Abstract
Background:
The reported neurologic complication rate following surgery for complex adult spinal deformity (ASD) is variable due to several factors. Most series have been retrospective with heterogeneous patient populations and use of nonuniform neurologic assessments. The aim of this study was to prospectively document lower extremity motor function by means of the American Spinal Injury Association (ASIA) lower extremity motor score (LEMS) before and through 2 years after surgical correction of complex ASD.
Methods:
The Scoli-RISK-1 study enrolled 272 patients with ASD, from 15 centers, who had undergone primary or revision surgery for a major Cobb angle of ≥80°, corrective osteotomy for congenital spinal deformity or as a revision procedure for any type of deformity, and/or a complex 3-column osteotomy.
Results:
One of 272 patients lacked preoperative data and was excluded from the analysis, and 62 (22.9%) of the remaining 271 patients, who were included, lacked a 2-year postoperative assessment. Patients with no preoperative motor impairment (normal LEMS group; n = 203) had a small but significant decline from the mean preoperative LEMS value (50) to that at 2 years postoperatively (49.66 [95% confidence interval = 49.46 to 49.85]; p = 0.002). Patients who did have a motor deficit preoperatively (n = 68; mean LEMS, 43.79) had significant LEMS improvement at 6 months (47.21, p < 0.001) and 2 years (46.12, p = 0.003) postoperatively. The overall percentage of patients (in both groups combined) who had a postoperative LEMS decline, compared with the preoperative value, was 23.0% at discharge, 17.1% at 6 weeks, 9.9% at 6 months, and 10.0% at 2 years.
Conclusions:
The percentage of patients who had a LEMS decline (compared with the preoperative score) after undergoing complex spinal reconstructive surgery for ASD was 23.0% at discharge, which improved to 10.0% at 2 years postoperatively. These rates are higher than previously reported, which we concluded was due to the prospective, strict nature of the LEMS testing of patients with these challenging deformities.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Adult spinal deformity (ASD) is a condition often requiring complex surgical interventions to improve the patient’s quality of life. Procedures for surgical correction continue to evolve with pedicle screw fixation and complex osteotomies. Neurologic impairment of the lower extremities may be present before surgery or as a postoperative complication. Neurologic deficits after ASD surgery have been reported in numerous studies; however, the true prevalence is difficult to ascertain because of the extreme heterogeneity of the populations being evaluated and treatments rendered. The majority of these reports were on patients with mild or moderate deformities (<70°)1-28 or did not present information about curve severity29-39. Very few studies have included results regarding neurologic deficits after spinal reconstruction in patients with more severe spinal deformity (curves of >70° or sagittal imbalance of >10 cm27,40-46). Additionally, most of the reports were retrospective, and often the methods for determining neurologic deficits were not well described. The aim of the present analysis was to prospectively document lower extremity motor function using the American Spinal Injury Association (ASIA) lower extremity motor score (LEMS), a validated outcome instrument47,48, before and after surgical correction of complex ASD and to report changes in motor function through 2 years postoperatively.
Materials and Methods
This prospective, observational, international, multicenter study evaluated neurologic complications associated with surgical correction of complex ASD, defined as surgery for a major Cobb angle of ≥80° in the coronal and/or sagittal plane, corrective osteotomy for congenital spinal deformity or as a revision procedure for any type of deformity, 3-column osteotomy (i.e., pedicle subtraction osteotomy and vertebral column resection between and including C7 and L5), reconstruction for deformity-induced myelopathy, or deformity reconstruction with concomitant spinal cord decompression due to deformity and ossification of the ligamentum flavum or posterior longitudinal ligament.
The study was conducted in 15 spinal deformity centers in North America (9), Europe (3), and Asia (3). The ethics committees or institutional review boards granted approval at all sites, and patients signed informed consent forms prior to enrollment. The study is registered with ClinicalTrials.gov (NCT01305343).
The operating surgeon decided on the surgical approach, instrumentation, corrective maneuvers, and use of bone grafts or substitutes. Patients were between 18 and 80 years old and had ASD with the major deformity apex in the cervicothoracic or thoracolumbar region. Detailed inclusion and exclusion criteria are listed in Table I.
TABLE I.
Study Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
| Signed informed consent | Unlikely to comply with follow-up |
| Age 18 to 80 yr, inclusive | Recent history (≤3 mo) of substance dependency or psychosocial disturbance |
Diagnosis of ASD with apex of major deformity in cervicothoracic or thoracolumbar region (apex between C7 and L2, inclusive) with any of following deformity characteristics:
|
Active malignancy |
| Active, overt bacterial infection (systemic or local) | |
| Recent history (≤3 mo) of substantial spinal trauma/injury/fracture/malignancy in spinal region | |
| Complete, long-term paraplegia | |
| Pregnant or nursing or unwilling to agree not to become pregnant for 6 mo postop. | |
| 3-column spinal osteotomy (pedicle subtraction osteotomy and vertebral column resection) from C7 to L5, inclusive | Incarcerated |
| Preop. myelopathy due to spinal deformity | Institutionalized |
| Ossification of ligamentum flavum or posterior longitudinal ligament and deformity needing concomitant reconstruction along with decompression of spinal cord |
An ASIA neurologic examination48 was performed by an ASIA-certified examiner (each site had multiple certified examiners performing this function) within 6 weeks preoperatively; at hospital discharge; and at 6 weeks, 6 months, and 2 years postoperatively. Standing coronal and sagittal radiographs were made and patient-reported outcomes as well as adverse events were recorded at each visit.
The primary outcome measure was the change in the ASIA LEMS48 at each time point. The LEMS evaluates motor function on a scale of 0 (no motor function) to 5 (full motor function) for 5 lower extremity muscle groups with a 50-point maximum (25 per side).
