Supplemental Digital Content is Available in the Text.
Naldemedine treatment of opioid-induced constipation was well tolerated for 52 weeks, did not precipitate opioid withdrawal, and increased bowel movements and quality of life.
Keywords: Chronic pain, Naldemedine, Opioid-induced constipation, Peripherally acting µ-opioid, Receptor antagonist
Abstract
The long-term safety of naldemedine, a peripherally acting µ-opioid receptor antagonist, was evaluated in patients with opioid-induced constipation and chronic noncancer pain in a 52-week, randomized, double-blind, phase 3 study. Eligible adults who could be on a routine laxative regimen were randomized 1:1 to receive once-daily oral naldemedine 0.2 mg (n = 623) or placebo (n = 623). The primary endpoint was summary measures of treatment-emergent adverse events (AEs). Additional endpoints included opioid withdrawal on the Clinical Opiate Withdrawal Scale and the Subjective Opiate Withdrawal Scale, pain intensity on Numeric Rating Scale, frequency of bowel movements, and constipation-related symptoms and quality of life on the Patient Assessment of Constipation Symptoms and Patient Assessment of Constipation Quality of Life scales, respectively. Treatment-emergent AEs (naldemedine, 68.4% vs placebo, 72.1%; difference: −3.6% [95% confidence interval: −8.7 to 1.5]) and treatment-emergent AEs leading to study discontinuation (6.3% vs 5.8%; difference: 0.5% [−2.2 to 3.1)] were reported for similar proportions of patients. Diarrhea was reported more frequently with naldemedine (11.0%) vs placebo (5.3%; difference: 5.6% [2.6-8.6]). There were no meaningful differences between groups in opioid withdrawal or pain intensity. Sustained significant improvements in bowel movement frequency and overall constipation-related symptoms and quality of life were observed with naldemedine (P ≤ 0.0001 vs placebo at all time points). Naldemedine was generally well tolerated for 52 weeks and did not interfere with opioid-mediated analgesia or precipitate opioid withdrawal. Naldemedine significantly increased bowel movement frequency, improved symptomatic burden of opioid-induced constipation, and increased patients' quality of life vs placebo.
1. Introduction
Long-term opioid therapy is a common component of chronic noncancer pain management.4,8 Use of opioid analgesics has been associated with the serious risks of abuse, addiction, and overdose, as well as the less serious but debilitating side effect of opioid-induced constipation (OIC).3,27 Approximately 40% to 60% of patients receiving opioids for chronic noncancer pain are affected by OIC,12,21 and its symptoms do not attenuate with duration of opioid therapy.2,25,27,30 One-third of patients with OIC report skipping, reducing, or stopping use of opioids—despite experiencing an increase in pain—in an effort to have a bowel movement.2,15 Opioid-induced constipation may also lead to complications such as obstipation, colonic distention, ileus, and bowel perforation.10 Unsurprisingly, the quality of life of patients with OIC is significantly poorer than those unaffected by it.2
Opioid-induced constipation is characterized by a reduction in bowel movement frequency, an increase in straining, a sensation of incomplete evacuation, and/or hard stool consistency after initiating opioid therapy.6,22 Opioids bind to peripheral µ-opioid receptors in the submucosal and myenteric plexuses of the enteric nervous system within the gastrointestinal tract. This alters neural output from the enteric neurons, leading to impaired motility, decreased fluid secretion, and increased fluid absorption in the gastrointestinal tract. Together, these physiological changes manifest in OIC.6 Management strategies such as laxatives and lifestyle modifications do not target the underlying mechanism of OIC and often do not provide adequate relief.5,13 In addition, the side effects introduced by laxatives further diminish the quality of life of these patients.14
Peripherally acting μ-opioid receptor antagonists (PAMORAs) are a class of drugs that target the mechanism underlying OIC without affecting the centrally mediated analgesic effects of opioids.32 Peripherally acting μ-opioid receptor antagonists bind to and markedly reduce opioid stimulation of peripheral µ-opioid receptors in the gastrointestinal tract. Naldemedine (also known as S-297995) is a PAMORA approved as a once-daily oral drug for the relief of OIC in adult patients with cancer and those with chronic noncancer pain, including patients with chronic pain related to previous cancer, or its treatment which does not require frequent dosage escalation.29 Naldemedine is an amide derivative of the opioid receptor antagonist naltrexone with an added side chain that increases its polarity and lowers its lipid solubility, reducing its ability to cross the blood–brain barrier.29 Results of 2 identically designed, 12-week, placebo-controlled, double-blind, phase 3 trials conducted in patients with OIC and chronic noncancer pain (COMPOSE-1 and COMPOSE-2) demonstrated that once-daily oral naldemedine at a dose of 0.2 mg effectively treated OIC and was generally well tolerated.16
There are limited published studies assessing the impacts of long-term use of PAMORAs in managing symptoms of OIC. Here, we report the long-term safety and tolerability of naldemedine 0.2 mg for OIC in patients with chronic noncancer pain who received a stable dose of opioids over the course of 1 year. Effects of long-term use of naldemedine on bowel movements, symptomatic burden of OIC, and quality of life are also described.
