Abstract
In T lymphocytes, expression of miR-148a is induced by T-bet and Twist1, and is specific for pro-inflammatory Th1 cells. In these cells, miR-148a inhibits the expression of the pro-apoptotic protein Bim and promotes their survival. Here we use sequence-specific cholesterol-modified oligonucleotides against miR-148a (antagomir-148a) for the selective elimination of pro-inflammatory Th1 cells in vivo. In the murine model of transfer colitis, antagomir-148a treatment reduced the number of pro-inflammatory Th1 cells in the colon of colitic mice by 50% and inhibited miR-148a expression by 71% in the remaining Th1 cells. Expression of Bim protein in colonic Th1 cells was increased. Antagomir-148a-mediated reduction of Th1 cells resulted in a significant amelioration of colitis. The effect of antagomir-148a was selective for chronic inflammation. Antigen-specific memory Th cells that were generated by an acute immune reaction to nitrophenylacetyl-coupled chicken gamma globulin (NP-CGG) were not affected by treatment with antagomir-148a, both during the effector and the memory phase. In addition, antibody titers to NP-CGG were not altered. Thus, antagomir-148a might qualify as an effective drug to selectively deplete pro-inflammatory Th1 cells of chronic inflammation without affecting the protective immunological memory.
Keywords: Inflammatory bowel disease, Pro-inflammatory Th1 cells, Chronic inflammation, miRNA-148a, Oligonucleotide therapy, Pre-clinical study, Antagomirs
Highlights
-
•
Th1 cells expressing miR-148a mediate colitis in a murine model of IBD.
-
•
Antagomir-148a inhibits colitis by selectively depleting Th1 cells from the colon.
-
•
Antagomir-148a does not affect the protective immunological memory.
1. Introduction
In the murine model of transfer colitis [1], chronic inflammation of the gut can be induced by effector memory T helper type 1 (Th1) cells [2], [3], [4]. It is likely that Th lymphocytes are not only able to induce, but also can maintain inflammatory bowel diseases, since treatment of Crohn's disease patients with depleting anti-cluster of differentiation 4 (CD4) antibodies did reduce disease activity significantly [5]. However, therapeutic ablation of CD4+ Th lymphocytes not only in Crohn's disease, but also in other chronic inflammatory diseases, has been abandoned. Anti-CD4 induces long-lasting lymphopenia and immunodeficiency, since also protective Th lymphocytes are depleted [5], [6], [7]. Here we describe the in vivo use of microRNA-148a (miR-148a)-specific antagomirs (antagomir-148a) for the selective depletion of pro-inflammatory Th1 cells in the murine model of transfer colitis.
Recently, we have identified miR-148a as a microRNA (miRNA) specifically upregulated in repeatedly activated Th1 cells, as compared to Th2 and Th17 cells [8]. The T-box transcription factor T-bet and the transcription factor twist-related protein 1 (Twist1), two signature proteins of Th1 cells adapted to chronic inflammation, induce the upregulation of miR-148a. Both Twist1 and miR-148a are highly expressed in effector/memory Th cells isolated from inflamed tissues of patients with chronic inflammatory diseases, including Crohn's disease and rheumatoid arthritis [8], [9]. A target of miR-148a is Bcl2l11, encoding the pro-apoptotic protein Bcl2-interacting protein (Bim) [8], [10], [11], [12]. Specific inhibition of miR-148a in repeatedly activated murine Th1 lymphocytes in vitro, results in enhanced Bim expression and negatively affects their survival [8].
Antagomirs are 20–25 nucleotides long oligonucleotides complementary to the mature form of distinct miRNAs and conjugated to cholesterol at their 3′ end. They easily penetrate into cells and inhibit the activity of their target miRNAs, both in vitro and in vivo [13], [14]. Here we show that in the murine transfer colitis model, systemic administration of antagomir-148a efficiently targets pro-inflammatory Th1 cells in the inflamed gut, reducing their numbers by 50%, and resulting in a mild but significant amelioration of inflammation. This effect was selective for chronic inflammation, since neither the maintenance nor the functionality of protective CD4+ memory Th cells of the bone marrow (BM) and the spleen were affected in healthy mice that were immunized with NP-CGG to elicit vaccine-like immune responses. Thus, antagomirs represent a novel class of therapeutic molecules that can be used to target pro-inflammatory Th cells, which have adapted to chronic inflammation by the selective expression of distinct miRNAs, here miR-148a.
2. Material and methods
2.1. Mice
C57BL/6 mice were bred and housed under specific pathogen-free (SPF) conditions at the animal facility of the DRFZ Berlin. Recombination activating gene-deficient (Rag1−/−) mice were bred under SPF conditions at Charles River and housed in our animal facility during experiments. All animal experiments were performed in accordance with institutional, state, and federal guidelines. All experiments were approved by the federal state institution “Landesamt für Gesundheit und Soziales” (G0300/11, G0008/13, T0192/10) in Berlin, Germany.
2.2. Cell culture
Naive CD4+ Th cells were isolated from spleens of 6–12 weeks old C57BL/6 mice. First, regulatory T cells were removed by staining with cyanine 5 (Cy5)-coupled anti-CD25 antibodies and subsequent magnetic separation with anti-Cy5 microbeads (Miltenyi Biotec). Afterwards, Th cells were stained with anti-CD4 fluorescein isothiocyanate (FITC) and purified by magnetic-activated cell sorting (MACS®) using anti-FITC microbeads (Miltenyi Biotec). Finally, naive CD4+CD62L+ T cells were isolated by labeling of Th cells with anti-CD62L microbeads (Miltenyi Biotec). To generate Th1 cells, naive Th cells were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (3 μg/ml each, BD Biosciences) in the presence of CD90-depleted, irradiated (30 Gy) splenocytes, as well as anti-interleukin 4 (IL-4) (10 μg/ml, clone 11B11) and the Th1-polarizing cytokine IL-12 (5 ng/ml, R&D Systems). All T cell cultures were conducted in “RPMI complete medium” (pH 7.2), i.e. Roswell Park Memorial Institute-1640 (RPMI) medium (Life Technologies GmbH) containing 10% fetal calf serum (Th. Geyer), 100 units/ml penicillin, 0.1 mg/ml streptomycin and 10 μM β-mercaptoethanol (all from Life Technologies GmbH). To avoid activation-induced cell death, Th cells were removed from anti-CD3/anti-CD28-coated plates before 48 h of stimulation and transferred to new tissue culture plates. After 5 days of culture, viable Th cells were purified by gradient separation with Ficoll histopaque (Sigma-Aldrich), washed with PBS/BSA (i.e. phosphate-buffered saline (PBS) including 0.2% bovine serum albumin (BSA), pH 7.2) and re-stimulated for additional 5 days under the same conditions, except that IL-2 (10 ng/ml, R&D Systems) was added to the cultures. Polarization of Th cells to Th1 lymphocytes was determined by measuring interferon-γ (IFN-γ) expression by flow cytometry following phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation.
2.3. Antagomirs
Lyophilized antagomirs were custom-synthesized according to Krutzfeldt et al. (2005) [13]. Antagomirs were generated by Dharmacon, GE Healthcare, for in vivo applications. Purification of both antagomir-148a and antagomir-Scrambled (antagomir-Scr) was performed by high performance liquid chromatography (HPLC) and contained similarly low concentrations of endotoxins, with ≤0.218 EU/mg (endotoxin units per milligram) for antagomir-148 and ≤ 0.2 EU/mg for antagomir-Scr. Antagomir sequences are as follows: antagomir-Scr 5-mU(*)mC(*)mAmCmGmCmAmGmAmUmUmCmAmUmA-mA(*)mC(*)mG(*)mU(*)-3-Chol [15]; and antagomir-148a 5-mA(*)mC(*)mAmAmAmGmUmUmCmUmGmUmAmGmUmGmCmAmC(*)mU(*)mG(*)mA(*)-3-Chol [8]. All ribonucleotides were 2-O-methyl modified (mN) and (*) represents a phosphorothioate modification of the backbone. At the 3′-end of the oligonucleotides, a cholesterol (Chol) molecule was added. Lyophilized antagomirs were dissolved in PBS (pH 7.2) at the desired concentration at room temperature for 30 min with slight shaking [14].
2.3.1. Colitis induction and antagomir treatment
Two weeks prior to colitis induction, Rag1−/− recipient mice were colonized by oral gavage with fecal bacteria suspensions containing segmented filamentous bacteria (SFB) and Helicobacter species (H. spp.) [16]. In order to confirm whether the Rag1−/− mice were colonized successfully, PCRs of fecal DNA with the following primers were conducted: H. spp. Fw: 5′-ctatgacgggtatccggc-3′, Rv: 5′-attccacctacctctccca-3′, SFB Fw: 5′-gacgctgaggcatgagagcat-3′, Rv: 5′-gacggcacggattgttattca-3´. In order to ensure comparable compositions of the intestinal microbiota in antagomir-148a- and in antagomir-Scr-treated (i.e. control) groups throughout the experiments, mice of both groups were co-housed in identical cages during the experiment.
Colitis was induced as published before with small modifications [1]. In brief, repeatedly activated Th1 cells were resuspended in PBS (pH 7.2) in order to transfer 4 × 105 cells into Rag1−/− recipients via intravenous (i.v.) injections into the lateral tail vein. On days 2, 4 and 6 after Th1 cell transfer, mice were injected i.v. with 50 mg/kg antagomir-Scr or with antagomir-148a. During ongoing experiments, health conditions of mice were monitored according to the guidelines of the “Landesamt für Gesundheit und Soziales” in Berlin and experiments were terminated according to the same guidelines. The severity of inflammation was evaluated by measuring the length and the weight of the colon to calculate its weight-to-length ratio, which for healthy colons of mice of that age typically is in the range of 35–40 and which increases with higher degrees of inflammation [17], [18], [19].
