Abstract
Introduction:
Neuroprotection with therapeutic hypothermia (TH) is standard of care for neonatal encephalopathy (NE) and decreases death and neurodevelopmental disability. TH initiated shortly after birth insult results in greater neuroprotection compared with delayed initiation.
Methods:
Quality improvement methodology was used to improve temperature control during transport to a level IV neonatal intensive care unit. We included neonates with NE transported to a single institution for TH from 2010 to 2016. The quality improvement interventions were 2-fold. Review of the Transport Body Cooling Protocol revealed a suboptimal temperature goal of 34–35°C; this protocol was revised to 33–34°C. The second intervention was the implementation of an active cooling protocol. Clinical characteristics were compared using 2-sample t tests for continuous variables and Fisher’s exact tests for categorical variables; statistical process control chart was used to monitor admission temperatures.
Results:
We obtained baseline data for 78 neonates admitted from 2010 to 2014. These data were compared with postintervention data for 26 patients admitted between 2015 and 2016. Distance transported, NE severity, and seizures were similar between the 2 groups. The use of active cooling increased from 8% preimplementation to 31% postimplementation (P < 0.01). After implementation of the 2 interventions, more infants were admitted within the goal temperature of 33–34°C, 58% versus 22% (P < 0.01), and the average neonatal intensive care unit admission temperature improved from 34.4 ± 0.8°C to 33.8 ± 0.8°C (P < 0.01).
Conclusion:
Increased utilization of active cooling during transport for TH improves the percentage of neonates admitted within the target temperature range. However, 42% of neonates remained outside the target temperature range, supporting the need for additional tools to improve admission temperatures.
INTRODUCTION
Background
Neonatal encephalopathy (NE) is an acute neurologic injury at birth that affects 1–2 term neonates per 1,000 live births.1 Neuroprotection with therapeutic hypothermia (TH) for neonates with moderate-severe encephalopathy has proven to decrease death and moderate-to-severe neurodevelopmental disability at 18–24 months of age in multiple randomized clinical trials.2 As such, TH initiated within 6 hours after birth and continued for 72 hours is now considered the standard of care.3–5 Shorter time to initiation of TH may confer greater neuroprotection as demonstrated in experimental models of NE and asphyxiated neonates.6,7 Therefore, initiation of TH and attainment of target core temperatures during transport has the potential to improve neurodevelopment in infants with NE.
Problem Identified
Since 2010, Children’s Hospital Colorado (CHCO) has treated over 100 outborn neonates for NE with TH. Due to a large and geographically diverse referral area, the Flight for Life (FFL) team transports neonates up to 560 miles from the referral birth hospital to the CHCO neonatal intensive care unit (NICU). We initiated this quality improvement (QI) project after a review of our initial transport data, which revealed that 82% of neonates were admitted outside of the goal core body temperature range of 33–34°C. Also, only 8% of neonates were actively cooled during transport because of the lack of a standardized approach for active cooling utilizing gel packs.
Although studies have shown that implementation of servo-controlled mattresses for active cooling is superior to cooling with gel packs, there are potential drawbacks including cost and feasibility related to weight and power supply considerations, especially during helicopter transport.8,9 To avoid these concerns, we targeted our initiative toward increasing and standardizing the utilization of active cooling with gel packs during transport rather than via a servo-controlled mattress. These data provided an opportunity to evaluate if standardizing the mode of active cooling with gel packs during transport and revising the Transport Body Cooling Protocol would increase the number of neonates admitted to the CHCO NICU within the goal temperature range.
METHODS
Study Approval
This project involved revision of an existing transport protocol as well as the implementation of a formalized active-cooling protocol. Although this project did not necessitate approval by the Colorado Multiple Institutional Review Board, we obtained approval from the Organizational Research Risk and Quality Improvement Review Panel at CHCO (#1703–11).