2. Methods
2.1. Study design
This was a randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial (COMPOSE-3) that evaluated the long-term safety and tolerability of once-daily oral naldemedine 0.2 mg for 52 weeks in patients with chronic noncancer pain, on a stable opioid therapy, and who could be on a routine laxative regimen but still had OIC (ClinicalTrials.gov, Clinical trial identifier NCT01965652). This study was performed at 195 sites in North America, Europe, Africa, and Asia-Pacific from September 24, 2013, to January 12, 2016. COMPOSE-3 was conducted in accordance with all local laws, the International Council for Harmonization and Good Clinical Practice guidelines, and the ethical principles outlined in the Declaration of Helsinki and was approved by each institutional review board (see Table, Supplemental digital content 1, which lists the names of the Institutional Review Boards; available online at http://links.lww.com/PAIN/A541). All patients provided written informed consent.
2.2. Patients and treatment
Eligible patients (aged 18-80 years) had chronic noncancer pain for ≥3 months, were receiving a stable daily dose of opioids (≥30 mg oral morphine equivalents) for ≥1 month before screening, and had OIC. The diagnostic threshold for OIC was ≤4 total spontaneous bowel movements (defined as a bowel movement not induced by rescue laxatives) over the 14–consecutive-day qualifying period of the 28-day screening period and ≤3 spontaneous bowel movements in any given week of the qualifying period. Patients on a routine laxative regimen (defined as using an over-the-counter [OTC] laxative at least once per week) at screening were allowed to remain on the stable laxative regimen for the duration of the study. Patients were not required to be on a routine laxative regimen for study inclusion. All patients had access to rescue laxatives (rescue was defined as any laxative taken for the first time during the treatment period). Patients with any structural abnormalities or medical conditions that may have contributed to the symptoms of constipation (other than the opioid therapy itself) were excluded.
Eligible patients were randomized 1:1 to receive once-daily oral naldemedine (SYMPROIC; Shionogi Inc, Florham Park, NJ) 0.2 mg or placebo for 52 weeks. Patients were randomized with an automated interactive voice or web response system and stratified by their daily dose of opioids during the qualifying period (30-100 mg or >100 mg of oral morphine equivalents). During the study, the dose of opioids should have been kept stable; however, investigators were allowed to change the dose of opioids as needed to ensure adequate management of pain. Treatment assignments for study drug administration were concealed by the use of identical packaging, labeling, dosing instructions, appearance, taste, color, and odor. All patients, investigators, and study site personnel were blinded to treatment assignments until the end of the study.
2.3. Assessments
The primary endpoint was summary measures of treatment-emergent adverse events. These included incidences of treatment-emergent adverse events, serious treatment-emergent adverse events, and treatment-emergent adverse events leading to study discontinuation. Adverse events were collected and followed from the time of informed consent through 14 days after the last dose of study treatment. An adverse event was considered treatment emergent if it occurred after the first dose of study drug administration or at any time up to 14 days after the last dose of study drug. Treatment-emergent adverse events of opioid withdrawal were identified using the Medical Dictionary for Regulatory Activities (version 16.0) standardized query “drug withdrawal.” A treatment-emergent adverse event was considered possible opioid-withdrawal syndrome if ≥3 events potentially related to opioid withdrawal occurred within the same day or the next day.
Opioid withdrawal was assessed using the 11-item Clinical Opiate Withdrawal Scale36 and the 16-item Subjective Opiate Withdrawal Scale.17 Site personnel evaluated level of opioid withdrawal on the Clinical Opiate Withdrawal Scale; scores were totaled to assess overall severity of withdrawal (5-12 = mild; 13-24 = moderate; 25-36 = moderately severe; and >36 = severe). A score ≥5 on the Clinical Opiate Withdrawal Scale was considered elevated and clinically significant. Patients assessed level of opioid withdrawal on the Subjective Opiate Withdrawal Scale; the rating scale for each question ranged from 0 to 4 (0 = not at all; 1 = a little; 2 = moderate; 3 = quite a bit; and 4 = extreme). Lower scores in either scale indicate a lesser severity of opioid withdrawal. Pain intensity was assessed by patients using the 11-point Numeric Rating Scale with 0 indicating no pain and 10 representing the worse pain possible. The Clinical and Subjective Opiate Withdrawal Scales and the Numeric Rating Scale were administered on weeks 1, 2, 6, 12, 24, 36, 48, and 52. The Clinical Opiate Withdrawal Scale was also administered on day 1 at predose, and at 60, 90, and 120 minutes postdose.
Secondary efficacy endpoints included change from baseline in the frequency of bowel movements on weeks 12, 24, 36, and 52. Patients recorded their bowel movements for the week before each study visit in a “Bowel Habits” paper diary. Constipation-related symptoms and quality of life were assessed using 2 questionnaires: Patient Assessment of Constipation Symptoms28 and Patient Assessment of Constipation Quality of Life.23 The overall score for Patient Assessment of Constipation Symptoms questionnaire comprised scores from 3 domains with multiple items: abdominal, rectal, and stool symptoms. The overall score for Patient Assessment of Constipation Quality of Life questionnaire comprised scores from 4 domains with multiple items: physical discomfort, psychosocial discomfort, satisfaction, and worries and concerns. In both measures, lower scores reflect more favorable self-assessments of symptoms or quality of life. The changes from baseline in overall scores for the Patient Assessment of Constipation Symptoms and Patient Assessment of Constipation Quality of Life questionnaires were assessed on weeks 2, 12, 24, 36, and 52.