For analysis, the patients were divided into 2 groups: those with a normal preoperative LEMS (50) and those with an abnormal preoperative LEMS (<50). Differences in demographic characteristics and surgical approach between the normal and abnormal groups were analyzed using the Fisher exact test for categorical variables and the t test for continuous variables. Changes in the LEMS were analyzed using a mixed model for repeated measures with an unstructured covariance. This model has advantages over simple imputation using the last, or baseline, observation carried forward method and has been recommended for handling missing data in longitudinal clinical trials49. Missing values are dealt with by taking into account data derived from the same patient at other time points to calculate estimates. P values and 95% confidence intervals (CIs) for changes from baseline within each group were derived from the mixed model and adjusted for multiple testing using the simulation-based adjustment. Changes in the LEMS from baseline were classified as “maintenance,” “improvement,” or “decline” and compared between the 2 groups with the Fisher exact test or chi square test. The statistical analysis was performed using SAS software, version 9.2 (SAS Institute). The study utilized the web-based online data-capture system eCRF (OpenClinica). Additionally, a clinical end point committee evaluated all neurologic and non-neurologic complications, which were assessed and adjudicated to ensure their accuracy.
Results
From September 2011 to October 2012, 43 surgeons enrolled 272 consecutive patients. One patient lacked a preoperative LEMS; therefore, only 271 patients could be included in the group analyses. Preoperatively, 203 patients (74.9%) had no lower extremity motor impairment (LEMS of 50; normal group) and 68 (25.1%) had a LEMS of <50 (abnormal group). Thirteen patients (4.8%) had no or incomplete LEMS data at 6 weeks; 19 (7.0%), at 6 months; and 62 (22.9%), at 2 years.
The study population included 182 women and 89 men. The mean age (and standard deviation) was 56.9 ± 15.3 years. The procedures included primary or revision surgery for ASD with a major Cobb angle of ≥80° in the coronal or sagittal plane in 29.0% of the patients, revision surgery including osteotomy in 60.7%, and/or a 3-column osteotomy in 75.7%, emphasizing the complex nature of these patients’ conditions and their surgical treatments (Table II).
TABLE II.
Surgical Procedures
| Procedure (Combinations Possible)* | No. (%) (N = 272) |
| Primary or revision surgery for scoliosis, kyphosis, or kyphoscoliosis with major Cobb angle of ≥80° in coronal or sagittal plane | 79 (29.0) |
| Osteotomy (any PSO, VCR, and/or SPO) | 259 (95.2) |
| Revision surgery including osteotomy (any PSO, VCR, and/or SPO) | 165 (60.7) |
| 3-column osteotomy | 206 (75.7) |
| Spinal reconstruction due to deformity with preop. myelopathy | 12 (4.4) |
| Spinal reconstruction and decompression of spinal cord due to deformity and ossification of ligamentum flavum or posterior longitudinal ligament | 5 (1.8) |
PSO = pedicle subtraction osteotomy, VCR = vertebral column resection, and SPO = Smith-Petersen osteotomy.
The normal group included a higher percentage of women than the abnormal group (70.4% and 57.4%, p = 0.053) and a lower percentage with previous spine surgery (57.6% and 76.5%, p = 0.006). No significant differences between groups were seen with respect to age, race, smoking status, surgical approach, or prevalence of multistage surgery (Table III).
TABLE III.
Demographics and Surgical Approach by Group
| No. (%)* |
|||
| Characteristic | Normal Group | Abnormal Group | P Value |
| Sex | N = 203 | N = 68 | 0.053 |
| Male | 60 (29.6) | 29 (42.6) | |
| Female | 143 (70.4) | 39 (57.4) | |
| Age (yr) | N = 203 | N = 68 | 0.236 |
| Mean ± standard deviation | 56.3 ± 15.9 | 58.8 ± 13.2 | |
| Median (Q1, Q3) | 60.0 (49, 68) | 60.5 (53, 69) | |
| Range | 18-80 | 19-81† | |
| Race | N = 202 | N = 68 | 0.051 |
| White | 163 (80.7) | 51 (75.0) | |
| Black | 2 (1.0) | 0 (0) | |
| Native American | 1 (0.5) | 0 (0) | |
| East Asian | 36 (17.8) | 14 (20.6) | |
| Other | 0 (0) | 3 (4.4) | |
| Smoker | N = 202 | N = 67 | 0.478 |
| No | 184 (91.1) | 59 (88.1) | |
| Yes | 18 (8.9) | 8 (11.9) | |
| Previous spine surgery | N = 203 | N = 68 | 0.006 |
| No | 86 (42.4) | 16 (23.5) | |
| Yes | 117 (57.6) | 52 (76.5) | |
| Surgical approach | N = 203 | N = 68 | 0.869 |
| Posterior only | 154 (75.9) | 53 (77.9) | |
| Anterior + posterior | 49 (24.1) | 15 (22.1) | |
| No. of surgical stages | N = 203 | N = 68 | 1.000 |
| 1 | 165 (81.3) | 55 (80.9) | |
| ≥2 | 38 (18.7) | 13 (19.1) | |
Unless otherwise indicated.
One patient was enrolled at age 80 and turned 81 on the day of surgery.
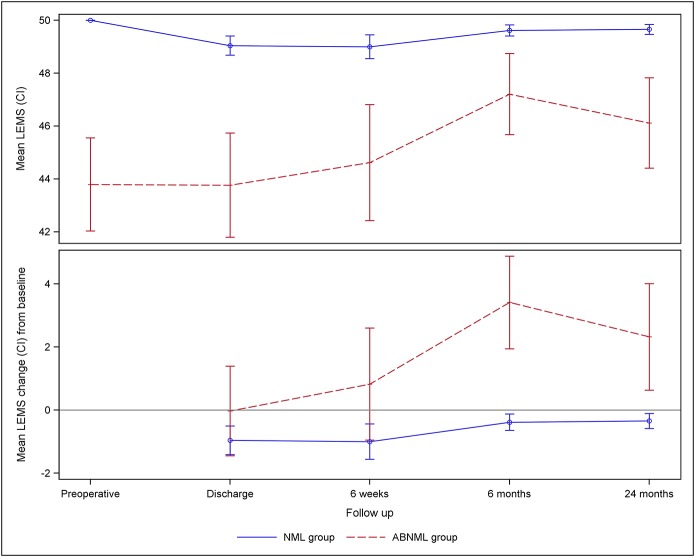
The proportion of patients with a postoperative LEMS decline was similar in the 2 groups at all time points; this decline was most pronounced at discharge, with gradual improvement over time (Table IV). Compared with baseline, 23.0% of all patients experienced a LEMS decline at discharge, with this rate decreasing to 17.1% at 6 weeks and to 9.9% at 6 months and remaining stable at 10.0% at 2 years. Compared with baseline, the LEMS declined for 22.1% of the patients in the normal group and 25.8% in the abnormal group at discharge; 11.1% and 6.5%, respectively, at 6 months; and 9.2% and 13.0%, respectively, at 2 years. There was a small significant decline in the mean LEMS at all follow-up assessments as compared with baseline (p = 0.001 up to six months and p = 0.002 at 2 years) in the normal group, whereas the abnormal group had a significant improvement at 6 months (p < 0.001) and 2 years (p = 0.003) (Fig. 1 and Table V).