2.3.2. Antagomir treatment during and after vaccine-like immune responses
C57BL/6 mice were intraperitoneally (i.p.) immunized with 100 μg NP-CGG and 100 μl incomplete Freund's adjuvant (IFA). Three weeks later, mice were boosted i.p. with 10 μg NP-CGG and 100 μl IFA. One, 3 and 5 days after boost, mice were injected i.v. with 50 mg/kg antagomir-148a or antagomir-Scr. On day 7 after boost, mice were sacrificed to analyze immune cell populations by flow cytometry. Non-immunized control mice were age-matched littermates of immunized mice but were not immunized with NP-CGG and were not treated with antagomirs.
In order to determine T memory cells in the memory phase after immunization and also to generate NP-CGG-specific antibody titers by long-lived plasma cells, C57BL/6 mice were immunized as described above, but were boosted twice i.p. with 10 μg NP-CGG and 100 μl IFA with a 3 week interval in between. Mice were left untreated for additional 3 weeks before they were treated 3 times every second day i.v. with 50 mg/kg antagomir-148a or antagomir-Scr. Two days after the third treatment, sera of mice were collected to determine antibody titers by Enzyme-linked Immunosorbent Assay (ELISA). Subsequently, the mice were sacrificed, in order to analyze immune cell populations from the spleen and the bone marrow by flow cytometry. Non-immunized control mice were age-matched littermates of immunized mice but were not immunized with NP-CGG and were not treated with antagomirs.
2.3.3. Isolation of leukocytes from the colonic mucosa
Purification of leukocytes from the colon was performed as described before [20]. Fat tissues were removed from intestines before the colons were opened longitudinally and washed with PBS (pH 7.2). The epithelial layers were removed by 2 cycles of incubation in Ca2+/Mg2+-free Hank's Balanced Salt Solution (HBSS, pH 7.2) with 5 mM ethylenediaminetetraacetic acid (EDTA) and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, pH 7.2) for 20 min at 37 °C while gently spinning at 100 rotations per minute (rpm). Afterwards, the intestines were cut into small pieces and digested 3 times 20 min each at 37 °C with HBSS (pH 7.2) containing Ca2+/Mg2+ and 0.5 mg/ml Collagenase D (Roche), 0.5 mg/ml DNase I (Sigma-Aldrich) and 0.5 U/ml Dispase (BD Biosciences) while agitated gently at 300 rpm. After digestion, cells were filtered (100 μm cell strainers, BD) into single cell suspensions and washed with PBS/BSA (pH 7.2) prior to antibody staining for flow cytometry or cell sorting.
2.4. Flow cytometry
Erythrocytes of single cell suspensions from spleen and BM were lysed. T cell cultures or single cell suspensions of leukocytes isolated from BM, inguinal lymph nodes (iLNs), spleen or colon were stained in PBS/BSA (pH 7.2) with EDTA (2 mM) after blocking with anti-mouse CD16/CD32 (2.4G2) antibodies. Surface antibody stainings were performed for 15 min at 4 °C.
For intracellular cytokine stainings, cells were re-stimulated for 4 h with 10 ng/ml PMA and 1 μg/ml ionomycin in RPMI complete medium at 37 °C, 5% CO2. After 1 h of incubation, Brefeldin A (Biolegend) was added to the cultures to reach a final concentration of 5 μg/ml. Cells were washed and stained with fixable viability dye in PBS (pH 7.2) for 15 min at 4 °C using the Zombie Yellow™ Fixable Viability Kit from Biolegend. Subsequently, the cells were fixed and stained intracellularly using the Fixation/Permeabilization Solution Kit from BD Biosciences. To avoid unspecific stainings, cells were blocked again with anti-mouse CD16/CD32 (2.4G2) after fixation and permeabilization of the cells. For detection of NP-CGG-specific T lymphocytes from BM, cells and splenocytes isolated from one mouse were stimulated together in one well (1:1 ratio) for 6 h at 37 °C, 5% CO2. In order to distinguish splenocytes from BM cells, splenocytes were labeled with CFSE (1 μM, 10 min at 37 °C, 5% CO2) prior to stimulation with NP-CGG. After the first 2 h of stimulation, Brefeldin A (5 μg/ml final concentration) was added to the cultures. Subsequently, cells were stained intracellularly for cytokines and CD40L as described for cells stimulated with PMA/ionomycin above.
Transcription factor stainings were performed using the forkhead-box-protein P3 (FoxP3) staining buffer kit (eBioscience) according to the manufacturer's instructions. For intracellular Bim and B-cell lymphoma 2 (Bcl-2) stainings, cells were fixed over-night using the FoxP3 staining buffer set, then washed and blocked with 130 μg/ml rat immunoglobulin G2a (IgG2a) and 130 μg/ml armenian hamster IgG in addition to anti-mouse CD16/CD32 for 30 min in the fridge. Afterwards, cells were stained for Bim and Bcl-2 at 4 °C over-night. Cells were recorded on a Fluorescence-activated Cell Sorting (FACS) Canto II instrument (BD Biosciences) or on a MACSQuant (Miltenyi Biotec). The following antibodies and reagents were used: Bcl-2 phycoerythrin (PE) (3F11, BD Biosciences), Bim Alexa Fluor 647 (14A8, Merck Millipore, Darmstadt, Germany), CD3 allophycocyanin (APC)-eFluor 780 (145-2C11, eBioscience), CD1d-PBS57-loaded tetramer PE (NIH tetramer core facility), CD4 PE-Cy7 (RM4-5, eBioscience), CD8 Cy5 (53–6.72, own conjugate), CD40L Alexa Fluor 647 (MR1, eBioscience), CD40L PE (MR1, Biolegend), CD44 Pacific Blue (IM7, own conjugate), CD64 APC (X54-5/7.1, eBioscience), CD69 FITC (H1.2F3, own conjugate), CD138 brilliant violet 421 (BV421) (281-2, Biolegend) FoxP3 eFluor 450 (FJK-16 s, eBioscience), GATA-binding protein-3 (GATA-3) peridin chlorophyll protein complex (PerCP)-eFluor710 (TWAJ, eBioscience), IL-2 PE and APC (JES6-5H4, Biolegend), IL-10 PE (JES5-16E3, eBioscience), IL-17A FITC (TC11-18H10.1, Biolegend), IL-22 APC (IL22JOP, eBioscience), Ki-67 Alexa Fluor 488 (B56, eBioscience), Ly-6C Pacific Blue (HK1.4, Biolegend), Ly-6G PerCP-Cy5.5 (1A8, Biolegend), propidium iodide (PI, Sigma Aldrich), ROR-γt PE (Q31-378, BD Biosciences), T-bet Alexa Fluor 647 (4B10, Biolegend), tumor necrosis factor-α (TNF-α) Alexa Fluor 405 (MP6-XT22, own conjugate). The gating of the flow cytometric data was performed according to the guidelines for the use of flow cytometry and cell sorting in immunological studies [21].
2.5. Cell sorting and quantitative PCR
CD3+CD4+ Th cells from the colonic lamina propria were sorted using a FACSAria I sorter (BD Biosciences). Cells were lysed and total RNA was purified with the Quick-RNA™ MiniPrep kit (Zymo Research). Mature miR-148a and U6 small nuclear RNA (snRNA) were detected by quantitative PCR with the Taqman MicroRNA Reverse Transcription kit in combination with TaqMan MicroRNA Assays (Applied Biosystems) according to the manufacturer's recommendations. For normalization, the expression values were compared to values of snU6 RNA by the change-in-threshold method (2−ΔCT).
2.6. Enzyme-linked Immunosorbent Assays
Maxisorp ELISA 96-well plates were coated with 2 μg/ml NP-CGG in PBS (pH 7.2) (over-night at 4 °C). Afterwards, the plates were blocked for 1 h at room temperature with PBS (pH 7.2) including 2% BSA and 0.05% Tween and rinsed twice with tap water. The sera were initially diluted by a factor of 200, followed by 3 serial 1:100 dilutions in PBS (pH 7.2) with 2% BSA + 0.05% Tween. The sera then were transferred to the NP-CGG-coated plate and incubated for 2–3 h at room temperature. Subsequently, the plates were washed again with tap water and incubated with alkaline phosphatase-labeled antibodies (1 μg/ml) for 1 h at room temperature. The plates were washed again and 50 μl developing solution (one tablet of nitrophenyl phosphate in 5 ml 1 M diethanolamine, pH 9.8 (Sigma-Aldrich)) was added to each well. After 10, 15, 30 and 45 min, the absorbance at 405 nm was determined by a plate reader.
2.7. Histology
Organs were dissected from mice and fixed in 4% paraformaldehyde at 4 °C over-night. Subsequently, the organs were washed with PBS (pH 7.2), dewatered and embedded in paraffin. Tissue sections were prepared and stained with hematoxylin and eosin.
2.8. Statistics
If not stated otherwise, the Mann–Whitney test for unpaired data was used for all statistical analyses with *, ** and *** representing p values of <0.05, <0.01 or <0.001, respectively. The program GraphPad Prism was used for all statistical analyses.