2.4. Statistical analysis
A sample size of 1200 patients (600 per treatment arm) was planned to fully meet or exceed the International Council for Harmonization E1 guidelines: The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-term Treatment of Non-Life-Threatening Conditions.20 Safety assessments were conducted on the safety population, defined as all randomized patients who received ≥1 dose of the study drug. Efficacy assessments were conducted on the intent-to-treat population, defined as all randomized patients.
The significance level for statistical tests was set at 0.05 (2 sided). The mixed-effects model for repeated measures was used to analyze differences between treatment groups in the change from baseline in the frequency of bowel movements and overall scores for Patient Assessment of Constipation Symptoms and Patient Assessment of Constipation Quality of Life. The mixed-effects model for repeated measures included opioid dose strata as a covariate and group, time, and the time-by-treatment group interaction as fixed effects. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
3. Results
3.1. Patients
Of 2414 patients screened, 1246 were randomized to receive naldemedine (n = 623) or placebo (n = 623; Fig. 1). Patient demographics and baseline characteristics were similar between treatment groups in the safety population (Table 1). Although randomization was not stratified by baseline laxative use, similar proportions of patients in each treatment group were on a routine laxative regimen (naldemedine, 50.2%; placebo, 54.0%) or were not (naldemedine, 30.0%; placebo, 29.5%) during the study. The remaining patients (naldemedine, 19.8%; placebo, 16.5%) did not meet the criteria for being considered on, or not on, routine laxatives. At randomization, the mean duration of opioid therapy for patients in the naldemedine and placebo groups was 62.6 months and 57.0 months, respectively, and most patients were in the stratum of ≥30 to ≤100 mg total daily dose of opioid in both the naldemedine (60.9%) and the placebo (59.5%) groups. In each treatment group, 66.3% of patients completed the study (Fig. 1).
Figure 1.
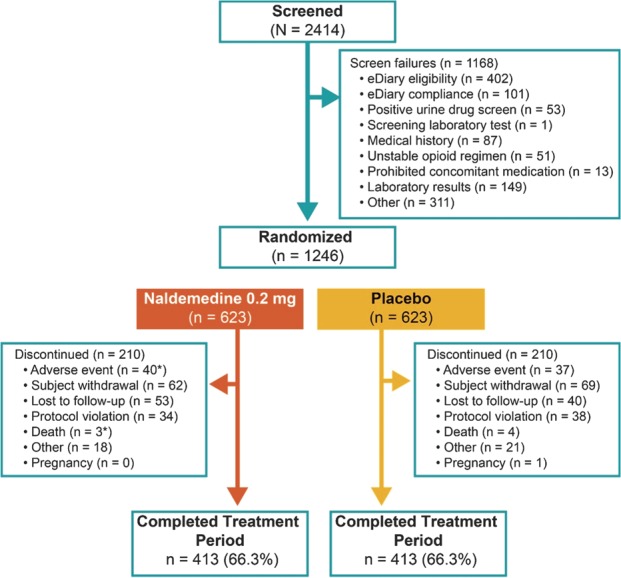
Patient disposition (CONSORT diagram). *One patient in the naldemedine group died 22 days after being removed from the study by the investigator because of an adverse event, which was recorded as the primary reason for discontinuation.
Table 1.
Patient demographic and baseline characteristics.
3.2. Safety and tolerability
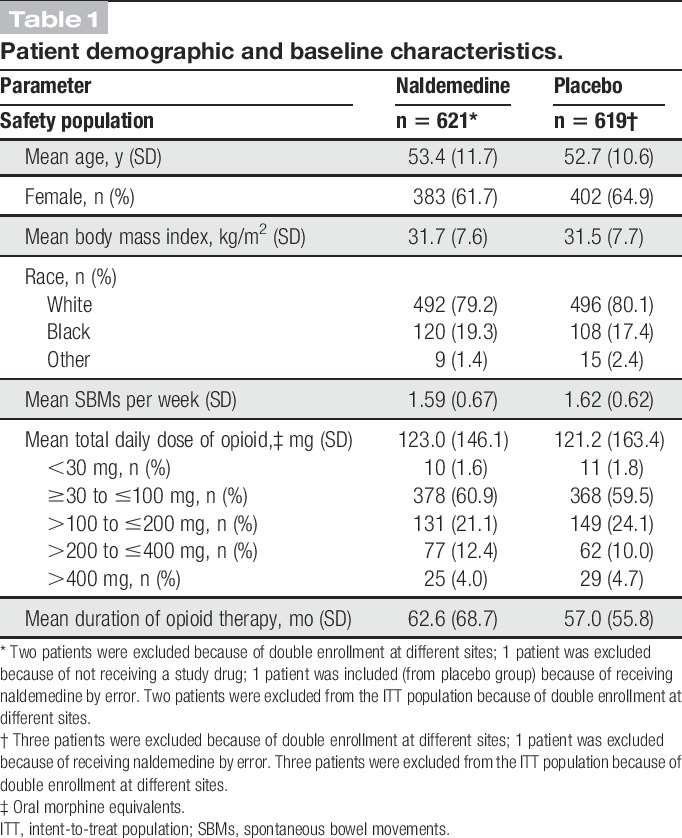
The proportions of patients who reported a treatment-emergent adverse event (primary endpoint) were similar between treatment groups (naldemedine, 68.4%; placebo, 72.1%; difference of proportion: −3.6%; 95% confidence interval: −8.7 to 1.5; Table 2). Diarrhea was the most common treatment-emergent adverse event in the naldemedine group (11.0%) and was reported more frequently compared with placebo (5.3%). A greater proportion of patients treated with naldemedine (vs placebo) reported other gastrointestinal-related treatment-emergent adverse events, including abdominal pain (8.2% vs 3.1%) and vomiting (6.0% vs 3.1%). However, most events were mild to moderate in severity, and the number of patients who discontinued treatment because of a gastrointestinal-related treatment-emergent adverse event in either group was low (naldemedine, 3.7%; placebo, 1.6%). The proportions of patients who discontinued treatment due to any treatment-emergent adverse event were low and similar between groups (naldemedine, 6.3%; placebo, 5.8%). The incidences of treatment-related serious adverse events, serious treatment-emergent adverse events leading to study discontinuation, and major adverse cardiac events were also low (<2%) and similar between treatment groups.
