Supplemental Digital Content is available in the text
Keywords: breast cancer, cause-specific mortality, ductal carcinoma in situ, mortality, population
Abstract
Objective:
To assess cause-specific mortality in women treated for ductal carcinoma in situ (DCIS).
Background:
From screening and treatment perspective, it is relevant to weigh the low breast cancer mortality after DCIS against mortality from other causes and expected mortality in the general population.
Methods:
We conducted a population-based cohort study comprising 9799 Dutch women treated for primary DCIS between 1989 and 2004 and estimated standardized mortality ratios (SMRs).
Results:
After a median follow up of 9.8 years, 1429 patients had died of whom 284 caused by breast cancer (2.9% of total cohort). DCIS patients <50 years experienced higher mortality compared with women in the general population (SMR 1.7; 95% confidence interval, CI: 1.4–2.0), whereas patients >50 had significantly lower mortality (SMR 0.9; 95% CI: 0.8–0.9). Overall, the risk of dying from general diseases and cancer other than breast cancer was lower than in the general population, whereas breast cancer mortality was increased. The SMR for breast cancer decreased from 7.5 (95% CI: 5.9–9.3) to 2.8 (95% CI: 2.4–3.2) for women aged <50 and >50 years, respectively. The cumulative breast cancer mortality 10 years after DCIS was 2.3% for women <50 years and 1.4% for women >50 years treated for DCIS between 1999 and 2004.
Conclusions:
DCIS patients >50 years had lower risk of dying from all causes combined compared with the general female population, which may reflect differences in health behavior. Women with DCIS had higher risk of dying from breast cancer than the general population, but absolute 10-year risks were low.
Ductal carcinoma in situ (DCIS) is a proliferation of neoplastic cells confined to the ductolobular system—that is, without invading the surrounding breast tissue. It is a heterogeneous disease entity ranging from indolent, harmless DCIS to aggressive lesions with high invasive potential.1 As such, some DCIS lesions may progress into invasive breast cancer (IBC), and ultimately to fatal metastatic disease, whereas many DCIS lesions will never become invasive.2 The low risk of death from breast cancer among DCIS patients3,4 may be because of effective treatment, the potentially indolent and slow-growing nature of most DCIS, or both.
Most DCIS cases are picked up by breast cancer screening mammograms.5 Women with screen-detected breast lesions are generally in good health and do not experience any breast changes or symptoms. It is not surprising that inaccurate risk perceptions and anxiety in women diagnosed with DCIS are frequent.6,7 DCIS patients are generally told that they do not have cancer and have normal life expectancy, but may experience short- and long-term morbidity from the invasive treatment, which is in many respects similar to that for women with IBC. Therefore, the treatment decision-making process in DCIS is complex and controversial.
To fine-tune current practice and reduce confusing perceptions, there is an ongoing need to provide accurate information to DCIS patients and their health-care providers about the risks involved. Several population-based studies have studied breast cancer-specific mortality among DCIS patients,4,8–10 but only a few assessed competing causes of death or comprehensively compared these with that of the general population.3,11 Therefore, we assessed the likelihood of breast cancer-related death in DCIS patients and compared cause-specific mortality with rates expected based on mortality in the general population.
METHODS
Data Collection and Patient Selection
The Netherlands Cancer Registry (NCR) identified all women who were diagnosed with noninvasive breast cancer as first primary neoplasm between 1 January 1989 and 31 December 2004 in the Netherlands.12 The NCR provided date of birth, diagnosis and death, topography, morphology, grade, stage, type of surgery, and whether radiotherapy, chemotherapy, and/or hormonal therapy were administered, and data on subsequent neoplasms. Follow up on subsequent malignancies and vital status was complete until at least 1 January 2010. Linkage with the nationwide network and registry of histology and cytopathology in the Netherlands (PALGA) was used to validate and complete missing data on surgery type and to exclude patients with Paget disease, which the NCR had not registered as such.13 From an initial pool of 12,305 women we excluded those who were diagnosed at time of death (n = 4), whose DCIS was not histologically confirmed (n = 21), whose morphology was not pure DCIS (n = 2,094), who were diagnosed with an IBC or second breast carcinoma in situ within 4 months of DCIS diagnosis (n = 146), who received chemotherapy or hormonal therapy as part of DCIS treatment (not recommended in the Netherlands) (n = 109), and who were not surgically treated or for whom surgery type remained unknown (n = 132).
To obtain information on cause of death the cohort was linked with the nationwide cause of death registry at Statistics Netherlands. For analyses, age was categorized or subdivided into 2 groups of <50 and ≥50 years based on eligibility for the Dutch population-based breast cancer screening program.14 Between 1989 and 1997, women >69 years, and between 1998 and 2004, women >75 years were not eligible for screening, but these women were added to the ≥50 years group in our analyses.
Initial DCIS treatment was defined as the treatment strategy for the primary DCIS within 3 months of diagnosis and was subdivided into 3 categories: BCS alone, breast-conserving surgery (BCS) plus radiotherapy, and mastectomy. We categorized year of diagnosis into 2 periods, reflecting the gradual implementation of the screening program: 1989 to 1998 (implementation phase) and 1999 to 2004 (implementation completed), and the treatment guideline shift from mastectomy toward BCS plus radiotherapy for screen-detected smaller DCIS. Grade was classified according to the method by Holland et al.15 Information on grade was available for 24% of the women diagnosed between 1989 and 1998 and for 83% of the women diagnosed between 1999 and 2004.
All data were coded and anonymous to the researchers. The study was approved by the review boards of the NCR, PALGA, and Statistics Netherlands.
