We quantify the impacts of poaching, Ebola, and habitat degradation on western lowland gorillas and central chimpanzees.
Abstract
We present a range-wide assessment of sympatric western lowland gorillas Gorilla gorilla gorilla and central chimpanzees Pan troglodytes troglodytes using the largest survey data set ever assembled for these taxa: 59 sites in five countries surveyed between 2003 and 2013, totaling 61,000 person-days of fieldwork. We used spatial modeling to investigate major drivers of great ape distribution and population trends. We predicted density across each taxon’s geographic range, allowing us to estimate overall abundance: 361,900 gorillas and 128,700 chimpanzees in Western Equatorial Africa—substantially higher than previous estimates. These two subspecies represent close to 99% of all gorillas and one-third of all chimpanzees. Annual population decline of gorillas was estimated at 2.7%, maintaining them as Critically Endangered on the International Union for Conservation of Nature and Natural Resources (IUCN) Red List. We quantified the threats to each taxon, of which the three greatest were poaching, disease, and habitat degradation. Gorillas and chimpanzees are found at higher densities where forest is intact, wildlife laws are enforced, human influence is low, and disease impacts have been low. Strategic use of the results of these analyses could conserve the majority of gorillas and chimpanzees. With around 80% of both subspecies occurring outside protected areas, their conservation requires reinforcement of anti-poaching efforts both inside and outside protected areas (particularly where habitat quality is high and human impact is low), diligent disease control measures (including training, advocacy, and research into Ebola virus disease), and the preservation of high-quality habitat through integrated land-use planning and implementation of best practices by the extractive and agricultural industries.
INTRODUCTION
To conserve great apes effectively, we need to have reliable estimates of their distribution, density, and abundance, and also to understand the factors driving their population trends in space and time. The objective of this study was to undertake a range-wide, data-driven assessment of these parameters of population status for sympatric African great apes, western lowland gorillas Gorilla gorilla gorilla, and central chimpanzees Pan troglodytes troglodytes. The geographic range of central chimpanzees overlaps with that of western lowland gorillas by 97% (1). Western lowland gorillas occur in six countries of Western Equatorial Africa (WEA): Angola (Cabinda enclave), Cameroon, Central African Republic (CAR), mainland Equatorial Guinea, Gabon, and Republic of Congo (Congo). Chimpanzees (P. troglodytes) inhabit 21 countries and occupy a much wider ecological niche than the gorillas (G. gorilla and G. beringei), which are found in 10 countries. Henceforth, “gorilla” refers to the western lowland gorilla subspecies and “chimpanzee” refers to the central chimpanzee subspecies.
All great apes are protected by national laws and international conventions; thus, it is illegal to kill, capture, or trade in live individuals or their body parts wherever they occur. Despite this legal protection, the combination of poaching with Ebola virus disease (EVD) has been catastrophic for gorillas and chimpanzees (2). Another emerging threat is industrial-scale forest conversion for agricultural plantations, especially oil palm plantations—a type of land use inimical to great apes (3) and other forest-dwelling wildlife. Massive development corridors planned for the continent are proceeding apace, fragmenting and making accessible large areas of previously remote forest, which will, in turn, draw many more people into these sensitive ecosystems (4).
WEA’s protected areas (PAs) cover over 650,000 km2 or 12.2% of the terrestrial surface area (5). This is below the 2020 Aichi Target 11 of at least 17% per country (www.cbd.int/sp/targets/). Instead, large conservation landscapes with PAs at their core, usually surrounded by logging concessions, swamp forests, or community lands, maximize the area of habitat available to wildlife (1, 5).
Wildlife monitoring provides empirical data on population trends, distribution, and the key ecological and human factors affecting them. These data are used to inform conservation actions and evaluate their success, for both effective site management and to identify the best sites to designate as new PAs. Large-mammal population monitoring in African forests involves conducting extensive line-transect and reconnaissance surveys on foot (6, 7), covering a wide range of habitats under differing degrees of human impact. Data on large mammal sightings and signs are collected, including night nests built by great apes and the dung of elephants and ungulates. Up to this point, the nest data have been used to estimate the abundance of great apes at local scales, highlighting some large tracts of forest where gorillas and chimpanzees are abundant.
Our extensive data set, collated through the collaborative efforts of numerous organizations, comprises great ape nest counts from 59 sites in five countries surveyed over 11 years. A preliminary examination of these data provided the science to underpin a regional conservation planning process. The resulting International Union for Conservation of Nature (IUCN) action plan (1) identified several large landscapes in WEA that are key for the conservation of great apes. From the outset, this planning process involved decision makers and other government representatives in producing a set of detailed, budgeted, time-specific actions to be implemented at each site in each conservation landscape. This paper presents our analysis of the region-wide data set and discusses the implications of our results for conservation planning and the maintenance of intact forests in WEA.