Fig. 1.
A line graph showing the mean LEMS and the mean change in LEMS from baseline over time in the normal (NML) and abnormal (ABNML) groups.
TABLE IV.
Change in LEMS Compared with Preoperative Score by Group
| No. (%) |
||||
| Change in LEMS Compared with Baseline | Total | Normal Group | Abnormal Group | P Value |
| Discharge | N = 265 | N = 199 | N = 66 | 0.542* |
| Maintenance/improvement | 204 (77.0) | 155 (77.9) | 49 (74.2) | |
| Decline | 61 (23.0) | 44 (22.1) | 17 (25.8) | |
| 6 wk | N = 258 | N = 194 | N = 64 | 0.677* |
| Maintenance/improvement | 214 (82.9) | 162 (83.5) | 52 (81.3) | |
| Decline | 44 (17.1) | 32 (16.5) | 12 (18.8) | |
| 6 mo | N = 252 | N = 190 | N = 62 | 0.293* |
| Maintenance/improvement | 227 (90.1) | 169 (89.0) | 58 (93.5) | |
| Decline | 25 (9.9) | 21 (11.1) | 4 (6.5) | |
| 2 yr | N = 209 | N = 163 | N = 46 | 0.417† |
| Maintenance/improvement | 188 (90.0) | 148 (90.8) | 40 (87.0) | |
| Decline | 21 (10.0) | 15 (9.2) | 6 (13.0) | |
Chi square test.
Fisher exact test.
TABLE V.
LEMS at Follow-up Time Points by Group*
| Normal Group |
Abnormal Group |
|||||
| Mean LEM score (95%CI) | Mean Change in LEMS compared to preoperative (95%CI) | P Value† | Mean LEM score (95%CI) | Mean Change in LEMS compared to preoperative (95%CI) | P Value† | |
| Preop. | 50.00 (50.00, 50.00) | 43.79 (42.03, 45.56) | ||||
| Discharge | 49.04 (48.68, 49.40) | −0.96 (−1.41, −0.51) | <0.001 | 43.77 (41.80, 45.74) | −0.03 (−1.45, 1.39) | 1.000 |
| 6 wk | 49.00 (48.55, 49.45) | −1.00 (−1.56, −0.44) | <0.001 | 44.62 (42.42, 46.81) | 0.83 (−0.95, 2.60) | 0.628 |
| 6 mo | 49.62 (49.41, 49.82) | −0.38 (−0.65, −0.12) | 0.001 | 47.21 (45.67, 48.74) | 3.41 (1.95, 4.88) | <0.001 |
| 2 yr | 49.66 (49.46, 49.85) | −0.34 (−0.58, −0.11) | 0.002 | 46.12 (44.41, 47.83) | 2.32 (0.63, 4.01) | 0.003 |
Means, 95% CIs, and p values derived from a mixed model for repeated measures.
The p values are for the difference compared with the preoperative LEMS.
Analysis of the LEMS over time in both groups revealed a significant time effect (p < 0.001). The pattern of changes significantly differed between the 2 groups (group*time effect, p < 0.001). Most declines in the LEMS, compared with preoperatively, were ≤5 points, with 72.1%, 65.9%, 76.0%, and 76.2% of the declines being ≤5 points at discharge, 6 weeks, 6 months, and 2 years, respectively (Table VI).
TABLE VI.
Change in LEMS Compared with Preoperative Score in Ordinal Categories
| No. (%) |
|||
| Change in LEMS Compared with Preop. | Total | Normal Group | Abnormal Group |
| Discharge | N = 265 | N = 199 | N = 66 |
| Decline | 23.0% | ||
| Decline >10 | 4 (1.5) | 2 (1.0) | 2 (3.0) |
| Decline 6-10 | 13 (4.9) | 9 (4.5) | 4 (6.1) |
| Decline 1-5 | 44 (16.6) | 33 (16.6) | 11 (16.7) |
| Maintenance | 170 (64.2) | 155 (77.9) | 15 (22.7) |
| Improvement 1-5 | 31 (11.7) | 0 (0) | 31 (47.0) |
| Improvement 6-10 | 2 (0.8) | 0 (0) | 2 (3.0) |
| Improvement >10 | 1 (0.4) | 0 (0) | 1 (1.5) |
| 6 wk | N = 258 | N = 194 | N = 64 |
| Decline | 17.1% | ||
| Decline >10 | 6 (2.3) | 3 (1.5) | 3 (4.7) |
| Decline 6-10 | 9 (3.5) | 6 (3.1) | 3 (4.7) |
| Decline 1-5 | 29 (11.2) | 23 (11.9) | 6 (9.4) |
| Maintenance | 172 (66.7) | 162 (83.5) | 10 (15.6) |
| Improvement 1-5 | 31 (12.0) | 0 (0) | 31 (48.3) |
| Improvement 6-10 | 10 (3.9) | 0 (0) | 10 (15.6) |
| Improvement >10 | 1 (0.4) | 0 (0) | 1 (1.6) |
| 6 mo | N = 252 | N = 190 | N = 62 |
| Decline | 9.9% | ||
| Decline >10 | 2 (0.8) | 1 (0.5) | 1 (1.6) |
| Decline 6-10 | 4 (1.6) | 4 (2.1) | 0 (0) |
| Decline 1-5 | 19 (7.5) | 16 (8.4) | 3 (4.8) |
| Maintenance | 175 (69.4) | 169 (88.9) | 6 (9.7) |
| Improvement 1-5 | 37 (14.7) | 0 (0) | 37 (59.7) |
| Improvement 6-10 | 12 (4.8) | 0 (0) | 12 (19.4) |
| Improvement >10 | 3 (1.2) | 0 (0) | 3 (4.8) |
| 2 yr | N = 209 | N = 163 | N = 46 |
| Decline | 10.0% | ||
| Decline >10 | 0 (0) | 0 (0) | 0 (0) |
| Decline 6-10 | 5 (2.4) | 3 (1.8) | 2 (4.4) |
| Decline 1-5 | 16 (7.7) | 12 (7.4) | 4 (8.7) |
| Maintenance | 153 (73.2) | 148 (90.8) | 5 (10.9) |
| Improvement 1-5 | 26 (12.4) | 0 (0) | 26 (56.5) |
| Improvement 6-10 | 9 (4.3) | 0 (0) | 9 (19.6) |
| Improvement >10 | 0 (0) | 0 (0) | 0 (0) |
One patient with congenital kyphoscoliosis who had a 3-column osteotomy demonstrated loss of signals on spinal cord monitoring and acute paraplegia immediate postoperatively. The patient showed slow but steady recovery of the LEMS, which improved to 30 by discharge, 40 at 6 weeks, and 50 (normal) at 6 months postoperatively. A 60-year-old patient who had had a posterior spinal fusion for adult lumbar scoliosis required revision surgery at 7 weeks postoperatively due to a fall causing proximal junctional kyphosis and acute paraplegia. Following the revision surgery, the clinical paraplegia persisted and the patient died 23 months after the index surgery (Table VII). Importantly, no patient in this study experienced permanent postoperative paraplegia following their index surgical procedure.
