Abstract
Many previous studies have demonstrated that the blindness patients have has functional and anatomical abnormalities in the visual and other vision-related cortex. However, changes in the brain function in late monocular blindness (MB) at rest are largely unknown. In this study, we investigated the underlying regional homogeneity (ReHo) of brain-activity abnormalities in patients with late MB and their relationship with clinical features. A total of 32 patients with MB (25 male and seven female) and 32 healthy controls (HCs) (25 male and seven female) closely matched in age, sex, and education underwent resting-state functional MRI scans. The ReHo method was used to assess local features of spontaneous brain activities. Patients with MB were distinguishable from HCs using the receiver operating characteristic curve. The relationship between the mean ReHo in brain regions and the behavioral performance was calculated using correlation analysis. Compared with HCs, patients with MB showed significantly decreased ReHo values in the right rectal gyrus, right cuneus, right anterior cingulate, and right lateral occipital cortex and increased ReHo values in the right inferior temporal gyrus, right frontal middle orbital, left posterior cingulate/precuneus, and left middle frontal gyrus. However, there was no significant relationship between the different mean ReHo values in the brain regions and the clinical features. Late MB involves abnormalities of the visual cortex and other vision-related brain regions, which may reflect brain dysfunction in these regions.
Keywords: functional MRI, monocular blindness, regional homogeneity, resting state
Introduction
Blindness, known as the loss of response to external light stimulus, is one of the most serious eye conditions. A previous study demonstrated that there were 32.4 million people suffering from blindness in 2010 globally 1. The prevalence of blindness was 0.33% in urban Southern China 2. Blindness can be divided into early blindness and late blindness roughly. It can be caused by a variety of factors such as ocular trauma 3, cataract 4, and glaucoma 5. At present, drugs and surgery are effective for reversible blindness caused by cataract 6 and optic neuritis 7. However, there is no effective treatment for irreversible blindness. Blindness greatly affects the daily life in the patients 8. Blindness has also become a serious social problem. Around $5.5 billion/year is spent for the medical care for blind patients in the USA 9.
Functional MRI (fMRI) has been successfully used to evaluate the changes in brain activities in blindness. In the early blindness, the superior colliculus (visual subcortical) was reorganized with the auditory system 10. Meanwhile, the blindness showed enhanced processing of auditory motion 11. In addition, it has been shown that the occipital cortex is thicker in early blindness patients 12, whereas the interactions between the visual and other sensory cortices are weaker 13. Late blindness also leads to the altered cerebral function. Gray matter volume in the visual areas is markedly decreased in late blindness 14. Moreover, compared with late blindness, early blindness has increased functional connectivity in the ventral visual stream 15. Bola et al. 16 demonstrated that the blindness showed the impairment of synchronization in brain networks and more specifically in temporal patterns 17. In our previous study, we demonstrated that the late monocular blindness (MB) showed the lower brain amplitude of low-frequency fluctuation in the left cerebellum anterior lobe, right parahippocampal gyrus, right cuneus, and left precentral gyrus 18. Meanwhile, another research reported that the macaque monkeys with MB showed decreased fractional anisotropy and increased mean diffusivity in the disease side optic tracts compared with the normal optic tracts 19. However, synchronous neural activities in MB are less studied.
The regional homogeneity (ReHo) method, a resting-state fMRI measurement method, is thought to be a reliable and sensitive measurement, which can be used to evaluate the coherence of the blood oxygen level-dependent signal among neighboring voxels of the whole brain at rest. Kendall’s coefficient of concordance is used to calculate the similarity of the time series of the voxel with those of its nearest neighbors. The major advantage of ReHo is the ability to detect spontaneous hemodynamic responses of resting-state fMRI 20,21. In our previous studies, the ReHo method has been successfully used to assess the neurological conditions in certain eye diseases such as optic neuritis 22 and comitant strabismus 23.
Here, we hypothesized that the ReHo values of resting-state brain activity would be different between the MB and the healthy control (HC) groups, which might underlie the mechanism related to the dysfunction of the visual cortex. The aim of our study was to investigate brain synchronous neural activity changes in patients with late MB and investigate its relationship with the behavioral performances.