Clinical Setting
The CHCO NICU is a level IV regional unit with 84 beds and approximately 1,300 admissions annually. Greater than 90% of infants are outborn, and these infants are transported from a wide and geographically diverse catchment area. Board-certified neonatologists provide care for all neonates. The unit participates in the Children’s Hospital Neonatal Database (CHND), which collects prospective data on all neonates admitted to participating regional NICUs.10 Dedicated data abstractors collect and verify data at key points during NICU admission, as specified by CHND.
Intervention
After identification of the problem, we established a multidisciplinary team in 2014 to further investigate and evaluate the transport cooling process. This team included neonatologists, an FFL transport nurse, a local CHND data abstractor, and QI experts. As FFL transports the majority of infants with NE to the NICU at CHCO, we targeted our intervention to include only the FFL transport team and excluded the few infants who were transported by other transport teams. The QI team’s purpose was to review, revise, and standardize the Transport Body Cooling Protocol (Fig. 1). This process included a 2-fold intervention: (1) revision of the protocol to include the optimal rectal temperature of 33–34°C for whole-body cooling during transport, previously inaccurately listed as 34–35°C; (2) standardization of an active cooling protocol for neonates during transport who were unable to achieve goal temperature with passive cooling alone. Incorrect target temperature was listed in the initial transport protocol due to differences in core temperature goals during selective head cooling and total body cooling. Upon implementation of the initial cooling transport protocol for FFL, our NICU utilized selective head cooling for TH, with a target core temperature of 34–35°C. As the target core temperature for whole-body cooling is 33–34°C, the target temperature during transport was adjusted accordingly.4 FFL and nursing leaders provided education to the transport team members on these new transport guidelines.
Fig. 1.
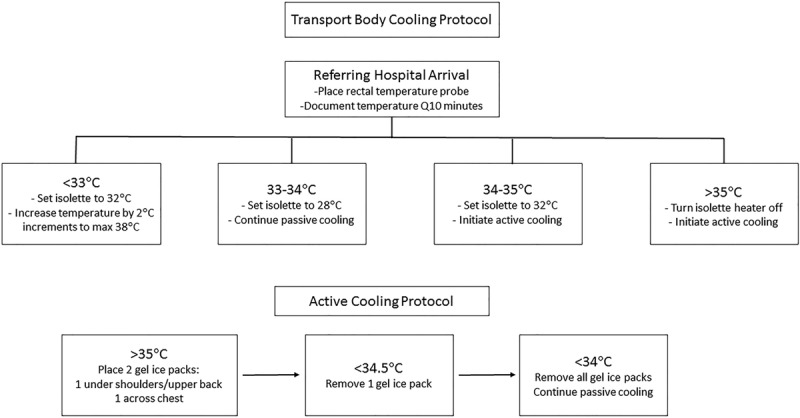
Transport Body Cooling Protocol including criteria for starting passive or active cooling along with guidelines for isolette temperature regulation. Active Cooling Protocol outlines the process for applying and removing gel ice packs depending on infant rectal temperature during transport.
Selected electronic medical chart review revealed inconsistent obtainment and documentation of the neonate’s admission temperature. The first documented admission temperature for many infants often occurred more than 1–2 hours postadmission. This observation resulted in NICU nursing education and standardization of the neonate’s last transport rectal temperature as the NICU admission temperature in the electronic medical record. Transport staff documented the last rectal temperature upon arrival to the NICU, before removal of the transport temperature probe.
Nursing and flight team leaders provided ongoing education to team members based on a review of protocol adherence every 3 months. Also, new FFL and nursing team members underwent education upon hiring. The clinical nurse manager of the NICU disseminated information to all bedside nurses regarding compliance with using the last transport temperature as the first documented admission temperature to ensure consistency.
The primary outcome was the percentage of infants with NE admitted to the CHCO NICU with a temperature within the target range of 33–34°C with a goal of 50% improvement from baseline over a 24-month period. The corresponding process measure was the percentage of infants with a core body temperature of > 35°C who underwent active cooling during transport. The balancing measures for this study included the percentage of infants who arrived with a core temperature of < 33°C and the incidence of skin injuries associated with active cooling.
