Supplemental digital content is available in the text.
Abstract
Background and Objectives
This study is a meta-analysis of randomized controlled trials comparing the efficacy of transcutaneous electrical nerve stimulation (TENS) to a control and to other nerve stimulation therapies (NSTs) for the treatment of chronic back pain.
Methods
Citations were identified in MEDLINE, the Cochrane Library, Google Scholar, and ClinicalTrials.gov through June 2014 using the following keywords: nerve stimulation therapy, transcutaneous electrical nerve stimulation, back pain, chronic pain. Control treatments included sham, placebo, or medication only. Other NSTs included electroacupuncture, percutaneous electrical nerve stimulation, and percutaneous neuromodulation therapy.
Results
Twelve randomized controlled trials including 700 patients were included in the analysis. The efficacy of TENS was similar to that of control treatment for providing pain relief (standardized difference in means [SDM] = −0.20; 95% confidence interval [CI], −0.58 to 0.18; P = 0.293). Other types of NSTs were more effective than TENS in providing pain relief (SDM = 0.86; 95% CI, 0.15–1.57; P = 0.017). Transcutaneous electrical nerve stimulation was more effective than control treatment in improving functional disability only in patients with follow-up of less than 6 weeks (SDM = −1.24; 95% CI, −1.83 to −0.65; P < 0.001). There was no difference in functional disability outcomes between TENS and other NSTs.
Conclusions
These results suggest that TENS does not improve symptoms of lower back pain, but may offer short-term improvement of functional disability.
Chronic low back is a debilitating condition that results from factors such as physical activity, trauma, and inflammatory conditions. The Global Burden of Disease studies ranked chronic back pain (CBP) as the first cause of years lived with a disability and the sixth cause of disability-adjusted life-years.1 Such high rankings may arise in part from the prevalence of CBP: recent estimates indicate that approximately 1 in 5 to 1 in 10 individuals in representative populations in the United States and Germany are afflicted with CBP, respectively.2,3 As might be expected, the rate of CBP increases with age.4,5 Perhaps less expectedly, recent studies have found an association of CBP with smoking, body mass index, and depression.6–8 Given that the worldwide population is getting both older and heavier, successful management of this condition is becoming increasingly important.
Although patients with CBP are frequently managed with pharmacological therapy, lack of efficacy and adverse events lead many to discontinue treatment. For these patients, nonpharmacological approaches such as physical therapy and exercise may have some benefit. Nerve stimulation therapy (NST), which alters the activity of peripheral and central components of the nervous system, has also been used to treat CBP.9 One of the oldest of the NSTs is electroacupuncture (EA), which has been used to provide pain relief for several decades.10–12 Neural stimulation with EA is delivered by needles inserted into the skin, soft tissue, or muscles, at sites that will maximize pain relief. Percutaneous electrical nerve stimulation (PENS) and percutaneous neuromodulation therapy (PNT), as their names imply, also provide stimulation via needles or electrodes that pierce the skin. An alternative procedure also approved to treat chronic pain is transcutaneous electrical nerve stimulation (TENS). Although the neuromodulation elicited by TENS is similar to that of percutaneous techniques,13 TENS is delivered through the skin by surface electrodes encased in a patch.
Although TENS is widely used for pain management, evidence for its effectiveness is controversial. As a result, insurance coverage for this technique in the United States is currently restricted to patients enrolled in a randomized controlled trial (RCT). A 1995 assessment by the Canadian Coordinating Office for Health Technology Assessment found no benefit of TENS for chronic pain.14 A later assessment, conducted in 2010 by the American Academy of Neurology, reached a similar conclusion.15 The American Academy of Neurology's assessment was based on the evidence of 5 studies, only 2 of which were RCTs. Moreover, the assessment did not compare the effectiveness of TENS and other NSTs.
Thus, the purpose of this study was to conduct a meta-analysis of RCTs that compared TENS to sham TENS and to other therapies including EA, PENS, and PNT for the treatment of CBP.
METHODS
Search Strategy
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines.16 We searched MEDLINE, Cochrane, Google Scholar, and ClinicalTrials.gov databases through June 30, 2014, using the following search terms: nerve stimulation therapy (electroacupuncture, percutaneous electrical nerve stimulation, and percutaneous neuromodulation therapy), transcutaneous electrical nerve stimulation, back pain, and chronic pain. Duplicate citations were eliminated after the preliminary search results were obtained. To identify the final studies that would be included in the meta-analysis, the remaining citations were screened by a 2-step process. First, the title and abstract of each article were examined, and citations not meeting the inclusion criteria and meeting the exclusion criteria were discarded. Second, we obtained full-text copies of the remaining citations, and these were examined to determine which met all of the inclusion criteria and none of the exclusion criteria. Two independent reviewers identified eligible studies by using the search strategy described previously. If any uncertainties existed regarding eligibility, a third reviewer was consulted. The reference lists of the relevant studies were hand searched to identify other studies that met the inclusion criteria.