TABLE VII.
Data on Patients with >10-Point Decline in LEMS
| LEMS |
Change in LEMS Compared with Preop. |
|||||||||
| Case | Group | Preop. | Discharge | 6 Wk | 6 Mo | 2 Yr | Discharge | 6 Wk | 6 Mo | 2 Yr |
| 1 | Abnormal | 43 | 28 | 50 | 50 | 50 | −15 | 7 | 7 | 7 |
| 2 | Normal | 50 | 28 | Not avail. | 39 | Not avail. | −22 | Not avail. | −11 | Not avail. |
| 3 | Normal | 50 | 30 | 40 | 50 | 50 | −20 | −10 | 0 | 0 |
| 4 | Abnormal | 49 | 29 | 32 | 33 | 40 | −20 | −17 | −16 | −9 |
Discussion
This prospective study analyzed lower extremity motor function after complex spinal reconstruction in patients with severe ASD. To our knowledge, this is the largest series of patients with such severe deformities whose neurologic function was documented prospectively with a validated outcome instrument.
Loss of neurologic function is one of the most important complications following complex ASD surgery. The decreased mobility directly impacts the patient’s quality of life and may lead to additional adverse events such as lower extremity deep vein thrombosis, pulmonary embolism, and pneumonia.
We found a decline in the LEMS at hospital discharge, compared with preoperatively, for 23.0% of the patients who underwent correction of ASD. As motor function recovered over time, this rate decreased to 17.1% at 6 weeks and then to 9.9% at 6 months, and then remained stable at 10.0% at 2 years. These rates of perioperative motor decline are by far the highest reported to date, for multiple reasons: our “high-risk” study population of patients with ASD who underwent complex reconstructions; the prospective study design with frequent and structured data-collection time points; and the sensitivity to change of the ASIA neurologic examination, in which a single muscle-group grade of 4/5 results in a decreased LEMS. Thus, relatively minor weaknesses could produce a “motor decline” rating (i.e., a change in the LEMS of 50 to 49).
Several reports that specifically address complications subsequent to spinal deformity reconstruction in large series of patients have been published, even though the severity of the deformity in those publications may not be comparable with that in our population (Table VIII)5,19,31,35. In what we believe is the largest review of new neurologic deficits—after 108,419 spinal procedures—the Scoliosis Research Society (SRS) Morbidity and Mortality Committee stratified their analyses by primary diagnosis and reported a prevalence of neurologic deficits of 1.8% after surgery for scoliosis and 3.4% after surgery for kyphosis35. The neurologic deficit rate was 41% higher after revisions than after primary procedures. Of note, the study depended on voluntary surgeon/center retrospective data entry, without prospective performance of neurologic assessment with a validated instrument. Therefore, selection and detection bias are distinct possibilities.
TABLE VIII.
Literature Review Summary of Neurologic Complication Rates Following Spinal Deformity Surgery
| Study | Year Published | No. of Patients | Study Type | Single Vs. Multicenter | Type of Deformity | Surgical Procedures | Neurologic Complication Rate |
| Charosky et al.5 | 2012 | 306 | Retrospective review of prospectively collected data | Multicenter | Adult degenerative scoliosis | Primary | 7% with 2 cases (0.6%) of late cord-level deficits |
| Buchowski et al.31 | 2007 | 108 | Retrospective radiographic review of prospectively collected clinical data | Single center | Scoliosis and kyphosis | Pedicle subtraction osteotomy | 11.1% (12), 2.8% (3) with permanent neurologic deficits |
| Kim et al.19 | 2012 | 233 | Retrospective | Single center | Scoliosis and kyphosis | Posterior vertebral column resection vs. decancellation osteotomy | Transient: 31.8% after posterior vertebral column resection vs. 7.5% after decancellation osteotomy; permanent: 3.3% vs. 1.2% |
| Bomback et al.40 | 2007 | 34 | Retrospective case-control | Single center | Thoracic curves >50° | Thoracoscopic surgery vs. thoracotomy | No neurologic complications in either group |
| Shapiro et al.43 | 2003 | 16 | Retrospective case series | Single center | Adult idiopathic thoracolumbar and/or lumbar scoliosis | Anterior and posterior spinal reconstructive surgery | No neurologic complications |
| Suk et al.44 | 2005 | 16 | Retrospective | Single center | Severe rigid scoliosis | Posterior vertebral column resection | 1 patient developed complete paralysis |
| Xie et al.46 | 2012 | 28 | Retrospective | Single center | Severe rigid scoliosis with curves >100° | Posterior vertebral column resection | 17.9% |
| Lenke et al.41 | 2010 | 43 | Retrospective | Single center | Severe scoliosis, global kyphosis, angular kyphosis, or kyphoscoliosis | Posterior vertebral column resection | 4.7% (2) with postop. nerve root deficits |
| Swank et al.45 | 1981 | 222 | Retrospective | Single center | Idiopathic scoliosis, congenital scoliosis, and paralytic scoliosis | Primary | 1 patient became paraparetic postop. |
| Qiu et al.42 | 2008 | 1,373 | Retrospective | Single center | Scoliosis | Anterior, posterior, and anteroposterior techniques | 3.7% |
In a retrospective study of 306 patients who underwent primary surgery for adult scoliosis with a mean deformity of 50°, Charosky et al. reported a 7% rate of new neurologic deficits, with 2 patients (0.6%) developing late cord-level deficits5. Buchowski et al. assessed early complications in 108 adults who underwent a pedicle subtraction osteotomy for an average +13-cm fixed sagittal imbalance and a mean lordosis of −17°31. Twelve patients (11.1%) developed new neurologic deficits, which were permanent in 3 (2.8%). Kim et al. performed a retrospective study of 233 patients with a mean local kyphosis of 51°, thoracic scoliosis of 64°, and thoracolumbar or lumbar scoliosis of 50°19. They found the prevalence of transient neurologic deficits to be 31.8% in those treated with a posterior vertebral column resection and 7.5% in those treated with a decancellation osteotomy whereas the prevalences of permanent neurologic deficits were 3.3% and 1.2%, respectively. Most patients who had postoperative paraplegia had had a preoperative neurologic deficit and >5 levels fused.