Data Collection
We obtained the following baseline characteristics on all neonates with NE admitted to the CHCO NICU: gestational age, birth weight, gender, admission date, referring hospital distance from CHCO NICU (miles), NE severity (mild, moderate, severe), presence of preadmission or admission seizures, and mode of cooling during admission. We combined these data from FFL transport data, which included the neonate’s temperature at the referring hospital upon FFL arrival, mode of cooling during transport (passive or active), and admission temperature at CHCO NICU. Transported neonates were stratified into 2 groups based upon the distance between the referring hospital and CHCO NICU: 10–31 miles represented neonates admitted from within the metro area, and > 31 miles represented neonates transported from outside the larger metro area. Sixteen infants were transported > 100 miles although due to the overall small number, these infants were not analyzed separately. We excluded infants transported < 10 miles as they represent referrals from only 1 hospital on the same campus as CHCO and therefore had minimal transport times.
Data Analysis
Demographic and clinical characteristics of the neonates in the pre- and postintervention groups were compared using 2-sample t tests for continuous variables and by Fisher’s exact tests for categorical variables. Variables were assessed for skewness using histograms and comparison of the mean and median and, when appropriate, were log-transformed. We set significance at 0.05. Data analysis was performed using R version 3.1.1 software (R Foundation for Statistical Computing, Vienna, Austria). Statistical process control charts were generated using QI Macros (KnowWare International, Denver, Colo.).
RESULTS
Baseline Neonate Characteristics
CHCO NICU admitted 113 neonates with NE for continued management with TH. Nine neonates were excluded due to admission to our NICU more than 6 hours after birth, leaving 104 infants for further analysis. The 9 excluded infants with NE had been undergoing TH at an outside facility and required later transport to our regional NICU for a higher level of care primarily due to unanticipated cardiopulmonary decompensation. As this study focuses on initial cooling efforts during transport, these infants were excluded from analysis. The number of neonates with NE transported by FFL to CHCO NICU for TH ranged from as few as 10 in 2016 to as many as 27 in 2012, with an average of 17 neonates admitted annually during the study period.
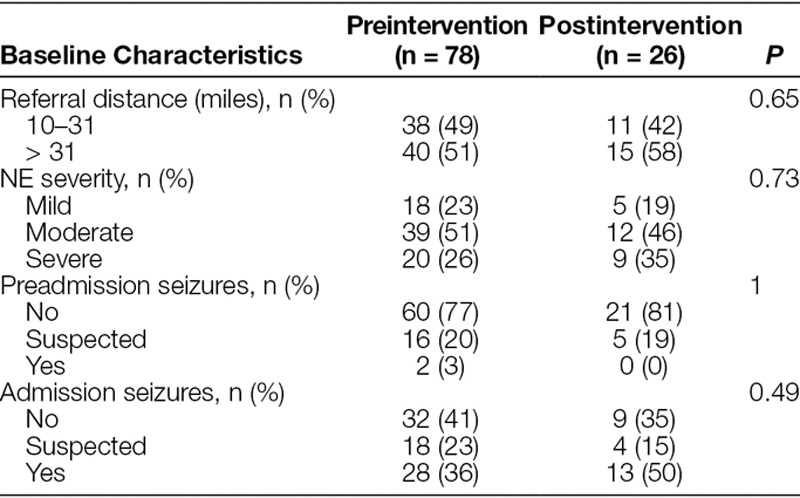
The baseline preintervention population includes 78 neonates admitted for TH from January 2010 until the implementation of the revised Transport Body Cooling Protocol in December 2014. These infants had a mean gestational age of 38.8 ± 1.89 weeks, birthweight of 3,082 ± 586 g, and 40% were female. After standardization of transport cooling, 26 neonates were admitted from January 2015 toDecember 2016 and represented the postintervention cohort. The postintervention group had a mean gestational age of 39.2 ± 1.89 weeks, birthweight of 3,356 ± 690 g, and 31% were female. Overall, neonates were referred from 25 hospitals within a 4-state region and were transported up to 560 miles (Fig. 2). There was no significant difference in the evaluated baseline clinical characteristics of these neonates pre/postintervention (Table 1).
Fig. 2.