3. Results
3.1. Antagomir-148a depletes pro-inflammatory Th1 cells in inflamed colons of mice with colitis
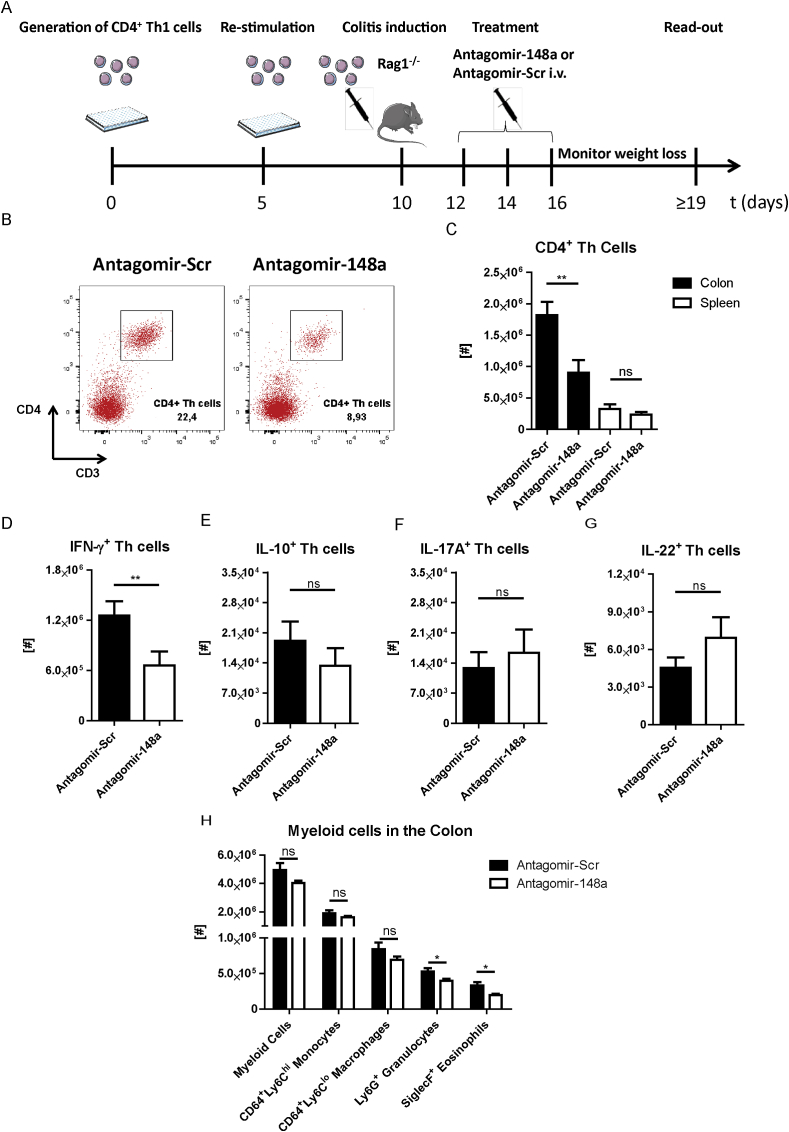
Th1 cells adapt to repeated stimulation by upregulating the expression of miR-148a in vitro, which promotes their survival [8]. To investigate whether such pro-inflammatory Th1 cells can be targeted by inhibiting miR-148a function in vivo, we used the Th cell-dependent transfer colitis model [1]. In this model, the induction of colitis requires transfer of Th cells into immunodeficient mice (e.g., Rag1-deficient mice). Accordingly, we induced inflammation of the colon by adoptive transfer of repeatedly activated Th1 cells into Rag1-deficient mice (Fig. 1A). Within 11 days after transfer, the recipient mice developed colitis as shown by weight loss and diarrhea. On days 2, 4 and 6 after adoptive transfer, 50 mg/kg miR-148a-specific antagomirs (antagomir-148a) or antagomirs with a non-targeting control sequence (antagomir-Scr) were injected i.v. into these mice (Fig. 1A). The experiment was terminated before the first mouse had lost 20% of its initial body weight and cells from inflamed colons and spleens were analyzed. More than 90% of Th cells in the inflamed colons were T-bet-expressing Th1 cells, with less than 5% of Th cells expressing ROR-γt, GATA-3 or FoxP3 (Supplemental Fig. 1A). Approximately 70% of all Th cells expressed IFN-γ, of which ∼20% also expressed TNF-α (Supplemental Fig. 1B). In contrast, fewer than 5% of Th cells expressed IL-17A, IL-22 or IL-10 (Supplemental Fig. 1B). Compared to mice treated with antagomir-Scr, antagomir-148a-treated mice showed a 50% depletion of CD4+ Th cells in the colon (Fig. 1B and C). In the spleen, Th cell numbers were not significantly different (Fig. 1C). In the colons of antagomir-148a-treated mice, the number of IFN-γ+ Th1 cells was reduced by 47% (Fig. 1D). In contrast, IL-10- (Fig. 1E), IL-17A- (Fig. 1F) or IL-22- (Fig. 1G) producing Th cells were not reduced. Antagomir-148a treatment also did not affect the numbers of total CD3-negative myeloid cells, Ly6ChiCD64+ monocytes or Ly6CloCD64+ macrophages in the colon, while the numbers of Ly6G+ neutrophilic and SiglecF+ eosinophilic granulocytes were reduced by 25% and 37%, respectively (Fig. 1H).
Fig. 1.
Systemic antagomir-148a treatment of colitic mice depletes IFN-γ-expressing Th1 cells selectively in the inflamed colon. (A) Schematic overview depicting the experimental procedure for inducing colitis by adoptive transfer of repeatedly activated Th1 cells into Rag1−/− mice and subsequent antagomir treatment. (B) Representative dot plots showing the frequencies of CD3+CD4+ Th cells isolated from inflamed colons of colitic mice that were treated with antagomir-Scr or antagomir-148a. Displayed frequencies are percentages of Th cells among isolated viable cells. (C) Total cell numbers of viable CD3+CD4+ Th cells that were isolated from the colons and spleens of colitic mice following antagomir-148a or antagomir-Scr treatment as shown in (A). (D–G) Lymphocytes from colitic mice were isolated from the inflamed colons and stimulated with PMA/ionomycin in the presence of Brefeldin A. Shown are the absolute cell numbers of CD3+CD4+ Th cells that expressed IFN-γ (D), IL-10 (E), IL-17A (F) or IL-22 (G). (H) Quantification of different myeloid subsets isolated from inflamed colonic mucosae of colitic mice. Depicted data are pooled from two independent experiments with n = 12 and n = 13 for antagomir-148a- and antagomir-Scr-treated mice, respectively.
3.2. Antagomir-148a reduces the expression of miR-148a while enhancing the expression of Bim in colonic Th1 cells
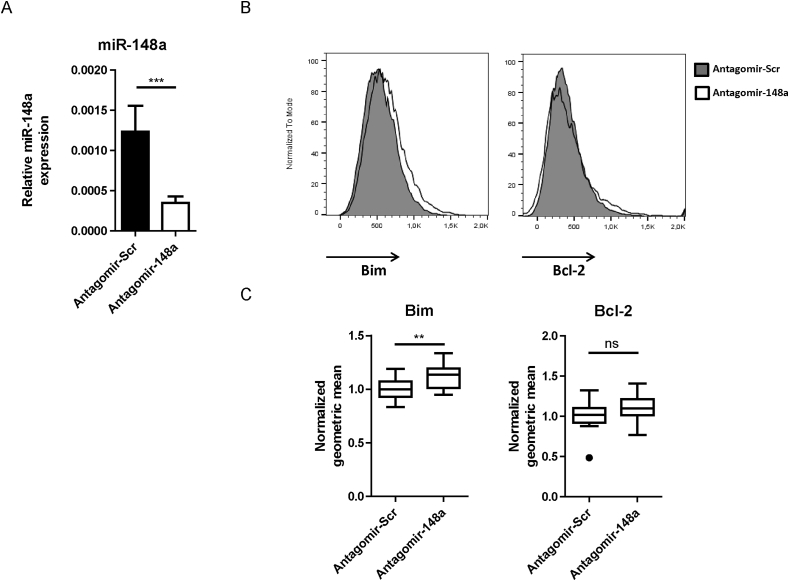
From the inflamed colons of colitic mice, Th cells surviving the antagomir-148a treatment were isolated on days 9 or 11 after T cell transfer, and analyzed for expression of miR-148a, Bim and Bcl-2. The expression of miR-148a, as determined by quantitative PCR, was reduced by 71%, when compared to Th cells isolated from the colons of mice treated with antagomir-Scr (Fig. 2A). Expression of the pro-apoptotic protein Bim, one of the targets of miR-148a, was upregulated by 15%, as determined by cytometric immunofluorescence of individual Th cells (Fig. 2B and C, left panels). In the same cells, expression of the anti-apoptotic protein Bcl-2 was not affected (Fig. 2B and C, right panels), showing that inhibition of miR-148a skews the balance of pro- and anti-apoptotic molecules in Th cells in favor of pro-apoptotic proteins, as has been shown before in vitro [8]. The unimodal staining for Bim in CD4+ T cells from the colon shows that all T cells were affected by the antagomir treatment, and not just a subpopulation (Fig. 2B).
Fig. 2.
Systemic antagomir-148a treatment efficiently inhibits miR-148a expression and results in upregulation of Bim protein in Th cells of the inflamed intestinal mucosa in colitic mice. Colitic Rag1-deficient mice were treated with antagomir-148a or antagomir-Scr as displayed in Fig. 1A. (A) On the last day of the experiment, CD3+CD4+ Th cells were isolated from the colonic laminae propriae by FACS. RNA from the sorted cells was purified and miR-148a expression was measured by TaqMan™ PCR. (B, C) Colonic Th cells were analyzed by flow cytometry following intracellular staining of the pro-apoptotic molecule Bim (B, C left panels) and the anti-apoptotic molecule Bcl-2 (B, C right panels). Shown are representative histograms (B) and the quantification of geometric means normalized to antagomir-Scr-treated mice (C). Data in (A) are pooled from two independent experiments with n = 10 and n = 8 mice, while data in (C) are pooled from two independent experiments with n = 12 and n = 13 mice that were treated with antagomir-148a or antagomir-Scr, respectively.