Selection Criteria
Studies included in this meta-analysis met the following inclusion criteria: (1) the study was an RCT; (2) enrolled patients were 18 years or older, and women were not pregnant; (3) patients were being treated for CBP; (4) the intervention involved TENS; and (5) the control group was either a negative control (ie, sham control, placebo, or medication only) or an active control (ie, other types of NSTs). Studies were excluded if they did not provide numerical data regarding the degree of pain or disability, and if they were non-English or non-Chinese publications. Letters, comments, editorials, and case reports were also excluded. Chronic pain was defined as pain lasting more than 12 weeks.
Data Extraction
Data extraction was also performed by 2 independent reviewers, and a third reviewer was consulted to resolve any uncertainties. The following information was extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, demographic data and diagnoses of the enrolled patients, information on the intervention(s), length of follow-up, and numerical pain and/or disability data from before and after the intervention.
Quality Assessment
We used the Cochrane Risk of Bias Tool to assess the quality of the included studies. Recommendations for judging risk of bias are provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions.17 Quality assessment was also performed by 2 independent reviewers, and any uncertainties were resolved by consulting a third reviewer.
Outcome Measures
The primary outcomes were the difference between the 2 interventions in the mean change in pain from baseline to after the intervention, for TENS versus control, and for TENS versus other NSTs. The secondary outcome was the difference between groups in improvement of functional disability.
Several different disability scores were evaluated. The Roland-Morris Disability Questionnaire (RMDQ) evaluates items associated with daily function and physical activities that may be affected by lower back pain, such as housework, sleeping, mobility, dressing, getting help, appetite, irritability, and pain severity. Although it is called a “disability” scale, it contains elements of impairment, disability, and handicap according to the International Classification of Functioning, Disability and Health. Scores range from 0 (no disability) to 24 (maximal disability). The Oswestry Disability Index (ODI) has 1 item regarding pain and 9 items regarding the activities of daily living including personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and traveling. The total ODI score ranges from 0 (no disability) to 100 (maximum disability). The Quebec Back Pain Disability Scale (QBPDS) contains assessments of elementary daily activities that patients with back pain might perceive difficult to perform. Items can be classified into 6 domains of activity affected by back pain: bed/rest (items 1–3), sitting/standing (items 4–6), ambulation (items 7–9), movement (items 10–12), bending/stooping (items 13–16), and handling of large/heavy objects (items 17–20). Scores range from 0 (no disability) to 100 (maximal disability).
Statistical Analysis
For the primary and secondary outcomes, the means and SDs were calculated and compared between the 2 interventions. Because the outcomes were determined by various instruments, a standardized difference in means (SDM) with a corresponding 95% confidence interval (CI; lower and upper limits) was calculated for the outcomes of each individual study and for studies combined. A χ2-based test of homogeneity was performed, and the inconsistency index (I2) and Q statistics were determined. If I2 was greater than 50% or greater than 75%, the trials were considered to be heterogeneous or highly heterogeneous, respectively. If I2 was less than 25%, the studies were considered to be homogeneous. If the I2 statistic was greater than 50%, a random-effects model of analysis was used.18 Otherwise, a fixed-effects model (Mantel-Haenszel method) was used. The combined effects were calculated, and a 2-sided P < 0.05 was considered to indicate statistical significance. Sensitivity analysis was carried out using the leave-one-out approach. Publication bias was not assessed because more than 10 studies are required to detect funnel plot asymmetry.19 Subgroup analysis was performed to evaluate treatment efficacy according to follow-up duration (<6 and ≥6 weeks). All analyses were performed using Comprehensive Meta-analysis Statistical Software, version 2.0 (Biostat, Englewood, New Jersey).
RESULTS
Literature Search
A flow diagram of study selection is presented in Figure 1, Supplemental Digital Content 1, http://links.lww.com/AAP/A237. The literature search initially identified 398 citations. Of these, 357 were excluded after screening the title and abstract. Review of the full text of the remaining 41 citations resulted in exclusion of 29. Thus, ultimately 12 RCTs published from 1986 through 2011 were included in the meta-analysis.20–31
Study Characteristics
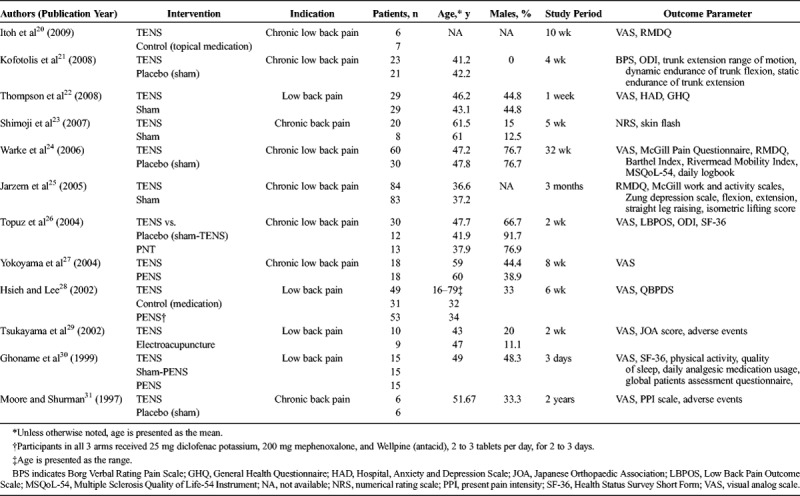
A summary of the characteristics of the included studies is provided in Table 1. A total of 700 patients were enrolled in the 12 studies: 350 received TENS, 81 underwent another type of NST, and 269 were in control groups (sham and other controls). Nerve stimulation therapies included EA (1 study of 9 patients), PENS (3 studies of 86 patients), and PNT (1 study of 13 patients). The mean age, reported for all but 2 studies, ranged from 36.6 to 61.5 years. The percentage of men in the TENS arm ranged from 0% to 76.7%. The studies were performed in Asia (Hong Kong, Japan), Europe, the United States, Canada, and South America. The study periods varied widely, with the shortest being 3 days and the longest 8 months.