Table 2.
Overall measures of safety (safety population).
Similar proportions of patients in each treatment group reported treatment-emergent adverse events of opioid withdrawal (naldemedine, 1.8%; placebo, 1.1%). A greater incidence of treatment-emergent adverse events of possible opioid withdrawal was observed with naldemedine (2.4%) vs placebo (0.6%); however, all such events in the naldemedine group were defined by at least 1 term of gastrointestinal-related treatment-emergent adverse events. Mean overall scores on the Clinical Opiate Withdrawal Scale were similar between groups and remained relatively stable over the course of the 52-week treatment period (Fig. 2A). Low and equal proportions of patients in both treatment groups (4.5%) had an elevated score on the Clinical Opiate Withdrawal Scale (naldemedine, n = 28; placebo, n = 28). There was a similar degree of decrease from baseline in the mean overall Subjective Opiate Withdrawal Scale scores in both groups (Fig. 2B); these decreases were small and the differences between treatment groups were not considered clinically meaningful. The mean Numeric Rating Scale scores for pain assessment were similar between treatment groups and were generally stable for the extent of the study (Fig. 3). Similar proportions of patients in both treatment groups (36.6% and 36.3% in the naldemedine and placebo groups, respectively) had an increase of ≥2 on the Numeric Rating Scale at least once during the treatment period. In addition, throughout the course of the study, the dose of opioid analgesic remained generally unchanged relative to baseline values in both treatment groups (Fig. 4).
Figure 2.
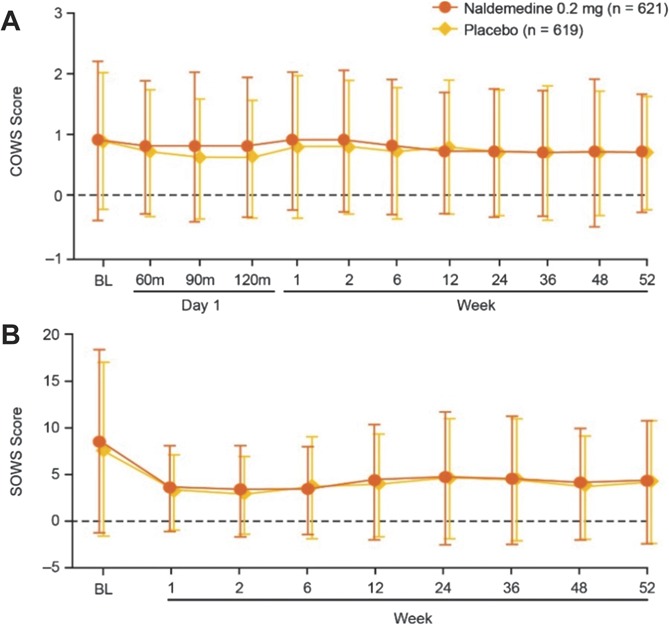
Assessments of opioid withdrawal. Patients treated with naldemedine or placebo were evaluated for opioid withdrawal using the Clinical Opiate Withdrawal Scale (A), and Subjective Opiate Withdrawal Scale (B) (safety population; mean ± SD). BL, baseline; COWS, Clinical Opiate Withdrawal Scale; SOWS, Subjective Opiate Withdrawal Scale.
Figure 3.
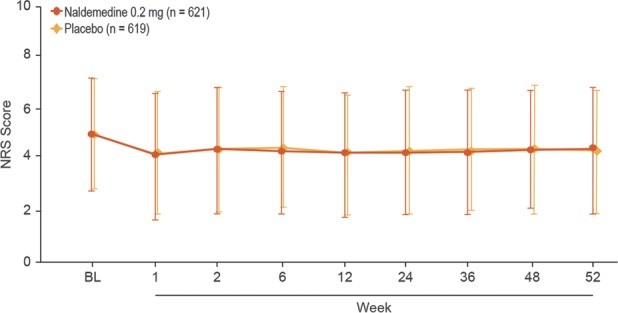
Assessment of pain intensity. Patients treated with naldemedine or placebo were evaluated for pain intensity using the numeric rating scale (safety population; mean ± SD). BL, baseline; NRS, numeric rating scale.