RESULTS
Abundance estimates
We estimate that, in 2013, gorillas numbered 361,919 weaned individuals [95% confidence interval (CI), 302,973 to 460,093], and chimpanzees numbered 128,760 weaned individuals (95% CI, 114,208 to 317,039). We used the top-ranked models to map gorilla and chimpanzee distribution as density surfaces (Fig. 1) and to estimate range-wide and country-specific population sizes (Table 1).
Fig. 1. Modeled estimated densities (per square kilometer) of (A) western lowland gorillas and (B) central chimpanzees across their geographic range in 2013.
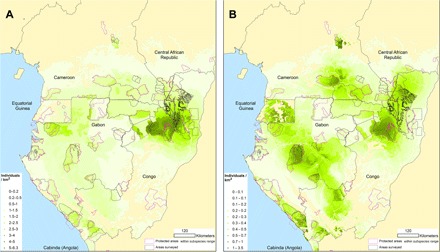
Table 1.
Regional and country-specific abundance estimates for western lowland gorillas and central chimpanzees (with 95% CIs), plus areas of forest, PAs, and FSC-certified concessions. Also shown by country: percentage of regional gorilla and chimpanzee populations; percentage loss of gorillas 2005–2013; area of forest as given by Verhegghen et al. (53) apart from Angola, for which Global Forest Watch data (http://www.globalforestwatch.org/country/AGO/4) were used; and percentage of total regional forest cover per country. Information on PAs and FSC certification was downloaded from the WRI Interactive Congo Basin Forest Atlases (http://www.wri.org/our-work/project/congo-basin-forest-atlases), apart from Angola (Cabinda), for which we used the legal document for the creation of Mayombe National Park (http://extwprlegs1.fao.org/docs/pdf/ang118430.pdf). For Cameroon, the area south of the Sanaga River only is included, which is within the range of western lowland gorillas and central chimpanzees.
| Subspecies |
Year/ measurement |
Cameroon | CAR | Congo |
Equatorial Guinea |
Gabon | Angola | Regional totals |
| Gorilla g. gorilla | 2005 | 49,379 (45,497–127,517) |
5,985 (4,862–12,938) |
264,655 (242,633–309,516) |
2,325 (1,510–3,476) |
123,869 (95,667–242,293) |
3,086 (2,188–6,355) |
449,300 (424,244–595,564) |
| 2013 | 38,654 (34,331–112,881) |
4,695 (3,872–9,705) |
215,799 (180,814–263,913) |
1,872 (1,082–3,165) |
99,245 (67,117–178,390) |
1,652 (1,174–3,311) |
361,919 (302,973–460,093) |
|
| % of total | 10.7 | 1.3 | 59.6 | 0.5 | 27.4 | 0.5 | 100 | |
| % loss | 21.7 | 21.6 | 18.5 | 19.5 | 19.9 | — | 19.4 | |
| Pan t. troglodytes | 2005–2013 | 21,489 (18,575–40,408) |
2,843 (1,194–4,855) |
55,397 (42,433–64,824) |
4,290 (2,894–7,985) |
43,037 (36,869–60,476) |
1,705 (1,027–4,801) |
128,760 (114,208–317,039) |
| % of total | 16.7 | 2.2 | 43.0 | 3.3 | 33.5 | 1.3 | 100 | |
| Forested area | km2 | 117,445 | 69,888 | 213,100 | 21,560 | 224,600 | 6,045 | 652,638 |
| % of total | 18.0 | 10.7 | 32.7 | 3.3 | 34.4 | 0.9 | 100 | |
| PAs (IUCN categories I–VI) |
km2 | 18,853 | 5,467 | 32,632 | 4,346 | 34,216 | 1,930 | 97,444 |
| % of total | 19.3 | 5.6 | 33.5 | 4.5 | 35.1 | 2.0 | 100 | |
| FSC-certified concessions |
km2 | 9,105 | 0 | 26,069 | 0 | 20,677 | 0 | 55,851 |
| % of total | 16.3 | 0 | 46.7 | 0 | 37.0 | 0 | 100 |
Most of these great apes reside in two countries—Congo and Gabon (Fig. 2 and Table 1). Congo harbors 60% of all gorillas and 43% of all chimpanzees in 32.7% of the total forest domain in WEA, whereas Gabon, with the highest percentage of the region’s forests (34.4%), hosts 27% of the gorilla population and 34% of the chimpanzees. Both taxa occur at particularly high densities in northern Congo and southern Cameroon.
Fig. 2. Forest cover and great ape populations by country.