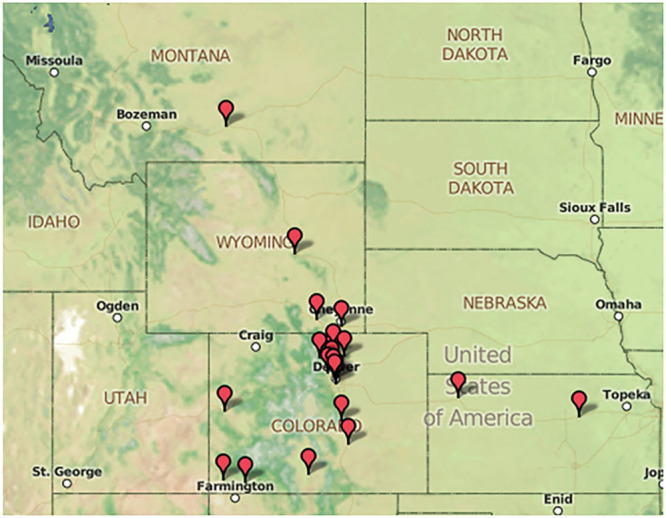
Map representation of referrals from 25 hospitals in a 4-state area for management of NE with TH at CHCO NICU.
Table 1.
Baseline Characteristics of Infants Transported for Therapeutic Hypothermia
Transport Cooling
There was no significant difference in the neonate’s temperature documented at the time FFL arrived at the referring hospital between the preintervention and postintervention groups (34.5 ± 1.2°C versus 34.5 ± 1°C; P = 0.87). Also, there was no significant difference in the geometric mean distance that neonates were transported between the 2 groups; 35 miles (95% CI, 28–43) versus 45 miles (95% CI, 31–64), P = 0.29.
Compared with the preintervention group, significantly more neonates in the postintervention group were managed by the active cooling method (8% versus 31%; P ≤ 0.01). They were significantly more likely to arrive at CHCO NICU within the goal target temperature of 33–34°C (22% versus 58%; P < 0.01). Figure 3 represents the median admission temperature by admission year. In the postintervention group, there was a narrowing of the interquartile range (IQR) of admission temperatures between the first postintervention year, 2015 (IQR, 33.2–34.4°C) and the second postintervention year, 2016 (IQR, 33.6–34.0°C). This narrowing demonstrates improvement over time with ongoing education and use of active cooling during transport. Figure 4 demonstrates 7 successive points below the baseline mean during the postintervention period and indicates special cause variation.
Fig. 3.
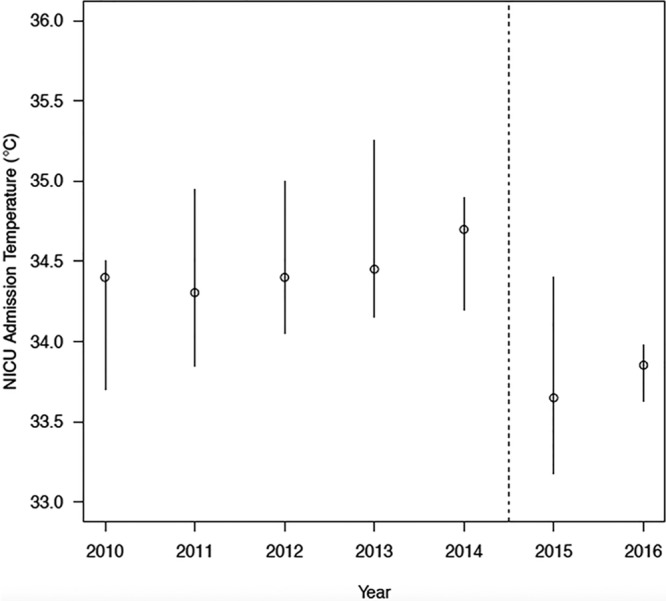
Decreased median admission temperature postintervention. Graphic representation of the median (open circle) and IQR (vertical bar) of NICU admission temperature. Dotted line (—) represents implementation time point for active cooling protocol.
Fig. 4.