Statistical Analysis
To estimate cause-specific excess mortality, we compared observed deaths in the study population with expected number of deaths in the Dutch female population, taking into account the person-years of observation in the study cohort. Expected numbers were calculated based on the corresponding sex-, age-, and calendar period-specific mortality rates in the general Dutch female population provided by Statistics Netherlands. We estimated standardized mortality ratios (SMRs) as ratios of observed and expected numbers of death. Absolute excess mortality (AEM) was calculated as the observed number of deaths minus the number expected, divided by the number of person-years at risk, and multiplied by 10,000.16 We stratified results for major causes of death by age group, treatment type, period of diagnosis, occurrence of subsequent IBC, and follow-up interval. In addition, we estimated SMRs for breast cancer by grade for women diagnosed between 1999 and 2004. Tests for homogeneity and trends of SMRs were performed within collapsed person-time Poisson regression models. We evaluated the likelihood of a model with a continuous variable or a variable representing the classes of a categorical variable as discrete value, respectively, against the likelihood of a model without that variable.
We estimated the absolute risk of breast cancer mortality using death caused by other causes as a competing event. To quantify the effects of DCIS treatment, age at DCIS diagnosis, period of DCIS diagnosis and DCIS grade on breast cancer mortality, within cohort comparisons were performed using competing risk regression models with death caused by causes other than breast cancer treated as competing risk.17 We performed univariable and multivariable analyses to calculate unadjusted and adjusted subdistribution hazard ratios (SHR) and 95% confidence intervals (95% CI). In addition, we performed regression analysis by diagnostic period. To evaluate the impact of a subsequent ipsilateral or contralateral IBC on breast cancer mortality we added these as time-dependent variables in a second multivariable-adjusted model. Women who developed IBC contributed person-time to the “no subsequent IBC” group until IBC diagnosis, and subsequently to the “subsequent IBC” group.
Time at risk started at DCIS diagnosis in all analyses and ended at date of death, emigration, or 1 January 2010, whichever occurred first, unless stated differently. Stata/Se 14.0 (StataCorp LP, College Station, TX) was used for statistical analysis, and a P < 0.05 was considered statistically significant.
RESULTS
Our cohort comprised 9799 women treated for primary unilateral pure DCIS between 1989 and 2004 in the Netherlands. Median age at diagnosis was 57.4 years (interquartile range = 50.6–66.1 years). Half of the women underwent mastectomy. Of the women treated by BCS, 50% received radiotherapy, mostly between 1999 and 2004 (Table 1). Median follow up time was 9.8 years (interquartile range = 6.9–13.5 years). During follow up, 926 women were diagnosed with IBC and 1429 died. In total, 368 women died of cardiovascular diseases, 284 women of breast cancer, and 333 women of malignant disease other than breast cancer (respectively 26%, 20%, and 23% of all deaths) (Table 2).
TABLE 1.
Characteristics of the Study Population by Treatment
| DCIS treatment | BCS alone | BCS + RT | Mastectomy | Total |
| Number of DCIS patients (%) | 2558 | 2574 | 4667 | 9799 |
| Age at DCIS diagnosis, median (IQR), y | 58.7 (51.1–66.9) | 57.2 (51.2–65.1) | 56.9 (49.8–66.2) | 57.4 (50.6–66.1) |
| Age at DCIS diagnosis, y | ||||
| <40 | 106 (4.1) | 92 (3.6) | 359 (7.7) | 557 (5.7) |
| 40–49 | 376 (14.7) | 371 (14.4) | 857 (18.4) | 1604 (16.4) |
| 50–59 | 908 (35.5) | 1067 (41.5) | 1508 (32.3) | 3483 (35.5) |
| 60–69 | 750 (29.3) | 730 (28.4) | 1190 (25.5) | 2670 (27.3) |
| 70–75 | 250 (9.8) | 266 (10.3) | 457 (9.8) | 973 (9.9) |
| >75 | 168 (6.6) | 48 (1.9) | 296 (6.3) | 512 (5.2) |
| Period of DCIS diagnosis | ||||
| 1989–1998 | 1605 (62.7) | 736 (28.6) | 2499 (53.6) | 4840 (49.4) |
| 1999–2004 | 953 (37.3) | 1838 (71.4) | 2168 (46.5) | 4959 (50.6) |
| DCIS grade | ||||
| 1 | 412 (16.1) | 243 (9.4) | 262 (5.6) | 917 (9.4) |
| 2 | 339 (13.3) | 660 (25.6) | 684 (14.7) | 1683 (17.2) |
| 3 | 336 (14.1) | 881 (34.2) | 1442 (30.9) | 2659 (27.1) |
| Unknown* | 1471 (57.5) | 790 (30.7) | 2279 (48.8) | 4540 (46.3) |
| Follow-up time, median (IQR), y | 11.1 (8.4–14.4) | 8.1 (6.2–11.0) | 10.2 (7.0–14.0) | 9.8 (6.9–13.5) |
| Follow-up time, y | ||||
| 0–4 | 180 (7.0) | 96 (3.7) | 282 (6.0) | 558 (5.7) |
| 5–9 | 810 (31.7) | 1670 (64.9) | 1988 (42.6) | 4468 (45.6) |
| 10–14 | 1044 (40.8) | 588 (22.8) | 1493 (32.0) | 3125 (31.9) |
| ≥15 | 524 (20.5) | 220 (8.6) | 904 (19.4) | 1648 (16.8) |
| Subsequent invasive breast cancer | ||||
| No | 2146 (83.9) | 2355 (91.5) | 4372 (93.7) | 8873 (90.6) |
| Yes | 412 (16.1) | 219 (8.5) | 295 (6.3)† | 926 (9.5) |
| Ipsilateral only | 274 (10.7) | 99 (3.9) | 62 (1.3) | 435 (4.4) |
| Contralateral only | 104 (4.1) | 113 (4.4) | 226 (4.8) | 443 (4.5) |
| Ipsilateral and contralateral | 34 (1.3) | 7 (0.3) | 6 (0.1) | 47 (0.5) |
| Subsequent invasive cancer‡ | ||||
| No | 1944 (76.0) | 2190 (85.1) | 4048 (86.7) | 8182 (83.5) |
| Yes | 614 (24.0) | 384 (14.9) | 619 (13.3) | 1617 (16.5) |
| Only invasive breast cancer | 373 (14.0) | 208 (8.0) | 281 (6.0) | 862 (8.0) |
| Any other subsequent invasive cancer and no IBC | 202 (7.9) | 165 (6.4) | 324 (6.9) | 691 (7.1) |
| Any other subsequent invasive cancer and IBC | 39 (1.5) | 11 (0.4) | 14 (0.3) | 64 (0.7) |
| Vital status at end of follow up | ||||
| Alive | 1995 (78.0) | 2308 (89.7) | 3835 (82.2) | 8138 (83.1) |
| Dead | 491 (19.2) | 229 (8.9) | 709 (15.2) | 1429 (14.6) |
| Emigrated | 72 (2.8) | 37 (1.4) | 123 (2.6) | 232 (2.4) |
BCS indicates breast conserving surgery; DCIS, ductal carcinoma in situ; IBC, invasive breast cancer; IQR, interquartile range; RT, radiotherapy.