TABLE 1.
Characteristics of Studies Included in the Meta-Analysis
Treatment Effect: Pain
TENS Versus Control
Pain relief in patients who received TENS versus control groups is summarized in Table 2. Nine studies reported complete numerical data (mean and SD) for pain scores before and after the intervention for patients who received TENS or the sham control/placebo and were included in the meta-analysis. There was evidence of heterogeneity among the studies (Q statistic = 20.242, I2 = 60.48%, P = 0.009); therefore, a random-effects model of analysis was used. The combined SDM indicated that pain relief did not differ significantly between the 2 groups (SDM = −0.20; 95% CI, −0.58 to 0.18; P = 0.293) (Fig. 1A).When subdivided by follow-up duration, there was no significant difference in pain relief between the TENS group and control group for studies with a follow-up period of less than 6 weeks (P = 0.209). A similar result was found for studies with a follow-up period of 6 weeks or longer (P = 0.818).
TABLE 2.
Summary of Pain Scores Reported in the Included Studies
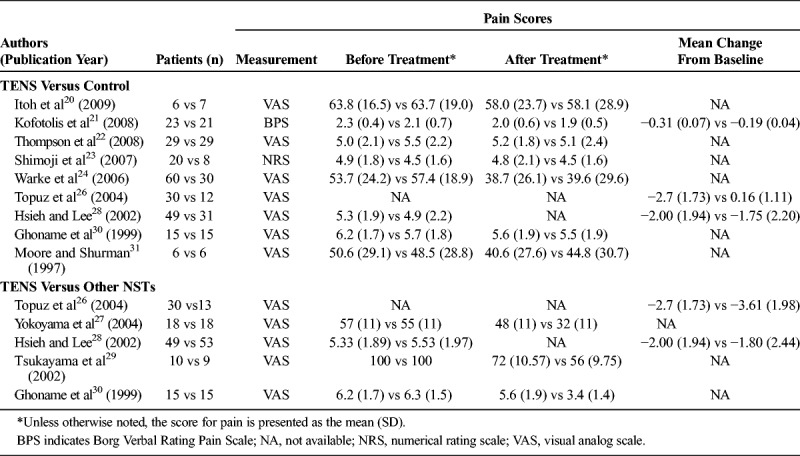
FIGURE 1.
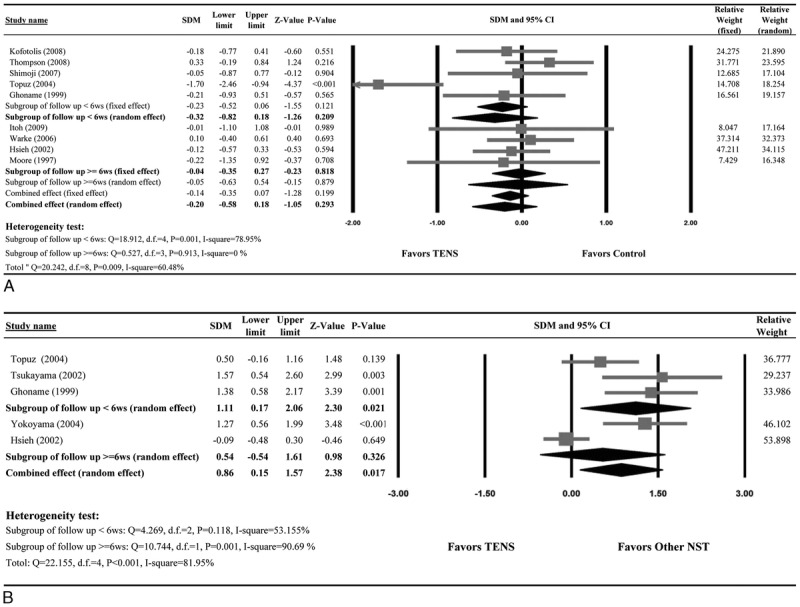
Meta-analysis of pain relief. Forest plot comparing the difference in pain relief between patients who underwent treatment with (A) TENS or a control or (B) TENS or another NST.
