Regenerative medicine in stroke involves therapies that induce tissue repair and recovery. This is a distinct approach from reducing stroke damage: restoring blood flow, reducing cell death or limiting secondary progression of injury. These three areas have a very concise or limited focus: restoring blood flow involves lysing or removing clots. Reducing cell death means neuroprotection. Limiting secondary damage involves modulating process of inflammation or delayed apoptosis. In contrast, tissue regeneration after stroke relates to many potential therapeutic targets, such as enhancing angiogenesis, neurogenesis, or gliogenesis; promoting axonal sprouting; stabilizing injured synaptic connections; or modulating excitatory/inhibitory balance in brain circuits. Single molecular targets may promote one specific tissue repair process, but clinical success is likely to occur if many of these reparative events are stimulated by one therapeutic treatment. This concept has informed the stem cell field in stroke. In experiments with transplantation of stem/progenitor cells in stroke, tissue repair can occur through direct formation of or replacement to neurons or glia, production of growth factors and cytokines, and/or stimulation of the cellular progenitors that lead to angiogenesis, neurogenesis and gliogenesis.
Tissue repair and recovery after stroke has been shown with the first wave of studies in the field: the application of the easiest to produce stem or progenitor cells, such as adult progenitor cells (mesenchymal stromal cells, multipotent adult progenitor cells, hematopoetic stem/progenitor cells) or very early neural precursor cells that are differentiated from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs). With adult progenitor cells, isolation and expansion of the cells is relatively straightforward and application to stroke has progressed into two clinical trial efforts (Athersys, SanBio). The differentiation of ESCs or iPSCs into a very early neural precursor is a default cellular program, and can be done with relatively simple methods. As a result, ESC- and iPSC-NPC delivery into stroke models has been studied for many years. However, the field has recently advanced in its ability to take pluripotent stem cells and differentiate these into more specific neurons and glia. This review will discuss evolving approaches in stroke regenerative medicine: from ESC- or iPSC-NPC transplantation to transplants of stem cell-derived neurons or glia that are more differentiated and more closely related to the actual damaged cells in the brain after stroke. This review will cover first ESC and iPSC-derived neuronal cells, and then ESC and iPSC-derived glial cells (Fig. 1).
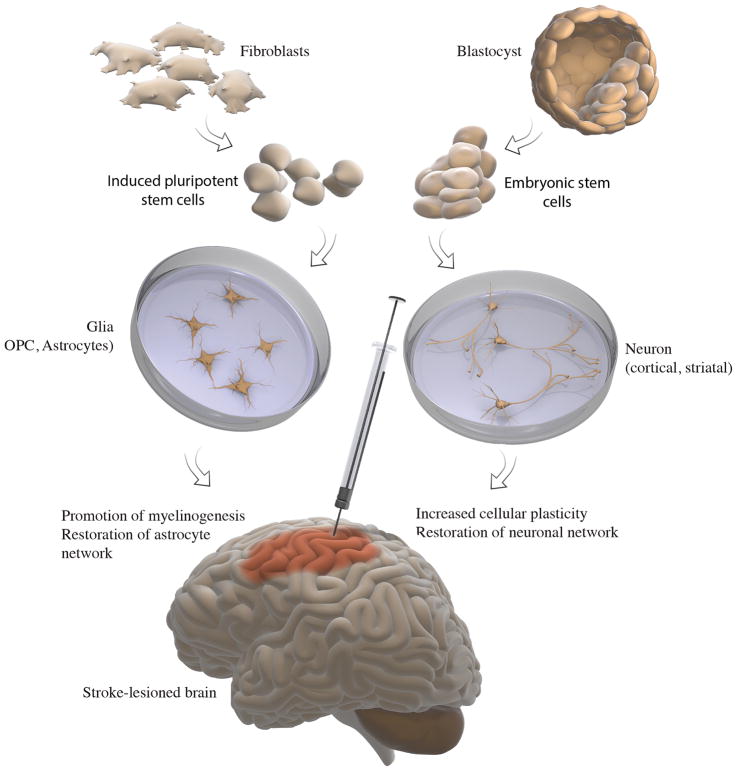
Fig. 1. Application of pluripotent stem cells in cell therapy for stroke-injured brain.
(i) Pluripotent stem cells can be derived from blastocyst (embryonic stem cells, ESCs) or through reprogramming of post-mitotic somatic cells, most commonly fibroblasts, generating induced pluripotent stem cells (iPSCs). (ii) Both ESCs and iPSCs can be treated in vitro to generate glia and neurons. (iii) Transplantation of glial and neuronal cells at early stages of their development into stroke-injured brain can lead to functional recovery through promotion of myelinogenesis and/or restoration of astrocyte network (glia) or by increasing cellular plasticity and/or restoring neuronal network (neurons).
