Abstract
Several medications have been shown to cause disulfiram-like reactions in patients concomitantly exposed to ethanol, including specific cephalosporin antibiotics that possess a methylthiotetrazole substituent. Within the cephalosporin class, there are few reports of disulfiram-like reactions with ceftriaxone. This case report is the first to involve a pediatric patient, and it describes a mild but likely disulfiram-like reaction manifesting as facial flushing in an 8-year-old male upon receiving a ceftriaxone infusion preceded by a dose of prednisolone elixir (5% ethanol by volume) for presumed community-acquired pneumonia thought to be complicated by an asthma exacerbation. The patient's flushing resolved with intravenous diphenhydramine, did not reappear, and was diagnosed as an allergy to ceftriaxone. Upon further evaluation, a hypersensitivity reaction was considered unlikely, and the allergy history was revised. The patient's antibiotic treatment was switched to azithromycin without steroids, and he had no further issues. This case suggests there is benefit in increased monitoring of pediatric patients receiving certain cephalosporins along with alcohol-containing medications, and it demonstrates how disulfiram reactions can easily be misinterpreted as hypersensitivity reactions. Aside from just alcohol-cephalosporin interactions, this case underscores the need for general vigilance when using alcohol-containing drug preparations in pediatric patients in an effort to prevent adverse effects and potential drug interactions.
Keywords: ceftriaxone, disulfiram, drug interactions, ethanol, flushing
Introduction
Disulfiram-like reactions between certain medications and ethanol have been well described in the literature. The reaction's mechanism involves disulfiram or the offending drug inhibiting aldehyde dehydrogenase (ALDH), the enzyme responsible for converting acetaldehyde—an ethanol metabolite—to acetate.1 The resulting increase in noxious serum acetaldehyde leads to clinical effects that range in severity and that are proportional to the amount of exposure to alcohol and the offending drug. Mild reactions manifest as vasodilation resulting in flushing and headache, while moderate to severe reactions can progress from nausea and vomiting to hypotension, dysrhythmia, and death.2,3
Aside from nitroimidazoles like metronidazole, perhaps the second most-recognized antimicrobial class associated with disulfiram-like reactions is cephalosporin antibiotics, specifically those that contain a methylthiotetrazole (MTT) substituent (e.g., cefotetan, cefoperazone, cefamandole). This group has been demonstrated to inhibit ALDH activity both in vitro and in vivo.4,5 Ceftriaxone is a third-generation cephalosporin that is commonly used for respiratory, genitourinary, and central nervous system infections in pediatrics, but it does not possess an MTT side chain. Instead, ceftriaxone's structure replaces the MTT substituent with a distinct but related methylthiodioxotriazine (MTDT) ring system that contributes to the compound's antimicrobial activity while increasing both its serum protein-binding ability and elimination half-life (Figure).
Figure.
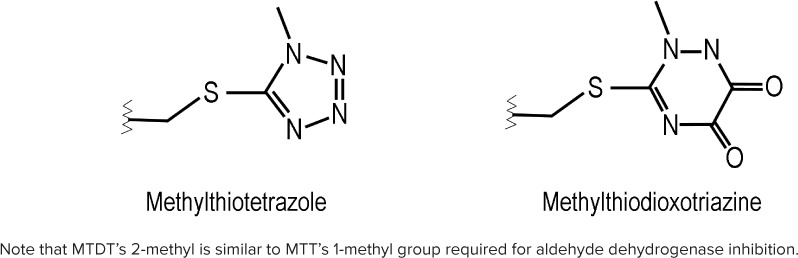
Comparison of methylthiotetrazole (MTT) and methylthiodioxotriazine (MTDT) structures.
Disulfiram-like reactions between ceftriaxone and ethanol are exceedingly rare. Aside from a recent retrospective review, there is only one relevant case in the literature that dates back to ceftriaxone's clinical trials in the early 1980s.6–9 To our knowledge, there are no reports of such reactions involving pediatric patients. This report describes a possible ceftriaxone-related disulfiram-like reaction in a pediatric patient after exposure to an ethanol-containing medication. The reaction in this case emphasizes vigilance when using alcohol-containing drug preparations in pediatric patients, and it demonstrates the potential for disulfiram-like reactions to be misinterpreted as hypersensitivity reactions in patients of all ages.