TENS Versus Other NSTs
Pain relief in patients who received TENS versus other types of NST is summarized in Table 2. Five studies provided complete pain score data for before and after the intervention and were included in the meta-analysis. Because of evidence of heterogeneity among the studies (Q statistic = 22.155, I2 = 81.95%, P < 0.001), a random-effects model of analysis was used. The combined SDM indicated that other types of NSTs were significantly more effective than TENS in providing pain relief (0.86; 95% CI, 0.15–1.57; P = 0.017) (Fig. 1B). In patients with a follow-up period of less than 6 weeks, other types of NSTs were significantly more effective than TENS in providing pain relief (SDM = 1.11; 95% CI, 0.17–2.06; P = 0.021). However, no significant difference in the pain relief between the 2 groups was found in patients with a follow-up period of 6 weeks or longer (SDM = 0.54; 95% CI, −0.54 to 1.61; P = 0.326) (Fig. 1B).
Treatment Effect: Functional Disability
TENS Versus Control
Data of disability level of patients who received TENS versus control are summarized in Table 3. Six studies provided complete numerical data for the disability level before and after the intervention and were included in the meta-analysis. Evidence of heterogeneity was present (Q statistic = 25.036, I2 = 80.03%, P = 0.001); thus, a random-effects model of analysis was used. The combined SDM (−0.60; 95% CI, −0.67 to −0.02; P = 0.328) indicated there was no significant difference in the improvement of functional disability between patients who received TENS and control patients. For patients with follow-up period of less than 6 weeks, TENS was significantly more effective than sham control/placebo in improving functional disability (SDM = −1.24; 95% CI, −1.83 to −0.65; P < 0.001). No significant difference in functional disability between the 2 groups was seen for patients with a follow-up period of 6 weeks or longer (SDM = −0.04; 95% CI, −0.26 to 0.18; P = 0.707) (Fig. 2A).
TABLE 3.
Summary of Disability Outcomes of the Included Studies
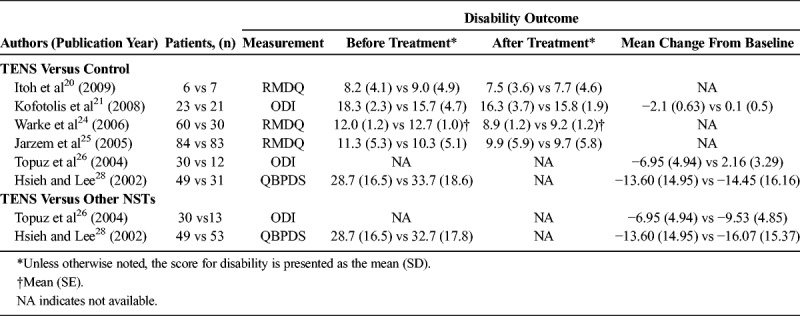
FIGURE 2.
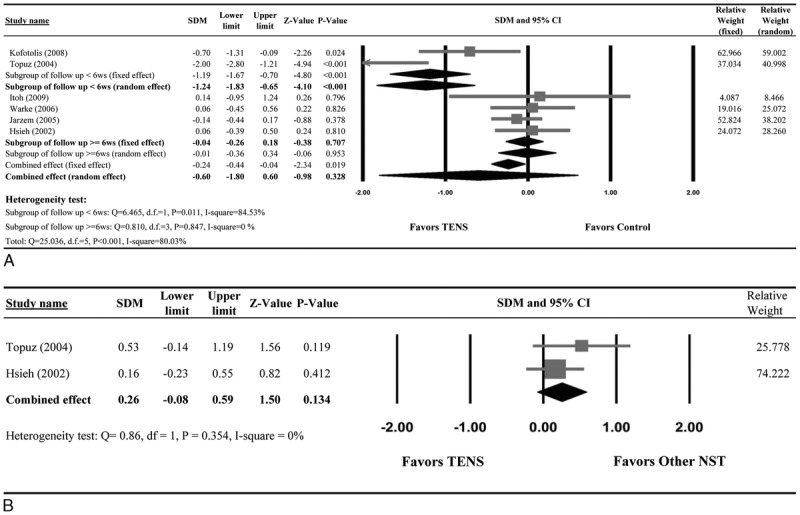
Meta-analysis of disability level. Forest plot comparing the change in disability level between patients who underwent treatment with (A) TENS or a control or (B) TENS or another NST.
TENS Versus the Other NSTs
Data of disability level of patients who received TENS versus other NSTs are summarized in Table 3. Only 2 studies provided disability data from before and after the intervention. The duration of follow-up in both studies was less than 6 weeks. The combined SDM (0.26; 95% CI, −0.08 to 0.59; P = 0.134) indicated no difference in improvement between the 2 groups (Fig. 2B).
Sensitivity Analysis and Publication Bias
To determine the reliability of the results, sensitivity analysis using the leave-one-out approach, in which the analysis was performed with each study removed in turn, was conducted (Table 1, Supplemental Digital Content 2, http://links.lww.com/AAP/A238). The direction and magnitude of each SDM did not vary markedly with the removal of each study in turn, indicating the meta-analysis had good reliability and the results were not overly influenced by any single study. However, in the analysis of TENS versus other NSTs with respect to pain, although the pooled SDM remained greater than 0, P values became nonsignificant when 3 studies were removed (Yokoyama et al,27 Tsukayama et al,29 and Ghoname et al30). No sensitivity analysis was performed for TENS versus other NSTs with respect to disability because only 2 studies were included in the analysis.