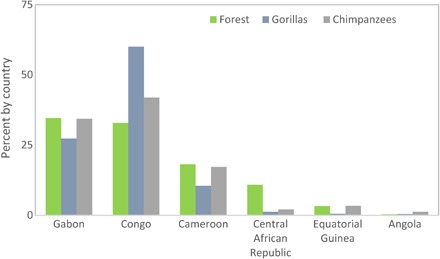
Percentage of total forest cover within the range of western lowland gorillas and central chimpanzees, and percentage of gorilla and chimpanzee populations by country. Arranged in descending order of percentage forest cover.
Population trends
Gorilla populations in WEA declined by 19.4% between 2005 and 2013, with an average annual loss of ca. 2.7%. Nationally, with the exception of Angola (where no survey data were collected), estimated gorilla losses ranged from 18.5 to 21.7% (Table 1). We did not detect a statistically significant change in chimpanzee numbers over the same period.
Predicted density and distribution
Around 13 and 51% of the combined ranges of both subspecies are in PAs or timber concessions, respectively. Sites staffed with wildlife guards—most PAs and all Forest Stewardship Council (FSC)–certified logging concessions—cover only 12.7 and 7.4%, respectively, of the great apes’ range. An additional 3.1% of their geographic range is noncertified concessions protected by guards. Thus, a total of 23.2% of the gorilla and chimpanzee range is protected by guards. The remaining 76.8% is unprotected, leaving the majority of great apes highly vulnerable to poaching.
Gorillas occur at their highest densities in PAs, FSC-certified logging concessions, and swamp forests, and their stronghold is northern Congo. Other areas of importance are southeast Cameroon, north and central Gabon, and parts of southern Congo. Only 22.6% of gorillas in WEA live in PAs, but 20.3% of these are not guarded. A further 21.4% are in FSC-certified logging concessions and another 1.8% in noncertified concessions protected by guards. Overall, 58.7% of gorillas in WEA are not protected by guards.
Chimpanzees have a different pattern of distribution. They occur at low densities in swamp forests and at their highest densities along the Monts de Cristal-Monts du Chaillu mountain range that runs northwest to southeast from Equatorial Guinea through central Gabon to southern Congo. They are relatively abundant along the southern coast of Gabon and contiguous coast of Congo. A third key area is the forests of south-central Cameroon and northern Congo. Only about 19.3% of chimpanzees in WEA are found in PAs, but of these, 16.3% are in PAs with no guards. A further 14.1% of chimpanzees inhabit FSC-certified concessions and are protected by guards, and about 3.7% are in guarded, noncertified concessions. Therefore, the majority (65.8%) of chimpanzees are not protected by guards.
Key drivers of density and distribution
Several human-related variables [Guards, Human Influence Index (HII), Distance to road, Human population density (HPD), Eat gorilla, and Eat chimpanzee] and two natural variables (Canopy height and Ebola) were important predictors of great ape density and distribution (fig. S1 and table S1). For gorillas, the most important predictors—in descending order—were Guards, Ebola, Distance to road, Canopy height, Time (year of survey), HPD, Eat gorilla, and Elevation. For chimpanzees, the predictors—again in descending order of importance—were Eat chimpanzee, Canopy height, Ebola, HII, Guards, Slope, and Elevation (see Materials and Methods for definitions of variables).
Human-related variables
At the time of survey, guards were absent from 37% of the 59 sites. Over half of the surveys (51%) were carried out in PAs; the rest were in logging concessions or in relatively undisturbed areas with few human settlements, such as the vast swamps of northern Congo. Densities of both subspecies were significantly higher in areas where guards were present compared with those where they were not. In areas without guards, gorilla density declined almost linearly as proximity to roads increased (Fig. 3A), but if guards were present, gorilla density gradually rose with increasing proximity to roads (Fig. 3B). Chimpanzee density declined sharply with increased proximity to roads where there were no guards, but if guards were present, Distance to road had no significant influence (fig. S2).
Fig. 3. Estimated conditional dependence of western lowland gorilla nest density on human-related variables for the top-ranked model.

The y axis (nest density) is on the scale of the linear predictor. Estimates (solid lines) with CIs (dashed lines) are shown for (A) proximity to roads without guards, (B) proximity to roads if guards are present, and (C) human population density.
Gorilla density declined as human population density increased (Fig. 3C), except in Equatorial Guinea, where we found no significant relationship. In Cameroon, Congo, and Gabon, chimpanzees tended to occur in areas with low or moderate human influence, and their density dropped rapidly after a medium degree of human influence was reached (Fig. 4A). In CAR (Fig. 4B) and Equatorial Guinea (Fig. 4C), there was an almost linear decline in chimpanzee density with increasing human influence, although this relationship was not significant for Equatorial Guinea (where there are no areas of low human influence).