We do not know of any published prospective studies of a large patient population with deformities comparable with those in our series. A prospective study with predefined data-collection time points strongly increases data accuracy and result validity50. This is particularly important for analyses of neurologic deficits, which may be subtle and/or transient and thus remain undetected in retrospective studies. Understanding the risk of such defects is fundamental to patients’ ability to provide informed consent and to clinical decision-making.
There have been only a few retrospective reports in the literature on populations with deformity severity and interventions comparable with ours, with only 2 comprising >100 patients42,45 and several others consisting of small series40,41,43,44,46. Bomback et al. performed a case-control study, which included children and adults, comparing 17 patients treated with video-assisted thoracoscopic surgery with 17 treated with thoracotomy40. The mean major coronal curves were 81° and 101° in the respective treatment groups. No neurologic complications occurred in either group. Shapiro et al. reviewed records of 16 children and adults with a mean major curve of 71° and reported that no major neurologic deficits occurred43. Suk et al. studied 16 consecutive patients who underwent posterior vertebral column resection for severe rigid scoliosis with a mean major curve of 109°44. One patient with Beals syndrome and a preoperative Frankel grade of C developed complete paralysis (Frankel grade A) postoperatively. The autopsy showed no definite pathological finding or abnormality accounting for the neurologic deterioration. Xie et al. reported a neurologic complication rate of 17.9% in 28 adults who had undergone posterior vertebral column resection for correction of severe, rigid spinal curves of >100°46. These complications included 1 case of late-onset paralysis in a patient without any preoperative or immediate postoperative neurologic deficit who developed complete spinal cord dysfunction 8 hours later. The patient received medical therapy with methylprednisolone, and the neurologic function gradually improved to normal after 2 weeks. Lenke et al. reviewed the cases of 43 children and adults with severe spinal deformities who underwent posterior vertebral column resection41. Twelve patients had preoperative Cobb angles of >75°. Although 7 patients (18%) lost monitoring signals intraoperatively, the signals returned to baseline following surgical intervention. No patients developed any spinal cord-related neurologic dysfunction. Two patients (4.7%) had postoperative nerve root deficits, which resolved at 2 weeks and 6 months.
Two larger retrospective studies of populations comparable with ours have been published42,45. Swank et al. reviewed the charts of 222 patients with an average initial curve of 80° who had undergone scoliosis correction45. One patient in this series became paraparetic postoperatively. A myelogram detected a block at L1. The patient underwent decompression/laminectomy and neurologic function improved, but urinary retention, anal pain, and loss of sensation over the penis and perineum remained. In a large single-center study, Qiu et al. reviewed data on 1,373 patients (the majority [1,074] of whom were <18 years old) and analyzed neurologic deficits stratified by curve severity and procedure type42. The prevalence of neurologic deficits was 3.7% for major curves with a preoperative Cobb angle of ≥90° and only 1.5% for smaller curves (p = 0.015). The neurologic deficit prevalence was 0.9% after anterior procedures versus 1.2% after posterior procedures, and 3.4% after combined anterior and posterior procedures. The influence of procedure type was significant (p = 0.028), while the influence of age was insignificant (neurologic deficit rate of 1.9% in patients <18 years old versus 2% in those ≥18 years old; p = 0.87).
Two important aspects of all of these publications are the variability in the prevalences of the neurologic deficits and the fact that these prevalences always appeared lower than those in our study. We believe that the reasons for both are multifactorial. The high variability can be explained by the heterogeneity of individual study populations and the multitude of treatment modalities employed. Retrospective evaluations without preset data-collection time points or standardized/validated outcome instruments increased the variability. Additionally, selection and detection bias may have played larger roles in these cohort studies than in our multicenter cohort.
Several additional factors likely contributed to the lower prevalences of neurologic decline reported in other studies. First, rigorous data collection as well as the use of the ASIA LEMS instrument may have allowed us to detect more subtle changes than retrospective studies can. Second, our study population was more homogeneous, with all patients having severe deformity (a major curve of ≥80°) and/or being treated with a complex operation (e.g., 3-column osteotomy). Although different procedures were performed in our series, all were complex reconstructions in a population at a high risk for neurologic deterioration. The high risk involved in complex osteotomy surgery for correction of a large deformity or procedures such as 3-column osteotomy (pedicle subtraction osteotomy and vertebral column resection) with direct manipulation of neural elements and acute changes in spinal canal alignment and/or shape has been emphasized by other authors44.