Quality Assessment
Figure 3A shows the potential risks of bias for the individual studies. Although most studies had bias in 1 or more categories, 3 studies received positive assessments for all categories analyzed. The most significant bias came from the performance category, because several of the included studies (5 of 12) did not apply a sham control or placebo control to sufficiently blind the participants. The included studies had an overall high risk of performance and attrition bias, as well as a high risk of bias due to lack of an intention-to-treat analysis (Fig. 3B).
FIGURE 3.
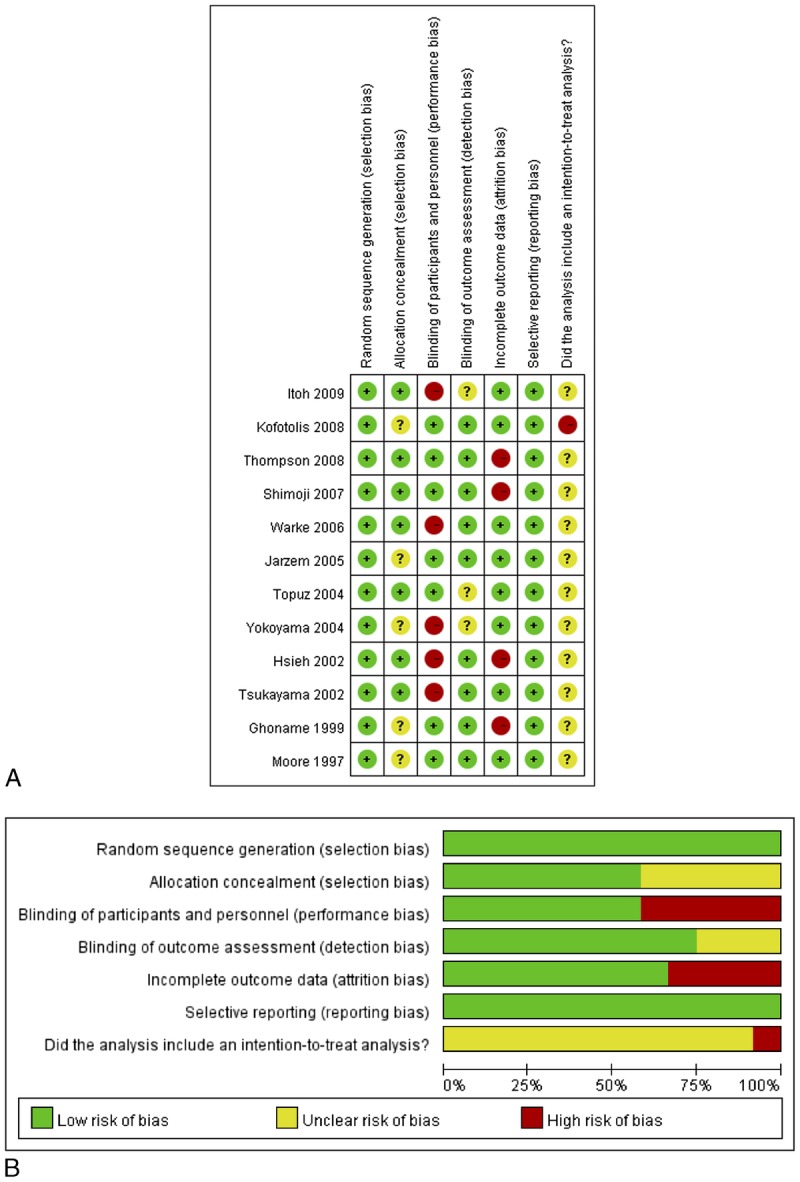
Quality assessment results. The green circles indicate lack of bias; red circles indicate the presence of bias. A, Risk of bias for each included study. The studies were assessed for 5 types of bias and for the use of an intent-to-treat analysis. B, The overall summary of bias of the 16 studies.
DISCUSSION
In this meta-analysis, we evaluated the efficacy of TENS for the treatment of CBP. A total of 12 studies enrolling 700 patients from 8 countries were included in the analysis. Moreover, the analysis included several RCTs whose results were published after the most recent systematic review of this topic. The results indicated that pain relief was not different between patients treated with TENS versus control patients and that other NSTs (including EA, PENS, and PNT) were more effective in providing pain relief than TENS. Overall, TENS did not provide improvement in disability when compared with control treatment, but TENS was more effective in improving functional disability within 6 weeks after the treatment. The difference in improvement of disability between TENS and other NSTs was not conclusive because only 2 studies were included in the analysis.