Fig. 4. Estimated conditional dependence of central chimpanzee nest density on the HII for the top-ranked model.
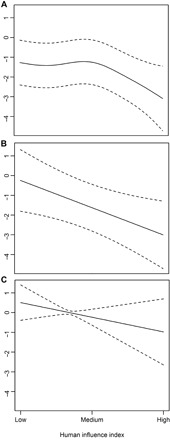
The y axis (nest density) is on the scale of the linear predictor. Estimates (solid lines) with CIs (dashed lines) are shown for (A) Cameroon, Congo, and Gabon combined; (B) CAR; and (C) Equatorial Guinea.
Gorilla and chimpanzee densities were significantly higher in areas where local communities have taboos against eating their meat. At the time of survey, most people at 10% of the 59 sites did not eat chimpanzees, and at 5% of sites, most people did not eat gorillas.
Natural variables
Habitat was an important predictor of both gorilla and chimpanzee densities. Intact forest landscapes (IFLs) are nested in hinterland forests (see Materials and Methods for definitions); just over 30% of the combined range of gorillas and chimpanzees is classified as IFLs and 48% as hinterland forest. More than half (52%) of all gorillas occurred in IFLs, and 67% in hinterland forests. Gorillas were found at higher densities in areas where the canopy height was >25 m (table S1), but were absent or occurred at low densities where canopy height was <15 m. Chimpanzee densities were strongly correlated with canopy height, and no nests were recorded in forests with canopy heights <15 m. Indeed, 41% of all chimpanzees were in IFLs and 60% in hinterland forests. Conversely, chimpanzees occurred at low densities in swamp forests, where canopy height tends to be lower than in terra firma forests. Gorilla and chimpanzee densities were significantly higher in areas not affected by EVD in the previous 20 years; they were only marginally influenced by Slope and Elevation.
Model selection and validation
For each taxon, the final model selected (see table S1) had the largest Akaike’s information criterion (AIC) weight in its set (8). The final model for gorillas had an AIC weight of just over 0.60 and was 1.7 times more likely to be the best model compared to the next-best model in terms of AIC weight ranking. For chimpanzees, the final model had an AIC weight of 0.59 and was 3.1 times more likely to be the best model. On the basis of predictions from these models, great ape abundance estimates for each of the sites surveyed closely resembled those generated by design-based analyses of the 82 surveys at 59 sites.
For both gorillas and chimpanzees, the models selected included the variables Guards, Canopy height, Elevation, Eat gorilla/Eat chimpanzee, Ebola, and geographic location (Latitude, Longitude). Country was always a better predictor than Transparency. For gorillas, human influence was best captured by Distance to road and HPD (Fig. 3), whereas for chimpanzees, it was best captured by HII. The final chimpanzee model selected included Slope and an interaction term between HII and Country; a single smooth function was fitted for Cameroon, Congo, and Gabon combined, because the relationship was similar across these three countries and the combination improved the AIC value; separate smooth functions were fitted for CAR and Equatorial Guinea (Fig. 4). All of these variables, detailed in Table 2, influenced great ape distribution and densities in the way predicted a priori.
Table 2. Description of spatial variables, data sources, method of calculation, and predicted influence on great ape density.
|
Variable type |
Variable name | Description | Data source |
Method of calculating variable value and variable summary statistics |
Predicted influence on great ape density |
|||||
| Survey site–specific |
Great ape sign |
Number of gorilla or chimpanzee nests per sampling unit (transect or recce segment) |
Survey data sets | Counts per sampling unit | Response variable (note that effectively nest density was the response variable, as effort (kilometers walked) and detectability (effective strip half-width) associated with each count were included as an offset term in each model) |
|||||
| Gorilla | Chimpanzee | |||||||||
| Min. | 0 | 0 | ||||||||
| 1st Qu. | 0 | 0 | ||||||||
| Median | 0 | 0 | ||||||||
| Mean | 2.254 | 1.219 | ||||||||
| 3rd Qu. | 1 | 1 | ||||||||
| Max. | 114 | 58 | ||||||||
|
Time (year of survey) |
Calendar year | Survey data sets and reports |
Calendar year survey was conducted | Gorilla or chimpanzee density negatively associated with surveys completed in recent years |
||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |||||
| 2003 | 2005 | 2006 | 2007 | 2010 | 2013 | |||||
| Guards | Whether or not a site has guards |
Knowledge of sites | Yes or no | Gorilla or chimpanzee density positively associated with sites that have guards |
||||||
| N: | 2407 | |||||||||
| Y: | 3412 | |||||||||
|
Canopy height |
Forest canopy height |
M. Simard, N. Pinto, J. B. Fisher, A. Baccini, Mapping forest canopy height globally with spaceborne lidar. J. Geophys. Res. 116, G04021 (2011) |
0–62 m high across the region, 1-km spatial resolution; from 2005 satellite data |
Gorilla or chimpanzee density positively associated with areas with taller tree cover (intact forest) |