Patients and methods
Patients
A total of 32 patients with MB (25 male and seven female, all with right eye blindness) were recruited from the Ophthalmology Department of the First Affiliated Hospital of Nanchang University Hospital. The diagnostic criteria of MB were as follows: (a) late stage of MB (in 18 patients it was caused by ocular trauma and in 14 patients it was due to keratitis); (b) normal contralateral eye without any ocular diseases (cataracts, glaucoma, optic neuritis, and retinal degeneration). The exclusion criteria were as follows: (i) congenital blindness; (ii) impaired contralateral eye vision; (iii) blindness caused by eye diseases (cataracts, glaucoma, optic neuritis, macular degeneration, and ocular ischemic diseases); (iv) a history of surgery in both eyes; (v) long-term medical treatment of blindness; (vi) psychiatric disorders (depression, bipolar disorder, and sleep disorder), and cerebral infarction diseases (cerebral hemorrhage, cerebral infarction, and cerebral vascular malformations).
Thirty-two (25 male and seven female) HCs with age, sex, and education status matched to participants in the MB group were also recruited for this study. All HCs met the following criteria: (i) no abnormalities in the brain parenchyma on cranial MRI; (ii) no ocular disease with uncorrected or corrected visual acuity (VA) more than 0.8; (iii) no psychiatric disorders; and (iv) be able to be scanned with MRI (e.g. no cardiac pacemaker or implanted metal devices). All research methods followed the Declaration of Helsinki and were approved by the principles of medical ethics. All volunteers participated voluntarily and were informed of the purposes, methods, and potential risks before signing an informed consent form.
MRI parameters
MRI scanning was performed on a 3 T MR scanner (Trio; Siemens, Munich, Germany). The functional data were obtained with spoiled gradient-recalled echo sequence with the following parameters: repetition time=1900 ms, echo time=2.26 ms, thickness=1.0 mm, gap=0.5 mm, acquisition matrix=256×256, field of view=250×250 mm, and flip angle=9°; 176 structural images were obtained. Finally, 240 functional images (repetition time=2000 ms, echo time=30 ms, thickness=4.0 mm, gap=1.2 mm, acquisition matrix=64×64, flip angle=90°, field of view=220×220 mm, and 30 axial slices with gradient-recalled echo-planar imaging pulse sequence) covering the whole brain were obtained.
Functional MRI data analysis
The 240 functional images were analyzed as described previously 22. Briefly, the data were filtered using MRIcro (Nottingham University, Nottingham, UK) and preprocessed using SPM8 (The MathWorks Inc., Natick, Massachusetts, USA) and DPARSFA (Institute of Psychology, CAS, Beijing, People’s Republic of China) software. On the basis of Kendall’s coefficient of concordance, ReHo computation was performed with the REST software (State Key Laboratory of Cognitive Neuroscience and Learning, Beijing, China), as previously described 22.
Statistical analysis
For fMRI data, two-sample t-test was performed to examine the voxel-wise difference between the MB and HC groups using the REST toolbox; State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, China (The statistical threshold was set at the voxel level with P<0.05 for multiple comparisons using Gaussian random field theory voxels with P<0.01 and cluster size>40 voxels, AlphaSim corrected.). These voxels were regarded as the regions of interest showing significant difference between the two groups.
For behavioral performances, effect size measures were used for continuous data. Cohen’s d and Gates’ δ were calculated corrected for multiple comparisons.
SPSS, version 20.0 (IBM Corporation, Armonk, New York, USA) statistical software was used for all statistical analyses.
Brain–behavior correlation analysis
The relationship between the mean ReHo value and their clinical features was calculated using the correlation analysis (P<0.05 was considered statistically significant).
Clinical data analysis
The cumulative clinical measurements, including the duration of the onset of MB and best-corrected VA were recorded and analyzed in the study with independent sample t-test (P<0.05 as significantly different).