This meta-analysis comparing the efficacy of TENS and other NSTs provides important insights regarding the use of TENS, PENS, and PNT. While our inclusion criteria allowed inclusion of studies that tested EA, only 1 of 5 (relative weight, 16.475) was assessed in the meta-analysis of pain relief; the remaining studies used PNT or PENS (combined relative weight, 83.525). Therefore, further analysis of the comparative efficacy of TENS and EA should be undertaken. At present, we cannot state with certainty why treatment with non-TENS NSTs was more effective than TENS for relieving pain. One possibility is that the percutaneous delivery of the electrical stimulation is superior to a transcutaneous approach. Alternatively, the better performance of PNT and PENS over TENS may arise from the experimental protocols used in the included studies. The efficacy of these procedures depends on parameters including stimulus intensity, duration, and frequency.32–35 Only 1 of 4 PENS/PNT studies, that of Topuz et al,26 included a description of the intensity of both TENS and PENS. The 4 studies also differed in length. Notably, we found no significant difference between TENS and PENS/PNT for the 2 short-term studies, those of Hsieh and Lee28 (1 treatment) and Topuz et al26 (2 weeks), but we found a significant benefit to patients who received PENS/PNT in the 2 relatively long-term studies, those of Ghoname et al30 (3 weeks) and Yokoyama et al27 (8 weeks). Bennett et al36 stressed the importance of eliminating all potential sources of bias, which our quality assessment showed to be significant.
In a 2000 Cochrane review, Milne et al37 reported an analysis of TENS versus placebo for chronic low back pain. The authors analyzed 5 RCTs (Jarzem38 [1997], Moore and Shurman31 [1997], Marchand39 [1993], Gemignani40 [1991], Deyo et al41 [1990]) that enrolled 323 subjects into the placebo and TENS arms. Three of the 5 trials analyzed by Milne and colleagues37 comprised approximately 85% of the total subjects. The trial by Moore and Shurman31 (1997) was included in the present analysis. Another Cochrane review by Khadilkar et al42 published in 2008 examined the same question by analyzing 4 RCTs (Jarzem et al25 [2005], Topuz et al26 [2004], Cheing43 [1999], Deyo et al41 [1990]) including 585 patients. Two of the trials in that analysis (Jarzem et al [2005],25 Topuz et al26 [2004]) were included in the present analysis. The studies included in the 2 analyses were different because of slightly different inclusion criteria. Khadilkar et al42 excluded the study by Gemignani40 because of a mixed sample of acute, subacute, and chronic low-back pain, and the study was confined to patients with ankylosing spondylitis (inflammatory arthritis); they excluded the study of Marchand39 because the study included patients with inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis) and other specific diagnoses, for which exact numbers were not provided; they excluded the study by Moore and Shurman31 because of a mixed sample of upper, middle, and low-back pain. Khadilkar et al42 concluded that there was conflicting evidence for a superior effect of TENS versus placebo, whereas Milne et al37 concluded that the pain relief provided by TENS is similar to that of placebo.
Both Khadilkar et al42 and Milne et al37 found no evidence of superiority of TENS over placebo with respect to disability. Our results differ in that we found a significant difference in TENS versus placebo in the ability to improve disability in patients within a 6-week follow-up period. A potential explanation is different scales were used to measure disability. Khadilkar et al42 and Milne et al37 included trials that used the ODI and Roland Disability Index, respectively, whereas the studies in our analysis also used other scales such as the QBPDS. Interestingly, of the studies in our meta-analysis that examined disability, the two that found a significant benefit of TENS both used the ODI.21,26 However, these studies contributed approximately only 10% of the total patients. In addition, all of these scales are validated and frequently used. Therefore, we do not believe that our opposing conclusion stems from analyzing data generated from several types of scales. Instead, we believe that the much larger number of subjects and RCTs in our meta-analysis has allowed us to identify a clinically significance of TENS treatment in improving disability.
The most recent meta-analysis examining TENS for chronic low back pain was performed by Jauregui et al44 in 2016. A visual analog scale for back pain was the primary outcome, and the analysis included 9 level I and 4 level II studies that included a total of 267 patients with a mean duration of treatment of 6 weeks and mean follow-up of 7 weeks. The authors found that TENS significantly reduced pain, with pretreatment to posttreatment SDM of 0.844. While the overall results were different than our results, interestingly, patients treated for less than 5 weeks had a significant reduction in pain, whereas those treated for more than 5 weeks did not.
Examination of more subjective parameters such as satisfaction with TENS treatment and outcome and overall perception of the treatment would add value to determination of the value of TENS treatment in CBP. However, only 2 studies included in the current analysis reported such data, and the measures were different in the 2 studies. Warke et al24 provided a questionnaire at the conclusion of the trial, and the majority of participants (69%) felt that the TENS had helped their low back pain during the trial, and 80.8% stated that they would consider using TENS again. Ghoname et al30 reported that PENS was the preferred treatment in 91% of patients, and 80% stated they would be willing to pay money out of pocket to continue PENS. In a study not included in the meta-analysis, Deyo et al41 reported that 56% of patients in a sham TENS group and 68% in a true TENS group stated they wished to continue TENS. Thus, even though the data are limited, it seems that patients believe that TENS treatment is valuable. Interestingly, in our literature review, we did not find any studies specifically focusing on patient satisfaction with TENS.