Method of derivation of neuronal cells from ESCs and iPSCs
ESCs and iPSCs, being pluripotent in nature, can give rise to different types of neurons both in vitro and after intracerebral transplantation. There are several methods which have been developed to obtain neurons from these pluripotent cells. The most commonly used protocols for the generation of NPCs from pluripotent cells involve several steps, including generation of embryoid bodies and treatment with a neuroectoderm inducer retinoic acid, or by inhibition of TGFβ and BMP. The procedures may also involve co-culturing with other cells and manipulations with gene expression1. These methods are often complicated and involve use of undefined culture medium with corresponding variable outcome. Neurons can be also generated by using of monolayer cultures of neural progenitors derived from pluripotent cells2. The resulting NPCs can be further expanded by growth factors either as attached monolayers or as floating neurospheres.
Long-term self-renewing neuroepithelial-like stem cells (lt-NESCs) can be also generated both from ESCs and iPSCs3. They are generated from neural rosette-like structures developed from emryoid bodies and can be continuously expanded in the presence of FGF2 and EGF. These cells have stable neuronal and glial differentiation competence with hindbrain specification. Most importantly, lt-NESCs have capacity to generate functionally mature human neurons. These cells resemble NPCs but with greater commitment in their molecular profile to neurons that, in development, will form hindbrain structures.
Different types of neurons derived from ESCs and iPSCs
Pluripotent stem cell-derived neuronal progenitors can be driven with various treatments to differentiate into specific neuronal subtypes i.e., spinal motor4, cerebellar5, dopaminergic4, or cortical interneurons6 and projection neurons7. The first convincing study demonstrating neuronal differentiation of mouse ESCs grafted in the stroke-lesioned brain was carried out on rats using endothelin-induced middle cerebral artery occlusion8. This study showed that grafted cells can partially survive for 12 weeks after transplantation and differentiate with high yield (25–30%) into immunohistochemically mature neurons of diverse neurotransmitter-subtypes such as cholinergic (1.4%), serotonergic (1.8%) and GABAergic neurons as well as striatal neurons expressing substance P (1.4%) or DARPP32 (6.4%). A small portion of grafted cells also differentiated into glial cells (8%). Importantly, grafted cells exhibited electrophysiological characteristics of mature neurons. Moreover, the authors also observed spontaneous excitatory post-synaptic currents in graft-derived cells indicating on their capacity to receive synaptic input. Similar results have been obtained with primate ESC-NPCs, transplanted into mice, with differentiation into several distinct subclasses of neurons and axonal extension from the transplanted cells to distant sites in the brain9.
Human-derived ESCs have been widely used in recent years for the generation of different types of neurons4,10,11. In study carried out by Daadi et al. ESC-derived NPCs uniformly expressed nestin, vimentin and radial glial markers. When these ESC-NPCs were implanted into the ischemic striatum in rats they migrated towards the lesion, and enhanced recovery of motor control10. After transplantation, the ES-NPCs stopped proliferating and efficiently (60%) differentiated into Tuj1+ neurons, with 50% being GABAergic and only 2% glutamatergic. The same group also demonstrated10 that the grafted cells exhibited synaptophysin staining and showed ultrastructural and electrophysiological evidence for synaptic connections. Human ESC-NPCs were also transplanted into the stroke-lesioned cortex of rats12. After 2 months, the majority of the transplanted cells were nestin+. A portion of transplanted cells expressed neuronal markers, whereas only a few cells co-localized with astroglial or oligodendrocyte markers. In the same model of cortical stroke, human ESC-NPCs transplanted into the basal ganglia 1 week after the insult at 3 weeks after transplantation showed differentiation into neurons (65%), astrocytes (15%) and oligodendrocytes (8%)13.
Involvement of basal ganglia lesion is very common during middle cerebral occlusion in humans. It is possible to direct human ESCs to cells with specific markers of striatal neurons that are enriched in the striatum, such as DARPP32+ striatal GABAergic neurons11. When transplanted into the quinolinic acid-lesioned striatum of mice these cells differentiated into DARPP32+ GABAergic neurons, which projected to the substantia nigra, received glutamatergic and dopaminergic inputs, and improved impaired motor function. These data bring the opportunity to generate specific striatal projection neurons which could be used as a source for intracerebral transplantation in the stroke-damaged brain. However, predominantly striatal lesions are quite rare in stroke patients, the resulting functional impairments are mild, and spontaneous recovery is efficient 14. Therefore, stem cell-based therapy will most likely be needed particularly for patients with cortical or combined cortico-subcortical lesions.
Although several studies used mouse iPSCs as a source for the intracerebral transplantation in mice15 and rat16 models of stroke, the majority of studies have been carried out using human iPSCs. The reprogrammed human iPSCs have been transplanted directly as iPSCs17. However, more often, before intracerebral transplantation in animal models of stroke, iPSCs are pre-differentiated towards NSPC phenotype18–22 or transformed into lt-NESC23–26. These in vitro treatments are carried out with 2 main goals: to bias the fate of the cells towards a neuronal phenotype and to avoid possible tumorogenicity by removing pluripotency. Transplanted iPSCs cells can be detected by human-specific antibodies or GFP (when iPSCs are pre-labelled with this marker) up to 10 weeks after transplantation with variable survival rate between the different studies, most likely due to factors such as host strain (i.e., nude rats vs. immunocompetent rats) and species23 Although, the survival time of the animals after intracerebral transplantation either iPSC-NSPC or iPSC-lt-NESCs in different studies varies from two21 up to ten 23 weeks, in all studies grafted cells expressed early or/and mature neuronal markers. Among early neuronal markers, grafted cells expressed nestin18–20, DCX23,24,26, and βIII tubulin19,20. In several studies, transplanted human iPSC-derived cells differentiated into mature neurons and showed immunoreactivity for general mature neuronal markers such as NeuN18,22,26, MAP218,20, HuD23,24,26 but also expressed more specific phenotypic makers such as GABA/GAD6518,26, glutamatergic marker kidney-type glutamate (KGA) 26, dopaminergic marker TH18 and maker for striatal projection neurons DARPP3218,26.