||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |||||
| 0 | 27 | 34 | 30.25 | 37 | 46 | |||||
| Slope | Slope of the terrain | Derived from Elevation (see below) |
0–25°, 90-m spatial resolution | Gorilla or chimpanzee density negatively associated with increasing slope |
||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |||||
| 0 | 1.066 | 2.203 | 3.287 | 4.043 | 25.309 | |||||
| Elevation | Elevation above sea level |
http://srtm.csi.cgiar.org/index.asp, version 4 |
0–1,126 m, 90-m spatial resolution | Gorilla or chimpanzee density negatively associated with increasing elevation |
||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |||||
| 0 | 333 | 493 | 451.9 | 607 | 1,126 | |||||
| HII | Aggregate score for a suite of variables (see data source) |
Last of the Wild data version 2 (http://sedac.ciesin.columbia.edu/wildareas/downloads.jsp#infl) |
HII value corresponding to sample unit location, 1-km spatial resolution |
Density negatively associated with increasing HII (corresponding to higher levels of human influence) |
||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |||||
| 1 | 3 | 5 | 6.485 | 9 | 25 | |||||
|
Distance to road |
Proximity to roads |
World Conservation Monitoring Centre data sets (Interactive Atlases for Central Africa) and local adjustments for errors, per year 2003–2013 |
Distance to the nearest road (km) in year of survey |
Gorilla or chimpanzee density positively associated with increasing distance from roads |
||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |||||
| 0 | 4.355 | 11.820 | 14.588 | 22.505 | 80.290 | |||||
| HPD | Number of people per km2 |
Gridded Population of the World version 4 (http://sedac.ciesin.columbia.edu/data/collection/gpw-v4) |
Population value (/km2) corresponding to sample unit location, 1-km spatial resolution |
Gorilla or chimpanzee density negatively associated with sites with a higher overall human population density |
||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |||||
| 0.598 | 1.470 | 3.427 | 3.951 | 5.230 | 30.543 | |||||
|
Eat gorilla/Eat chimpanzee |
Whether or not gorilla or chimpanzee meat is consumed |
Knowledge of areas | Yes or no | Gorilla or chimpanzee density positively associated with areas where they are not eaten (species-specific analysis) |
||||||
| Gorilla | Chimpanzee | |||||||||
| N: | 396 | 811 | ||||||||
| Y: | 5423 | 5008 | ||||||||
| Ebola | Suspected EVD outbreak occurred in previous 20 years |
Locations of known and suspected EVD outbreaks in Gabon and Congo |
Yes or no | Gorilla or chimpanzee density positively associated with sites where EVD outbreaks did not occur |
||||||
| N: | 5439 | |||||||||
| Y: | 380 | |||||||||
| Country- specific |
Country | Name of country | Not available | Unique value assigned per country | Gorilla or chimpanzee density positively associated with more developed, less corrupt countries where conservation is higher on the agenda |
|||||
| Sampling units per country: | ||||||||||
| Cameroon | 1961 | |||||||||
| CAR | 146 | |||||||||
| Congo | 1476 | |||||||||
| E. Guinea | 83 | |||||||||
| Gabon | 2153 | |||||||||
| Transparency | Degree of corruption |
Transparency International’s Corruption Perceptions Index (CPI) 2003–2013 (year-specific for survey site) (www.transparency.org/policy_research/surveys_indices/cpi) |
Ranges from 0 to 10. Smaller values indicate higher levels of corruption |
Gorilla or chimpanzee density negatively associated with a lower CPI (corresponding to higher levels of corruption) |
||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | |||||
| 1.80 | 2.20 | 2.45 | 2.52 | 3.00 | 3.50 | |||||
| Regional proxies |
Latitude | Latitude coordinate | Calculated in GIS | Approx. centroid of each transect or recce segment |
Not applicable | |||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max | |||||
| −4.582 | −1.974 | 0.991 | 0.748 | 2.594 | 6.151 | |||||
| Longitude | Longitude coordinate | Calculated in GIS | Approx. centroid of each transect | Not applicable | ||||||
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max | |||||
| 9.248 | 11.464 | 13.706 | 13.422 | 14.999 | 17.788 | |||||
In terms of covariates relevant to conservation management, guard presence or absence had the greatest predictive power on gorilla abundance, whereas the taboo against eating chimpanzees had the most explanatory power in the chimpanzee model (Fig. 4). In addition, both Canopy height and Ebola were important covariates in the top-ranked models for both taxa. The explanatory power of Canopy height was similar to that of Distance to road and HII for the gorilla and chimpanzee models, respectively, with less variability in the data explained by HPD for the former model. The explanatory power of Elevation and consumption of gorillas for the gorilla model, and of Elevation and Slope for the chimpanzee model, were much lower. Geographic location (Latitude and Longitude) acted as a proxy for other explanatory variables of great ape distribution that were not available at the scale of this analysis. Part of the variance in the data for both taxa was significantly explained by geographic location.
