrying the Basta resistance as a selectable marker (K. Richardson, unpublished data). The CaMV35S promoter and ocs3′ terminator cassette of pART7 was also excised with NotI and subcloned into pBART. This “empty vector” was used as a control in our transformation experiments.
PCR amplification of AtRAD1 from the uvh1-2 and Ler backgrounds was performed using genomic DNA extracted from both strains as template and a new set of primers (5′-CACTCTTCATCAATCAGTTTCTCCGATTTG-3′, 5′ primer) and (5′-CTAAGCAGCTTTGTGAATGAGC-TTCTAAAT-3′, 3′ primer). These amplified a 3,974-bp fragment containing the entire open reading frame. The PCR was performed using the Robocycler Gradient 96 with the following parameters: one cycle of denaturation at 95°C for 5 min followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 4 min 30 s, followed by a final extension at 72°C for 10 min using Pfu Turbo DNA Polymerase (Stratagene) following manufacturer's conditions. The PCR products obtained from each strain were then subcloned into pCR-Blunt (Invitrogen) and used for sequencing the AtRAD1 genes from both uvh1-2 and Ler backgrounds. To assure that the sequence information obtained for AtRAD1 uvh1-2 was accurate and no mutations were introduced in the gene by the Pfu Turbo DNA polymerase during amplification by PCR, we subcloned two independently amplified PCR products and fully sequenced one of them. The second clone was used to sequence only the region where the mutation was located. Indeed, the mutation was present in the second clone confirming it was not a product of a PCR mistake.
Transformation of Arabidopsis with AtRAD1
The pBART constructs above described were transformed into Agrobacterium tumefaciens by electroporation (Sambrook et al., 1989). Arabidopsis plants were transformed via A. tumefaciens following the in planta protocol (Clough and Bent, 1998). The vector pBART contains the BART gene that confers resistance to the herbicide Basta (AKA Finale, AgrEvo Environmental Health, Montvale, NJ), making it possible to select transformants on soil by spraying T1 seedlings with the herbicide. A. tumefaciens-treated (T0) plants were allowed to self-pollinate and were individually harvested. Approximately 3,000 T1 seeds from each transformed individual were screened by spraying seedlings with Basta at a dilution of 1:1,000 every 4 d after germination for a period of 1 month. Transformants survived after herbicide spraying, whereas non-transformants were killed. Transformation efficiency varied from 0% to 0.4% of all seed.
Analysis of the Transformants by PCR
To determine whether transformants positively selected by Basta contained the appropriate transgenic construct, DNA was extracted from either the T1 plant, using leaf material via the cetyl-trimethyl-ammonium bromide method (Ausubel et al., 1992), or T2 seeds (Edwards et al., 1991). PCR was performed with two primer combinations designed to amplify two different PCR products from either the sense or antisense constructs. The oligo sequences used were the following: 5′-CTGCCGACAGTGGTCCCAAAGATG-3′(which anneals to the CaMV35S region), 5′-GAGAACCGGAGGTGTAAAGCGAGTA-3′ (which anneals to the AtRAD1 gene and produces a PCR fragment of 535 bp when used with the CaMV35S primer and with a template that has the AtRAD1 sense construct), and 5′-GCCGGAATCTTCATCCTCAAT-3′(which anneals to the AtRAD1 gene and produces a PCR fragment of 628 bp when used with the CaMV35S primer and with a template that has the RAD1 antisense construct). The empty vector construct was checked using the primer that anneals to the CaMV35S region (upper primer) and a second primer that anneals to the ocs3′ region of the pART7 vector: 5′-TTCTCGGGGCAGCAAGTCGGTTAC-3′ (lower primer) producing a 836-bp fragment. PCR was performed using the Robocycler with the following parameters: one cycle of denaturation at 95°C for 5 min followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min using Taq DNA Polymerase (Promega, Madison, WI; or Amersham- Pharmacia Biotech, Piscataway, NJ) following manufacturer's conditions.