Different treatment of cells before or during transplantation could affect their differentiation in the host brain. The attempt by Lam and colleagues21 to improve survival of transplanted iPSC-NPCs to the infarct cavity of stroked mice through encapsulation in a hyaluronic acid hydrogel matrix did not lead to increased number of cells in the graft but favored DCX+ neuroblast formation at 1 week after transplantation. Differentiating iPSC-lt-NESCs towards neurons with a cortical phenotype before intracerebral transplantation in stroke-subjected rats resulted in more efficient conversion to mature neurons with morphological and immunohistochemical (increased number of Tbr1+ cells) characteristics of a cortical phenotype and higher axonal projection density at 2 months after transplantation26. These published studies clearly indicate that if human iPSCs are transformed into iPSC-NPCs or iPSC-lt-NESCs after transplantation in the stroke-damaged brain they become prone to develop into cells with a neuronal phenotype. Differentiating these cells into more specific subtypes of neurons promotes greater integration into the brain.
In the majority of rodent studies, transplantation in stroke-damaged brain has been carried out within 1–2 days after the insult. However, several studies have demonstrated that a positive effect of stem cell transplantation on functional recovery might occur also when cells are implanted at 1 week after stroke20,21,23,27. Moreover, it was shown that transplantation of human ESC-derived NPCs both in young and aged rats improved stroke-impaired behavior when delivered intracerebrally at 3 weeks after the insult 28. Also, delayed transplantation (at 6 weeks after stroke) of NPCs derived from human fetal striatum did not influence cell proliferation, magnitude of migration, or neuronal differentiation in the grafts29. It is conceivable, that the most suitable time for transplantation after stroke in humans will be from several weeks up to 3 months. However, this prediction needs to be supported by further experimental and clinical data.
Degree of behavioral improvement of ES- or iPS neuronal cell transplant
The analysis of all published papers, which performed ESC-NPC and iPSC-NPC transplantation after stroke and carried out the assessment of the behavioral/functional recovery revealed that virtually in all studies some degree of improvement due to cell implantation was observed. A beneficial effect has been seen with early transplantation, less than three days from the stroke, and later transplantation times. These improvements were observed in general neurological score27, in motor12,13,19,23,24,26,27, in sensorimotor10,12,19,22–24,27 and in memory function tests. This general improvement with ESC or iPSC transplantation implies that there may indeed be a general effect of a progenitor cell in its action on adjacent, injured tissue. In several studies in which iPSC or iPSC-NPC transplantation did not produce behavioral recovery, these cells formed tumors 20. It is conceivable that direct brain pathology caused by transplanted cell tumorigenesis prevented a beneficial effect on functional recovery. The formation of tumors from transplanted cells is a potential problem in all stem/progenitor therapies and a focus of the regulatory pathway of cell therapy
Mechanisms of action of ES- and iPSC-NPCs
The mechanisms underlying promotion of functional recovery in experimental stroke observed as a result of implantation of pluripotent stem cell-derived NPCs remain mostly unknown. Most of the studies indicate that the grafted cells promote functional improvement by mechanisms other than neuronal replacement - an effect of the transplant that is through induction of distinct tissue responses in the injured brain. More recent studies indicate that NPC transplants may differentiate into functional neurons and integrate into the post-stroke brain.
In their effect in inducing changes in the injured brain, a consistent finding in the transplantation field is that the ESC or iPSC-NPCs reduce secondary damage in stroke. After the acute cell detain in stroke, there is slowly progressive secondary element of tissue loss in connected brain structures. Several clinical30,31 and experimental32 studies demonstrated secondary alterations and cell loss after stroke in the areas functionally related to the lesion site. Analysis of postmortem material from patients with MCA infarction at least 4 months prior death demonstrates neuronal loss in the ipsilateral thalamus31 and substantia nigra30 and subcortical ischemic lesions induce thinning of connected cortical regions33. In rodent stroke models, iPSC-NPC transplantation reduced overall damage and tissue loss in the ischemic hemisphere, with transplantation both within days of the stroke or even the first week13,24.
A take-away point from these studies is that observed behavioral improvements seem to be related to a graft-exhibited paracrine effect in the remaining brain host tissue. The major argument in support of this assumption is that functional recovery is often observed much earlier than grafted cells differentiate to certain phenotype and thus will be able to exhibit their respective function. Multiple mechanisms have been proposed for this paracrine effect of stem cell mediated therapies. Among them neuroprotection, promotion of progenitor cell responses in the processes of angiogenesis and neurogenesis, and immunomodulation are most feasible mechanisms. Notably, all these mechanisms are based on the assumption that grafted cells through releasing different factors and molecules act on the surviving neurons of the host brain tissue, as well as glial and immune cells.