Some authors performed analyses stratified by deformity severity and procedure complexity35,42 and found that both factors increased the likelihood of a patient developing a neurologic deficit. Some authors reported cases of permanent paraplegia after the index procedure19,44, but that was not seen in any of our patients. Although 1 patient awoke with complete paraplegia, neurologic function improved by hospital discharge and was normal at 6 months. Thus, considering that we evaluated only the most complex ASD reconstructions and used standardized/validated outcome instruments with predefined data-collection time points, we believe that our results present an accurate risk of neurologic complications.
Our study also has limitations. We used heterogeneous diagnostic and treatment-related inclusion criteria. Also, some subjective bias is possible since the ASIA examination was performed by different examiners at multiple sites. We attempted to minimize this potential bias by having all examiners become ASIA-certified. Additionally, the availability of 2-year data was lower than anticipated (some patients were seen at 2 years but a LEMS was not obtained). We tried to mitigate the effect of missing data by using a mixed-effects model for repeated measures for the statistical analysis. Finally, we only studied patients who had undergone the most complex and neurologically risky procedures for their deformity; therefore, our results are valid only for this specific population and are difficult to compare with other studies. The surgeons and centers selected saw and treated a high volume of patients with severe deformity, so the results of this study may not be typical of those at centers and surgeon practices that do not routinely include this patient population.
The strengths of our study include its prospective/multicenter nature and use of the ASIA LEMS instrument preoperatively and at 4 postoperative time points. This neurologic assessment using a validated measurement system provided sensitivity with regard to the ability to detect subtle changes postoperatively. Although several studies of smaller series of patients with a deformity severity similar to that in our population have been published40,41,43,44,46, our large sample size allowed sufficient power to detect relatively rare events and our data-collection procedure ensured that any new postoperative lower extremity deficit was captured as a surgical complication, even if it was minor and transient.
In conclusion, this Scoli-RISK-1 study revealed that a decline in lower extremity motor function after complex ASD surgery is common and more frequent than previously reported. We identified such a decline in 23.0% of patients at discharge, with neurologic function recovering over time to a decline (compared with preoperatively) in 17.1% of the patients at 6 weeks, 9.9% at 6 months, and 10.0% at 2 years after the operation. Thus, many patients with a postoperative decline in LEMS showed motor function improvement (compared with the initial decline) by 6 months postoperatively. The neurologic complication rate in our study, determined with a strict protocol of neurologic evaluations, should be considered the accurate and expected neurologic outcome following complex ASD surgery at this time.
Acknowledgments
Note: The authors are grateful to the AO Clinical Investigation and Documentation for their support, especially Kathrin Espinoza for statistical assistance, Elke Rometsch for manuscript preparation, and Kathy Blanke, RN, for text editing.
Footnotes
The AOSpine International and SRS Scoli-RISK-1 Study Group includes Christopher P. Ames, MD; Oheneba Boachie-Adjei, MD; Mark B. Dekutoski, MD; Khaled M. Kebaish, MD; Stephen J. Lewis, MD, MSc, FRCSC; Yukihiro Matsuyama, MD, PhD; Hossein Mehdian, MD; Ferran Pellisé, MD, PhD; Yong Qiu, MD; and Frank J. Schwab, MD.
A commentary by Jeffrey L. Stambough, MD, MBA, is linked to the online version of this article at jbjs.org.
Disclosure: This study was supported organizationally and financially by the Scoliosis Research Society (SRS), Norton Healthcare, and AOSpine International. AOSpine is a clinical division of the AO Foundation—an independent medically guided nonprofit organization. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work and “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work (http://links.lww.com/JBJS/E641).
Contributor Information
Collaborators: Christopher P. Ames, Oheneba Boachie-Adjei, Mark B. Dekutoski, Khaled M. Kebaish, Stephen J. Lewis, Yukihiro Matsuyama, Hossein Mehdian, Ferran Pellisé, Yong Qiu, and Frank J. Schwab
References
- 1.Ahn UM, Ahn NU, Buchowski JM, Kebaish KM, Lee JH, Song ES, Lemma MA, Sieber AN, Kostuik JP. Functional outcome and radiographic correction after spinal osteotomy. Spine (Phila Pa 1976). 2002. June 15;27(12):1303-11. [DOI] [PubMed] [Google Scholar]
- 2.Berven SH, Deviren V, Smith JA, Hu SH, Bradford DS. Management of fixed sagittal plane deformity: outcome of combined anterior and posterior surgery. Spine (Phila Pa 1976). 2003. August 1;28(15):1710-5; discussion 1716. [DOI] [PubMed] [Google Scholar]
- 3.Boachie-Adjei O, Ferguson JA, Pigeon RG, Peskin MR. Transpedicular lumbar wedge resection osteotomy for fixed sagittal imbalance: surgical technique and early results. Spine (Phila Pa 1976). 2006. February 15;31(4):485-92. [DOI] [PubMed] [Google Scholar]
- 4.Chang KW, Cheng CW, Chen HC, Chang KI, Chen TC. Closing-opening wedge osteotomy for the treatment of sagittal imbalance. Spine (Phila Pa 1976). 2008. June 1;33(13):1470-7. [DOI] [PubMed] [Google Scholar]