3.3. Antagomir-148a ameliorates colitis
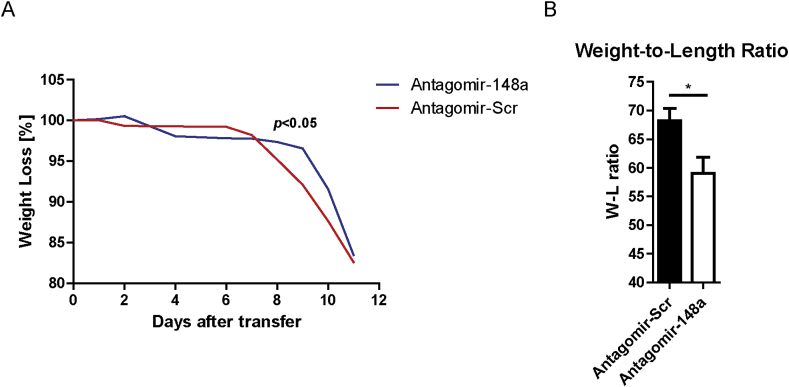
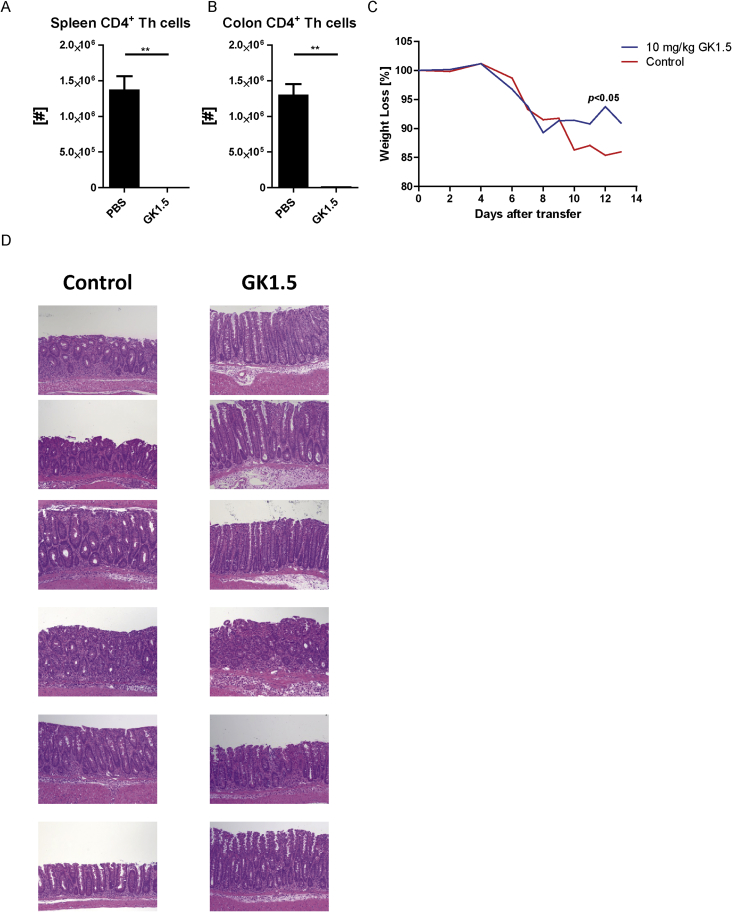
To quantify colitis pathology, we analyzed disease parameters such as body weight loss, the weight-to-length ratios of inflamed colons and the histology of the inflamed colons from mice treated as shown in Fig. 1A. Both mice treated with antagomir-148a and mice treated with antagomir-Scr showed signs of colonic inflammation, such as infiltration of immune cells, goblet cell loss, hyperplasia and crypt abscesses (Supplemental Fig. 2). However, and in conjunction with the ablation of Th1 cells, mice treated with antagomir-148a showed a significant weight loss delay during the inflammatory late phase of colitis, when compared to mice treated with antagomir-Scr (Fig. 3A). Antagomir-148a-treated mice showed a significant amelioration of colon inflammation as reflected by a 20% reduction of the colonic weight-to-length ratio (Fig. 3B). Of note, systemic ablation of CD4+ T lymphocytes by injection of anti-CD4 antibodies (clone GK1.5) causing a depletion of 99% of CD4+ T cells both in spleen and colon (Supplemental Figs. 3A and B), resulted in a similar amelioration of inflammation as determined by weight loss delay during the inflammatory late phase of colitis and histology (Supplemental Figs. 3C and D).
Fig. 3.
Antagomir-148a treatment moderately ameliorates pathology of colitis. Colitis was induced in Rag1−/− mice prior to their treatment with antagomirs as shown in Fig. 1A. (A) The weight loss relative to starting weight was monitored during the experiment. (B) At the end of the experiment, mice were sacrificed and the weight-to-length (W–L) ratios of the colons were determined. Displayed data are pooled from two independent experiments. The Two-Way ANOVA test was used to determine the overall statistical significance of total body-weight loss curves between antagomir-148a- and antagomir-Scr-treated mice. Bonferroni multiple comparison was used as a post-hoc test to evaluate the significance of single time points. Shown data are pooled from two independent experiments with n = 12 and n = 13 for antagomir-148a- and antagomir-Scr-treated mice, respectively. Depicted curves in (A) represent the median weight per designated group.
3.4. Antagomir-148a does not affect the immunological memory during the effector phase of an acute immune response
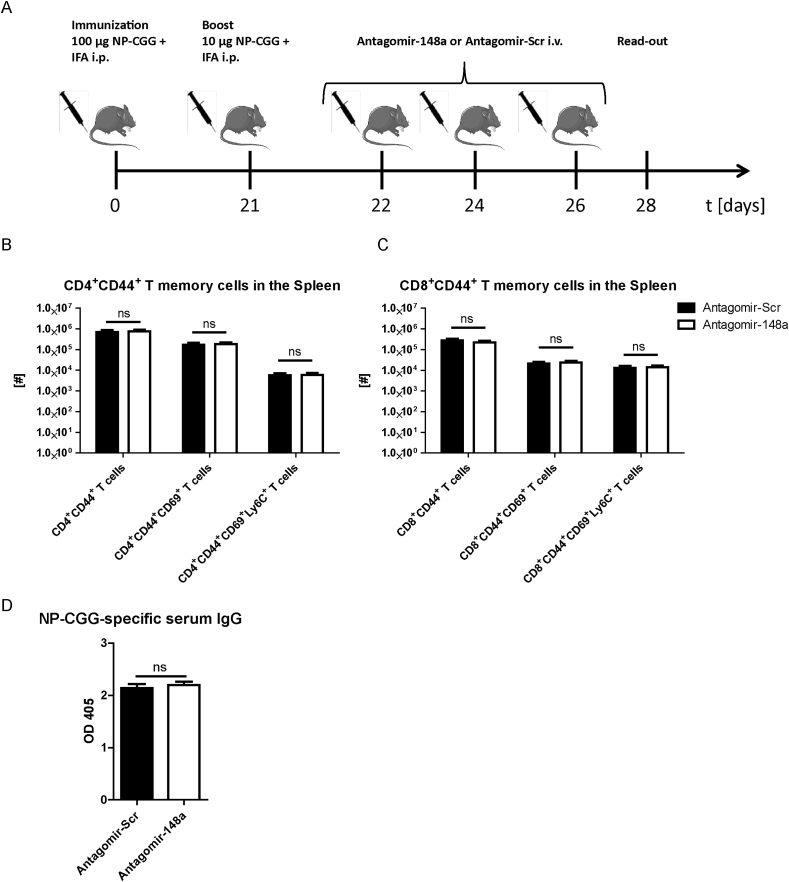
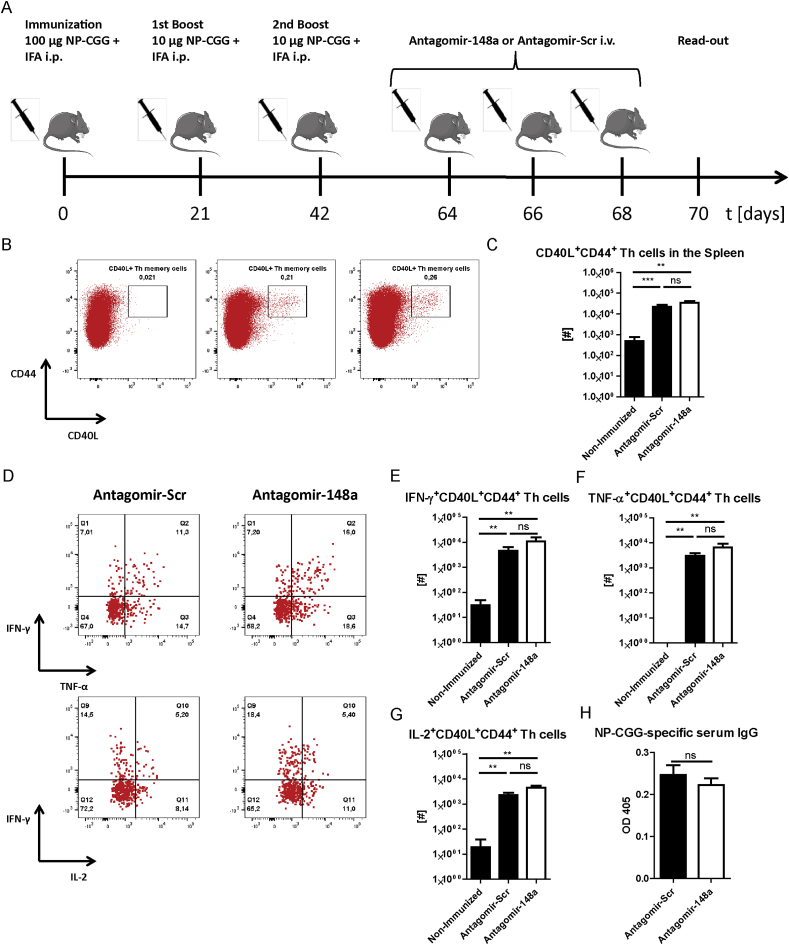
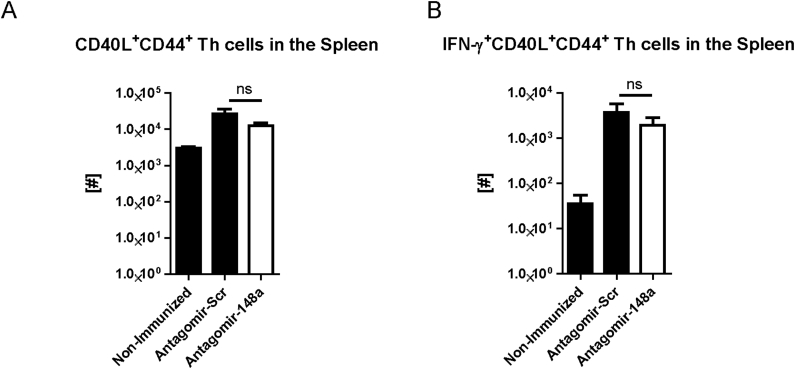
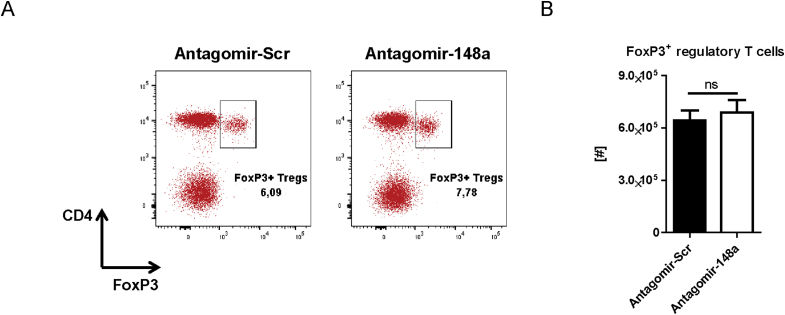
To investigate whether the reduction of Th cells by systemic inhibition of miR-148a is specific for pro-inflammatory Th1 cells and does not interfere with protective memory Th cells generated by vaccine-like immune responses, we immunized healthy C57BL/6 mice i.p. with 100 μg NP-CGG and IFA (Fig. 4A). After 21 days, we boosted the mice with 10 μg NP-CGG and IFA prior to treating them i.v. with either 50 mg/kg antagomir-148a or antagomir-Scr on days 1, 3 and 5 after the boost, i.e. during the effector phase of the immune response. On day 7 after boost, we determined the cell numbers of CD4+ and CD8+ memory T cell subsets in the spleen. The numbers of all investigated memory T cell subsets were unchanged upon antagomir-148a treatment and in antagomir-Scr-treated controls (Fig. 4B and C). Importantly, antagomir-148a treatment did also not reduce NP-CGG-specific CD40L+ memory Th cells as well as their functionality reflected by production of IFN-γ upon re-stimulation with NP-CGG (Supplemental Figs. 4A and B). MiR-148a is also expressed in plasmablasts and plasma cells in which its expression accounts for 20% of all expressed microRNAs [12], hence antagomir-148a treatment could potentially affect antibody-secreting cells. However, 6 days after initial antagomir-148a treatment, NP-CGG-specific immunoglobulin G (IgG) antibody titers in the serum were not altered (Fig. 4D). Of note, antagomir injections did not have any detrimental effects on the structure and architecture of highly perfused organs such as the liver, heart, kidney or lung (Supplemental Fig. 5). Finally, antagomir-148a treatment did also not change the numbers of FoxP3+ T regulatory cells in the spleen (Supplemental Fig. 6).
Fig. 4.