This paracrine effect of produced by transplanted human pluripotent stem cells might be due to secretion of plasticity-promoting trophic and other factors. Several studies implicated release of vascular endothelial growth factor (VEGF) from transplanted stem cells as mechanism for improved post-stroke recovery16,34,35. VEGF induction in the stroke-injured brain by ESC- or iPSC-NPC transplantation may be transient, but the improved behavioral effects are long lasting23,24 VEGF production by the transplanted cells themselves is a second mechanism of a paracrine effect, induced by the transplanted cells. Transplantation of a fetally-derived NPC produces VEGF-related effects in dendritic sprouting, axonal plasticity, and axonal transport36. It should be emphasized, although, that increased VEGF signaling is one possible explanation for the beneficial effects and other mechanisms or secreted factors, not explored in these studies, could be responsible for improved behavioral performance.
The plasticity of the post-stroke surviving brain tissue might be also increased at cellular level through promotion of post-stroke neurogenesis or effects on the immune response after stroke. iPSC-NPC transplantation in stroke promotes proliferation in the subventricular zone and migration of cells with markers of immature neurons to the site of stroke damage18,37. The exact cellular mechanism for enhanced post-stroke neurogenesis in behavioral recovery remains unclear. An inflammation-suppression capacity has been also shown for pluripotent stem cell-derived cells in animal models of stroke and this mechanism is widely considered as possible way for transplanted cells to promote functional recovery. Transplantation of iPSC-NPCs very early after stroke (24 hours) reduces inflammatory cytokine and chemokine production in the brain and secondary blood brain barrier opening19. Early transplantation of iPSC-lt-NESC or fetally-derived NPCs modulates microglial/macrophage responses to stroke24,37 and alter the balance of pro- and anti-inflammatory cytokine signaling
Transplanted ESC- or iPSC-NPCs may also differentiate into mature neurons and directly integrate into the post-stroke brain. Neuronal integration of grafted ESC- or iPSC-NPCs injured host neural network will most likely lead to optimum functional recovery after stroke, but direct evidence that neuronal replacement really occurs is virtually lacking. However, accumulating evidence indicate on potential of grafted ESC- or iPSC-NPC-derived neurons to reconstruct neuronal circuitry. It has been shown that transplanted ESC- or iPSC-NPCs show spontaneous postsynaptic currents indicative of neurons 8,10,23 and have ultrastructural evidence of synaptic formation10,25. Graft-derived neurons in the cortex exhibit AMPA receptor-mediated evoked currents by stimulating a cortical region remote from the transplant23 and respond electrophysiologically to peripheral stimulation25. Transplanted iPSC-NPCs generate long-distance connections, such as from striatum to globus pallidus23, thalamus9 or other distant sites27. In cortical stroke and iPSC-NPC transplantation, transplanted cells can extend their axons even into contralateral cortex26. Using rabies virus tracing of direct synaptic input, cortically transplanted iPSC-NPCs receive connections from adjacent intact cortex after stroke25. However, it has still unclear whether neuronal replacement and integration in injured circuitry of grafted cells contribute to the long-term recovery of impaired motor, sensory or cognitive functions following stroke. Modern methods such as optogenetics can be used to inhibit or stimulate the activity of grafted neurons at different stages of post-stroke recovery while animals are performing various behavioral tasks as it has been demonstrated in animal model of Parkinson’s disease38. This approach will be instrumental in determining the mechanisms underlying functional recovery and the significance of integration of grafted cells in host neural circuitry39.
Pluripotent-derived glial cells
Human induced pluripotent stem cells (hiPSCs) have been efficiently differentiated to astrocytes40 and oligodendrocyte progenitor cell (OPCs)41,42. Demyelinating diseases, injuries, and conditions, including pediatric leukodystrophies, white matter stroke, radiation-induced damage after cancer therapy, and spinal cord injury (SCI), are characterized by the loss or dysfunction of oligodendrocytes and the primarily death of glial cells. A more OPC- or astrocyte-based therapy is ideally suited for brain repair due to the completely different cellular constituents of all of these diseases.
Replicating the white matter ischemic damage seen in humans has proven to be relatively difficult in experimental animals. Overall, rodent stroke models have many well-recognized limits, such as differences in tolerance to cerebral edema, a small region of subcortical white matter to model lacunar infarction, and important molecular differences in thrombotic, inflammatory, and DNA repair cascades compared with humans43. Although it is not possible to duplicate all components of human white matter stroke in an animal model, it is essential to control infarct location. Different kinds of vasoconstrictor drugs (i.e. L-NIO or ET-1) are used to significantly reduce local blood flow to levels that produce ischemic injury, when injected directly into parenchyma, and to induce precise and reproducible focal ischemic lesions in gray or white matter without disruption of the BBB. Although these white matter stroke models would not be suitable to model vasogenic edema, the histological studies show significant similarities to human white matter stroke. Axonal injury is another hallmark of white matter stroke that is replicated by vasoconstrictor-induced ischemia44. Compared to the rodent, the pig brain has greater anatomical and physiological similarities to humans with respect to gray to white matter composition, blood flow, gyral patterning, metabolism, and size - key factors that directly affect injury evolution, tissue recovery and treatment development45. The development of primate and higher mammal stroke models is an important goal but without institutional change in animal facilities and costs, rodent models will continue to provide the predominant basic science research into the mechanisms of neuroprotection and neural repair after stroke.