Results
Demographics and visual measurements
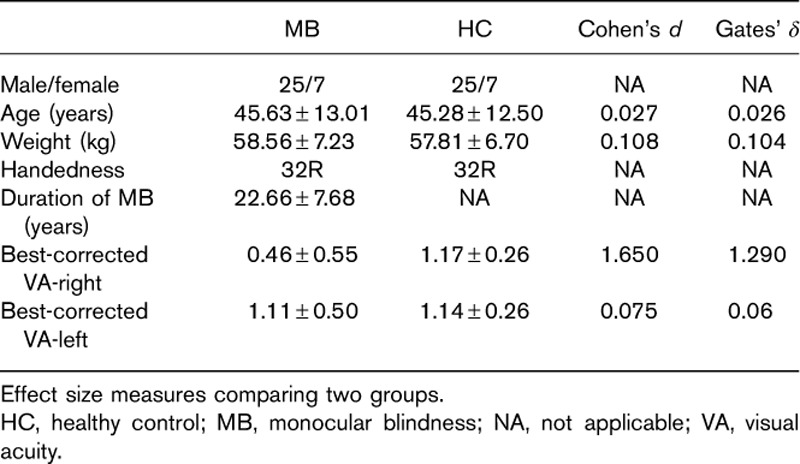
There were low Cohen’s d in weight (0.108), age (0.108), and best-corrected VA-left (P=0.719) between the two groups. There was high Cohen’s d in best-corrected VA-right (0.075) between the two groups (Table 1).
Table 1.
Demographics and clinical measurements
Regional homogeneity differences
Compared with HCs, MB patients showed lower ReHo values in the right rectal gyrus, right cuneus, right anterior cingulate, and right lateral occipital cortex [Fig. 1 (dark grey) and Table 2]. In contrast, higher ReHo values in the MB group were observed in the right inferior temporal gyrus (ITG), right frontal middle orbital, left posterior cingulate/precuneus, and left middle frontal gyrus [Fig. 1 (light grey) and Table 2]. The mean values of altered ReHo between the MB and HC groups are shown in Fig. 2. In the MB group, there was no significant correlation between the mean ReHo values of the different brain areas and the clinical manifestations (P>0.05).
Fig. 1.
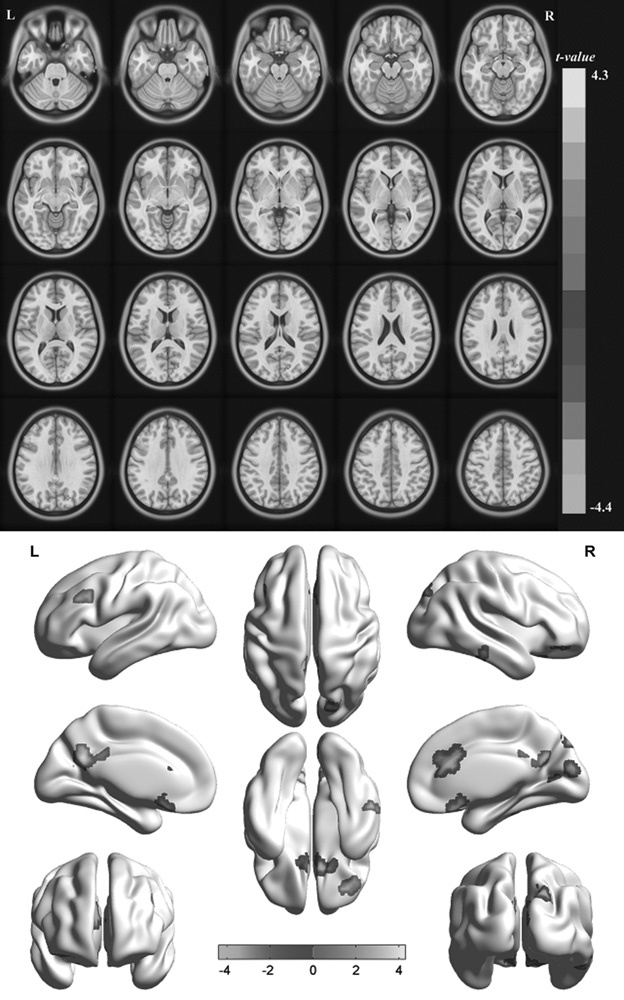
Spontaneous brain activity in the monocular blindness and healthy control groups. Significant activity differences were observed in the right rectal gyrus, right cuneus, right anterior cingulate, right lateral occipital cortex, right inferior temporal gyrus, right frontal middle orbital, left posterior cingulate/precuneus, and left middle frontal gyrus. Black denotes higher regional homogeneity values. [P<0.01 for multiple comparisons using Gaussian random field theory (z>2.3, P<0.01, cluster>40 voxels, AlphaSim corrected)].