Figure 4.
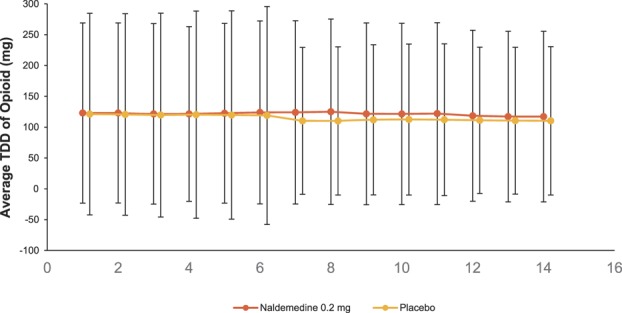
Total daily dose of opioid. Patients were assessed every 4 weeks (safety population; mean ± SD). Baseline was the 14–consecutive-calendar-day qualifying period during the screening period. Number of patients at each visit is shown. BL, baseline; TDD, total daily dose.
A total of 8 deaths (naldemedine, n = 4; placebo, n = 4) occurred during this 52-week study (Table, Supplemental Digital Content 2, available online at http://links.lww.com/PAIN/A541); however, only 1 death in the naldemedine group and 3 deaths in the placebo group were considered treatment emergent. A death was not considered treatment emergent if the patient died >14 days after their last known dose. No deaths in either treatment group were considered to be related to the study drug by the investigator.
3.3. Efficacy
The intent-to-treat population comprised 621 and 620 patients in the naldemedine and placebo treatment groups, respectively. At baseline, mean (SD) bowel movements per week were 2.02 (0.95) and 2.02 (0.94), respectively. There was a significant and sustained increase from baseline in the frequency of bowel movements with naldemedine vs placebo throughout the 52-week treatment period (nominal P ≤ 0.0001 at all assessments; Fig. 5). Among those on a routine laxative regimen, the proportion of patients requiring rescue laxatives (defined as any laxatives different from the regular regimen at screening or taken for the first time during the treatment period) was numerically lower with naldemedine vs placebo (8.0% vs 14.0%); a similar trend was observed for patients not on a routine laxative regimen (7.0% vs 13.1%). A significant and sustained decrease from baseline (improvement) with naldemedine vs placebo was observed in mean overall scores for Patient Assessment of Constipation Symptoms and Patient Assessment of Constipation Quality of Life questionnaires (both nominal P < 0.0001 at all assessments; Fig. 6).
Figure 5.
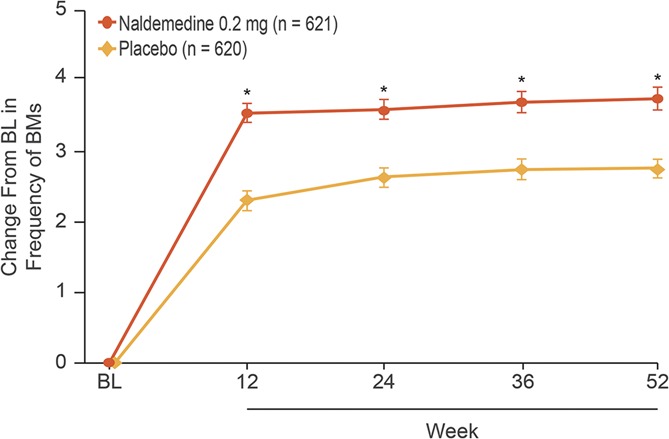
Changes from baseline in frequency of bowel movements (intent-to-treat population; least squares mean ± SE). *P ≤ 0.0001 vs placebo. BL, baseline; BM, bowel movement. BL value for both treatment groups: 2.02 BMs per week.
Figure 6.
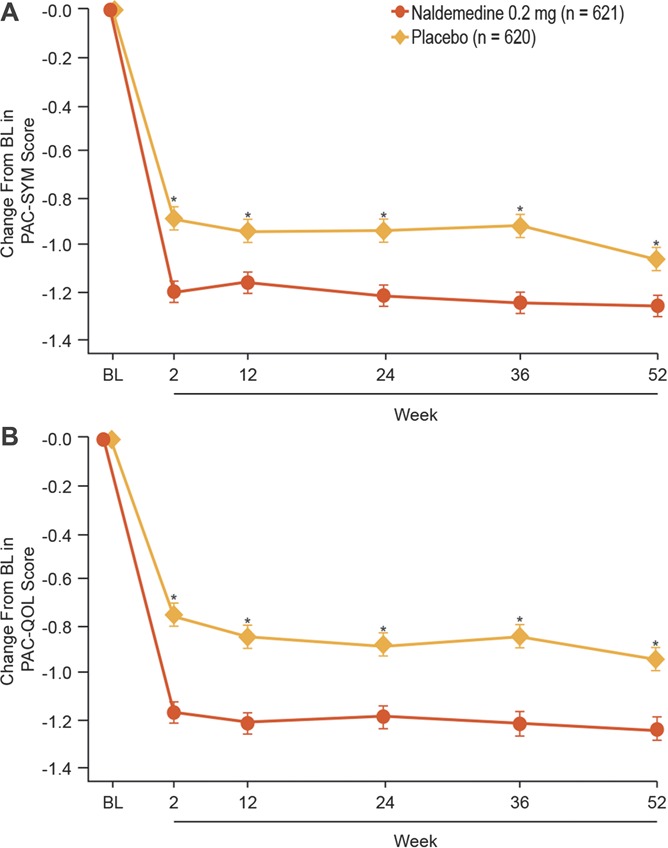
Change from baseline in (A) Patient Assessment of Constipation Symptoms and (B) Patient Assessment of Constipation Quality of Life scores (intent-to-treat population; least squares mean ± SE). *P ≤ 0.0001 vs placebo. BL, baseline; PAC-QOL, Patient Assessment of Constipation Quality of Life; PAC-SYM, Patient Assessment of Constipation Symptoms.