Induced pluripotent stem cell-derived OPCs (iPSC-OPCs)
Pre-differentiation into the oligodendroglial lineage has been shown to be more efficient for remyelination-mediated repair than grafting undifferentiated or uncommitted cells. Human pluripotent stem cell-derived OPCs are capable of rescuing brain function through remyelination in a mouse model of congenital hypomyelination46, promote functional recovery in a rat model of radiation-induced brain trauma47, and yield encouraging initial clinical results for cervical spinal cord injury48. Moreover, ESC-OPC transplantation is the focus of a clinical trial in spinal cord injury49. These results suggest that re-myelination is a target for a neural repair therapy in many brain diseases, and may also be a target in stroke, where white matter injury and oligodendrocyte loss are prominent44.
However, differentiating pluripotent stem cells along the oligodendrocyte lineage has been a long-standing challenge in the field46. Several protocols for the differentiation of human iPSCs to OPCs have been published50. The process is lengthy, usually taking more than 3 months. This might hinder the clinical utility of a human OPC therapy, especially if the goal is to use autologous cell transplants, since the time window for beneficial cell transplantation might be shorter than the differentiation protocols. Longer differentiation times are needed for greater lineage commitment or to generate mature oligodendrocytes, which lose the ability to migrate and remyelinate spared axons.
Induced pluripotent stem cell-derived astrocytes (iPSC-Astros)
Astrocytes have a central role in brain development and function, and so have gained increasing attention as an source for a stem cell-based therapy for stroke, multiple sclerosis, congenital or early myelin loss in periventricular leukomalacia, and the hereditary and metabolic disorders of myelin loss, the pediatric leukodystrophies51. Astrocytes normally provide trophic and tropic support to neurons, and have important functions in protecting neurons from toxic levels of glutamate and potassium. Additionally, normal astrocytes have the ability to migrate along white matter tracts after transplantation into the brain; this migratory capacity may be useful in disseminating a transplant to widespread areas of the post-stroke brain. iPSC-Astros differentiated by using chemically defined, xeno-free protocols can be maintained at an immature stage in culture40,52. Moreover, iPSC-derived immature astrocytes can be further differentiated to astrocytes with defined mature phenotypes40. However, it remains unclear how precisely engrafted glial progenitors can recapitulate the pleomorphism of the host glial network they are intended to replace. In particular, the extent to which the development of an astroglial morphological and functional phenotype in the adult brain is cell-autonomous or context-dependent remains unclear. Interestingly, several studies have proven that iPSC-derived immature astroglial transplants promote myelinogenesis and improve behavioral outcome in animal models of periventricular leukomalacia. These results implicate a novel strategy for promoting myelinogenesis by iPSC-derived immature astroglia that may be extended from these non-stroke conditions into stroke.
Advantages and limitations of ESC- and iPSC-neural cells
Currently, both ESC- and iPSC-derived cells are considered as potential source for cell therapy in stroke. However, there is still ethical controversy on clinical use of ESCs. Further, ESCs are by definition foreign to the transplant recipient - they are an allogeneic transplant and likely will need some degree of immunosuppression. iPSCs have an advantage as compared to ESCs by providing a potential source of patient-specific cells for transplantation. iPSCs being derived from skin biopsy have virtually no ethical concerns in contrast to ESCs obtained from human embryos. However, both viral DNA constructs, which are permanently integrated into the host genome, and the use of the c-myc oncogene as one of the transcription factors to produce iPSCs increase the chance of tumorogenecity53. Recently developed non-integrating reprogramming methods based on episomal vectors, synthetic mRNAs, and Sendai viruses54 allows efficient production of iPSCs from various somatic cells for potential future applications in clinical settings that avoid these problem genome integration problems. Some groups have developed iPSCs without c-myc, by using nanog and lin-28 instead20 or only Sox2 and Oct415. Such human iPSCs that are free of vectors and transgenes have been used to generate NPCs with subsequent transplantation in stroke model20,22. In these studies no tumors were detected after 4 weeks20 or 12 months22 after transplantation in stroke-lesioned brain.