Table 2.
Brain areas with significantly different regional homogeneity values between groups
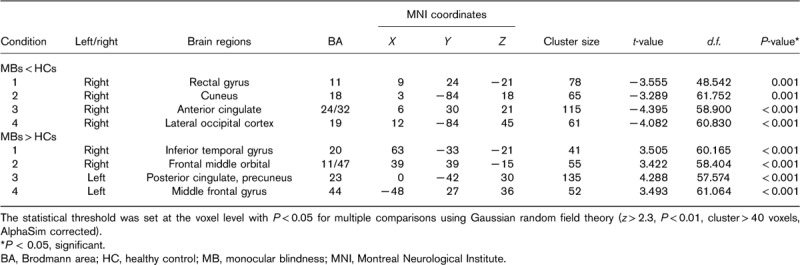
Fig. 2.
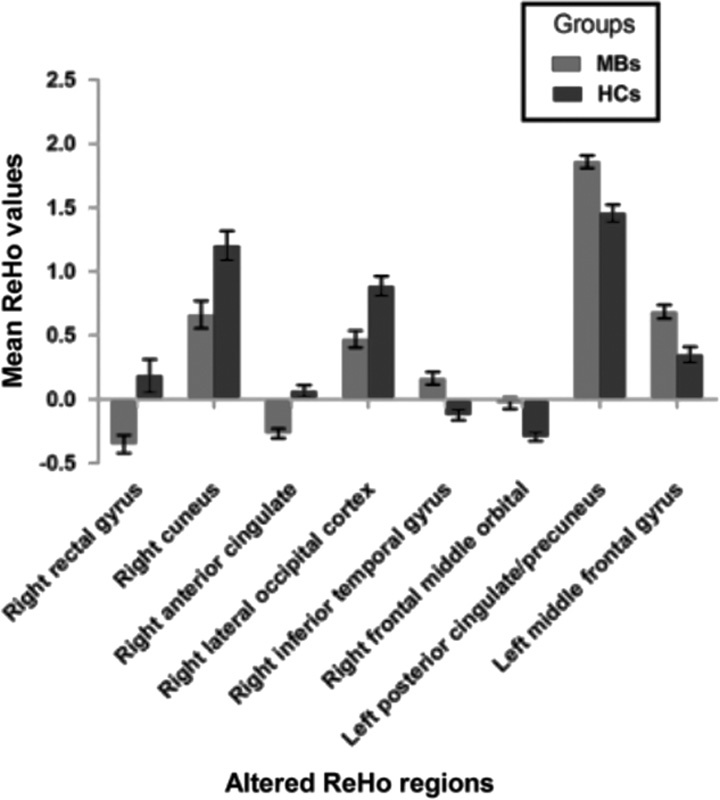
The mean values of altered ReHo values between the MB and HC groups. HC, healthy control; MB, monocular blindness; ReHo, regional homogeneity.
Receiver operating characteristic curve
We hypothesized that the ReHo differences between the MB and HC groups might be useful diagnostic markers. The mean ReHo values of the different brain regions were analyzed using the receiver operating characteristic (ROC) curves. The areas under the ROC curve were as follows: right rectal gyrus, 0.771; right cuneus, 0.715; right anterior cingulate, 0.828; and right lateral occipital cortex (0.756) (MBs<HCs) (Fig. 3a); the areas under the ROC curve for ReHo values were as follows: right ITG, 0.778; right frontal middle orbita, 0.795; left posterior cingulate/precuneus, 0.793; and left middle frontal gyrus, 0.782 (MBs>HCs) (Fig. 3b).
Fig. 3.