Scenario-based predictive modeling
Predictive modeling was used to further examine the importance of predictor variables on gorilla and chimpanzee density and distribution across a wide range of conditions.
Gorillas
Presence or absence of guards, proximity to roads, and Ebola were critical determinants of gorilla nest density. First, to isolate the effect of roads and examine the interaction between roads and guards, we contrasted scenarios in Congo at two distances from a road where guards were either absent or present under the following set of conditions: low Elevation, relatively low HPD (~1.5 people/km2), gorillas are eaten, and no Ebola. When there were no guards, gorilla nest density was predicted to be 55% higher far from a road (25 km) than near to a road (0.5 km). The effect of protection was clear: In areas without guards, even far from a road (25 km), gorilla nest density was predicted to be 52% lower than nest density in areas with guards, but only 0.5 km from a road.
Second, to examine the influence of Ebola on predicted gorilla density, we compared an area with guards 0.5 km from a road in Congo to an area in Gabon under the same set of conditions, except that an EVD outbreak had occurred. The gorilla nest density predicted was 75% lower in the areas affected by Ebola, which comprise about 8% of the taxon’s total range.
Chimpanzees
The presence or absence of guards, the degree of human influence, and cultural taboos against eating chimpanzees were critical determinants of chimpanzee nest density. First, to isolate the effects of human influence, we compared an area in Congo with low human influence to a similar area in CAR with low-to-medium human influence under the following set of conditions: The areas were equivalent in terms of Guards (present), Slope (flat), Elevation (low), consumption (humans eat chimpanzees), and Ebola (none documented). The model predicted that chimpanzee nest density would be 22% lower at the CAR site. Second, the positive effect of guards became clearer with a similar comparison between the same low human influence area in Congo with guards, and an area in Equatorial Guinea with medium human influence without guards. This time, predicted chimpanzee nest density at the Equatorial Guinea site was 38% lower than at the Congo site. Third, if we compare the same area in Congo with an area along the Gabon coast where chimpanzees are not eaten, then the predicted chimpanzee nest density in the Gabon site is higher than in the Congo site: 88% at low and 61% at medium-to-high human influence.
DISCUSSION
Predicted abundance and temporal trends
The larger-than-previous estimates for both gorillas [~361,900 compared to 150,000 to 250,000 individuals (2)] and chimpanzees [~128,700, updating an estimate of 70,000 to 117,000 (2)] result from the fact that our predictions cover the entire geographic ranges of these taxa—not only the areas actually surveyed but also the land in between. In the past, no corrections were made for animals in areas that had not been surveyed.
Together, the other gorilla subspecies are estimated to number fewer than 5000 individuals, and they inhabit much smaller geographic ranges than the western lowland gorilla (2). Although our analysis shows that there are many more gorillas remaining in WEA than previously thought, they are declining precipitously, by 2.7% per year over the time frame examined (2005 to 2013). At this rate, the reduction in the gorilla population would be expected to exceed 80% over three generations (2). Therefore, western lowland gorillas are classified as “Critically Endangered” under the IUCN Red List of Threatened Species criteria.
The number of central chimpanzees is also somewhat higher than previous estimates, and using population estimates taken from the Red List (2), they comprise roughly one-third of the species’ total. Although it is unlikely that the chimpanzee population remained stable, any change in their numbers between 2005 and 2013 was not statistically significant. Consequently, the subspecies remains “Endangered” on the Red List.
Monitoring great apes over large geographic scales is currently limited to surveys of the nests they build, rather than direct observations of the animals. Using estimates of nest density to derive estimates of great ape density, distribution, and abundance has the potential to introduce inaccuracies from the different analysis components. The first is the potentially incorrect attribution of a nest to species (gorilla or chimpanzee) based on its characteristics. However, the models we have used for this attribution have shown a high degree of accuracy (see Materials and Methods for details). Second, the conversion rates used to obtain great ape density from nest density, namely, nest creation and nest decay, have the potential to introduce bias. We used a nest-building rate obtained through a long-term study (9). The model used to obtain spatially explicit nest decay rates came from a long-term study that accounted for habitat and nest characteristics, as well as variation in rainfall (10). Models themselves are also prone to biases due to mis-specification, as well as inaccurate or missing explanatory variables. We used the best explanatory variable layers available at the regional scale. In addition, we had the added advantage of being able to validate our model-based results using the design-based estimates for each survey, which do not rely on fitting an unbiased model. Thus, even with these caveats, we believe that our analysis provides reliable information for long-term conservation planning for these great ape subspecies. Although poaching, disease, and forest loss and degradation are known to be the principal threats to all great ape taxa, this regional analysis demonstrates for the first time the quantitative effects on gorilla and chimpanzee densities in a spatially explicit manner.