The challenges of developing autologous neural therapies
When considering the pros and cons of application of iPSCs as an autologous source for stroke patients, there are several factors which needs to be taken into account (Table 1). Firstly, the risk for stroke in 75–84 years old is 25-fold higher than the risk for 45–54 years old people55. The vast majority of stroke patients are older than 75 years and it is unclear how reproducibly and efficiently one can generate iPSCs from aged sources. Although, some studies show successful generation of iPSCs from aged humans since the major bulk of the existing pre-clinical studies are based on iPSCs derived from embryonic, postnatal or young/non-aged fibroblasts further investigations are needed. It is of great importance to determine whether iPSCs derived from aged patients are similarly beneficial for post-stroke functional recovery. Secondly, many studies in the field have transplanted iPSC-derived cells in acute (directly after stroke) or sub-acute (24 h to 1 week after onset of insult) time points, as noted above. The efficient generation and expansion of iPSCs from an aged patient’s skin fibroblasts within this timeframe based on existing technologies is not feasible. Currently, generation of well-characterized iPSCs, pre-differentiated towards desired a neuronal phenotype and produced in a sufficient number of cells for transplantation might take at least 7 weeks20,23,26.
Table 1.
Pros and Cons of different pluripotent sources for stem cell therapy in stroke patients
| Pros | Cons | |
|---|---|---|
| Autologous iPSCs | - No need for immunosupression - Ethically non-controversial |
- Long time for generation, validation and expansion - Old age of the patient as a donor for fibroblasts |
| Allogeneic iPSCs (HLA matched) | - Minimal need (?) for immunosupression - Ethically non-controversial - Freely available on demand - Easily expandable |
- High expensive to generate and validate all HLA haplotypes. - Need for special facilities for storage and expansion of lines |
| ESCs | - Less genetic manipulation - Freely available on demand - Easily expandable |
- Ethically controversial |
Recently, generation of functional neurons with different phenotype has been demonstrated through direct conversion from fibroblasts (termed induced neurons, iNs)26,56 and this process is much faster than iPSC production. Forced expression of the three neurodevelopmental transcription factors Ascl1, Brn2, and Myt1l is sufficient to convert mouse fibroblasts into iNs with morphology and elecrophysiological properties closely resembling that of mature primary neurons. Importantly, iNs can efficiently survive intracerebral transplantation and develop morphological properties of mature neurons57. However, currently the efficiency of direct conversion is relatively low. This means that direct neuronal conversion of ESC or iPSC cells may not be a process that can be scaled up to the billions or trillions of cells that would be necessary for a clinical therapy. Small molecules can be used to improve efficiency of iN cell conversion57, and to convert human58 and mouse fibroblasts59 to functional neurons, suggesting that this field may evolve as viable source for a transplantation therapy.
Isolation, and validation of iPSCs and development of NPCs or lt-NESCs for individual stroke patients based on currently available methodology might be too complicated and time consuming procedure which might fail to be useful within existing therapeutic window. However, a new compelling alternative to using patient-specific cells for transplantation could be to create an iPSC bank which can then be used for allografting trials in patients. Such a bank will provide iPSC lines generated under GMP conditions, well-characterized, comprehensively tested and cryopreserved with all potential HLA haplotypes matching the population of respective countries60. It has been reported, that in non-human primates autologous transplantation without immunosuppression of iPSC-derived neural cells is beneficial in terms of the immune response and cell survival compared to allogeneic grafts 61. Importantly, the same team recently demonstrated that haploptype-matching reduces the immune response and increases the survival of grafted dopaminergic neurons in cynomolgus macaques62. However, MHC matching did not completely evade the immune response. Therefore, it was proposed that MHC matching might not be sufficient to avoid immunosuppression but could reduce the dose and duration of the immunosuppressive treatment. Establishment of iPSC banks has been considered in several countries including Japan, USA, and UK. The most advanced iPSC bank is located in Japan63 and by 2022 is expected to have about 60 iPSC lines covering all HLA haplotypes for the entire population of Japan. An iPSC bank is likely to be extremely useful also for the treatment of stroke patients, considerably reducing both the costs and the time between the insult and cell transplantation.
Conclusions
Pluripotent stem cells can differentiated into immature neurons (NPCs) and more differentiated and specific neuronal subtypes as well as astrocytes and oligodendrocyte precursor cells. Most experimental studies in stroke have been performed with transplantation of NPCs. However, transplantation of ESCs or iPSCs that have been differentiated into more committed cortical or striatal subtypes of neurons show substantial synaptic integration into the post-stroke brain and may respond to cues specific to their brain region. Transplanted NPCs and more committed or mature neurons promotes repair and recovery through a paracrine effect on injured brain, reducing secondary tissue loss and promoting angiogenesis, neurogenesis, gliogenesis and modulating neuroinflammation. Stroke damages not just neurons of course, and astrocyte and OPC therapies promote remyelination and recovery in many brain injury models, providing new directions in stroke. ESCs as a source for a cell therapy in stroke have ethical and practical limitations that may be overcome by iPSC approaches, particularly in the generation of iPSC haplobanks.
Acknowledgments
Sources of Funding
This work was supported by Swedish Research Council, Swedish Brain Foundation, Torsten Söderberg Foundation, Region Skåne, Sparbanksstiftelsen Färs & Frosta, California Institute of Regenerative Medicine, AHA grant 14BFSC17760005, United States and NIH, grants NS085019 and NS081055 (NINDS) and DISC1-08723 (CIRM).
Footnotes
Disclosures
None.