This study has certain limitations. First, our analysis included a limited number of studies comparing TENS and other NSTs. Second, the length of the intervention varied among the studies, and subgroup analysis by length of follow-up showed different results in some comparisons. This variance raises an important issue regarding the need for experimental standards in future trials. Third, the comorbidities of the enrolled patients could differ. If so, this would explain a certain degree of the heterogeneity of the included studies and could lessen the general applicability of the results.
In conclusion, we have conducted a meta-analysis of studies that reported the efficacy of TENS and other NSTs for the treatment of patients with CBP. The results indicated that pain relief was not different between patients treated with TENS versus control patients and that other non-TENS NSTs (eg, PENS, PNT) were more effective in providing pain relief than TENS. Overall, TENS did not provide an improvement in disability when compared with control treatment; but in patients followed up for less than 6 weeks TENS was more effective than control treatment in improving functional disability. The difference in improvement of disability between TENS and other NSTs was not conclusive. Additional RCTs comparing the efficacy of TENS and other approved procedures are warranted.
Supplementary Material
Footnotes
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.rapm.org).
REFERENCES
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuhauser H, Ellert U, Ziese T. Chronic back pain in the general population in Germany 2002/2003: prevalence and highly affected population groups [in German]. Gesundheitswesen. 2005;67:685–693. [DOI] [PubMed] [Google Scholar]
- 3.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knauer SR, Freburger JK, Carey TS. Chronic low back pain among older adults: a population-based perspective. J Aging Health. 2010;22:1213–1234. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs JM, Hammerman-Rozenberg R, Cohen A, Stessman J. Chronic back pain among the elderly: prevalence, associations, and predictors. Spine (Phila Pa 1976). 2006;31:E203–E207. [DOI] [PubMed] [Google Scholar]
- 6.Heuch I, Heuch I, Hagen K, Zwart JA. Body mass index as a risk factor for developing chronic low back pain: a follow-up in the Nord-Trondelag health study. Spine (Phila Pa 1976). 2013;38:133–139. [DOI] [PubMed] [Google Scholar]
- 7.Alkherayf F, Agbi C. Cigarette smoking and chronic low back pain in the adult population. Clin Invest Med. 2009;32:E360–E367. [DOI] [PubMed] [Google Scholar]
- 8.Suri P, Boyko EJ, Smith NL, et al. Modifiable risk factors for chronic back pain: insights using the co-twin control design. Spine J. 2017;17:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroeling P, Gross A, Goldsmith CH, et al. Electrotherapy for neck pain. Cochrane Database Syst Rev. 2009;CD004251. [DOI] [PubMed] [Google Scholar]
- 10.Lee PK, Modell JH, Andersen TW, Saga SA. Incidence of prolonged pain relief following acupuncture. Anesth Analg. 1976;55:229–231. [DOI] [PubMed] [Google Scholar]
- 11.Han FY, Lin JH, Ch'en K'C, Sun JK. Electroacupuncture for dental anesthesia in exodontia: preliminary report of 100 cases. Chin Med J. 1960;80:100–102. [PubMed] [Google Scholar]
- 12.Cummings M. Percutaneous electrical nerve stimulation—electroacupuncture by another name? A comparative review. Acupunct Med. 2001;19:32–35. [DOI] [PubMed] [Google Scholar]
- 13.Peacock J. Tens and acupuncture therapy for soft tissue pain. Anaesth Int Care Med. 2013;14:502. [Google Scholar]
- 14.Reeve J, Corabian P. Transcutaneous Electrical Nerve Stimulation (TENS) and Pain Management. Ottowa, Ontario, Canada: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 1995. [Google Scholar]
- 15.Dubinsky RM, Miyasaki J. Assessment: efficacy of transcutaneous electric nerve stimulation in the treatment of pain in neurologic disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;74:173–176. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. Accessed September 12, 2016.