Antagomir-148a treatment during the effector response of an acute immune reaction does not reduce memory T cell populations or circulating antigen-specific IgG titers. (A) Schematic diagram showing the experimental procedure for eliciting a vaccine-like induced immune response and subsequent antagomir treatment during the effector phase after boosting with NP-CGG and IFA. (B, C) Cell numbers of splenic memory T cell populations in immunized mice that were treated with antagomir-148a or antagomir-Scr as shown in Figure 4A. (D) Blood sera of mice that were treated as shown in (A) were collected on day 28 and NP-CGG-specific antibody titers were determined by ELISA. Data in (B) and (C) are pooled from two independent experiments with n = 11 antagomir-Scr-treated mice and n = 12 antagomir-148a-treated mice. Data in (D) are from one experiment with n = 7 for both antagomir-148a- and antagomir-Scr-treated mice.
3.5. Antagomir-148a does not affect the resting immunological memory
To test whether antagomir-148a affects “protective”, resting memory Th cells generated by vaccine-like immune responses, we immunized C57Bl/6 mice with NP-CGG and IFA and injected them with antagomir-148a in the memory phase following contraction of the immune response as indicated in Fig. 5A. We measured the absolute numbers of splenic NP-CGG-specific Th memory cells by determining CD44+CD40L+ Th memory cells upon re-stimulation with NP-CGG ex vivo. Antagomir-148a treatment did not reduce the frequencies (Fig. 5B) or absolute cell numbers (Fig. 5C) of CD40L+ Th memory cells. Regarding cytokine expression, NP-CGG-specific memory Th cells were not affected in producing IFN-γ (Fig. 5D and E), TNF-α (Fig. 5D and F) or IL-2 (Fig. 5D and G) after treatment with antagomir-148a. In accordance to the unaltered pool of NP-CGG-specific memory Th cells, we also did not observe differences in the serum titers of NP-CGG-specific IgG in mice that were treated with antagomir-148a or antagomir-Scr (Fig. 5H).
Fig. 5.
Antagomir-148a treatment in the memory phase of acute immune responses does not alter the abundance and function of memory Th cells. (A) Schematic diagram displaying antagomir treatment of mice in the memory phase following immunizations to elicit acute immune responses. (B–H) Mice were immunized with NP-CGG and IFA as depicted in (A) or were left without immunization and antagomir treatment (non-immunized controls). On day 70, splenocytes were re-stimulated with NP-CGG before they were stained for flow cytometric analysis. Shown are representative dot plots with frequencies of NP-CGG-specific CD44+CD40L+ memory T cells among CD3+CD4+ Th cells (B) and the absolute cell numbers of CD40L+ NP-CGG-specific Th memory cells in the spleen (C). (D–G) Shown are representative dot plots depicting the frequencies (D) and graphs with the absolute cell numbers (E–G) of cytokine-expressing cells among NP-CGG-specific memory Th cells in the spleen. (H) Antibody titers of NP-CGG-specific IgG from blood sera of mice treated as shown in (A) were determined by ELISA. Data in (B–G) are pooled from two independent experiments with n = 10 (antagomir-148a), n = 9 (antagomir-Scr) and n = 6 (non-immunized) mice. Data in (H) are from one experiment with n = 7 for antagomir-148a- and antagomir-Scr-treated mice each. Dot plots in (B) and (D) are representative for 2 independent experiments.
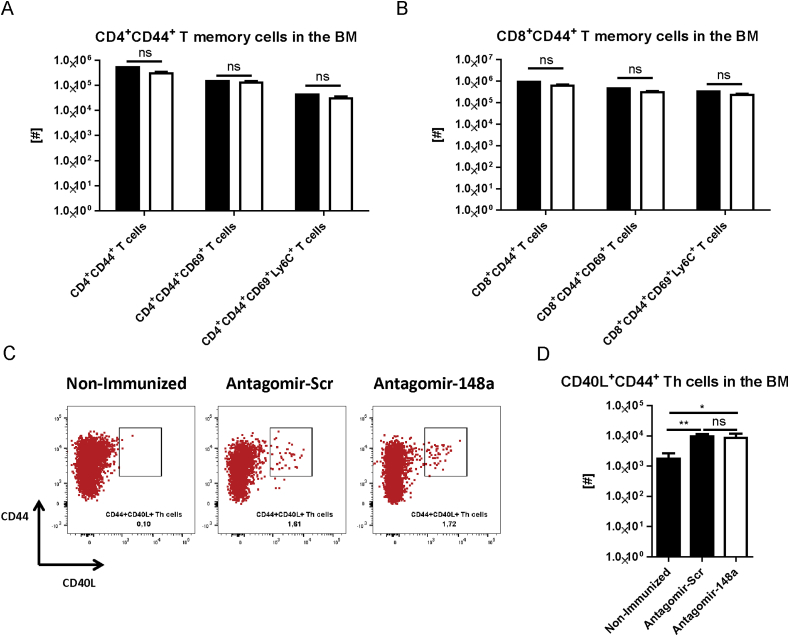
We have previously shown that protective memory Th cells that had been generated by vaccine-like induced immune responses migrate to the bone marrow and express the markers CD69 and Ly6C [22], [23]. In order to test whether antagomir-148a treatment affects the resting memory T cell pool that has been accumulated by natural exposure to antigens, we determined the absolute numbers of BM CD4+ memory T cells expressing CD69 and/or Ly6C, 6 days after the first antagomir treatment as depicted in Fig. 5A. No differences were detectable between mice treated with antagomir-Scr and antagomir-148a (Fig. 6A). This was also true for CD8+ T memory cells in the bone marrow (Fig. 6B) and both for CD4+ and CD8+ memory T cell populations in the spleen and in lymph nodes (Supplemental Figs. 7A–D). Following PMA/ionomycin stimulation, the frequencies of IFN-γ- and TNF-α-expressing Th lymphocytes among memory Th cells were not significantly different in BM, spleen and lymph nodes of mice treated with antagomir-148a or with antagomir-Scr (Supplemental Fig. 7 E–J). Of note, also resting NP-CGG-specific BM memory Th cells that were generated by controlled immunization as shown in Fig. 5A were unaltered after antagomir-148a treatment (Fig. 6C and D). Together, these data show that antagomir-148a does not ablate resting memory Th cells that had been generated in “protective” immune responses with a finite presence of antigen, as opposed to Th1 cells of a chronic immune reaction.