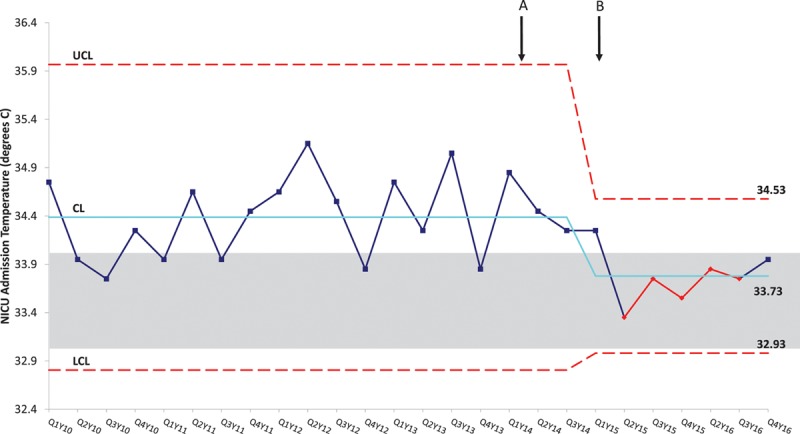
Statistical Process Control chart demonstrates 7 successive points below the baseline mean during the postintervention period, indicating special cause. Implementation of interventions including education of using the last transport temperature as the NICU admission temperature (A) and implementation of active cooling (B) are marked. The solid line represents the baseline average and dotted lines represent the control limits while the grayed box outlines the target temperature of 33–34°C. CL, Control Limit; LCL, Lower Control Limit; QY, Quarter/Year; UCL, Upper Control Limit.
There was no significant difference in average admission temperature postintervention between the 2 transportation distance groups (10–31 miles and > 31 miles), 33.7 ± 1.1°C versus 33.8 ± 0.6°C, P = 0.67. Of the 11 babies that arrived outside the goal temperature range in the postintervention period, 55% were transported 10–31 miles, 27% were transported 32–99 miles, and 18% were transported > 100 miles. In contrast, of the 61 infants who arrived outside the goal temperature range in the preintervention group, 49% were transported 10–31 miles, 36% were transported 32–99 miles, and 15% were transported > 100 miles. Due to overall small numbers in the group transported > 100 miles, we did not perform separate statistical analysis of these infants.
Documentation regarding protocol compliance was maintained throughout the project by FFL. In the postintervention group, we achieved 81% compliance to the active cooling protocol overall, with 13 months at 100% compliance.
Overall, the majority of infants (88%) who arrived outside the target range had a core temperature of > 34°C. The balancing measure of neonates with an admission temperature of less than 33°C was also evaluated, and while there was a slight increase in the percentage of infants who arrived less than 33°C in the postintervention group, the difference was not statistically significant (6% versus 12%; P = 0.41). The infants in the postintervention group who arrived below the target temperature arrived between 31.8°C and 32.8°C. Additionally, there were no reported cases of skin injuries due to active cooling with gel packs.
DISCUSSION
Implementation of the revised Transport Body Cooling Protocol along with standardization of active cooling during transport resulted in a significant improvement in the number of neonates who were admitted within the goal temperature range of 33–34°C. This population represents neonates who were transported within a large and diverse geographical area and included both ground and air (fixed-wing plane and helicopter) transport.
Although we demonstrated a significant improvement in achievement of goal admission temperatures by 164%, there remain a large percentage of neonates admitted to our institution outside of the desired temperature range with the majority of infants being > 34°C.
Protocol noncompliance is notable in 5 postintervention infants who were admitted with temperatures > 34°C, yet did not undergo active cooling during transport. Upon further review of the transport of these 5 infants, we noted that they were all transported by different flight nurses. Therefore, additional widespread education rather than targeted individual education is necessary to improve compliance with implementation of active cooling during transport.