*1989–1998: 76% unknown versus 1999 and 2004: 17% unknown.
†1 patient with unknown laterality of subsequent invasive breast cancer.
‡Most common (n ≥ 40) subsequent invasive cancers other than invasive breast cancer: malignant neoplasm of colon (C18, n = 125); malignant neoplasm of bronchus and lung (C34, n = 104), leukemia and related conditions (C42, n = 54); other malignant neoplasms of skin (other than melanoma) (C44, n = 117); malignant neoplasm of corpus uteri (C54, n = 58).
TABLE 2.
Major Causes of Death and Standardized Mortality Ratios in Population-based Cohort of DCIS Patients
| Cause | ICD-10 | O | E | % | SMR (95% CI) | AEM |
| All causes | A00-Y89 | 1429 | 1557.2 | 100 | 0.92 (0.87–0.97) | −12.6 |
| Malignant neoplasm of breast | C50 | 284 | 85.4 | 20 | 3.33 (2.95–3.74) | 19.6 |
| Malignant neoplasm other than breast | C00-97 (excl. C50) | 333 | 406.6 | 23 | 0.82 (0.73–0.91) | −7.62 |
| Malignant neoplasm of digestive tract and peritoneum | C15-26, 48 | 137 | 143.6 | 10 | 0.95 (0.80–1.13) | −0.7 |
| Malignant neoplasm of lung, bronchus, and trachea | C33-34 | 72 | 96.9 | 5 | 0.74 (0.58–0.94) | −2.5 |
| Diseases of circulatory system | I00–99 | 368 | 477.1 | 26 | 0.77 (0.69–0.85) | −10.8 |
| Myocardial infarction | I21–22 | 85 | 98.3 | 6 | 0.87 (0.69–1.07) | −1.3 |
| Other heart disease | I30–33, 39–52 | 88 | 133.6 | 6 | 0.66 (0.53–0.81) | −4.5 |
| Cerebrovascular disease | I60–69 | 107 | 129.4 | 7 | 0.83 (0.68–1.00) | −2.2 |
| Diseases of respiratory system | J00-99 | 103 | 140.7 | 7 | 0.73 (0.60–0.89) | −3.7 |
AEM indicates absolute excess mortality per 10,000 patients per year; CI, confidence interval; E, expected number of deaths; O, observed number of deaths; SMR, standardized mortality ratio.
Cause-specific Mortality: Comparison With the General Population
DCIS patients had a significantly lower risk of dying than women in the general population (SMR 0.92; 95% CI: 0.87–0.97) (Table 2). Specifically, they experienced significantly lower mortality from diseases of the circulatory (SMR 0.77; 95% CI: 0.69–0.85), respiratory (SMR 0.73; 95% CI: 0.60–0.89), and digestive system (SMR 0.74; 95% CI: 0.55–0.98); mental and behavioral disorders (SMR 0.7; 95% CI: 0.52–0.90); and endocrine, nutritional, and metabolic diseases (SMR 0.69; 95% CI: 0.49–0.94). With regard to cancer mortality, DCIS patients had higher risk of breast cancer mortality (SMR 3.33; 95% CI: 2.95–3.74), but lower risk of death from all other cancer combined (SMR 0.82; 95% CI: 0.73–0.91) and from lung (SMR 0.74; 95% CI: 0.58–0.94) and urogenital cancers (SMR 0.62; 95% CI: 0.45–0.83) individually (Fig. 1). For most other categories the observed number of deaths was lower than the expected number; however, these differences were not statistically significant (See Table, Supplemental Digital Content 1, which demonstrates an extended list of causes of death).
FIGURE 1.