Irradiation of Arabidopsis Seedlings with UV-C
Seeds were sown in Sunshine mix number 2 at a density of 1 seed cm−2 and kept at 4°C for 2 d. Flats were subsequently brought to the growth chamber at 22°C under cool-white lamps filtered through Mylar. Two-week-old seedlings were irradiated with a germicidal lamp (UV-C) at 200 J m−2 with a fluence rate of 1.67 mW cm−2 measured with a UV-C-specific probe (UV Products, San Gabriel, CA). Irradiated seedlings were stored in the absence of light for 3 d and then brought back to the growth chamber to be scored for UV sensitivity.
Root-Bending Assay
Seeds were sown on nutritive agar plates at a density of approximately 30 seeds/100 mm2 plate and then kept at 4°C for 2 d. The plates were placed in a vertical position to prevent the root tips from growing into the agar. The plates were then brought to the growth chamber where they were placed under non-photoreactivating conditions (cool-white lamps filtered with orange polyvinyl chloride) at the vertical position. Two-day-old seedlings were then irradiated with UV-B for a final dose of 0, 1, 2, 3, and 4 kJ m−2 using a UV transilluminator (Fisher Scientific, Pittsburgh) filtered with 0.005 mL of cellulose acetate with a flux rate of 3.53 mW cm−2 measured with a UV-B-specific probe (UV Products). Plates were then rotated by 90° and kept in the dark. Two days later new root growth (visible as growth at a right angle to previous growth) was measured using an eye piece micrometer. Data obtained was statistically analyzed using the t statistic (ts), which measures the difference between the sample means expressed in relation to the se of the difference.
γ-Irradiation Assay
Approximately 30 seeds of Columbia, uvh1-1, and T2 seeds of two independently transformed AtRAD1 uvh-1 lines, were soaked in water in a microfuge tube and left at 4°C overnight before irradiation. Seeds were irradiated in water with γ-radiation provided by a 137Cs irradiator (Institute of Toxicology and Environmental Health, University of California, Davis) at a dose rate of 922 rad/min. Seeds were immediately sown on soil after irradiation and leaf formation was scored 19 d after sowing.
6-4 Product Repair Assay
Seeds were sown on vertically oriented nutritive agar plates at a density of approximately 1,000 seeds (12.5 mg)/plate and kept at 4°C for 2 d. Twenty plates were used for each one of the lines used in the experiment: T4 seedlings of AtRAD1 uvh1-1 (line 3-1), uvh1-1, and Columbia (three sets of 20 plates each). Plates were stored in the growth chamber in the same conditions described earlier for the root-bending assay. Five-day-old seedlings were irradiated with UV-B provided by a UV transilluminator in a similar way as described above for the root-bending assay at a fluence rate of 3.85 mW cm−2 measured with a UV-B-specific probe (UVP). Seedlings from one-half of each of the three sets were harvested from the plates immediately after irradiation and frozen with liquid nitrogen for further DNA extraction (0-h time point). The remaining one-half of the plates were kept in the dark for 24 h and then seedlings were harvested and DNA extracted according to via the cetyl-trimethyl-ammonium bromide method (24-h time point). DNA concentration was measured using the OliGreen ssDNA Quantification Reagent (Molecular Probes, Eugene, OR) following manufacturer's protocol. The 6-4 products were quantified using a lesion-specific radioimmunoassay method (Mitchell, 1996). The results represent an average of two measurements ± sd.
ACKNOWLEGMENTS
We thank Dr. D. Mount (University of Arizona) for providing us the uvh1-1 mutant used in our experiments and also for releasing the uvh1-1 XPF cDNA sequence used in our sequence analyses. We also thank the Kazusa DNA Research Institute for providing us with the P1 clone MEE6.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. 98–35301–6058).