Case
An 8-year-old Hispanic male (26.6 kg, 134 cm) presented to an outpatient clinic after 5 days of cough, shortness of breath, sore throat, and subjective fever. His past medical history included a benign systolic heart murmur in addition to moderate persistent asthma requiring several past emergency department visits for exacerbations resulting in corticosteroid therapy, including prednisolone administration. His home medications included an albuterol inhaler (2 inhalations every 6 hours, as needed; ProAir HFA, Teva Pharmaceutical, North Wales, PA), daily oral cetirizine (5 mg), and a fluticasone/salmeterol 100/50 mcg/dose inhaler (1 inhalation by mouth twice daily; Advair Diskus, GlaxoSmithKline, Research Triangle Park, NC), which he was incorrectly using only once daily. He was not regularly using his prescribed intranasal fluticasone (50 mcg each nostril daily) or oral montelukast (5 mg daily) regimens. This patient only reported seasonal and canine dander allergies, and he had no recorded history of exposure to beta-lactam antibiotics. After arrival to the clinic, he received 40 mg (1.5 mg/kg, 13.3 mL) of oral prednisolone elixir (5% ethanol v/v) in addition to alcohol-free acetaminophen (10 mg/kg by mouth) and ibuprofen (10 mg/kg by mouth) liquid for pain and fever management. He also received 3 consecutive doses of nebulized albuterol-ipratropium. A complete blood count, C-reactive protein, and streptococcal antigen test were obtained (Table), and chest radiographs confirmed a right basilar pneumonia.
Table.
Baseline Patient Laboratory Values
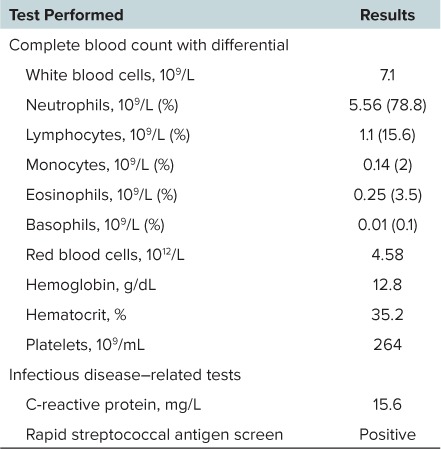
Approximately 2.5 hours after his prednisolone dose, the patient received ceftriaxone, 1000 mg (37.6 mg/kg) intravenously over 30 minutes, along with a normal saline infusion at 125 mL/hr. Within 30 minutes after completing the antibiotic infusion, the patient was noted to have facial flushing without any observed urticaria, swelling, itching, or difficulty breathing. As a result of concern for an allergic reaction, the provider gave the patient diphenhydramine 25 mg (0.9 mg/kg) intravenously, after which the flushing symptoms resolved. Subsequently, the prescriber entered ceftriaxone as an allergen in the patient's medical record. Of note, the patient's vital signs were stable and unremarkable during his clinic stay, with the exception of persistent tachycardia, which appeared after his bronchodilator administration, and increased respiratory rates, which were attributed to his illness.
The patient was then transferred from the clinic to our inpatient facility, where it was thought that his clinical presentation was most consistent with atypical pneumonia rather than community-acquired pneumonia or acute asthma exacerbation; for this reason, he did not receive any further doses of steroids or cephalosporins. During his admission, he received acetaminophen (10 mg/kg by mouth every 4 hours as needed), ibuprofen (10 mg/kg by mouth every 6 hours as needed), albuterol treatments (2 puffs every 4 hours as needed), maintenance intravenous fluids (dextrose 5% and sodium chloride 0.9% with 20 mEq/L potassium chloride at 67 mL/hr), and a loading dose of oral azithromycin (10 mg/kg). Aside from the first flushing episode, the patient did not experience any other reactions during his inpatient stay, and it was concluded that the patient's initial reaction was unlikely to be immune-related. Thereafter, ceftriaxone was removed from the patient's allergy history. He was discharged from our facility within 24 hours of his initial clinic encounter, and he was prescribed 4 more days of azithromycin therapy (5 mg/kg by mouth daily) for his pneumonia in addition to his current home medications.
Approximately 2 weeks after discharge, the patient was evaluated at a follow-up visit and reported no issues. Approximately 2 months later, he returned to the same outpatient clinic for another asthma exacerbation, at which time he received a dose of 28.2 mg (1 mg/kg, 9.4 mL) oral prednisolone elixir with nebulized albuterol and ipratropium without any reported reactions.