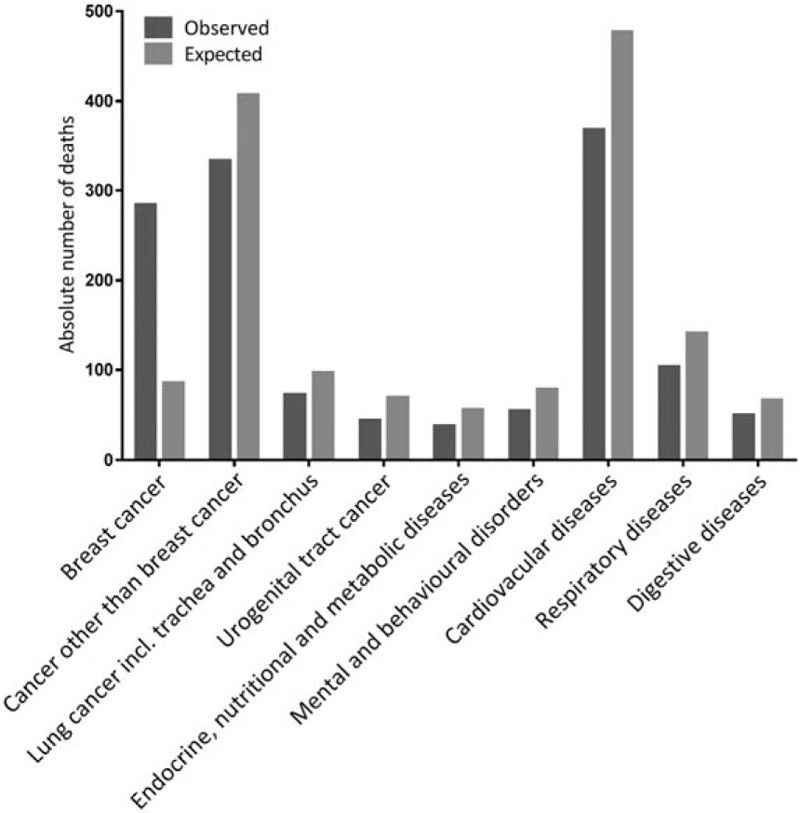
Observed and expected number of deaths from various disease categories in population-based cohort of 9799 DCIS patients (P < 0.05).
Standardized mortality ratios for all causes and breast cancer differed by age at diagnosis, diagnostic period, and follow-up interval (Table 3; Supplemental Digital Content 2, which demonstrates SMRs by subsequent IBC and follow-up interval). Compared with the general female population, women with DCIS <50 years had increased risk of dying from all causes combined (SMR 1.70; 95% CI: 1.42–2.03), whereas DCIS patients >50 years had lower risk (SMR 0.88; 95% CI: 0.83–0.93). With regard to breast cancer mortality women <50 years had higher risk of dying compared with the general population than women >50 years (SMR 7.46; 95% CI: 5.89–9.32 and SMR 2.76; 95% CI: 2.39–3.16, respectively; Phomogeneity< 0.001). When studying smaller age groups, we observed that the SMR for breast cancer decreased with increasing age (SMR 23.20; 95% CI: 15.65–33.11 to SMR 1.91; 95% CI: 1.11–3.06 for women aged <40 years and >75 years, respectively; Ptrend < 0.001).
TABLE 3.
Standardized Mortality Ratios of Major Causes of Death by Age, Diagnostic Period and treatment
| All Causes | Breast Cancer | |||||||
| O | SMR (95% CI) | AEM | P | O | SMR (95% CI) | AEM | P | |
| Number of deaths | 1429 | 0.92 (0.87–0.97) | −12.6 | 284 | 3.33 (2.95–3.74) | 19.6 | ||
| Age at diagnosis, y | <0.001 | <0.001 | ||||||
| <40 | 39 | 4.70 (3.34–6.42) | 48.5 | 30 | 23.20 (15.65–33.11) | 45.4 | ||
| 40–49 | 83 | 1.31 (1.04–1.63) | 10.6 | 47 | 5.21 (3.83–6.92) | 20.5 | ||
| 50–59 | 258 | 1.05 (0.93–1.19) | 3.4 | 87 | 3.30 (2.64–4.07) | 16.6 | ||
| 60–69 | 469 | 0.89 (0.81–0.97) | −21.3 | 76 | 2.63 (2.07–3.29) | 16.7 | ||
| 70–75 | 284 | 0.91 (0.81–1.03) | −33.8 | 27 | 2.48 (1.63–3.60) | 20.3 | ||
| >75 | 296 | 0.74 (0.66–0.83) | −279.2 | 17 | 1.91 (1.11–3.06) | 21.8 | ||
| Period of diagnosis | 0.004 | <0.001 | ||||||
| 1989–1998 | 1028 | 0.96 (0.91–1.03) | −5.9 | 223 | 3.97 (3.47–4.53) | 26.1 | ||
| 1999–2004 | 401 | 0.82 (0.74–0.90) | −24.2 | 61 | 2.09 (1.60–2.68) | 8.5 | ||
| DCIS treatment | 0.085 | 0.206 | ||||||
| BCS + RT | 229 | 0.86 (0.75–0.98) | −16.6 | 54 | 3.01 (2.26–3.92) | 15.6 | ||
| BCS alone | 491 | 1.00 (0.91–1.09) | −0.8 | 99 | 3.86 (3.14–4.70) | 25.5 | ||
| Mastectomy | 709 | 0.89 (0.83–0.96) | −17.7 | 131 | 3.13 (2.62–3.72) | 18.0 | ||
| Other Cancer | Circulatory Diseases | |||||||
| O | SMR (95% CI) | AEM | P | O | SMR (95% CI) | AEM | P | |
| Number of deaths | 333 | 368 | ||||||
| Age at diagnosis, y | 0.011 | 0.036 | ||||||
| <40 | 5 | 1.70 (0.55–3.96) | 3.24 | 0 | 0.00 (0.00–2.88) | −2.03 | ||
| 40–49 | 23 | 0.85 (0.54–1.28) | −2.2 | 0 | 0.00 (0.00–0.37) | −5.31 | ||
| 50–59 | 94 | 0.90 (0.73–1.10) | −2.9 | 32 | 0.68 (0.47–0.96) | −4.1 | ||
| 60–69 | 137 | 0.85 (0.72–1.01) | −8.3 | 115 | 0.73 (0.61–0.88) | −14.8 | ||
| 70–75 | 52 | 0.78 (0.58–1.02) | −18.3 | 99 | 0.92 (0.75–1.12) | −10.8 | ||
| >75 | 22 | 0.49 (0.30–0.73) | −62.7 | 122 | 0.79 (0.66–0.94) | −87.7 | ||
| Period of diagnosis | 0.111 | 0.782 | ||||||
| 1989–1998 | 230 | 0.87 (0.76–0.99) | −5.3 | 263 | 0.78 (0.69–0.88) | −11.7 | ||
| 1999–2004 | 103 | 0.72 (0.59–0.88) | −10.6 | 105 | 0.75 (0.62–0.91) | −9.2 | ||
| DCIS treatment | 0.265 | 0.551 | ||||||
| BCS + RT | 72 | 0.85 (0.66–1.07) | −5.6 | 51 | 0.70 (0.52–0.92) | −9.4 | ||
| BCS alone | 113 | 0.91 (0.75–1.10) | −3.7 | 128 | 0.82 (0.69–0.99) | −9.2 | ||
| Mastectomy | 148 | 0.75 (0.63–0.88) | −10.1 | 189 | 0.76 (0.65–0.87) | −12.3 | ||
AEM indicates absolute excess mortality per 10,000 patients per year; BCS, breast conserving surgery; CI, confidence interval; O, observed number of deaths; RT, radiotherapy; SMR, standardized mortality ratio.