4. Discussion
This is the first study to investigate the utility of a PAMORA over the course of 52 weeks in a randomized, placebo-controlled, and double-blind manner. In the United States and Europe, methylnaltrexone bromide24,31 and naloxegol9,33 are approved for treatment of OIC in patients with chronic noncancer pain. However, the 48-week and 52-week studies evaluating the safety and tolerability of methylnaltrexone bromide and naloxegol, respectively, were open-label and did not include a placebo group.33,35 Our results showed that once-daily oral naldemedine 0.2 mg compared with placebo was generally well tolerated for 52 weeks in patients concomitantly receiving long-term opioid therapy for chronic noncancer pain. Naldemedine treatment significantly increased the frequency of bowel movements and improved patient-reported outcome measures of constipation-related symptoms and quality of life compared with placebo at every time point assessed over the 52-week treatment period. The lack of meaningful differences between treatment groups in assessments of symptoms of opioid withdrawal and pain intensity over time supports that naldemedine exerts its therapeutic benefits peripherally with limited potential for interference with the centrally mediated effects of opioid therapy. This is consistent with the preclinical data of naldemedine showing that exposure required to cause centrally mediated symptoms of opioid withdrawal and antianalgesic effects is at least 30 times that required for the anticonstipation effect (Kanemasa T, Koike K, Arai T, Horita N, Chiba H, Kihara T, Hasegawa M. Effects of naldemedine, a PAMORA, in rat models of OIC. Poster presented at: Annual Meeting of the American College of Gastroenterology; October 16-21, 2015; Honolulu, HI).
In this study, the long-term safety profile of naldemedine was similar to that of placebo, with the exception of a higher incidence of gastrointestinal-related treatment-emergent adverse events with naldemedine. This observation builds on the results of the previous phase 3 trials of naldemedine of shorter duration (ie, 12 weeks)16 and is consistent with the mechanism of action of PAMORAs. Patients treated with naloxegol for 12 weeks or 52 weeks also reported a higher incidence of gastrointestinal-related treatment-emergent adverse events compared with, respectively, placebo or usual care.9,33 The most common treatment-emergent adverse events during 4 or 48 weeks of methylnaltrexone bromide treatment compared with, respectively, placebo or no comparator, were also gastrointestinal related.24,35 The antagonistic effects of naldemedine on peripheral µ-opioid receptors in the gastrointestinal tract result in physiological changes that may facilitate the occurrence of diarrhea.18 Despite the greater incidence of diarrhea, abdominal pain, nausea, and vomiting associated with naldemedine, gastrointestinal disorders led to only a few treatment discontinuations in this study. Moreover, study discontinuations owing to treatment-emergent adverse events were low and similar between treatment groups.
Among the main safety concerns of PAMORAs are the possible precipitation of opioid-withdrawal syndrome and the disruption of opioid analgesia. In this study, naldemedine treatment had no meaningful impact on assessments of opioid withdrawal or pain intensity; an observation that is consistent with results from shorter-term naldemedine studies.16,32 Furthermore, in this study, there were no treatment-emergent adverse events of possible opioid withdrawal in the naldemedine group that was defined solely by terms related to non-gastrointestinal disorders. These findings indicate that naldemedine has negligible effects on the central nervous system and limited potential for interference with opioid-mediated analgesia. Another safety concern with long-term use of PAMORAs is the potential increased risk of cardiovascular events.19 In the current long-term study, the incidence of major adverse cardiac events in the naldemedine group was low and similar to that in placebo.
The efficacy of naldemedine, as measured by change in the frequency of bowel movements, was durable and did not attenuate even after consistent treatment for 52 weeks. Naldemedine was associated with a numerically lower need for rescue laxatives compared with placebo, regardless of whether patients were on a routine laxative regimen. In the 12-week phase 3 trials in which patients were not allowed to use laxatives, an increase in frequency of bowel movements occurred after 1 week of naldemedine treatment and lasted throughout the course of the studies with little attenuation.7 The efficacy of naldemedine is further supported by corresponding improvements in patient-reported outcomes of constipation-related symptoms and quality of life. Opioid-induced constipation and its associated symptoms have been shown to have a significant negative impact on quality of life11; however, few studies have reported the long-term effect of treatment on this key component of treatment success.34
The strengths of this long-term study include the placebo-controlled, double-blind trial design and the large number of patients. Limitations of the study are that during its conduct over 52 weeks, assessments were performed only at specific time points and data regarding the opioid dose were captured as the dose prescribed, not as the actual patient daily intake. The latter could have been a useful measure because symptoms of OIC are opioid dose dependent.14 The above limitations were primarily attributable to logistic challenges associated with long-term studies.