Our study found that 77.4% of gorillas and 80.7% of chimpanzees in this region live outside the PA network. Clearly, PAs alone are not enough for their long-term survival; additional areas need to be targeted for gorilla and chimpanzee conservation, and the actions necessary to assure their protection must be implemented. We found that the densities of both subspecies are higher where guards are present in suitable high-canopy habitat, and where human influence is low in terms of the density of human settlements, human population size, infrastructure development, industrial agriculture, and extraction activities.
Impacts of human population density, access, poaching, and presence of guards
Over the past 60 years, the proportion of people living in cities in the WEA region has trebled, from around 20% in 1960 to around 60% today (in Gabon, 87% of people live in urban areas) (11). Many urban human populations, living far from wildlife, attribute cultural value to bushmeat, appreciate its taste, and are prepared to pay a premium for it. As the numbers of people residing in the towns and cities increase, demand to supply the commercial bushmeat trade—which includes ape meat—grows, increasing the pressure exerted on wildlife (12). In addition, the synergy of the negative impacts of natural resource exploitation and roads providing easy access to forest for hunters has been well documented (13), and when both are unregulated, great ape populations decline rapidly (14).
We have shown that great apes benefit from the presence of guards, with the positive impact for gorillas being greater than for chimpanzees. This difference may be explained by aspects of their socioecology, as synthesized by Williamson et al. (15): Gorillas live in stable groups, averaging 10 individuals with a single adult male. Chimpanzees live in “fission-fusion” communities, which do not move as a group, but are most often found in small parties or mother-infant pairs that can disperse quickly. Because of their larger social units, group cohesion, and being more terrestrial than chimpanzees, gorillas are both more vulnerable and easier to kill in large numbers with guns. Similarly, Grauer’s gorillas are easier to track than eastern chimpanzees, usually traveling shorter distances in a day and leaving denser trails (16).
Chimpanzees in heavily hunted areas are unlikely to be aggressive toward human adversaries, whereas adult male gorillas put themselves in danger by defending their females and offspring. Gorilla group stability is disrupted if the dominant male is killed by a poacher, and if a group subsequently disperses, then this will likely lead to further deaths through infanticide by an unrelated adult male (15).
In addition, in areas without guards, great ape densities are positively correlated with increasing distance from roads, as hunters’ efforts and travel costs mount (Fig. 3A and fig. S2A). However, where guards are present, gorilla density is high at roadsides, reflecting the gorillas’ liking for the abundant herbs that grow in light gaps created by removal or disturbance of the canopy along roads and for roadside timber storage. Gorilla density remains high up to 25 to 45 km from roads where there are guards, and then decreases (Fig. 3B). Beyond 45 km, only six of our sites were guarded, and an EVD outbreak had occurred in three of those (the maximum distance from a road in this data set was 80 km). There is no significant change in chimpanzee density with increasing proximity to roads when guards are present (fig. S2B), which could be because chimpanzees are able to move stealthily and elude poachers.
Gorillas are less abundant in areas with higher human population densities, again pointing to their vulnerability to poaching. Chimpanzee density is negatively affected by human influence, but the exact nature of this relationship varies by country. The exception for both taxa is Equatorial Guinea, where human variables do not significantly influence density of the few great apes remaining: Human population density is high almost throughout the country, and even the most remote areas are no more than ~15 km from a road. The effects of high human population density and proximity to roads are compounded by the lack of patrols in the PAs of Equatorial Guinea (17).
Chimpanzee density was not significantly influenced by Slope. In contrast, two other studies in heavily hunted montane areas found that chimpanzee densities were higher on steep slopes (18, 19). Hunters are less likely to use steep slopes, which require more effort to access than level land or medium slopes, but perhaps Slope had less influence than other factors in our analysis, because most of WEA has low human population densities and hunting has been less intense than in West Africa.
Impacts of forest loss and degradation
Although historically low compared to other parts of tropical Africa, rates of deforestation and degradation in Central Africa are increasing (20), as the human population in WEA grows by 2.7% annually and doubles every 28 years (it is currently around 43 million) (11). Old-growth forests are being slowly lost as the shifting agricultural edge around human settlements advances, but recent work in the Democratic Republic of Congo (DRC) has shown that a high proportion of shifting agriculture is on land that has been cleared previously, which is effectively a rotation system (21).