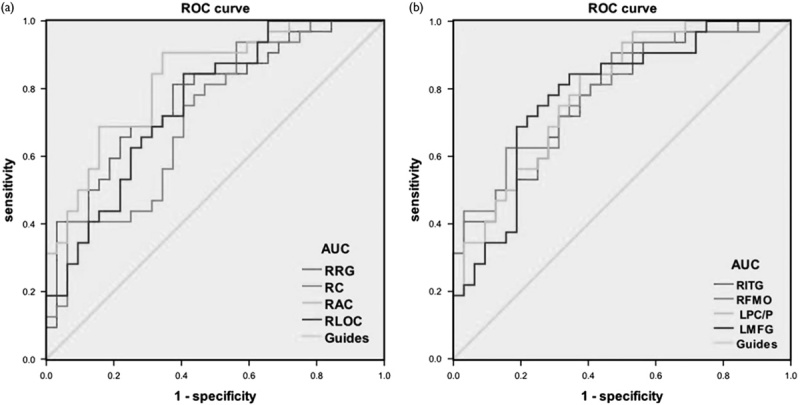
ROC curve analysis of the mean regional homogeneity values for altered brain regions. The AUCs were 0.771 (P<0.001; 95% CI: 0.657–0.886) for the RRG, 0.715 (P=0.003; 95% CI: 0.589–0.840) for the RC, 0.828 (P<0.001; 95% CI: 0.729–0.927) for the RAC, and 0.756 (P<0.001; 95% CI: 0.639–0.873) for the RLOC (MBs<HCs). (a) The AUCs were 0.778 (P<0.001; 95% CI: 0.667-0.889) for the RITG, 0.795 (P<0.001; 95% CI: 0.687–0.903) for the RFMO, 0.793 (P<0.001; 95% CI: 0.685–0.901) for the LPC/P, and 0.782 (P<0.001; 95% CI: 0.668–0.897) for the LMFG (MBs>HCs) (b). AUC, area under the curve; CI, confidence interval; HC, healthy control; LMFG, left middle frontal gyrus; LPC/P, left posterior cingulate/precuneus; MB, monocular blindness; RAC, right anterior cingulate; RC, right cuneus; RFMO, right frontal middle orbital; RITG, right inferior temporal gyrus; RLOC, right lateral occipital cortex; ROC, receiver operating characteristic; RRG, right rectal gyrus.
Discussion
In our study, compared with HCs, patients with MB showed significantly decreased ReHo values in the right rectal gyrus, right cuneus, right anterior cingulated, and right lateral occipital cortex, and increased ReHo values in the right ITG, right frontal middle orbital, left posterior cingulate/precuneus, and left middle frontal gyrus.
Analysis of the decreased regional homogeneity values in monocular blindness
The cuneus is located in the occipital lobe, playing an important role in visual processing 24. In addition, the cuneus has been shown to be involved in the visual imagery tasks 25. The dysfunction of the cuneus has been suggested in many diseases such as trigeminal neuralgia 26 and panic disorder 27. A previous study demonstrated that the blindness showed decreased regional gray matter in the cuneus 28. Consistent with that, in our study, we also found significantly decreased ReHo values in the right cuneus of the MB group. We speculated that the MB might be related to disrupted synchronous neural activity in the right cuneus.
The anterior cingulated cortex (ACC) located in the medial surface of the frontal lobes is a part of the limbic system. The ACC is involved in cognition 29 and emotion 30. Moreover, the ACC is responsible for error detection and behavior monitoring 31. Attention-deficit/hyperactivity disorder patients exhibit ACC functional deficits 32. Meanwhile, a previous study suggests that the right dorsal ACC plays an important role in the visual function 33. Abnormality of the ACC has been associated with many diseases such as depression 34, schizophrenia 35, and autism 36. In our study, we demonstrated that the MB group showed significantly decreased ReHo values in the right ACC, indicating that the synchronous neural activity was also disrupted in the right ACC in MB patients.