Fig. 6.
Antagomir-148a treatment does not alter the abundance of the accumulated, protective memory T cell pool in the bone marrow. Mice were treated as shown in Fig. 5A or left without immunization and antagomir treatment as non-immunized controls. (A, B) Subsequently, mice were sacrificed to determine the accumulated, resting pools of CD4+ (A) and CD8+ (B) T memory cells expressing CD69 and Ly6C in the bone marrow. (C, D) Representative dot plots of bone marrow cells following re-stimulation with NP-CGG to determine the frequencies (C) and the absolute cell numbers (D) of NP-CGG-specific CD44+CD40L+ Th memory cells. Dot plots in (C) are gated on CD4+ Th cells and representative for 2 independent experiments. Data shown in (A) and (B) are pooled from 2 independent experiments with n = 9 antagomir-Scr- and n = 10 antagomir-148a-treated mice. Data in (D) are from 2 independent experiments with n = 6 non-immunized mice, as well as n = 9 and n = 10 antagomir-Scr- and antagomir-148a-treated mice, respectively.
4. Discussion
The role of pro-inflammatory Th1 lymphocytes in chronic inflammatory diseases is still controversially discussed [24], [25], [26]. Generic depletion of bulk Th lymphocytes by anti-CD4 treatment has been reported to ameliorate chronic inflammatory bowel disease [5]. However, this treatment strategy has been abandoned due to two significant drawbacks. First, this treatment has been shown to induce a long-lasting lymphopenia in patients [5]. Second, Th cell depletion by anti-CD4 antibodies does not distinguish between pathogenic and protective memory Th cell subsets [5]. To date, no therapeutic option for the selective ablation of pathogenic Th1 lymphocytes driving chronic inflammation is available.
In the murine model of transfer colitis, intestinal inflammation can be induced and maintained by Th1 cells [2], [3], [4]. Th lymphocytes isolated from the inflamed guts of patients suffering from Crohn's disease are enriched for Th1 cells expressing high levels of the master transcription factor T-bet, activated signal transducer and activator of transcription-4 (STAT4) and, upon re-stimulation, the effector cytokine IFN-γ [27], suggesting a direct involvement of Th1 cells in the pathogenesis of the disease [24]. They also express high levels of TWIST1, unlike Th lymphocytes isolated from healthy colon and blood [9]. Expression of Twist1 - an E-box binding transcription factor - increments after repeated activation of Th1 lymphocytes [9]. Together with T-bet, Twist1 induces the expression of miR-148a. In repeatedly activated Th1 cells, miR-148a represses Bim and thus promotes their survival [8]. Here we demonstrate that systemic treatment of colitic mice with antagomir-148a selectively ablates Th1 cells from their inflamed colons. Memory T cells of spleen and bone marrow, including Th1 memory cells, are not depleted in healthy mice after immunization. Finally, humoral immune responses were also not altered by this treatment.
MiR-148a is a member of the miR-148/-152 family, together with miR-148b and miR-152 [14], [28]. Expression of miR-148a has been reported for kidney glomerular cells [29], dendritic cells [30], thrombocytes [31], plasma cells [12], B cells [11], repeatedly activated Th1 lymphocytes [8], and a variety of cancer cells, e.g. in renal cell carcinoma [32]. Multiple miR-148a targets have been identified including the gene Bcl2l11 encoding for the pro-apoptotic protein Bim [8], [10], [11], [12]. Knocking down the physiological expression of miR-148a results in enhanced expression of Bim in glioblastoma cells [10], plasma cells [12] and repeatedly activated Th1 cells [8]. By regulating expression of Bim, miR-148a favors the survival of cells expressing it. In vitro, antagomir-mediated inhibition of miR-148a impairs the viability of glioblastoma and repeatedly activated Th1 cells [8], [10]. Here we show, that antagomir-148a treatment of colitic mice resulted in an efficient knock-down of miR-148a in pro-inflammatory Th1 cells of the inflamed colon. Our results confirm a previous report that showed that antagomirs can be utilized as a systemic treatment in that they are resistant to degradation by RNases following injection into an organism [13]. Further, they demonstrate that antagomir-148a penetrates into pro-inflammatory Th1 lymphocytes residing in the inflamed colon. In this manner, antagomir-148a treatment is suitable for manipulating gene expression in vivo also in the inflamed tissue. Indeed, expression of the miR-148a target Bim was increased in the remaining Th1 cells of the colon following inhibition of miR-148a by antagomir-148a injections. These results resemble what we previously have observed for repeatedly activated Th1 cells in vitro [8], suggesting that miR-148a also controls Bim expression in repeatedly activated Th1 cells in vivo. According to the higher expression of Bim, antagomir-148a led to a significant depletion of Th1 lymphocytes by 50% from the inflamed colon in vivo. Antagomir-148a-mediated depletion of Th cells was selective for cells residing in the inflamed tissue, whereas Th lymphocytes from the spleen were not affected.
Regarding disease pathology, antagomir-148a treatment resulted in alleviation of inflammation as determined by a reduced colonic weight-to-length ratio and delayed body weight loss in the chronic late phase of colitis, while regeneration of colonic tissue in terms of histopathology was not observed. Importantly, treatment of colitic mice with anti-CD4 antibodies after disease onset did not result in complete remission of mice either. Of note, in the model used here, colitis progressed very rapidly, and remission was not achieved, probably due to the composition of the microbiota, which contained segmented filamentous bacteria and helicobacter species, both shown to promote colitis [33], [34](data not shown). This is in contrast to a previously published report of complete regeneration of mice after antibody-mediated ablation of Th cells 11 days after treatment [35]. The function of the microbiota regarding differences in disease quality and severity has been noted before, for example, the microbiota dictates whether colitis is induced with the transfer of T-bet-deficient Th cells in Rag1−/− mice [16], [27]. In our study, disease progression reached a terminal stage between 10 and 13 days after Th lymphocyte transfer. Thus, the time for achieving complete remission as determined by tissue regeneration was probably not enough [35]. Nonetheless, with this model we could show that antagomir-148a treatment is capable of depleting pro-inflammatory Th1 cells with a history of repeated activation resulting in mild but significant amelioration of inflammation in the colon.
While the depletion of pro-inflammatory Th1 cells in ongoing inflammation was efficient, we wondered whether antagomir-148a treatment is selective for these cells and spares protective memory. Our data obtained from experiments with healthy, immunized mice do not indicate that systemic antagomir-148a treatment interferes with protective immune responses both during the effector phase and the memory phase. This conclusion is based on equal numbers and functionality (i.e. cytokine production) of NP-CGG-specific T memory cells and the generation of specific IgG antibodies. Importantly, not only the NP-CGG-specific T cell memory but the global pool of resting T cell memory in BM and secondary lymphoid organs, which had been generated by natural exposures to antigens during life, was also not affected. Moreover, FoxP3+ regulatory T cell numbers were also not altered by injection of antagomir-148a, indicating that the balance between immunity and tolerance was not skewed by this treatment. Taken together, these experiments confirm the selectivity of antagomir-148a for repeatedly activated Th1 cells during chronic inflammation. These data fit to what we observed previously in vitro, namely, that upregulated expression of miR-148a is confined to repeatedly activated Th1 cells to counterbalance the function of Bim which otherwise drives these cells into apoptosis. Further, our results corroborate the hypothesis that the mode of T cell activation (e.g., repeated or chronic activation of T cells) can be used as a discriminator for targeting cells found in chronic inflammation while sparing resting memory T cells that are important in protecting the host from recurring infections [4], [9].
The fact that we do not observe changes in the pool of memory T cells and protective antibody titers upon treatment with antagomir-148a is surprising to some degree, since miR-148a is expressed by dendritic cells (DCs) [30] and by plasma cells [12]. It has been shown that miR-148a represses the secretion of IL-12, IL-6, TNF-α, IFN-β and the upregulation of MHC-class II in mature DCs by targeting CaMKIIα (Calcium/calmodulin-dependent protein kinase II). As a consequence, the expansion of T cells was reduced [30]. We did not observe a significant increase in numbers of memory T cells after antagomir-148a treatment. The apparent refraction of bone marrow-derived dendritic cells to antagomir-148a treatment could be due to the fact that dendritic cells also express miR-148b and miR-152, which are inefficiently inhibited by antagomir-148a [14] and might not depend on the miR-148/-152 family. Bim is also a target of miR-148a in plasma cells. However, plasma cells in turn might be resistant to antagomir-148a treatment because they depend more on the Mcl-1/Noxa than Bcl2/Bim intrinsic apoptosis-pathway [36] for their survival and can potentially survive antagomir-148a treatment.
Functions of miR-148a have also been reported in kidney glomerular cells [29] and thrombocytes [37]. Histological analyses of kidney sections did not reveal any pathological changes of their architecture after antagomir-148a treatment, suggesting that the viability of the kidney glomerular cells was not affected. MiR-148a targets TULA-2 (T cell ubiquitin ligand-2) in thrombocytes and antagonizing miR-148a expression in platelets decreases FcγRIIa (Fc receptor for IgG IIA)-mediated activation and thrombosis in vivo [37]. However, tissue sections of highly perfused organs, such as the heart, kidney, lung and liver did not show any signs of tissue damage or hemorrhage in these organs in C57BL/6 mice.