- 5.Charosky S, Guigui P, Blamoutier A, Roussouly P, Chopin D; Study Group on Scoliosis. Complications and risk factors of primary adult scoliosis surgery: a multicenter study of 306 patients. Spine (Phila Pa 1976). 2012. April 15;37(8):693-700. [DOI] [PubMed] [Google Scholar]
- 6.Khan SN, Hofer MA, Gupta MC. Lumbar degenerative scoliosis: outcomes of combined anterior and posterior pelvis surgery with minimum 2-year follow-up. Orthopedics. 2009. April;32(4):pii:orthosupersite.com/view.asp?rID=38060. [PubMed] [Google Scholar]
- 7.Pateder DB, Kebaish KM, Cascio BM, Neubaeur P, Matusz DM, Kostuik JP. Posterior only versus combined anterior and posterior approaches to lumbar scoliosis in adults: a radiographic analysis. Spine (Phila Pa 1976). 2007. June 15;32(14):1551-4. [DOI] [PubMed] [Google Scholar]
- 8.Peelle MW, Boachie-Adjei O, Charles G, Kanazawa Y, Mesfin A. Lumbar curve response to selective thoracic fusion in adult idiopathic scoliosis. Spine J. 2008. Nov-Dec;8(6):897-903. Epub 2008 Feb 8. [DOI] [PubMed] [Google Scholar]
- 9.Rhee JM, Bridwell KH, Lenke LG, Baldus C, Blanke K, Edwards C, Berra A. Staged posterior surgery for severe adult spinal deformity. Spine (Phila Pa 1976). 2003. September 15;28(18):2116-21. [DOI] [PubMed] [Google Scholar]
- 10.Suk SI, Chung ER, Lee SM, Lee JH, Kim SS, Kim JH. Posterior vertebral column resection in fixed lumbosacral deformity. Spine (Phila Pa 1976). 2005. December 1;30(23):E703-10. [DOI] [PubMed] [Google Scholar]
- 11.Wang ST, Ma HL, Lin CF, Liu CL, Yu WK, Lo WH. Surgical treatment of adult idiopathic scoliosis - comparison of two instrumentations. Int Orthop. 2002;26(4):207-10. Epub 2002 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang BP, Ondra SL, Chen LA, Jung HS, Koski TR, Salehi SA. Clinical and radiographic outcomes of thoracic and lumbar pedicle subtraction osteotomy for fixed sagittal imbalance. J Neurosurg Spine. 2006. July;5(1):9-17. [DOI] [PubMed] [Google Scholar]
- 13.Ali RM, Boachie-Adjei O, Rawlins BA. Functional and radiographic outcomes after surgery for adult scoliosis using third-generation instrumentation techniques. Spine (Phila Pa 1976). 2003. June 1;28(11):1163-9; discussion 1169-70. [DOI] [PubMed] [Google Scholar]
- 14.Bess RS, Lenke LG, Bridwell KH, Cheh G, Mandel S, Sides B. Comparison of thoracic pedicle screw to hook instrumentation for the treatment of adult spinal deformity. Spine (Phila Pa 1976). 2007. March 1;32(5):555-61. [DOI] [PubMed] [Google Scholar]
- 15.Chang KW, Chen YY, Lin CC, Hsu HL, Pai KC. Closing wedge osteotomy versus opening wedge osteotomy in ankylosing spondylitis with thoracolumbar kyphotic deformity. Spine (Phila Pa 1976). 2005. July 15;30(14):1584-93. [DOI] [PubMed] [Google Scholar]
- 16.Deviren V, Patel VV, Metz LN, Berven SH, Hu SH, Bradford DS. Anterior arthrodesis with instrumentation for thoracolumbar scoliosis: comparison of efficacy in adults and adolescents. Spine (Phila Pa 1976). 2008. May 15;33(11):1219-23. [DOI] [PubMed] [Google Scholar]
- 17.Eck KR, Bridwell KH, Ungacta FF, Riew KD, Lapp MA, Lenke LG, Baldus C, Blanke K. Complications and results of long adult deformity fusions down to l4, l5, and the sacrum. Spine (Phila Pa 1976). 2001. May 1;26(9):E182-92. [DOI] [PubMed] [Google Scholar]
- 18.Emami A, Deviren V, Berven S, Smith JA, Hu SS, Bradford DS. Outcome and complications of long fusions to the sacrum in adult spine deformity: Luque-Galveston, combined iliac and sacral screws, and sacral fixation. Spine (Phila Pa 1976). 2002. April 1;27(7):776-86. [DOI] [PubMed] [Google Scholar]
- 19.Kim SS, Cho BC, Kim JH, Lim DJ, Park JY, Lee BJ, Suk SI. Complications of posterior vertebral resection for spinal deformity. Asian Spine J. 2012. December;6(4):257-65. Epub 2012 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YJ, Bridwell KH, Lenke LG, Cheh G, Baldus C. Results of lumbar pedicle subtraction osteotomies for fixed sagittal imbalance: a minimum 5-year follow-up study. Spine (Phila Pa 1976). 2007. September 15;32(20):2189-97. [DOI] [PubMed] [Google Scholar]
- 21.Kostuik JP, Hall BB. Spinal fusions to the sacrum in adults with scoliosis. Spine (Phila Pa 1976). 1983. Jul-Aug;8(5):489-500. [DOI] [PubMed] [Google Scholar]
- 22.Lapp MA, Bridwell KH, Lenke LG, Daniel Riew K, Linville DA, Eck KR, Ungacta FF. Long-term complications in adult spinal deformity patients having combined surgery a comparison of primary to revision patients. Spine (Phila Pa 1976). 2001. April 15;26(8):973-83. [DOI] [PubMed] [Google Scholar]
- 23.Simmons ED, Jr, Kowalski JM, Simmons EH. The results of surgical treatment for adult scoliosis. Spine (Phila Pa 1976). 1993. May;18(6):718-24. [DOI] [PubMed] [Google Scholar]
- 24.Smith JA, Deviren V, Berven S, Bradford DS. Does instrumented anterior scoliosis surgery lead to kyphosis, pseudarthrosis, or inadequate correction in adults? Spine (Phila Pa 1976). 2002. March 1;27(5):529-34. [DOI] [PubMed] [Google Scholar]
- 25.van Dam BE, Bradford DS, Lonstein JE, Moe JH, Ogilvie JW, Winter RB. Adult idiopathic scoliosis treated by posterior spinal fusion and Harrington instrumentation. Spine (Phila Pa 1976). 1987. Jan-Feb;12(1):32-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Zhang Y, Zhang X, Huang P, Xiao S, Wang Z, Liu Z, Liu B, Lu N, Mao K. A single posterior approach for multilevel modified vertebral column resection in adults with severe rigid congenital kyphoscoliosis: a retrospective study of 13 cases. Eur Spine J. 2008. March;17(3):361-72. Epub 2008 Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suk SI, Kim JH, Kim WJ, Lee SM, Chung ER, Nah KH. Posterior vertebral column resection for severe spinal deformities. Spine (Phila Pa 1976). 2002. November 1;27(21):2374-82. [DOI] [PubMed] [Google Scholar]