The occipital lobe is the anatomical region of the visual cortex, which is critical for visual processing. The occipital lobe contains the primary visual cortex (V1), visual area V2, visual area V3, visual area V4, and visual area V5 37. The V1 receives information from its ipsilateral lateral geniculate. The primate visual system can be divided into a ventral stream and a dorsal stream. Liu et al. 38 showed decreased functional connectivity within the occipital lobe. Another research reported that early blindness patients had significantly decreased gray matter volume in the early visual cortex 39. Meanwhile, blindness also correlates with reduced fractional anisotropy in the primary visual cortex using the diffusion tensor imaging method 40. In support of these findings, we also found that the MB group showed significantly decreased ReHo values in the right lateral occipital cortex. We speculated that MBs might lead to the dysfunction of the synchronous neural activity in the right lateral occipital cortex.
Analysis of the increased regional homogeneity values in monocular blindness
The ITG is located in contact with the inferior occipital gyrus below the middle temporal gyrus. The ITG is involved in the visual memory 41. In addition, the ITG plays an important role in the classification of visual shape 42. Abnormalities of the ITG are related to many diseases such as schizophrenia 43 and Alzheimer’s disease 44. In our study, we found that MB showed significantly increased ReHo values in the right ITG, which indicates the excessive activation of the right ITG in MB. Meanwhile, the area under the ROC curve of the RITG was 0.778. We speculated that high activities of the right ITG might reflect the compensation of the monocular vision loss in MB patients.
The frontal orbital is a part of the frontal cortex, which is below the brain areas BA 47. The BA 47 is involved in language and grammatical processing 45,46. Moreover, the BA 47 is also suggested to control the perception of musical structure 47. In our study, we found that MB showed significantly increased ReHo values in the right frontal middle orbital, which may reflect the enhancement of language understanding in MBs. The middle frontal gyrus is one-third of the frontal lobe, and is involved in attention 48,49 and inhibitory errors 50. The impairment of the middle frontal gyrus is related to many diseases such as attention-deficit hyperactivity disorder 51 and schizophrenia 52. In our study, we found that MB showed significantly increased ReHo values in the left middle frontal gyrus. We surmised that MB might lead to the dysfunction of the middle frontal gyrus.
Conclusion
In summary, our results showed abnormal spontaneous activities in many brain regions, which might reflect the altered synchronous neural activity in the visual cortex and other vision-related brain regions in MB. There are some limitations to our study. First, the noise during the MRI scanning might have some influence on the brain activity in all participants. Second, we did not differentiate different clinical outcomes of the MBs, such as right eye blindness and left eye blindness. Third, MBs can be caused by a variety of factors, such as ocular trauma and keratitis, which might affect the accuracy of the results. Future study should distinguish different types of MBs to more accurately assess brain activities and functional changes.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81160118, 81660158, and 81400372).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Xin Huang and Cheng-Long Ye contributed equally to the writing of this article.
References
- 1.Stevens GA, White RA, Flaxman SR, Price H, Jonas JB, Keeffe J, et al. Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990–2010. Ophthalmology 2013; 120:2377–2384. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Huang W, He M, Zheng Y, Huang S, Liu B, et al. Causes and five-year incidence of blindness and visual impairment in urban Southern China: the Liwan Eye Study. Invest Ophthalmol Vis Sci 2013; 54:4117–4121. [DOI] [PubMed] [Google Scholar]
- 3.Ojabo CO, Malu KN, Adeniyi OS. Open globe injuries in Nigerian children: epidemiological characteristics, etiological factors, and visual outcome. Middle East Afr J Ophthalmol 2015; 22:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci 2015; 56:6762–6769. [DOI] [PubMed] [Google Scholar]