In this study, an increase in the frequency of bowel movements from baseline and a reduction in the PAC-SYM or QOL scores were observed in the placebo group. One might interpret such placebo effects as an improvement of the symptom of OIC. However, it is important to note that the extent of such improvements was significantly larger for naldemedine compared with placebo at each time point measured, illustrating the importance of performing placebo-controlled clinical studies to demonstrate a true effect of an investigational treatment. Furthermore, the improvement from baseline in the placebo group cannot be attributed to OTC laxatives because patients in both groups were required to maintain a stable routine laxative regimen throughout the study. Although OTC laxatives might be an option for the treatment of OIC, our efficacy data suggest that naldemedine may be more effective than OTC laxatives for the treatment of OIC; however, future studies are warranted to confirm this hypothesis.
5. Conclusions
In patients with chronic noncancer pain and OIC, naldemedine 0.2 mg was shown to be generally well tolerated, with consecutive daily treatment for 52 weeks compared with placebo. Long-term naldemedine treatment elicited significant and durable improvements in the frequency of bowel movements, constipation-related symptoms, and patient quality of life. The findings of this study may provide reassurance to patients and physicians considering extended use of naldemedine in the management of OIC. In a recent consensus recommendation, it was noted that prophylactic and first-line OTC laxatives might not consistently and predictably lead to desired treatment responses in patients with OIC.1 Another recently published clinical guideline also recommended considering an opioid antagonist alongside OTC laxative or opioid switching in the treatment decision for OIC.26 Therefore, this study supports the addition of naldemedine to a patient's laxative regimen should patients still experience OIC after self-treating with OTC laxatives.
Conflict of interest statement
L.R. Webster and S. Nalamachu are consultants for Shionogi Inc and were clinical trial site investigators. In addition, L.R. Webster has received honoraria for: consultancy from AstraZeneca, BioDelivery Sciences International, CVS Caremark, Grünenthal USA, Mallinckrodt Pharmaceuticals, Nevro Corporation, and Synchrony Healthcare; for advisory board attendance from Depomed, Egalet, Inspirion Pharmaceuticals, Insys Therapeutics, Kaléo Pharma, Mallinckrodt Pharmaceuticals, Orexo, Proove Biosciences, Signature Therapeutics, Teva Pharmaceuticals, and Trevena; and travel expenses from AstraZeneca, BioDelivery Sciences International, Bristol-Myers Squibb, Depomed, Grünenthal USA, Inspirion Pharmaceuticals, Insys Therapeutics, Jazz Pharmaceuticals, Kaléo Pharma, Mallinckrodt Pharmaceuticals, Nektar Therapeutics, Nevro Corporation, Orexo, Proove Biosciences, and Trevena. In addition, S. Nalamachu is a consultant and speaker for AstraZeneca, Daiichi Sankyo, Purdue, and Salix. B. Morlion was a clinical trial site investigator for Shionogi Inc; a consultant for Astellas Pharma Europe Ltd, Boehringer Ingelheim International, Grünenthal, Mundipharma International, TEVA Pharmaceuticals Europe; and a speaker for Mundipharma International, Pfizer Inc, and Shionogi Inc. T. Yamada and Y. Baba are employees of Shionogi Inc. J. Reddy and J.C. Arjona Ferreira were employees of Shionogi Inc at the time of this study.
This study was sponsored by Shionogi Inc, Florham Park, NJ. Medical writing and editorial support, funded by Shionogi Inc, was provided by V. Ruvini Jayasinghe, PhD; Suhaida A. Selamat, PhD; and Shilpa Aggarwal, PhD, of Oxford PharmaGenesis Inc and by Linda Romagnano, PhD, of Peloton Advantage, LLC.
Presented in part at PAIN Week, September 06-10, 2016, Las Vegas, NV, USA.