5. Conclusions
In summary, we show here that antagomir-148a has an impressive therapeutic potential for the selective targeting of repeatedly activated Th1 cells in chronic inflammatory diseases. Applied systemically, antagomir-148a reaches intracellular concentrations sufficient to knock-down miR-148a efficiently in such Th1 cells, and depletes these cells from chronically inflamed tissue. Taken together, antagomir-148a could be used as a tool for treating chronic inflammatory diseases or other medical conditions that depend on dysregulated miR-148a expression without putting patients at risk to fall short of opportunistic infections due to impaired protective immunological memory.
Author contributions
P.M. designed the study and performed experiments, analyzed data and wrote the manuscript; G.P., F.S., J.Z., F.Z., K.L., S.S., M.W., C.H., R.R., M.B., G.A.H., C.L.T. did experiments and analyzed data; A.A.K. did histology; B.F.H., F.H., J.W., N.R., F.G.M. and H.-D.C. discussed the results, provided conceptual advice and commented on the manuscript; S.H. provided technical support; A.R. and M.F.M designed the study, supervised research and wrote the manuscript.
Conflicts of interest
The authors have declared that no conflict of interest exists.
Funding
This work was supported by the state of Berlin and the “European Regional Development Fund” to P.M, G.P., M.B., G.A.H. C.L.T. and M.F.M. (ERDF 2014–2020, EFRE 1.8/11, Deutsches Rheuma-Forschungszentrum); M.F.M. is supported by the “e:Bio Innovationswettbewerb Systembiologie” program of the Federal Ministry of Education. Supported by the German Research Council (DFG, SFB 650 and GRK1121), IMI JU-funded project BTCure and the European Research Council advanced grant ERC-2010-AdG_20100317 Grant 268987 to A.R.. F.S. was supported by Osteoimmune, a FP7 Marie Curie Initial Training Network (FP7-PEOPLE-2011-ITN-289150). P.M. was supported by EUTRAIN, a FP7 Marie Curie Initial Training Network for Early Stage Researchers funded by the European Union (FP7-PEOPLE-2011-ITN-289903). The DRFZ is a Leibniz Institute. G.A.H. was supported by GSC 203 Berlin-Brandenburg School for Regenerative Therapies, Charité – Universitätsmedizin Berlin, Berlin 13353, Germany. This work was supported by the “Leibniz ScienceCampus Chronic Inflammation” (www.chronische-entzuendung.org).
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank H. Schliemann, T. Geske, H. Hecker-Kia, A. Peddinghaus for technical assistance, T. Kaiser and J. Kirsch for cell sorting and Patrick Thiemann and Manuela Ohde for their assistance with animal care.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaut.2017.11.005.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental Fig. 1.
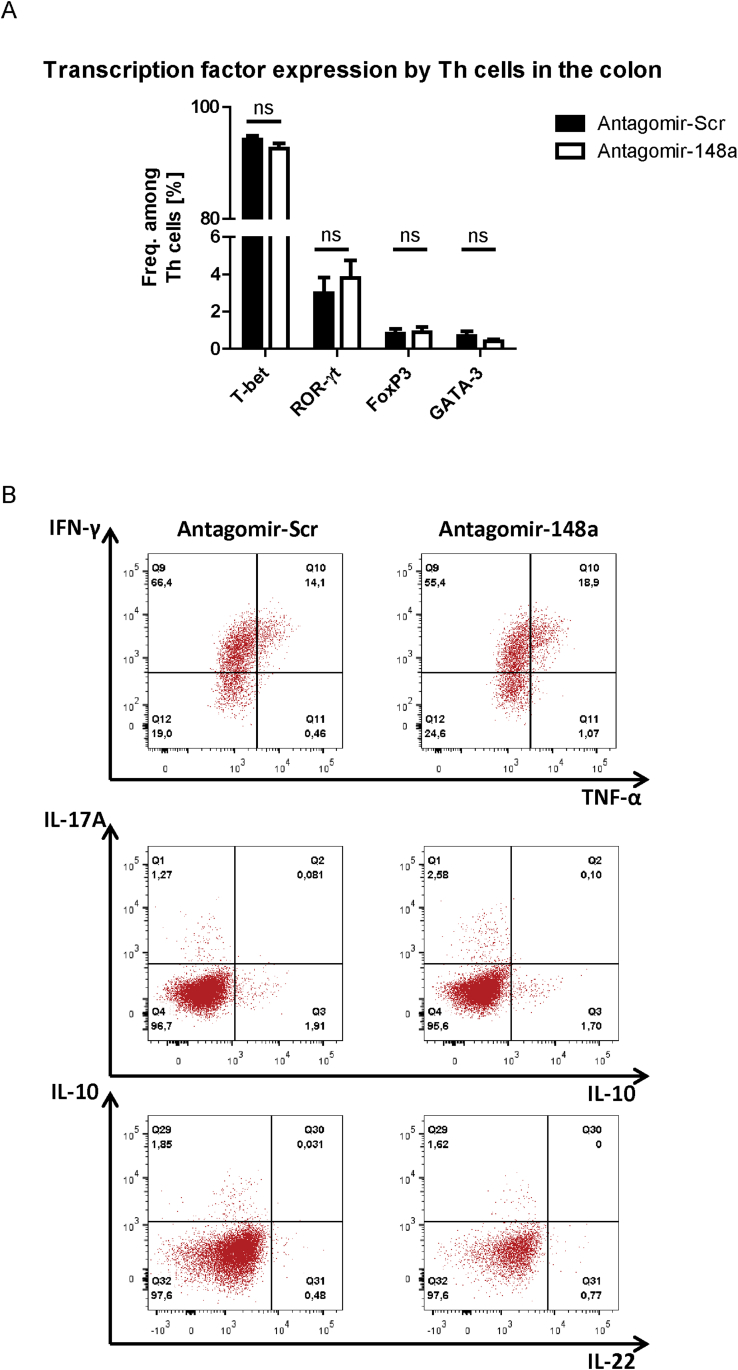
Adoptive transfer of repeatedly activated Th1 cells into Rag1-/- mice results in intestinal accumulation of pro-inflammatory Th cells that maintain their Th1 phenotype in the inflamed gut mucosa of colitic mice. Colitis was induced as shown in Figure 1A and lymphocytes were isolated from the inflamed colons of mice at the end of the experiment. (A) Lymphocytes were intracellularly stained with antibodies for determining transcription factor expression by flow cytometry. Shown are the frequencies of cells expressing the displayed transcription factors among CD4+ T lymphocytes. (B) Representative dot plots of colonic lymphocytes that were re-stimulated with PMA/ionomycin in the presence of Brefeldin A prior to intracellular staining of depicted cytokines. Data from (A) are pooled from two independent experiments with n=12 mice that received antagomir-148a injections and n=13 mice that were treated with antagomir-Scr. Dot plots in (B) are representative for two independent experiments.
Supplemental Fig. 2.
The gut mucosae of colitic mice that were treated with antagomir-148a or antagomir-Scr show histopathological symptoms of colonic inflammation. Colitis was induced as shown in Figure 1A. For hematoxylin and eosin staining of the colons, the organs were fixed in 4% paraformaldehyde at 4 °C in the ‘‘Swiss roll’’ formation over-night. Next, the fixed colons were washed with PBS, dewatered and embedded in paraffin. In both treatment groups the inflamed colons show infiltration by immune cells, goblet cell loss, hyperplasia and crypt abscesses. Data are representative for two independent experiments.
Supplemental Fig. 3.
Complete ablation of CD4+ Th cells after onset of colitis results in a slight but significant delay in body weight loss but not in complete regeneration of the gut mucosa. Colitis was induced by the adoptive transfer of naive CD45RBhiCD4+ Th cells. CD4+ T lymphocytes were depleted by i.p. injections of 10 mg/kg anti-CD4 (clone GK1.5) when the mice had lost ∼5% of their initial body weight. Lymphocytes were isolated from the inflamed colons of mice at the end of the experiment before the first mouse had lost more than 20% of its initial body weight. Numbers of splenic (A) and colonic (B) CD4+ Th cells are shown. The graph in (C) shows the relative body weight loss of colitic mice over time. (D) Tissue sections from the colons were prepared as described in the figure legend of Supplemental Figure 2. Hematoxylin and eosin staining of colons show infiltration by immune cells, goblet cell loss, hyperplasia and crypt abscesses for both treatment groups. The data shown in (A-C) are from n=6 mice treated with anti-CD4 antibodies and n=6 mice that were treated with PBS (control). Depicted curves in (C) represent the median weight per designated group.
Supplemental Fig. 4.
Antagomir-148a treatment during the effector phase of an acute immune response does not deplete antigen-specific memory Th cells and does not interfere with their effector function. Mice were immunized and injected with antagomirs as shown in Figure 4A or left untreated (non-immunized controls). Subsequently, mice were sacrificed to isolate splenocytes, which then were re-stimulated with NP-CGG and stained for flow cytometry. Shown are the absolute cell numbers of CD40L+ NP-CGG-specific Th memory cells (A) and the antigen-specific IFN-γ-expressing memory Th cells (B) following re-stimulation with NP-CGG. The data are from n=3 (non-immunized), n=4 (antagomir-Scr) and n=5 (antagomir-148a) mice, respectively.
Supplemental Fig. 5.
Antagomir treatment does not affect the architecture of highly perfused organs including liver, heart, kidney and lung. Mice were treated as shown in Figure 4A. Afterwards, the mice were sacrificed and tissues from liver, heart, kidney and lung were collected. The organs were fixed in 4% paraformaldehyde at 4 °C, washed with PBS, dewatered and embedded in paraffin. Tissue sections were prepared and stained with hematoxylin and eosin. Depicted are representative tissue sections for n=5 antagomir-148a- and antagomir-Scr-treated mice (heart) or for n=7 antagomir-148a- and antagomir-Scr-treated mice (liver, kidney, lung).