Although not statistically significant, there was a trend toward an increase in the percentage of infants who arrived with a core body temperature of < 33°C. Only 1 infant in this group inappropriately underwent active cooling during transport, whereass the remainder were either passively cooled or actively warmed during transport. These findings are concerning, given the recently published data that sustained deeper cooling to 32°C compared with 33–34°C demonstrated a trend toward increased risk of death or disability.11 To decrease noncompliance with the Transport Body Cooling Protocol, continued educational efforts aimed at improving the initial and ongoing education of transport team members are needed. Additional strategies for sustained improvement include ensuring flight and nursing team agreement with proposed changes; implementation of a new protocol that includes multidisciplinary input, and creating a small team dedicated to the project that meets regularly to review challenges and adjust the protocol as needed.
One limitation of this study is that it is a QI initiative in a single center institution and is not a controlled trial evaluating different modes of cooling during transport. Although not evident from the baseline clinical characteristics of the infants, there may be other unmeasured clinical or patient factors that impact the ability to achieve optimal cooling during transport and subsequent admission temperature. A second limitation is that we adjusted the goal admission temperature as 1 of the primary interventions. Subsequent improvement could be attributed to this fact alone. However, this is unlikely as noted in Figure 4. Implementation of the active cooling protocol resulted in ongoing improvement. Thus, the overall improvement may be attributable to both interventions.
Although the use of a standardized active cooling protocol with gel packs confers some benefit, our study reveals that the core temperature at the time of admission remained outside the target range in 42% of infants admitted to our NICU following our intervention. To enable sustainable change, the process of obtaining and implementing the use of servo-controlled mattress cooling for infants during transport with a goal of further improving admission temperatures is actively underway. The benefits of utilizing a servo-controlled mattress include potential improvement in both the number of infants arriving at the referral NICU within the target temperature range and a decreased time to achieve the optimal target temperature. Also, the process of active cooling via gel packs is time and labor intensive, and the use of a servo-controlled mattress will not only assist with temperature regulation but will also enable the flight nurse to focus their time on the cardiopulmonary needs of the neonate, which are often significant in these critically ill patients.
CONCLUSIONS
Implementation of a revised and standardized Transport Body Cooling Protocol was associated with a greater than 2-fold improvement in goal admission temperatures for neonates transferred from outside hospitals for ongoing management of TH. However, 42% of infants admitted to our NICU following our intervention remained outside the target temperature range. Further evaluation of active cooling modes via a servo-controlled mattress is needed to determine if its implementation will result in further improvement for these critically ill neonates.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Preliminary data presented at the Western Society for Pediatric Research, January 2017, Carmel, Calif., as an oral presentation and at the Pediatric Academic Societies, May 2017, San Francisco, Calif., in poster format.
Supported by the National Institutes of Health (5T32HD007186-37 to Bourque, SL, trainee).
Published online February 15, 2018
REFERENCES
- 1.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670.. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013(1):CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs SE, Morley CJ, Inder TE, et al. ; Infant Cooling Evaluation Collaboration. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700.. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, et al. ; National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584.. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi DV, Strohm B, Edwards AD, et al. ; TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358.. [DOI] [PubMed] [Google Scholar]
- 6.Gunn AJ, Gunn TR, Gunning MI, et al. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–1106.. [DOI] [PubMed] [Google Scholar]
- 7.Thoresen M, Tooley J, Liu X, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology. 2013;104:228–233.. [DOI] [PubMed] [Google Scholar]
- 8.Akula VP, Joe P, Thusu K, et al. A randomized clinical trial of therapeutic hypothermia mode during transport for neonatal encephalopathy. J Pediatr. 2015;166:856–61.e1.. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary R, Farrer K, Broster S, et al. Active versus passive cooling during neonatal transport. Pediatrics. 2013;132:841–846.. [DOI] [PubMed] [Google Scholar]
- 10.Murthy K, Dykes FD, Padula MA, et al. The Children’s Hospitals Neonatal Database: an overview of patient complexity, outcomes and variation in care. J Perinatol. 2014;34:582–586.. [DOI] [PubMed] [Google Scholar]
- 11.Shankaran S, Laptook AR, Pappas A, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA. 2017;318:57–67.. [DOI] [PMC free article] [PubMed] [Google Scholar]