P-values (for homogeneity and trends of SMRs) based on within collapsed person-time Poisson regression models.
Compared with the general population women diagnosed between 1999 and 2004 were less likely to die from any cause (SMR 0.82; 95% CI: 0.74–0.90), whereas women diagnosed earlier had a similar risk (SMR 0.96; 95% CI: 0.91–1.03). The SMR for breast cancer was lower for women diagnosed with DCIS between 1999 and 2004 (SMR 2.09; 95% CI: 1.60–2.68) than for those diagnosed between 1989 and 1998 (SMR 3.97; 95% CI: 3.47–4.53; Phomogeneity < 0.001). Compared with the general population DCIS patients had lower risk of dying from all causes combined only in the first 5 years after diagnosis, whereas the relative risk of dying from breast cancer increased with later follow-up interval. The SMRs for breast cancer by DCIS grade for women diagnosed between 1999 and 2004 were 0.95 (95% CI: 0.26–2.43) for DCIS grade 1, 1.91 (95% CI: 1.07–3.16) for DCIS grade 2, and 2.94 (95% CI: 2.04–4.11) for DCIS grade 3.
Of all women who died from breast cancer, 122 (43%) had been diagnosed with subsequent IBC after DCIS (See Table, Supplemental Digital Content 3, which demonstrates the characteristics of DCIS patients who died from breast cancer). Patients who developed IBC had a 26.6-fold (95% CI: 22.08–31.74) increased risk of dying from breast cancer compared with the general population (Supplemental Digital Content 2). For women with subsequent ipsilateral IBC the SMR was 31.64 (95% CI: 24.84–39.72), whereas for women with contralateral IBC the SMR was 17.10 (95% CI: 11.98–23.68). Distribution of age, DCIS grade, and diagnostic period was similar between women who did versus did not have a registered IBC, and died from breast cancer. Distribution of DCIS treatment was different among patients who died from breast cancer: women without subsequent IBC had more often undergone mastectomy, whereas women who experienced IBC had more often received adjuvant radiotherapy. In a subgroup analysis in which women who had died from breast cancer without experiencing IBC were censored, the SMR for breast cancer was 1.43 (95% CI: 1.19–1.71). When we stratified the subgroup analysis by diagnostic period, DCIS patients diagnosed between 1989 and 1998 had increased risk of breast cancer mortality compared with the general population (SMR 1.80; 95% CI: 1.46–2.18), whereas women diagnosed between 1999 and 2004 had similar risk (SMR 0.72; 95% CI: 0.44–1.10; Phomogeneity < 0.001).
Absolute Breast Cancer Mortality: Subgroups Comparisons
Absolute breast cancer mortality was 1.0% at 5 years after DCIS diagnosis, 2.5% at 10 years, and 4.0% at 15 years (See Table, Supplemental Digital Content 4, which demonstrates absolute breast cancer mortality). Women <50 years at DCIS diagnosis had an absolute risk of dying from breast cancer of 4.6% at 15 years, whereas the risk for women >50 years was 3.8% at 15 years. Stratified by treatment the absolute risk estimates at 15 years were 4.7% for BCS alone, 3.8% for BCS plus radiotherapy, and 3.6% for mastectomy (Fig. 2). Women diagnosed with DCIS between 1999 and 2004 had lower absolute risk than those diagnosed between 1989 and 1998 (10-year risk 1.5% vs. 3.1%, respectively; P < 0.001). The 10-year absolute risks for women treated between 1999 and 2004 stratified by age were: 2.3% and 1.4% for women <50 and >50 years, respectively.
FIGURE 2.

Fifteen-year absolute risks of breast cancer mortality by treatment (P < 0.05). With death caused by other causes as a competing event.
Using multivariable-adjusted competing risk regression analysis, women <40 years at DCIS diagnosis were at higher risk for death from breast cancer (hazard ratio, HR 1.99; 95% CI: 1.32–3.01) compared with DCIS patients aged 50 to 59 years, who in turn had the lowest risk of dying from breast cancer (See Table, Supplemental Digital Content 5, which demonstrates the multivariate regression analysis). Women diagnosed between 1999 and 2004 experienced lower breast cancer mortality than women diagnosed between 1989 and 1998 (HR 0.54; 95% CI: 0.39–0.74). Women who developed ipsilateral or contralateral IBC had higher risk of dying from breast cancer than women who did not (HR 14.65; 95% CI: 10.50–20.44 and HR 6.26; 95% CI: 4.40–8.89, respectively, model 2).