- 5.Pleet A, Sulewski M, Salowe RJ, Fertig R, Salinas J, Rhodes A, et al. Risk Factors associated with progression to blindness from primary open-angle glaucoma in an African-American population. Ophthalmic Epidemiol 2016; 23:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Xiao W. Congenital cataract: progress in surgical treatment and postoperative recovery of visual function. Eye Sci 2015; 30:38–47. [PubMed] [Google Scholar]
- 7.Pula JH, Macdonald CJ. Current options for the treatment of optic neuritis. Clin Ophthalmol 2012; 6:1211–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulton M, Haines L, Smyth D, Fielder A. Health-related quality of life of children with vision impairment or blindness. Dev Med Child Neurol 2006; 48:656–661. [DOI] [PubMed] [Google Scholar]
- 9.Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol 2007; 125:544–550. [DOI] [PubMed] [Google Scholar]
- 10.Coullon GS, Jiang F, Fine I, Watkins KE, Bridge H. Subcortical functional reorganization due to early blindness. J Neurophysiol 2015; 113:2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang F, Stecker GC, Boynton GM, Fine I. Early blindness results in developmental plasticity for auditory motion processing within auditory and occipital cortex. Front Hum Neurosci 2016; 10:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss P, Zatorre RJ. Early visual deprivation changes cortical anatomical covariance in dorsal-stream structures. Neuroimage 2015; 108:194–202. [DOI] [PubMed] [Google Scholar]
- 13.Burton H, Snyder AZ, Raichle ME. Resting state functional connectivity in early blind humans. Front Syst Neurosci 2014; 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang A, Tian J, Li R, Liu Y, Jiang T, Qin W, et al. Alterations of regional spontaneous brain activity and gray matter volume in the blind. Neural Plast 2015; 2015:141950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin W, Xuan Y, Liu Y, Jiang Y, Yu C. Functional connectivity density in congenitally and late blind subjects. Cereb Cortex 2015; 25:2507–2516. [DOI] [PubMed] [Google Scholar]
- 16.Bola M, Gall C, Moewes C, Fedorov A, Hinrichs H, Sabel BA. Brain functional connectivity network breakdown and restoration in blindness. Neurology 2014; 83:542–551. [DOI] [PubMed] [Google Scholar]
- 17.Bola M, Gall C, Sabel BA. Disturbed temporal dynamics of brain synchronization in vision loss. Cortex 2015; 67:134–146. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Huang X, Ye L, Wei R, Zhang Y, Zhong YL, et al. Altered spontaneous brain activity pattern in patients with late monocular blindness in middle-age using amplitude of low-frequency fluctuation: a resting-state functional MRI study. Clin Interv Aging 2016; 11:1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong YF, Tang ZH, Qiang JW, Wu LJ, Wang R, Wang J, et al. Changes in DTI parameters in the optic tracts of macaque monkeys with monocular blindness. Neurosci Lett 2017; 636:248–253. [DOI] [PubMed] [Google Scholar]
- 20.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004; 22:394–400. [DOI] [PubMed] [Google Scholar]
- 21.Tononi G, McIntosh AR, Russell DP, Edelman GM. Functional clustering: identifying strongly interactive brain regions in neuroimaging data. Neuroimage 1998; 7:133–149. [DOI] [PubMed] [Google Scholar]
- 22.Shao Y, Cai FQ, Zhong YL, Huang X, Zhang Y, Hu PH, et al. Altered intrinsic regional spontaneous brain activity in patients with optic neuritis: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat 2015; 11:3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Li SH, Zhou FQ, Zhang Y, Zhong YL, Cai FQ, et al. Altered intrinsic regional brain spontaneous activity in patients with comitant strabismus: a resting-state functional MRI study. Neuropsychiatr Dis Treat 2016; 12:1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanni S, Tanskanen T, Seppä M, Uutela K, Hari R. Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc Natl Acad Sci USA 2001; 98:2776–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaehle T, Jordan K, Wüstenberg T, Baudewiq J, Dechent P, Mast FW. The neural basis of the egocentric and allocentric spatial frame of reference. Brain Res 2007; 1137:92–103. [DOI] [PubMed] [Google Scholar]
- 26.Parise M, Kubo TT, Doring TM, Tukamoto G, Vincent M, Gasparetto EL. Cuneus and fusiform cortices thickness is reduced in trigeminal neuralgia. J Headache Pain 2014; 15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai CH, Wu YT. Decreased regional homogeneity in lingual gyrus, increased regional homogeneity in cuneus and correlations with panic symptom severity of first-episode, medication-naïve and late-onset panic disorder patients. Psychiatry Res 2013; 211:127–131. [DOI] [PubMed] [Google Scholar]