Discussion
The first case of a disulfiram-like reaction with ceftriaxone was observed during a study of 100 patients assessing the antibiotic's dosing regimens in bone and soft tissue infections in the early 1980s, and this single case was subsequently noted in multiple literature sources. A male patient was described as having an “Antabuse-like reaction” after consuming scotch, with a recurrence after ingesting wine several days later during his therapy. The specific signs and symptoms of his reaction were not noted, but they were significant enough to prematurely terminate his therapy.6–8 A recent Chinese retrospective review also revisited this link between ceftriaxone and disulfiram-like reactions in patients who ingested alcohol. Ren and colleagues9 analyzed 78 cephalosporin-induced disulfiram-like reactions in which drug hypersensitivity reactions were excluded based on cephalosporin skin testing. Twenty (25.6%) of the reactions occurred in patients who were receiving ceftriaxone. Of note, the high rates of ceftriaxone-related reactions observed in this group may be due to the study's Asian patient population, which generally experiences higher rates of sensitivity to ethanol and acetaldehyde due to genetic polymorphisms affecting ALDH activity.10
The related pharmacology of ceftriaxone's MTDT ring compared to MTT makes such reactions plausible. Aside from also being a nitrogenous heterocyclic ring, MTDT contains a 2-methyl group at a similar position to MTT's 1-methyl group (Figure), which has been determined in vitro to be necessary within the latter's structure for inhibiting ALDH.4 Rat models have shown that liberation of MTT from its parent cephalosporin is necessary for ALDH inhibition, and further disulfide bonding of their methylthiol groups may also be required.11,12 Ceftriaxone was not directly tested in these studies, and its exact transformation to inactive metabolites in humans is not clearly explained; however, ceftriaxone's methylthiol-containing ring system is indeed liberated during the antibiotic's metabolism in goats. If the same is true for the drug's human metabolism, it suggests that disulfiram-like reactions can occur.13
The pharmacokinetics of ethanol and its metabolites in this case supports the conclusion that this patient did not experience an adverse effect to alcohol alone. As stated earlier, some individuals naturally experience acetaldehyde-related flushing after ethanol exposure due to decreased ALDH activity attributed to genetic polymorphisms, with peak serum concentrations and effects of ethanol and acetaldehyde expected to occur within 1 hour after ingestion.10,14 The flushing reaction in this case occurred roughly 3 hours after ethanol exposure, making it less likely that the patient was solely having an adverse effect to the ethanol alone. Based on pediatric-modified blood alcohol concentration (BAC) calculations described by Donovan,15 our patient's peak BAC was estimated to have been 2 to 3 mg/dL after ingestion of the medication elixir based on its alcohol content (5% ethanol v/v), the dose volume (13.3 mL), and the patient's height and weight. Our patient's estimated BAC correlated with the reaction's dose-response relationship, in which mild reactions can occur with the actual disulfiram drug when blood ethanol concentrations are approximately 5 mg/dL.16
Some could argue that this patient's BAC would be too low by 2.5 hours post-exposure to cause such a reaction; however, data suggest that the concomitant administration of albuterol/ipratropium in this patient could have theoretically led to a delay in gastric emptying via the drug's combined anticholinergic-sympathomimetic action, thus delaying ethanol's absorption.17
Temporal correlations and the patient's past medical history support the theory that this was indeed a disulfiram-like reaction instead of a hypersensitivity reaction. Ren and colleagues9 found that all 78 cephalosporin-induced disulfiram-like reactions manifested within 60 minutes of exposure to either ethanol or cephalosporin, and our patient's reaction occurred roughly 30 minutes after he completed his ceftriaxone infusion. Furthermore, our patient's history of tolerance to prednisolone elixir and his lack of previous exposure to beta-lactam antibiotics make it less likely he could have been sensitized to these drugs to have an allergic reaction. This patient also did not experience any future reactions, despite ceftriaxone's long half-life of approximately 6 to 8 hours in children.18 If this was a true hypersensitivity reaction to ceftriaxone, it is reasonable to have expected a continued reaction as he cleared his diphenhydramine dose—with a half-life of 4 to 6 hours—given the antibiotic's extended elimination.19
Concomitant medications may have affected the patient's presentation of this reaction. Although the successful use of diphenhydramine in this case may support the theory that it was a hypersensitivity reaction, diphenhydramine is also used for managing disulfiram reactions, since many of the vasodilatory effects (e.g., flushing, headache, hypotension) appear histamine-related.16,20 Lastly, it is possible that this patient had other manifestations of a disulfiram reaction, such as tachycardia and headache, that were masked by his albuterol-ipratropium and acetaminophen/ibuprofen treatments, respectively.
Limitations with this case include a lack of objective laboratory data, such as ethanol or acetaldehyde concentrations, negative allergen skin tests, or elevated eosinophil counts, to support our conclusions. Furthermore, we were not able to rechallenge the patient with ceftriaxone and an alcohol-free prednisolone preparation as a result of changes in the patient's diagnosis, but this would also have supported our hypothesis. However, the temporal correlations, patient history, and nature of the reaction make it more likely that this was indeed a disulfiram reaction.
CONCLUSIONS
This case report is one of very few describing a possible disulfiram-like reaction following exposure to ethanol and ceftriaxone, and it is the first to describe such an interaction in a pediatric patient. There is some clinical and biochemical evidence to support such a reaction, yet reports are so few that only continued monitoring for similar incidents can be recommended at this time. However, this case demonstrates how disulfiram-like reactions can easily be misinterpreted as hypersensitivity reactions, and it underscores the need for vigilance when using alcohol-containing drug preparations in pediatric patients to prevent adverse effects and potential drug interactions.