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 20.Itoh K, Itoh S, Katsumi Y, Kitakoji H. A pilot study on using acupuncture and transcutaneous electrical nerve stimulation to treat chronic non-specific low back pain. Complement Ther Clin Pract. 2009;15:22–25. [DOI] [PubMed] [Google Scholar]
- 21.Kofotolis ND, Vlachopoulos SP, Kellis E. Sequentially allocated clinical trial of rhythmic stabilization exercises and TENS in women with chronic low back pain. Clin Rehabil. 2008;22:99–111. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JW, Bower S, Tyrer SP. A double blind randomised controlled clinical trial on the effect of transcutaneous spinal electroanalgesia (TSE) on low back pain. Eur J Pain. 2008;12:371–377. [DOI] [PubMed] [Google Scholar]
- 23.Shimoji K, Takahashi N, Nishio Y, Koyanagi M, Aida S. Pain relief by transcutaneous electric nerve stimulation with bidirectional modulated sine waves in patients with chronic back pain: a randomized, double-blind, sham-controlled study. Neuromodulation. 2007;10:42–51. [DOI] [PubMed] [Google Scholar]
- 24.Warke K, Al-Smadi J, Baxter D, Walsh DM, Lowe-Strong AS. Efficacy of transcutaneous electrical nerve stimulation (TENS) for chronic low-back pain in a multiple sclerosis population: a randomized, placebo-controlled clinical trial. Clin J Pain. 2006;22:812–819. [DOI] [PubMed] [Google Scholar]
- 25.Jarzem PF, Harvey EJ, Arcaro N, Kaczorowski J. Transcutaneous electrical nerve stimulation [TENS] for chronic low back pain. J Musculoskelet Pain. 2005;13:3–9. [Google Scholar]
- 26.Topuz O, Özfidan E, Ozgen M, Ardic F. Efficacy of transcutaneous electrical nerve stimulation and percutaneous neuromodulation therapy in chronic low back pain. J Back Musculoskelet Rehabili. 2004;17:127–133. [Google Scholar]
- 27.Yokoyama M, Sun X, Oku S, et al. Comparison of percutaneous electrical nerve stimulation with transcutaneous electrical nerve stimulation for long-term pain relief in patients with chronic low back pain. Anesth Analg. 2004;98:1552–1556. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh RL, Lee WC. One-shot percutaneous electrical nerve stimulation vs. transcutaneous electrical nerve stimulation for low back pain: comparison of therapeutic effects. Am J Phys Med Rehabil. 2002;81:838–843. [DOI] [PubMed] [Google Scholar]
- 29.Tsukayama H, Yamashita H, Amagai H, Tanno Y. Randomised controlled trial comparing the effectiveness of electroacupuncture and TENS for low back pain: a preliminary study for a pragmatic trial. Acupunct Med. 2002;20:175–180. [DOI] [PubMed] [Google Scholar]
- 30.Ghoname EA, Craig WF, White PF, et al. Percutaneous electrical nerve stimulation for low back pain: a randomized crossover study. JAMA. 1999;281:818–823. [DOI] [PubMed] [Google Scholar]
- 31.Moore SR, Shurman J. Combined neuromuscular electrical stimulation and transcutaneous electrical nerve stimulation for treatment of chronic back pain: a double-blind, repeated measures comparison. Arch Phys Med Rehabil. 1997;78:55–60. [DOI] [PubMed] [Google Scholar]
- 32.Tong KC, Lo SK, Cheing GL. Alternating frequencies of transcutaneous electric nerve stimulation: does it produce greater analgesic effects on mechanical and thermal pain thresholds? Arch Phys Med Rehabil. 2007;88:1344–1349. [DOI] [PubMed] [Google Scholar]
- 33.Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur J Pain. 2000;4:185–193. [DOI] [PubMed] [Google Scholar]
- 34.Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther. 2013;93:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen MF, Elden H, Janson ED, Lilja H, Stener-Victorin E. A comparison of high- versus low-intensity, high-frequency transcutaneous electric nerve stimulation for painful postpartum uterine contractions. Acta Obstet Gynecol Scand. 2007;86:310–314. [DOI] [PubMed] [Google Scholar]
- 36.Bennett MI, Hughes N, Johnson MI. Methodological quality in randomised controlled trials of transcutaneous electric nerve stimulation for pain: low fidelity may explain negative findings. Pain. 2011;152:1226–1232. [DOI] [PubMed] [Google Scholar]
- 37.Milne S, Welch V, Brosseau L, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic low back pain. Cochrane Database Syst Rev. 2001;CD003008. [DOI] [PubMed] [Google Scholar]
- 38.Jarzem P, Harvey EJ, Arcaro N, Kazarowski J. Transcutaneous Electrical Nerve Stimulation for Non-Acute Low Back Pain: A Randomized Double-Blind Study of Conventional, Nu-Wavefor, Acupuncture-Type and Sham Therapies. Presented at: American Academy of Orthopaedic Surgeons 1997 Annual Meeting; February 13–17, 1997; San Francisco, CA. [Google Scholar]
- 39.Marchand S, Charest J, Li J, Chenard JR, Lavignolle B, Laurencelle L. Is TENS purely a placebo effect? A controlled study on chronic low back pain. Pain. 1993;54:99–106. [DOI] [PubMed] [Google Scholar]
- 40.Gemigniani G. Transcutaneous electrical nerve stimulation in ankylosing spondylitis: a double-blind study. Arth Rheum. 1991;34:788–789. [DOI] [PubMed] [Google Scholar]
- 41.Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N Engl J Med. 1990;322:1627–1634. [DOI] [PubMed] [Google Scholar]
- 42.Khadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. Cochrane Database Syst Rev. 2008:CD003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheing GL, Hui-Chan CW. Transcutaneous electrical nerve stimulation: nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Arch Phys Med Rehabil. 1999;80:305–312. [DOI] [PubMed] [Google Scholar]
- 44.Jauregui JJ, Cherian JJ, Gwam CU, et al. A meta-analysis of transcutaneous electrical nerve stimulation for chronic low back pain. Surg Technol Int. 2016;28:296–302. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