Between 1989 and 1998, DCIS treatment by mastectomy was associated with lower breast cancer mortality (HR 0.69; 95% CI: 0.52–0.92), but between 1999 and 2004 women treated by mastectomy appeared to have similar risk compared with women treated by BCS alone (HR 1.06; 95% CI: 0.52–2.17) (Table 4) (Supplemental Digital Content 6, which demonstrates the multivariate regression analysis stratified by diagnostic period). However, there was no evidence for significant effect modification by period of diagnosis (Pinteraction = 0.098). When comparing BCS plus radiotherapy with BCS alone breast cancer mortality did not differ by period of diagnosis (HR 0.87; 95% CI: 0.58–1.30 for 1989–1998 and HR 1.06; 95%:CI 0.50–2.18 for 1999–2004; Pinteraction = 0.316). For women diagnosed with DCIS between 1999 and 2004 the risk of dying from breast cancer increased with higher grade (HR 1.99; 95% CI: 0.66–6.06 for grade 2 vs. grade 1 and HR 2.81; 95% CI: 0.99–7.94 for grade 3 vs. grade 1; Ptrend = 0.042).
TABLE 4.
Competing Risk Regression Analysis for Breast Cancer Mortality in Women Treated for DCIS
| Treatment | |||||
| Period of diagnosis | BCS alone | BCS + RT | Mastectomy | ||
| 1989–2004 | Adjusted SHR (95% CI) | Model 1 | ref | 0.87 (0.62–1.22) | 0.75 (0.57–0.97) |
| P | 0.417 | 0.027 | |||
| Adjusted SHR (95% CI) | Model 2 | ref | 1.34 (0.93–1.92) | 1.45 (1.06–1.98) | |
| P | 0.115 | 0.021 | |||
| 1989–1998 | Adjusted SHR (95% CI) | Model 1 | ref | 0.87 (0.58–1.30) | 0.69 (0.52–0.92) |
| P | 0.489 | 0.011 | |||
| Adjusted SHR (95% CI) | Model 2 | ref | 1.26 (0.83–1.92) | 1.30 (0.93–1.82) | |
| P | 0.282 | 0.128 | |||
| 1999–2004 | Adjusted SHR (95% CI) | Model 1 | ref | 1.04 (0.50–2.18) | 1.06 (0.52–2.17) |
| P | 0.911 | 0.864 | |||
| Adjusted SHR (95% CI) | Model 2 | ref | 2.12 (0.89–5.04) | 2.58 (1.07–6.19) | |
| P | 0.088 | 0.034 | |||
BCS indicates breast conserving surgery; CI, confidence interval; DCIS, ductal carcinoma in situ; NA, not applicable; RT, radiotherapy; SHR, subdistribution hazard ratio (with death due to other causes as a competing event).
Model 1: adjusted for age at DCIS diagnosis, period of DCIS diagnosis and DCIS grade.
Model 2: adjusted for age at DCIS diagnosis, period of DCIS diagnosis, DCIS grade and subsequent invasive breast cancer; with subsequent ipsilateral and contralateral invasive breast cancer as time-dependent variables.
DISCUSSION
In this large, nationwide study with 10 years of follow up, we evaluated cause-specific mortality in DCIS patients compared with the general population and we examined factors associated with mortality from specific causes. We observed that DCIS patients experienced lower mortality from diseases of the circulatory, respiratory and digestive system, mental and behavioral disorders, endocrine, nutritional and metabolic diseases, and cancer other than breast cancer. DCIS patients >50 years at diagnosis, which represent the majority of the DCIS population, had lower all-cause mortality than the general female population, whereas the relative risk of dying due to breast cancer was 2.8 times increased.
Women <50 years at diagnosis had a 1.7 times increased risk of dying compared with women in the general population, which could be because of their increased breast cancer mortality. Only 6 percent of the DCIS study population was <40 years at DCIS diagnosis, but these women had a 23-times greater risk of dying from breast cancer than expected. Importantly, this highly increased relative risk results from a very low expected number of breast cancer deaths in the general population <40 years. Also the results from our within-cohort analysis show that age <40 years was associated with increased breast cancer mortality. An explanation for this finding might be that younger women possibly more often have a larger extent of symptomatic DCIS resulting in a higher risk of unrecognized invasive disease. Our results are in line with a study by Narod et al4 and emphasize the importance of differential counselling of younger and older women diagnosed with DCIS. However, these young women and their DCIS may not optimally represent young patients and their noninvasive disease today (because of more opportunistic screening and awareness). Therefore, we want to stress that our results do not provide evidence that these women should be treated more intensively, for instance using hormonal treatment.