ABBREVIATIONS
- ALDH
aldehyde dehydrogenase
- BAC
blood alcohol concentration
- MTDT
methylthiodioxotriazine
- MTT
methylthiotetrazole
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org
REFERENCES
- 1. Zindel LR, Kranzler HR.. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J Stud Alcohol Drugs Suppl. 2014; 75 S17: 79– 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong H, Zhang J, Ren L, . et al. Unexpected death due to cefuroxime-induced disulfiram-like reaction. Indian J Pharmacol. 2013; 45 4: 399– 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cina SJ, Russell RA, Conradi SE.. Sudden death due to metronidazole/ethanol interaction. Am J Forensic Med Pathol. 1996; 17 4: 343– 346. [DOI] [PubMed] [Google Scholar]
- 4. Kamei C, Sugimoto Y, Tasaka K.. The effects of cephem antibiotics and related compounds on the aldehyde dehydrogenase in rat liver mitochondria. Biochem Pharmacol. 1987; 36 12: 1933– 1939. [DOI] [PubMed] [Google Scholar]
- 5. Fromtling RA, Gadebusch HH.. Ethanol-cephalosporin antibiotic interactions: an animal model for the detection of disulfiram (Antabuse)-like effects. Methods Find Exp Clin Pharmacol. 1983; 5 9: 595– 600. [PubMed] [Google Scholar]
- 6. Eron LJ, Park CH, Hixon DL, . et al. Ceftriaxone therapy of bone and soft tissue infections in hospital and outpatient settings. Antimicrob Agents Chemother. 1983; 23 5: 731– 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moskovitz BL. Clinical adverse effects during ceftriaxone therapy. Am J Med. 1984; 77 4C: 84– 88. [PubMed] [Google Scholar]
- 8. Billstein SA, Sudol TE.. Disulfiram-like reactions rare with ceftriaxone. Geriatrics. 1992; 47 4: 70. [PubMed] [Google Scholar]
- 9. Ren S, Cao Y, Zhang X, . et al. Cephalosporin induced disulfiram-like reaction: a retrospective review of 78 cases. Int Surg. 2014; 99 2: 142– 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomasson HR, Crabb DW, Edenberg HJ, . et al. Alcohol and aldehyde dehydrogenase polymorphisms and alcoholism. Behav Genet. 1993; 23 2: 131– 136. [DOI] [PubMed] [Google Scholar]
- 11. Matsubara T, Otsubo S, Ogawa A, . et al. Effects of beta-lactam antibiotics and N-methyltetrazolethiol on the alcohol-metabolizing system in rats. Jpn J Pharmacol. 1987; 45 3: 303– 315. [DOI] [PubMed] [Google Scholar]
- 12. Kitson TM. The effect of 5,5′-dithiobis(1-methyltetrazole) on cytoplasmic aldehyde dehydrogenase and its implications for cephalosporin-alcohol reactions. Alcohol Clin Exp Res. 1986; 10 1: 27– 32. [DOI] [PubMed] [Google Scholar]
- 13. Sar TK, Mandal TK, Patra PH, . et al. Disposition of ceftriaxone in hepatopathic goats following single-intramuscular dosing. Eur J Drug Metab Pharmacokinet. 2013; 38 4: 269– 273. [DOI] [PubMed] [Google Scholar]
- 14. Jones AW, Neiman J, Hillbom M.. Concentration-time profiles of ethanol and acetaldehyde in human volunteers treated with the alcohol-sensitizing drug, calcium carbimide. Br J Clin Pharmacol. 1988; 25 2: 213– 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donovan JE. Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics. 2009; 132 6: 975– 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rising Pharmaceutical, Inc. Disulfiram [package insert]. Allendale, NJ: Rising Pharmaceutical, Inc; 2016. [Google Scholar]
- 17. Rees MR, Clark RA, Holdsworth CD, . et al. The effect of beta-adrenoceptor agonists and antagonists on gastric emptying in man. Br J Clin Pharmacol. 1980; 10 6: 551– 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schaad UB, Stoeckel K.. Single-dose pharmacokinetics of ceftriaxone in infants and young children. Antimicrob Agents Chemother. 1982; 21 2: 248– 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simons KJ, Watson WT, Martin TJ, . et al. Diphenhydramine: pharmacokinetics and pharmacodynamics in elderly adults, young adults, and children. J Clin Pharmacol. 1990; 30 7: 665– 671. [DOI] [PubMed] [Google Scholar]
- 20. Stowell A, Johnsen J, Ripel A, . et al. Diphenhydramine and the calcium carbimide-ethanol reaction: a placebo-controlled clinical study. Clin Pharmacol Ther. 1986; 39 5: 521– 525. [DOI] [PubMed] [Google Scholar]