References
- 1.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 2.Erceg S, Lainez S, Ronaghi M, Stojkovic P, Perez-Arago MA, Moreno-Manzano V, et al. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One. 2008;3:e2122. doi: 10.1371/journal.pone.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falk A, Koch P, Kesavan J, Takashima Y, Ladewig J, Alexander M, et al. Capture of neuroepithelial-like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PLoS One. 2012;7:e29597. doi: 10.1371/journal.pone.0029597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacpoole SR, Bilican B, Webber DJ, Luzhynskaya A, He XL, Compston A, et al. Efficient derivation of npcs, spinal motor neurons and midbrain dopaminergic neurons from hescs at 3% oxygen. Nat Protoc. 2011;6:1229–1240. doi: 10.1038/nprot.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erceg S, Ronaghi M, Zipancic I, Lainez S, Rosello MG, Xiong C, et al. Efficient differentiation of human embryonic stem cells into functional cerebellar-like cells. Stem Cells Dev. 2010;19:1745–1756. doi: 10.1089/scd.2009.0498. [DOI] [PubMed] [Google Scholar]
- 6.Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486. S471. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, et al. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, et al. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- 10.Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, et al. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: Cell survival and functional recovery. Eur J Neurosci. 2009;29:562–574. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim DY, Park SH, Lee SU, Choi DH, Park HW, Paek SH, et al. Effect of human embryonic stem cell-derived neuronal precursor cell transplantation into the cerebral infarct model of rat with exercise. Neurosci Res. 2007;58:164–175. doi: 10.1016/j.neures.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Delavaran H, Sjunnesson H, Arvidsson A, Lindvall O, Norrving B, van Westen D, et al. Proximity of brain infarcts to regions of endogenous neurogenesis and involvement of striatum in ischaemic stroke. Eur J Neurol. 2013;20:473–479. doi: 10.1111/j.1468-1331.2012.03877.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu SP, Fu RH, Wu DC, Hsu CY, Chang CH, Lee W, et al. Mouse-induced pluripotent stem cells generated under hypoxic conditions in the absence of viral infection and oncogenic factors and used for ischemic stroke therapy. Stem Cells Dev. 2014;23:421–433. doi: 10.1089/scd.2013.0182. [DOI] [PubMed] [Google Scholar]
- 16.Chau MJ, Deveau TC, Song M, Gu X, Chen D, Wei L. Ipsc transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells. 2014;32:3075–3087. doi: 10.1002/stem.1802. [DOI] [PubMed] [Google Scholar]
- 17.Qin J, Ma X, Qi H, Song B, Wang Y, Wen X, et al. Transplantation of induced pluripotent stem cells alleviates cerebral inflammation and neural damage in hemorrhagic stroke. PLoS One. 2015;10:e0129881. doi: 10.1371/journal.pone.0129881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang DJ, Lee N, Park IH, Choi C, Jeon I, Kwon J, et al. Therapeutic potential of human induced pluripotent stem cells in experimental stroke. Cell Transplant. 2013;22:1427–1440. doi: 10.3727/096368912X657314. [DOI] [PubMed] [Google Scholar]
- 19.Eckert A, Huang L, Gonzalez R, Kim HS, Hamblin MH, Lee JP. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cells Transl Med. 2015;4:841–851. doi: 10.5966/sctm.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen MB, Yan H, Krishnaney-Davison R, Al Sawaf A, Zhang SC. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J Stroke Cerebrovasc Dis. 2013;22:304–308. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of ips-npcs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater. 2014;24:7053–7062. doi: 10.1002/adfm.201401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohamad O, Drury-Stewart D, Song M, Faulkner B, Chen D, Yu SP, et al. Vector-free and transgene-free human ips cells differentiate into functional neurons and enhance functional recovery after ischemic stroke in mice. PLoS One. 2013;8:e64160. doi: 10.1371/journal.pone.0064160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 24.Tatarishvili J, Oki K, Monni E, Koch P, Memanishvili T, Buga AM, et al. Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor Neurol Neurosci. 2014;32:547–558. doi: 10.3233/RNN-140404. [DOI] [PubMed] [Google Scholar]
- 25.Tornero D, Tsupykov O, Granmo M, Rodriguez C, Gronning-Hansen M, Thelin J, et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017;140:692–706. doi: 10.1093/brain/aww347. [DOI] [PubMed] [Google Scholar]
- 26.Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136:3561–3577. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- 27.Polentes J, Jendelova P, Cailleret M, Braun H, Romanyuk N, Tropel P, et al. Human induced pluripotent stem cells improve stroke outcome and reduce secondary degeneration in the recipient brain. Cell Transplant. 2012;21:2587–2602. doi: 10.3727/096368912X653228. [DOI] [PubMed] [Google Scholar]
- 28.Jin K, Mao X, Xie L, Greenberg RB, Peng B, Moore A, et al. Delayed transplantation of human neural precursor cells improves outcome from focal cerebral ischemia in aged rats. Aging Cell. 2010;9:1076–1083. doi: 10.1111/j.1474-9726.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, et al. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. JCBFM. 2011;31:235–242. doi: 10.1038/jcbfm.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forno LS. Reaction of the substantia nigra to massive basal ganglia infarction. Acta Neuropathol. 1983;62:96–102. doi: 10.1007/BF00684925. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa T, Yoshida Y, Okudera T, Noguchi K, Kado H, Uemura K. Secondary thalamic degeneration after cerebral infarction in the middle cerebral artery distribution: Evaluation with mr imaging. Radiology. 1997;204:255–262. doi: 10.1148/radiology.204.1.9205256. [DOI] [PubMed] [Google Scholar]