Intuitively, it is very unlikely that the better life expectancy among DCIS patients >50 years is related to the DCIS in itself. A more plausible explanation may be differences in lifestyle characteristics as DCIS patients seem to represent a generally healthy subgroup of the general population.3,11 In our study, women with DCIS had lower risk of dying caused by cardiovascular and respiratory disease and lung cancer, conditions that are largely caused by lifestyle factors. Notably, DCIS patients treated by radiotherapy were also at decreased risk for cardiovascular death compared with the general population, which has also been reported in a previous study in this cohort.18
DCIS is mostly detected by screening, and it has been suggested that women who adhere to mammographic screening may be more health-conscious, more often belong to higher socioeconomic classes and have lower comorbidity, resulting in a healthy screenee effect.3,18–20 This is in line with our finding that women diagnosed with DCIS between 1999 and 2004 (attendance of population-based screening 80%14) and women >50 years (eligible for screening) experienced lower mortality, whereas women diagnosed between 1989 and 1998 (implementation phase) had equal risk to their general population counterparts. However, a previous study that tried to account for this bias by adjusting the models for previous mammography use, comorbidity, and health care utilization seem to contradict a healthy screenee effect.21 They reported that women >66 years diagnosed with DCIS had similar comorbidity and visited primary care with similar frequency as their controls, and also concluded that a DCIS diagnosis in older women was associated with better survival. Nonetheless, in their discussion they state that “If there is a mortality risk for DCIS […], the risk is likely low and not strong enough to counterbalance a healthy user effect.” Another hypothesis is that DCIS patients may seek more medical attention and might adopt a healthier lifestyle after their diagnosis, allowing for prevention or earlier diagnosis and treatment of other diseases.
Similarly to previous studies,3,4 we observed that the 10-year absolute risk of breast cancer mortality in DCIS patients was low and declined for DCIS patients diagnosed in more recent years. This decline in absolute mortality may be because of a decrease in unrecognized IBC at DCIS diagnosis, as radiological and pathological assessment, and treatment selection have improved. Further, in more recent years, with breast screening fully implemented, more indolent DCIS could have been detected, resulting in over diagnosis.
Treatment effects on breast cancer mortality found in our study should be interpreted with caution, as confounding by indication may play a significant role. Between 1989 and 1998, women treated by mastectomy had lower breast cancer-specific mortality than women treated by BCS alone, whereas between 1999 and 2004, no difference was found. The results were stratified because we assumed that the DCIS cases diagnosed between 1999 and 2004 better reflect current DCIS cases (more screen-detected) than our study population diagnosed between 1989 and 1998. However, there was no statistically significant effect modification by period of diagnosis. Our findings could be explained by improved surgical treatment planning in the latter period, in which the incidence of DCIS increased rapidly and results from randomized controlled trials focusing on BCS were published.22–25 Moreover in a meta-analysis of the randomized controlled trials studying the effect of radiotherapy after BCS between 1985 and 1999, no difference between among groups was detected.26 Furthermore, a large observational study, including both BCS and mastectomy between 1998 and 2011, did not report association between treatment and breast cancer mortality.4 Importantly, the women in our study population were not treated with tamoxifen as part of DCIS treatment, because the clinical guidelines in the Netherlands do not recommend endocrine therapy for women with DCIS.
Remarkable, women who developed ipsilateral IBC appeared to have higher risk of dying from breast cancer than women who were diagnosed with contralateral IBC, a finding which is supported by a study from Narod et al.4 The difference in outcome after subsequent ipsilateral versus contralateral invasive disease might be explained by potentially more aggressiveness of subsequent ipsilateral IBC after treatment than of new primary tumor in the untreated contralateral breast.
In our study, 162 DCIS patients without subsequent IBC died from breast cancer. From all 8873 women with DCIS who did not develop IBC, this is only a small fraction (1.8%). When a woman with a history of DCIS dies from breast cancer, either the invasive component was unrecognized at the time of DCIS diagnosis, DCIS was left behind after treatment and progressed to IBC, or a new primary IBC developed. Therefore, among women with pure DCIS in our study, we hypothesize that true breast cancer deaths are the result of unrecognized, undetected, or unregistered IBC. Another possibility is that these women in fact did not die from breast cancer, but were registered on their death certificate as such. Also in 2 previous studies breast cancer deaths among women who did not have any IBC registered according to population-based cancer registry data were observed (9/2884 = 0.3% and 517/108,196 = 0.5%, respectively).4,8
Our study has several limitations. The interpretation of mortality statistics is usually complicated by uncertainties about the degree of misclassification of causes of death. However, Harteloh et al27 showed that for major causes of death, such as cancers, or acute myocardial infarction, reliability of cause of death statistics in the Netherlands was higher than 90%.
Moreover, we could not rely on pathology and clinical record review with respect to the diagnoses of primary DCIS and subsequent IBC because of the extensive and anonymous dataset. For example, some primary DCIS may have been unrecognized IBC during tumor sampling. Further, the Netherlands Cancer Registry might have missed some subsequent IBC, although their coverage is at least 96%.12,28,29 In addition, we had no information on estrogen receptor status, comedonecrosis and lesion size, all factors that predicted breast cancer mortality in a study from Narod et al.4 However, we were able to evaluate DCIS grade in women diagnosed between 1999 and 2004, and also found that the risk of dying from breast cancer increased with higher grade. Similar to the finding of Narod et al,4 we previously observed that high grade DCIS was, however, not associated with an increased risk of subsequent ipsilateral invasive breast cancer compared with low grade DCIS.30
Strengths of our study include its large size and population-based character. We were able to combine information on DCIS and subsequent IBC from the NCR with cause of death data from Statistics Netherlands. Furthermore, information from PALGA could be used to validate and complete treatment data. As a result, we had the unique opportunity to study a nationwide DCIS cohort with accurate and complete treatment information and follow-up.
In conclusion, DCIS patients >50 years had lower risk of dying compared with women in the general population, which may reflect differences in health behavior. Women diagnosed with primary DCIS had higher risk of dying from breast cancer than women in the general population, but absolute risks were low: cumulative breast cancer mortality 10 years after DCIS was 2.3% for women <50 years and 1.4% for women >50 years treated for DCIS between 1999 and 2004. The relative and absolute risk estimates provided in this study are important input for health care providers when counselling women diagnosed with DCIS.