- 28.Buttermann GR, Glazer PA, Hu SS, Bradford DS. Anterior and posterior allografts in symptomatic thoracolumbar deformity. J Spinal Disord. 2001. February;14(1):54-66. [DOI] [PubMed] [Google Scholar]
- 29.Bridwell KH, Lewis SJ, Edwards C, Lenke LG, Iffrig TM, Berra A, Baldus C, Blanke K. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine (Phila Pa 1976). 2003. September 15;28(18):2093-101. [DOI] [PubMed] [Google Scholar]
- 30.Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003. March;85(3):454-63. [DOI] [PubMed] [Google Scholar]
- 31.Buchowski JM, Bridwell KH, Lenke LG, Kuhns CA, Lehman RA, Jr, Kim YJ, Stewart D, Baldus C. Neurologic complications of lumbar pedicle subtraction osteotomy: a 10-year assessment. Spine (Phila Pa 1976). 2007. September 15;32(20):2245-52. [DOI] [PubMed] [Google Scholar]
- 32.Coe JD, Smith JS, Berven S, Arlet V, Donaldson W, Hanson D, Mudiyam R, Perra J, Owen J, Marks MC, Shaffrey CI. Complications of spinal fusion for Scheuermann kyphosis: a report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2010. January 1;35(1):99-103. [DOI] [PubMed] [Google Scholar]
- 33.Daubs MD, Lenke LG, Cheh G, Stobbs G, Bridwell KH. Adult spinal deformity surgery: complications and outcomes in patients over age 60. Spine (Phila Pa 1976). 2007. September 15;32(20):2238-44. [DOI] [PubMed] [Google Scholar]
- 34.DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine (Phila Pa 1976). 2006. September 1;31(19)(Suppl):S144-51. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton DK, Smith JS, Sansur CA, Glassman SD, Ames CP, Berven SH, Polly DW, Jr, Perra JH, Knapp DR, Boachie-Adjei O, McCarthy RE, Shaffrey CI; Scoliosis Research Society Morbidity and Mortality Committee. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: a report of the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2011. July 1;36(15):1218-28. [DOI] [PubMed] [Google Scholar]
- 36.Kim YB, Lenke LG, Kim YJ, Kim YW, Blanke K, Stobbs G, Bridwell KH. The morbidity of an anterior thoracolumbar approach: adult spinal deformity patients with greater than five-year follow-up. Spine (Phila Pa 1976). 2009. April 15;34(8):822-6. [DOI] [PubMed] [Google Scholar]
- 37.Murrey DB, Brigham CD, Kiebzak GM, Finger F, Chewning SJ. Transpedicular decompression and pedicle subtraction osteotomy (eggshell procedure): a retrospective review of 59 patients. Spine (Phila Pa 1976). 2002. November 1;27(21):2338-45. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya K, Bridwell KH, Kuklo TR, Lenke LG, Baldus C. Minimum 5-year analysis of L5-S1 fusion using sacropelvic fixation (bilateral S1 and iliac screws) for spinal deformity. Spine (Phila Pa 1976). 2006. February 1;31(3):303-8. [DOI] [PubMed] [Google Scholar]
- 39.Weistroffer JK, Perra JH, Lonstein JE, Schwender JD, Garvey TA, Transfeldt EE, Ogilvie JW, Denis F, Winter RB, Wroblewski JM. Complications in long fusions to the sacrum for adult scoliosis: minimum five-year analysis of fifty patients. Spine (Phila Pa 1976). 2008. June 1;33(13):1478-83. [DOI] [PubMed] [Google Scholar]
- 40.Bomback DA, Charles G, Widmann R, Boachie-Adjei O. Video-assisted thoracoscopic surgery compared with thoracotomy: early and late follow-up of radiographical and functional outcome. Spine J. 2007. Jul-Aug;7(4):399-405. Epub 2007 Feb 5. [DOI] [PubMed] [Google Scholar]
- 41.Lenke LG, Sides BA, Koester LA, Hensley M, Blanke KM. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010. March;468(3):687-99. Epub 2009 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu Y, Wang S, Wang B, Yu Y, Zhu F, Zhu Z. Incidence and risk factors of neurological deficits of surgical correction for scoliosis: analysis of 1373 cases at one Chinese institution. Spine (Phila Pa 1976). 2008. March 1;33(5):519-26. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro GS, Taira G, Boachie-Adjei O. Results of surgical treatment of adult idiopathic scoliosis with low back pain and spinal stenosis: a study of long-term clinical radiographic outcomes. Spine (Phila Pa 1976). 2003. February 15;28(4):358-63. [DOI] [PubMed] [Google Scholar]
- 44.Suk SI, Chung ER, Kim JH, Kim SS, Lee JS, Choi WK. Posterior vertebral column resection for severe rigid scoliosis. Spine (Phila Pa 1976). 2005. July 15;30(14):1682-7. [DOI] [PubMed] [Google Scholar]
- 45.Swank S, Lonstein JE, Moe JH, Winter RB, Bradford DS. Surgical treatment of adult scoliosis. A review of two hundred and twenty-two cases. J Bone Joint Surg Am. 1981. February;63(2):268-87. [PubMed] [Google Scholar]
- 46.Xie J, Wang Y, Zhao Z, Zhang Y, Si Y, Li T, Yang Z, Liu L. Posterior vertebral column resection for correction of rigid spinal deformity curves greater than 100°. J Neurosurg Spine. 2012. December;17(6):540-51. Epub 2012 Oct 12. [DOI] [PubMed] [Google Scholar]
- 47.Graves DE, Frankiewicz RG, Donovan WH. Construct validity and dimensional structure of the ASIA motor scale. J Spinal Cord Med. 2006;29(1):39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011. November;34(6):535-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallinckrodt CH, Lane PW, Schnell D, Peng Y, Mancuso JP. Recommendations for the primary analysis of continuous endpoints in longitudinal clinical trials. Drug Inf J. 2008;42(4):303-19. [Google Scholar]
- 50.Kelly MP, Lenke LG, Godzik J, Pellise F, Shaffrey CI, Smith JS, Lewis SJ, Ames CP, Carreon LY, Fehlings MG, Schwab F, Shimer AL. Retrospective analysis underestimates neurological deficits in complex spinal deformity surgery: a Scoli-RISK-1 Study. J Neurosurg Spine. 2017. July;27(1):68-73. Epub 2017 May 5. [DOI] [PubMed] [Google Scholar]