Supplemental Fig. 6.
Injection with antagomir-148a during an acute immune response does not deplete FoxP3+ regulatory T cells. Mice were immunized and injected with antagomirs as shown in Figure 4A. Subsequently, splenocytes were isolated from the mice and stained for flow cytometric analysis. Depicted frequencies and absolute cell numbers of CD4+FoxP3+ regulatory T cells (Tregs) were measured by flow cytometry.
Supplemental Fig. 7.
Antagomir-148a treatment in the memory phase of an immune response does not deplete immunological T cell memory in primary and secondary lymphoid organs. Mice were immunized and injected with antagomirs as shown in Figure 5A. Afterwards, lymphocytes from the BM, the spleen and from the two inguinal lymph nodes (iLNs) were collected. (A-D) Cell numbers of memory CD4+ and CD8+ T cells expressing CD69 or Ly6C in the spleen (A, B) or in iLNs (C, D) were determined by flow cytometry. (E-J) Isolated cells from the BM (E, F), spleen (G, H) and iLNs (I, J) were stimulated with PMA/ionomycin prior to intracellular staining for IFN-γ and TNF-α. Cell numbers of cytokine-expressing cells were measured by flow cytometry.
References
- 1.Powrie F., Leach M.W., Mauze S., Caddle L.B., Coffman R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 2.Feng T., Qin H., Wang L., Benveniste E.N., Elson C.O., Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal N., Oliver J.R., Wagner F.H., Lazenby A.S., Elson C.O., Weaver C.T. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J. Exp. Med. 2002;195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrecht I., Niesner U., Janke M., Menning A., Loddenkemper C., Kuhl A.A. Persistence of effector memory Th1 cells is regulated by Hopx. Eur. J. Immunol. 2010;40:2993–3006. doi: 10.1002/eji.201040936. [DOI] [PubMed] [Google Scholar]
- 5.Stronkhorst A., Radema S., Yong S.L., Bijl H., ten Berge I.J., Tytgat G.N. CD4 antibody treatment in patients with active Crohn's disease: a phase 1 dose finding study. Gut. 1997;40:320–327. doi: 10.1136/gut.40.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horneff G., Emmrich F., Reiter C., Kalden J.R., Burmester G.R. Persistent depletion of CD4+ T cells and inversion of the CD4/CD8 T cell ratio induced by anti-CD4 therapy. J. Rheumatol. 1992;19:1845–1850. [PubMed] [Google Scholar]
- 7.Goronzy J.J., Weyand C.M. Long-term immunomodulatory effects of T lymphocyte depletion in patients with systemic sclerosis. Arthritis Rheum. 1990;33:511–519. doi: 10.1002/art.1780330408. [DOI] [PubMed] [Google Scholar]
- 8.Haftmann C., Stittrich A.B., Zimmermann J., Fang Z., Hradilkova K., Bardua M. miR-148a is upregulated by Twist1 and T-bet and promotes Th1-cell survival by regulating the proapoptotic gene Bim. Eur. J. Immunol. 2015;45:1192–1205. doi: 10.1002/eji.201444633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niesner U., Albrecht I., Janke M., Doebis C., Loddenkemper C., Lexberg M.H. Autoregulation of Th1-mediated inflammation by twist1. J. Exp. Med. 2008;205:1889–1901. doi: 10.1084/jem.20072468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J., Zhang Y., Skalski M., Hayes J., Kefas B., Schiff D. microRNA-148a is a prognostic oncomiR that targets MIG6 and BIM to regulate EGFR and apoptosis in glioblastoma. Cancer Res. 2014;74:1541–1553. doi: 10.1158/0008-5472.CAN-13-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Martin A., Adams B.D., Lai M., Shepherd J., Salvador-Bernaldez M., Salvador J.M. The microRNA miR-148a functions as a critical regulator of B cell tolerance and autoimmunity. Nat. Immunol. 2016;17:433–440. doi: 10.1038/ni.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porstner M., Winkelmann R., Daum P., Schmid J., Pracht K., Corte-Real J. miR-148a promotes plasma cell differentiation and targets the germinal center transcription factors Mitf and Bach2. Eur. J. Immunol. 2015;45:1206–1215. doi: 10.1002/eji.201444637. [DOI] [PubMed] [Google Scholar]
- 13.Krutzfeldt J., Rajewsky N., Braich R., Rajeev K.G., Tuschl T., Manoharan M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 14.Haftmann C., Riedel R., Porstner M., Wittmann J., Chang H.D., Radbruch A. Direct uptake of Antagomirs and efficient knockdown of miRNA in primary B and T lymphocytes. J. Immunol. Methods. 2015;426:128–133. doi: 10.1016/j.jim.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stittrich A.B., Haftmann C., Sgouroudis E., Kuhl A.A., Hegazy A.N., Panse I. The microRNA miR-182 is induced by IL-2 and promotes clonal expansion of activated helper T lymphocytes. Nat. Immunol. 2010;11:1057–1062. doi: 10.1038/ni.1945. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann J., Kuhl A.A., Weber M., Grun J.R., Loffler J., Haftmann C. T-bet expression by Th cells promotes type 1 inflammation but is dispensable for colitis. Mucosal Immunol. 2016;9:1487–1499. doi: 10.1038/mi.2016.5. [DOI] [PubMed] [Google Scholar]
- 17.Charpentier C., Marion-Letellier R., Savoye G., Nicol L., Mulder P., Aziz M. Magnetic resonance colonography in rats with TNBS-induced colitis: a feasibility and validation study. Inflamm. Bowel Dis. 2012;18:1940–1949. doi: 10.1002/ibd.22897. [DOI] [PubMed] [Google Scholar]
- 18.Morteau O., Morham S.G., Sellon R., Dieleman L.A., Langenbach R., Smithies O. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J. Clin. Invest. 2000;105:469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostanin D.V., Bao J., Koboziev I., Gray L., Robinson-Jackson S.A., Kosloski-Davidson M. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanos S.L., Diefenbach A. Isolation of NK cells and NK-like cells from the intestinal lamina propria. Methods Mol. Biol. 2010;612:505–517. doi: 10.1007/978-1-60761-362-6_32. [DOI] [PubMed] [Google Scholar]
- 21.Cossarizza A., Chang H.D., Radbruch A., Andra I., Annunziato F., Bacher P. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 2017;47:1584–1797. doi: 10.1002/eji.201646632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokoyoda K., Zehentmeier S., Hegazy A.N., Albrecht I., Grun J.R., Lohning M. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Shinoda K., Tokoyoda K., Hanazawa A., Hayashizaki K., Zehentmeier S., Hosokawa H. Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc. Natl. Acad. Sci. U. S. A. 2012;109:7409–7414. doi: 10.1073/pnas.1118539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 25.Shale M., Schiering C., Powrie F. CD4(+) T-cell subsets in intestinal inflammation. Immunol. Rev. 2013;252:164–182. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damsker J.M., Hansen A.M., Caspi R.R. Th1 and Th17 cells: adversaries and collaborators. Ann. N. Y. Acad. Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neurath M.F., Weigmann B., Finotto S., Glickman J., Nieuwenhuis E., Iijima H. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J. Exp. Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y., Song Y.X., Wang Z.N. The microRNA-148/152 family: multi-faceted players. Mol. Cancer. 2013;12:43. doi: 10.1186/1476-4598-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qingjuan L., Xiaojuan F., Wei Z., Chao W., Pengpeng K., Hongbo L. miR-148a-3p overexpression contributes to glomerular cell proliferation by targeting PTEN in lupus nephritis. Am. J. Physiol. Cell Physiol. 2016;310:C470–C478. doi: 10.1152/ajpcell.00129.2015. [DOI] [PubMed] [Google Scholar]
- 30.Liu X., Zhan Z., Xu L., Ma F., Li D., Guo Z. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIalpha. J. Immunol. 2010;185:7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- 31.Nagalla S., Shaw C., Kong X., Kondkar A.A., Edelstein L.C., Ma L. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117:5189–5197. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jasinski-Bergner S., Stoehr C., Bukur J., Massa C., Braun J., Huttelmaier S. Clinical relevance of miR-mediated HLA-G regulation and the associated immune cell infiltration in renal cell carcinoma. Oncoimmunology. 2015;4 doi: 10.1080/2162402X.2015.1008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stepankova R., Powrie F., Kofronova O., Kozakova H., Hudcovic T., Hrncir T. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm. Bowel Dis. 2007;13:1202–1211. doi: 10.1002/ibd.20221. [DOI] [PubMed] [Google Scholar]
- 34.Cahill R.J., Foltz C.J., Fox J.G., Dangler C.A., Powrie F., Schauer D.B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect. Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brasseit J., Althaus-Steiner E., Faderl M., Dickgreber N., Saurer L., Genitsch V. CD4 T cells are required for both development and maintenance of disease in a new mouse model of reversible colitis. Mucosal Immunol. 2016;9:689–701. doi: 10.1038/mi.2015.93. [DOI] [PubMed] [Google Scholar]
- 36.Peperzak V., Vikstrom I., Walker J., Glaser S.P., LePage M., Coquery C.M. Mcl-1 is essential for the survival of plasma cells. Nat. Immunol. 2013;14:290–297. doi: 10.1038/ni.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y., Abraham S., Andre P., Edelstein L.C., Shaw C.A., Dangelmaier C.A. Anti-miR-148a regulates platelet FcgammaRIIA signaling and decreases thrombosis in vivo in mice. Blood. 2015;126:2871–2881. doi: 10.1182/blood-2015-02-631135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.