Supplementary Material
Acknowledgments
The authors would like to thank Naomi Boekel for performing the linkages, Statistics Netherlands for providing causes of death data and the registration teams of the Netherlands Comprehensive Cancer Organization for the collection of data for the Netherlands Cancer Registry. The authors also thank PALGA, the nationwide histopathology and cytopathology data network and archive, for providing pathology data.
Footnotes
Disclosure: J.W. and M.S. have joint last authorship of this study. The authors declared no conflict of interest. This work was supported by Pink Ribbon (grant No. 2011.WO19.C88, 2014-182 WO29, 2014-183 WO54 to Jelle Wesseling) and the Dutch Cancer Society (grant No. NKI2009-4363 to Marjanka K. Schmidt).
REFERENCES
- 1.Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, et al. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology 2010; 57:171–192. [DOI] [PubMed] [Google Scholar]
- 2.Erbas B, Provenzano E, Armes J, et al. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat 2006; 97:135–144. [DOI] [PubMed] [Google Scholar]
- 3.Ernster VL, Barclay J, Kerlikowske K, et al. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology, and end results program. Arch Intern Med 2000; 160:953–958. [DOI] [PubMed] [Google Scholar]
- 4.Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 2015; 1:888–896. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett JMS, Nofech-Moses S, Rakovitch E. Ductal carcinoma in situ of the breast: can biomarkers improve current management? Clin Chem 2014; 60:60–67. [DOI] [PubMed] [Google Scholar]
- 6.Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst 2008; 100:243–251. [DOI] [PubMed] [Google Scholar]
- 7.Ruddy KJ, Meyer ME, Giobbie-Hurder A, et al. Long-term risk perceptions of women with ductal carcinoma in situ. Oncologist 2013; 18:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falk RS, Hofvind S, Skaane P, et al. Second events following ductal carcinoma in situ of the breast: a register-based cohort study. Breast Cancer Res Treat 2011; 129:929–938. [DOI] [PubMed] [Google Scholar]
- 9.Sagara Y, Mallory MA, Wong S, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg 2015; 150:739–745. [DOI] [PubMed] [Google Scholar]
- 10.Wärnberg F, Bergh J, Holmberg L. Prognosis in women with a carcinoma in situ of the breast: a population-based study in Sweden. Cancer Epidemiol Biomarkers Prev 1999; 8:769–774. [PubMed] [Google Scholar]
- 11.Worni M, Akushevich I, Greenup R, et al. Trends in treatment patterns and outcomes for ductal carcinoma in situ. J Natl Cancer Inst 2015; 107:djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Sanden GA, Coebergh JW, Schouten LJ, et al. Cancer incidence in The Netherlands in 1989 and 1990: first results of the nationwide Netherlands cancer registry. Coordinating Committee for Regional Cancer Registries. Eur J Cancer 1995; 31A:1822–1829. [DOI] [PubMed] [Google Scholar]
- 13.Casparie M, Tiebosch ATMG, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007; 29:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Evaluation Team for Breast cancer screening NETB. Fracheboud J, van Luijt PA, Sankkatsing V, et al. National evaluation of breast cancer screening in the Netherlands 1990 –2012 Available at: http://wwwerasmusmcnl/mage/publications-collaborations/pub/national-evaluation-breastcancer?lang=en Accessed on January 5, 2017 [Google Scholar]
- 15.Holland R, Peterse JL, Millis RR, et al. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol 1994; 11:167–180. [PubMed] [Google Scholar]
- 16.IARC Scientific Publications, Day NE, Breslow NE. Statistical Methods in Cancer Research—Volume II—The Design and Analysis of Cohort Studies. 1987. [PubMed] [Google Scholar]
- 17.Fine JPG, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 18.Boekel NB, Schaapveld M, Gietema JA, et al. Cardiovascular morbidity and mortality after treatment for ductal carcinoma in situ of the breast. J Natl Cancer Inst 2014; 106:dju156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JR, Vogel VG. Who uses screening mammography regularly? Cancer Epidemiol Biomarkers Prev 1995; 4:901–906. [PubMed] [Google Scholar]
- 20.Hofer TP, Katz SJ. Healthy behaviors among women in the United States and Ontario: the effect on use of preventive care. Am J Public Health 1996; 86:1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schonberg MA, Marcantonio ER, Ngo L, et al. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol 2011; 29:1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 1998; 16:441–452. [DOI] [PubMed] [Google Scholar]
- 23.Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet 2000; 355:528–533. [DOI] [PubMed] [Google Scholar]
- 24.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet 2003; 362:95–102. [DOI] [PubMed] [Google Scholar]
- 25.Emdin SO, Granstrand B, Ringberg A, et al. SweDCIS: Radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol 2006; 45:536–543. [DOI] [PubMed] [Google Scholar]
- 26.Correa C, McGale P, Taylor C, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monographs 2010; 2010:162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harteloh P, de Bruin K, Kardaun J. The reliability of cause-of-death coding in The Netherlands. Eur J Epidemiol 2010; 25:531–538. [DOI] [PubMed] [Google Scholar]
- 28.Berkel J. General practitioners and completeness of cancer registry. J Epidemiol Community Health 1990; 44:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schouten LJ, Straatman H, Kiemeney LA, et al. The capture-recapture method for estimation of cancer registry completeness: a useful tool? Int J Epidemiol 1994; 23:1111–1116. [DOI] [PubMed] [Google Scholar]
- 30.Elshof LE, Schaapveld M, Schmidt MK, et al. Subsequent risk of ipsilateral and contralateral invasive breast cancer after treatment for ductal carcinoma in situ: incidence and the effect of radiotherapy in a population-based cohort of 10,090 women. Breast Cancer Res Treat 2016; 159:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