- 32.Tamura A, Kirino T, Sano K, Takagi K, Oka H. Atrophy of the ipsilateral substantia nigra following middle cerebral artery occlusion in the rat. Brain Res. 1990;510:154–157. doi: 10.1016/0006-8993(90)90744-v. [DOI] [PubMed] [Google Scholar]
- 33.Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology. 2015;84:1685–1692. doi: 10.1212/WNL.0000000000001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacigaluppi M, Russo GL, Peruzzotti-Jametti L, Rossi S, Sandrone S, Butti E, et al. Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter glt-1 in astrocytes. J Neurosci. 2016;36:10529–10544. doi: 10.1523/JNEUROSCI.1643-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun GH, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiology of disease. 2013;52:191–203. doi: 10.1016/j.nbd.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a parkinson’s disease model. Nat Biotechnol. 2015;33:204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter AR, Patel KR, Astafiev SV, Snyder AZ, Rengachary J, Strube MJ, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair. 2012;26:7–19. doi: 10.1177/1545968311411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, et al. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007;25:419–424. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 42.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 43.Carmichael ST. Rodent models of focal stroke: Size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sozmen EG, Hinman JD, Carmichael ST. Models that matter: White matter stroke models. Neurotherapeutics. 2012;9:349–358. doi: 10.1007/s13311-012-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker EW, Platt SR, Lau VW, Grace HE, Holmes SP, Wang L, et al. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke pig model. Sci Rep. 2017;7:10075. doi: 10.1038/s41598-017-10406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Chao F, Han F, Zhang G, Xi Q, Li J, et al. Pet demonstrates functional recovery after transplantation of induced pluripotent stem cells in a rat model of cerebral ischemic injury. J Nucl Med. 2013;54:785–792. doi: 10.2967/jnumed.112.111112. [DOI] [PubMed] [Google Scholar]
- 47.Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Priest CA, Manley NC, Denham J, Wirth ED, 3rd, Lebkowski JS. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med. 2015;10:939–958. doi: 10.2217/rme.15.57. [DOI] [PubMed] [Google Scholar]
- 49.Manley NC, Priest CA, Denham J, Wirth ED, 3rd, Lebkowski JS. Human embryonic stem cell-derived oligodendrocyte progenitor cells: Preclinical efficacy and safety in cervical spinal cord injury. Stem Cells Transl Med. 2017 doi: 10.1002/sctm.17-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Archer DR, Cuddon PA, Lipsitz D, Duncan ID. Myelination of the canine central nervous system by glial cell transplantation: A model for repair of human myelin disease. Nat Med. 1997;3:54–59. doi: 10.1038/nm0197-54. [DOI] [PubMed] [Google Scholar]
- 51.Goldman SA. Stem and progenitor cell-based therapy of the central nervous system: Hopes, hype, and wishful thinking. Cell Stem Cell. 2016;18:174–188. doi: 10.1016/j.stem.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C, Jiang P, Xue H, Peterson SE, Tran HT, McCann AE, et al. Role of astroglia in down’s syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat Commun. 2014;5:4430. doi: 10.1038/ncomms5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez F, Barragan Monasterio M, Tiscornia G, Montserrat Pulido N, Vassena R, Batlle Morera L, et al. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci U S A. 2009;106:8918–8922. doi: 10.1073/pnas.0901471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015;33:58–63. doi: 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markus H. Stroke: Causes and clinical features. Medicine. 40:484–489. [Google Scholar]
- 56.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereira M, Pfisterer U, Rylander D, Torper O, Lau S, Lundblad M, et al. Highly efficient generation of induced neurons from human fibroblasts that survive transplantation into the adult rat brain. Sci Rep. 2014;4:6330. doi: 10.1038/srep06330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, et al. Direct conversion of normal and alzheimer’s disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell. 2015;17:204–212. doi: 10.1016/j.stem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, et al. Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell. 2015;17:195–203. doi: 10.1016/j.stem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Nakatsuji N, Nakajima F, Tokunaga K. Hla-haplotype banking and ips cells. Nat Biotechnol. 2008;26:739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- 61.Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, et al. Direct comparison of autologous and allogeneic transplantation of ipsc-derived neural cells in the brain of a non-human primate. Stem Cell Reports. 2013;1:283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morizane A, Kikuchi T, Hayashi T, Mizuma H, Takara S, Doi H, et al. Mhc matching improves engraftment of ipsc-derived neurons in non-human primates. Nat Commun. 2017;8:385. doi: 10.1038/s41467-017-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cyranoski D. Stem-cell pioneer banks on future therapies. Nature. 2012;488:139. doi: 10.1038/488139a. [DOI] [PubMed] [Google Scholar]