- 28.Wan CY, Wood AG, Chen J, Wilson SJ, Reutens DC. The influence of preterm birth on structural alterations of the vision-deprived brain. Cortex 2013; 49:1100–1109. [DOI] [PubMed] [Google Scholar]
- 29.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 1999; 10:49–57. [DOI] [PubMed] [Google Scholar]
- 30.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4:215–222. [DOI] [PubMed] [Google Scholar]
- 31.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 1998; 280:747–749. [DOI] [PubMed] [Google Scholar]
- 32.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry 1999; 45:1542–1552. [DOI] [PubMed] [Google Scholar]
- 33.Shinoura N, Yamada R, Tabei Y, Shiode T, Itoi C, Saito S, et al. The right dorsal anterior cingulate cortex may play a role in anxiety disorder and visual function. Neurol Res 2013; 35:65–70. [DOI] [PubMed] [Google Scholar]
- 34.Philippi CL, Motzkin JC, Pujara MS, Koenigs M. Subclinical depression severity is associated with distinct patterns of functional connectivity for subregions of anterior cingulate cortex. J Psychiatr Res 2015; 71:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salgado-Pineda P, Landin-Romero R, Fakra E, Delaveau P, Amann BL, Blin O. Structural abnormalities in schizophrenia: further evidence on the key role of the anterior cingulate cortex. Neuropsychobiology 2014; 69:52–58. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Shi L, Cui X, Wang S, Luo X. Functional connectivity of the caudal anterior cingulate cortex is decreased in autism. PLoS One 2016; 11:e0151879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malikovic A, Vucetic B, Milisavljevic M, Tosevski J, Sazdanovic P, Milojevic B, et al. Occipital sulci of the human brain: variability and morphometry. Anat Sci Int 2012; 87:61–70. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, et al. Whole brain functional connectivity in the early blind. Brain 2007; 130 (Pt 8):2085–2096. [DOI] [PubMed] [Google Scholar]
- 39.Pan WJ, Wu G, Li CX, Lin F, Sun J, Lei H. Progressive atrophy in the optic pathway and visual cortex of early blind Chinese adults: a voxel-based morphometry magnetic resonance imaging study. Neuroimage 2007; 37:212–220. [DOI] [PubMed] [Google Scholar]
- 40.Reislev NL, Dyrby TB, Siebner HR, Kupers R, Ptito M. Simultaneous assessment of white matter changes in microstructure and connectedness in the blind brain. Neural Plast 2016; 2016:6029241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eskandar EN, Richmond BJ, Optican LM. Role of inferior temporal neurons in visual memory. I. Temporal encoding of information about visualimages, recalled images, and behavioral context. J Neurophysiol 1992; 68:1277–1295. [DOI] [PubMed] [Google Scholar]
- 42.McKee JL, Riesenhuber M, Miller EK, Freedman DJ. Task dependence of visual and category representations in prefrontal and inferior temporal cortices. J Neurosci 2014; 34:16065–16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry 2004; 161:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2011; 24:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright P, Randall B, Marslen-Wilson WD, Tyler LK. Dissociating linguistic and task-related activity in the left inferior frontal gyrus. J Cogn Neurosci 2011; 23:404–413. [DOI] [PubMed] [Google Scholar]
- 46.Sahin NT, Pinker S, Halgren E. Abstract grammatical processing of nouns and verbs in Broca's area: evidence from fMRI. Cortex 2006; 42:540–562. [DOI] [PubMed] [Google Scholar]
- 47.Levitin DJ, Menon V. Musical structure is processed in ‘language’ areas of the brain: a possible role for Brodmann area 47 intemporal coherence. Neuroimage 2003; 20:2142–2152. [DOI] [PubMed] [Google Scholar]
- 48.Andersson M, Ystad M, Lundervold A, Lundervold AJ. Correlations between measures of executive attention and cortical thickness of left posterior middlefrontal gyrus – a dichotic listening study. Behav Brain Funct 2009; 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Japee S, Holiday K, Satyshur MD, Mukai I, Unqerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci 2015; 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, et al. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend 2014; 141:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tafazoli S, O'Neill J, Bejjani A, Ly R, Salamon N, McCracken JK, et al. 1H MRSI of middle frontal gyrus in pediatric ADHD. J Psychiatr Res 2013; 47:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quan M, Lee SH, Kubicki M, Kikinis Z, Rathi Y, Seidman LJ, et al. White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr Res 2013; 145:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