Supplementary Material
Acknowledgements
The authors thank the patients and the investigators who participated in this study.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A541.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Argoff CE, Brennan MJ, Camilleri M, Davies A, Fudin J, Galluzzi KE, Gudin J, Lembo A, Stanos SP, Webster LR. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med 2015;16:2324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009;10:35–42. [DOI] [PubMed] [Google Scholar]
- [3].Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician 2008;11(2 suppl):S105–S20. [PubMed] [Google Scholar]
- [4].Blake H, Leighton P, van der Walt G, Ravenscroft A. Prescribing opioid analgesics for chronic non-malignant pain in general practice—a survey of attitudes and practice. Br J Pain 2015;9:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835–42. [DOI] [PubMed] [Google Scholar]
- [6].Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, Mawe GM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil 2014;26:1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Camilleri M, Tack J, Cai B, Yamada T, Arjona Ferreira J. Naldemedine treatment of opioid-induced constipation improved patient-reported outcomes in subjects with chronic noncancer pain [abstract K2]. J Manag Care Spec Pharm 2017;23(3 suppl):S80. [Google Scholar]
- [8].Cheung CW, Qiu Q, Choi SW, Moore B, Goucke R, Irwin M. Chronic opioid therapy for chronic non-cancer pain: a review and comparison of treatment guidelines. Pain Physician 2014;17:401–14. [PubMed] [Google Scholar]
- [9].Chey WD, Webster L, Sostek M, Lappalainen J, Barker PN, Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med 2014;370:2387–96. [DOI] [PubMed] [Google Scholar]
- [10].Cook SF, Lanza L, Zhou X, Sweeney CT, Goss D, Hollis K, Mangel AW, Fehnel SE. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224–32. [DOI] [PubMed] [Google Scholar]
- [11].Coyne KS, LoCasale RJ, Datto CJ, Sexton CC, Yeomans K, Tack J. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res 2014;6:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coyne KS, Margolis MK, Yeomans K, King FR, Chavoshi S, Payne KA, LoCasale RJ. Opioid-induced constipation among patients with chronic noncancer pain in the United States, Canada, Germany, and the United Kingdom: laxative use, response, and symptom burden over time. Pain Med 2015;16:1551–65. [DOI] [PubMed] [Google Scholar]
- [13].Coyne KS, Sexton C, LoCasale RJ, King FR, Margolis MK, Ahmedzai SH. Opioid-induced constipation among a convenience sample of patients with cancer pain. Front Oncol 2016;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Emmanuel A, Johnson M, McSkimming P, Dickerson S. Laxatives do not improve symptoms of opioid-induced constipation: results of a patient survey. Pain Med 2017;18:1932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gupta S, Patel H, Scopel J, Mody RR. Impact of constipation on opioid therapy management among long-term opioid users, based on a patient survey. J Opioid Manag 2015;11:325–38. [DOI] [PubMed] [Google Scholar]
- [16].Hale M, Wild J, Reddy J, Yamada T, Arjona Ferreira JC. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol 2017;2:555–64. [DOI] [PubMed] [Google Scholar]
- [17].Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 1987;13:293–308. [DOI] [PubMed] [Google Scholar]
- [18].Hansen MB. The enteric nervous system II: gastrointestinal functions. Pharmacol Toxicol 2003;92:249–57. [DOI] [PubMed] [Google Scholar]
- [19].Holzer P. New approaches to the treatment of opioid-induced constipation. Eur Rev Med Pharmacol Sci 2008;12(suppl 1):119–27. [PMC free article] [PubMed] [Google Scholar]
- [20].International Conference on Harmonisation Expert Working Group. ICH Harmonised Tripartite Guideline. The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-Term Treatment of Non-Life-Threatening Conditions E1. International Conference on Harmonisation; Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E1/Step4/E1_Guideline.pdf. Accessed August 23, 2017. [Google Scholar]
- [21].Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. PAIN 2004;112:372–80. [DOI] [PubMed] [Google Scholar]
- [22].Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel disorders. Gastroenterology 2016;150:1393–407. [DOI] [PubMed] [Google Scholar]
- [23].Marquis P, De La LC, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540–51. [DOI] [PubMed] [Google Scholar]
- [24].Michna E, Blonsky ER, Schulman S, Tzanis E, Manley A, Zhang H, Iyer S, Randazzo B. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain 2011;12:554–62. [DOI] [PubMed] [Google Scholar]
- [25].Morlion B, Clemens KE, Dunlop W. Quality of life and healthcare resource in patients receiving opioids for chronic pain: a review of the place of oxycodone/naloxone. Clin Drug Investig 2015;35:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Müler-Lissner S, Bassotti G, Coffin B, Drewes AM, Breivik H, Eisenberg E, Emmanuel A, Laroche F, Meissner W, Morlion B. Opioid-induced constipation and bowel dysfunction: a clinical guideline. Pain Med 2017;18:1837–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Poulsen JL, Brock C, Olesen AE, Nilsson M, Drewes AM. Evolving paradigms in the treatment of opioid-induced bowel dysfunction. Therap Adv Gastroenterol 2015;8:360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Slappendel R, Simpson K, Dubois D, Keininger DL. Validation of the PAC-SYM questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur J Pain 2006;10:209–17. [DOI] [PubMed] [Google Scholar]
- [29].Symproic. Symproic [package insert]. Florham Park: Shionogi Inc, 2017. [Google Scholar]
- [30].Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010;22:424–30, e96. [DOI] [PubMed] [Google Scholar]
- [31].Viscusi ER, Barrett AC, Paterson C, Forbes WP. Efficacy and safety of methylnaltrexone for opioid-induced constipation in patients with chronic noncancer pain: a placebo crossover analysis. Reg Anesth Pain Med 2016;41:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weber HC. Opioid-induced constipation in chronic noncancer pain. Curr Opin Endocrinol Diabetes Obes 2016;23:11–7. [DOI] [PubMed] [Google Scholar]
- [33].Webster L, Chey WD, Tack J, Lappalainen J, Diva U, Sostek M. Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther 2014;40:771–9. [DOI] [PubMed] [Google Scholar]
- [34].Webster L, Tummala R, Diva U, Lappalainen J. A 12-week extension study to assess the safety and tolerability of naloxegol in patients with noncancer pain and opioid-induced constipation. J Opioid Manag 2016;12:405–19. [DOI] [PubMed] [Google Scholar]
- [35].Webster LR, Michna E, Khan A, Israel RJ, Harper JR. Long-term safety and efficacy of subcutaneous methylnaltrexone in patients with opioid-induced constipation and chronic noncancer pain: a phase 3, open-label trial. Pain Med 2017;18:1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003;35:253–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.