LITERATURE CITED
- Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates/Wiley Interscience; 1992. [Google Scholar]
- Bardwell AJ, Bardwell L, Johnson DK, Friedberg EC. Yeast DNA recombination and repair proteins Rad1 and Rad10 constitute a complex in vivo mediated by localized hydrophobic domains. Mol Microbiol. 1993;8:1177–1188. doi: 10.1111/j.1365-2958.1993.tb01662.x. [DOI] [PubMed] [Google Scholar]
- Britt A. DNA repair in higher plants. In: Nickoloff J, Hoekstra M, editors. DNA Damage and Repair. Totowa, NJ: Humana Press; 1998. pp. 577–595. [Google Scholar]
- Britt A. Molecular genetics of DNA repair in higher plants. Trends Plant Biol. 1999;4:20–25. doi: 10.1016/s1360-1385(98)01355-7. [DOI] [PubMed] [Google Scholar]
- Britt AB, Chen J-J, Wykoff D, Mitchell D. A UV-sensitive mutant of Arabidopsis defective in the repair of pyrimidine-pyrimidinone (6-4) dimers. Science. 1993;261:1571–1574. doi: 10.1126/science.8372351. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Davies A, Friedberg E, Tomkinson A, Wood R, West S. Role of Rad1 and Rad10 proteins in nucleotide excision repair and recombination. J Biol Chem. 1995;270:24638–24641. doi: 10.1074/jbc.270.42.24638. [DOI] [PubMed] [Google Scholar]
- de Laat WL, Jaspers NGJ, Hoeijmakers JHJ. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13:768–785. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991;19:1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. Relationships between DNA repair and transcription. Annu Rev Biochem. 1996;65:15–42. doi: 10.1146/annurev.bi.65.070196.000311. [DOI] [PubMed] [Google Scholar]
- Gleave AP. A versatile binary vector system with a T-DNA organizational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol. 1992;20:1203–1207. doi: 10.1007/BF00028910. [DOI] [PubMed] [Google Scholar]
- Harlow GR, Jenkins ME, Pittalwala TS, Mount DW. Isolation of uvh1, an Arabidopsis mutant hypersensitive to ultraviolet light and ionizing radiation. Plant Cell. 1994;6:227–235. doi: 10.1105/tpc.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins ME, Harlow GR, Liu Z, Shotwell MA, Ma J, Mount DW. Radiation-sensitive mutants of Arabidopsis thaliana. Genetics. 1995;140:725–732. doi: 10.1093/genetics/140.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C-Z, Yen C-N, Cronin K, Mitchell D, Britt A. UV- and γ-radiation sensitive mutants of Arabidopsis. Genetics. 1997;147:1401–1409. doi: 10.1093/genetics/147.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz AR, Kirchheim B. Genetic Resources in Arabidopsis. Arabidopsis Information Service 24 Botanical Institute, J.W. Frankfurt: Goethe-University; 1987. [Google Scholar]
- Kuittinen H, Aguade M. Nucleotide variation at the chalcone isomerase locus in Arabidopsis thaliana. Genetics. 2000;155:863–872. doi: 10.1093/genetics/155.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZR, Hossain GH, Islas-Osuna MA, Mitchell DL, Mount DW. Repair of UV damage in plants by nucleotide excision repair: Arabidopsis UVH1 DNA repair gene is a homolog of Saccharomyces cerevisiae RAD1. Plant J. 2000;21:519–528. doi: 10.1046/j.1365-313x.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. Radioimmunoassay of DNA damaged by ultraviolet light. In: Pfeifer G, editor. Technologies for the Detection of DNA Damage and Mutations. New York: Plenum Press; 1996. pp. 73–83. [Google Scholar]
- Mitchell DL, Nairn RS. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989;49:805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sancar A. DNA excision repair. Annu Rev Biochem. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- Siede W, Friedberg AS, Friedberg EC. Evidence that the Rad1 and Rad10 proteins of Saccharomyces cerevisiae participate as a complex in nucleotide excision repair of UV radiation damage. J Bacteriol. 1993;175:6345–6347. doi: 10.1128/jb.175.19.6345-6347.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. DNA repair in eukaryotes. Annu Rev Biochem. 1996;65:135–167. doi: 10.1146/annurev.bi.65.070196.001031. [DOI] [PubMed] [Google Scholar]
- Yajima H, Takao M, Yasuhira S, Zhao JH, Ishii C, Inoue H, Yasui A. A eukaryotic gene encoding an endonuclease that specifically repairs DNA damaged by ultraviolet light. EMBO J. 1995;14:2393–2399. doi: 10.1002/j.1460-2075.1995.tb07234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]