Forests are also being degraded where there is selective logging. We used canopy height to reflect two things: selective logging in terra firma forest (overall forest height reduction) and swamp forest (which is naturally shorter than terra firma forest), or both. Gorillas and chimpanzees always require some forested habitat, and they prefer intact forest, with a medium (25 to 35 m) or high (>35 m) canopy. Chimpanzees occur at their highest densities where canopy height is >35 m. Gorilla densities were highest where the canopy was 25 to 35 m high (intact forest), which includes swamp forest, open canopy forest, and forest where the height reduction results from human activity (selective logging, but not agriculture). Chimpanzees depend on fruiting trees as a primary food source; in contrast, gorillas can tolerate and may even prefer more open forest conditions (22), including secondary and swamp forests in which understory herbs are abundant. In addition, chimpanzees nest almost exclusively in trees, usually high in the canopy, whereas gorillas often sleep at or near ground level (23). Finally, chimpanzees are highly territorial, and they are less flexible than gorillas in terms of shifting their home range when they are displaced by human activities. This can cause lethal conflict between neighboring chimpanzee communities (24).
Rates of deforestation are lower in than outside the conservation landscapes, especially in the PAs (5), and these landscapes hold the largest remaining populations of gorillas and chimpanzees. Nonetheless, most great ape habitat in the landscapes is in concessions that are logged repeatedly, with rotation times too short (often only 30 to 40 years) to allow regeneration of the tree species that the great apes rely on (25). By the third rotation, forest structure and composition have been radically changed (26). Empirical data have shown that chimpanzees were much less abundant in logged, unhunted forests than in unlogged, unhunted forests (14). This likely explains the higher densities of chimpanzees along the Monts de Cristal-Monts du Chaillu mountain chain, where logging has been less intense than in lower and flatter terrain, because of the comparative difficulty of access and higher costs of timber evacuation.
Central African forests are only moderately suitable for industrial agriculture (27). However, if economic incentives shift, then industrial agriculture, such as oil palm production, could expand dramatically (to date, less than 1% of gorilla and chimpanzee range has been allocated to palm oil concessions). In contrast to selective logging, large plantations require clear-cutting and would increase forest loss with catastrophic consequences for great apes (3) and other wildlife.
Impacts of cultural taboos
Taboos against eating gorillas exist in only 1% of their range (~7400 km2 in Mbam-Djerem, Deng Deng, and associated logging concessions in central Cameroon). This variable thus played a limited role in the range-wide analysis. In contrast, more chimpanzees benefit from such taboos, as reflected by their high densities in ~25,700 km2 of coastal Gabon and adjacent Congo (Mayumba and Conkouati; 5% of their range). Along this strip, chimpanzees are found wherever forest remains, although most has been selectively logged multiple times.
More people hold a belief or uphold a custom that prohibits them from killing and eating chimpanzees, as they bear a closer physical resemblance to humans than gorillas do. The positive association between great ape densities and taboos against eating them could be reinforced through conservation advocacy to the wider public.
Impacts of Ebola
The densities of gorillas and chimpanzees were significantly reduced where EVD had occurred in the previous 20 years, with little difference in magnitude of EVD impact on each subspecies (fig. S1). Mortality rates have reached 90 to 95% during the worst EVD outbreaks (28, 29), and large populations in Congo and Gabon were heavily affected by EVD before the surveys included in our study were conducted.
We found no significant evidence of population recovery in post-EVD areas, either because our model was not sensitive to small upward trends or because poaching was hampering recovery, or both. Without the pressure of poaching, some great ape populations begin to recover, and growth has been detected 10 to 20 years after an EVD outbreak at sites where anti-poaching activities were effective (30). In addition, illegal habitat modification in WEA is at lower levels than elsewhere; therefore, recovery is possible, albeit slow (total population recovery could take 75 to 131 years in the absence of poaching) (31). Where there is no effective anti-poaching or habitat protection, post-disease recovery of great ape populations will be even slower or impossible.
At present, EVD in wild great apes cannot be prevented. A review by Leendertz et al. (32) recommends that research into disease ecology and Ebola vaccine delivery should continue. In the meantime, great ape-to-human transmission of EVD can be avoided through appropriate and effective dissemination of information regarding the dangers implicit in the handling and eating of ape meat (33).
Industry, infrastructure development, integrated land-use planning, and conservation management
Often, the conditions where great apes can thrive are met only in well-managed PAs and in FSC-certified concessions with few, if any, people living in them. Around three-quarters of the combined range of gorillas and chimpanzees fall into neither of these categories, and although Equatorial Guinea, CAR, and Angola have PAs, at present there are no FSC-certified concessions in the great ape range. The unprotected range includes conservation areas, noncertified logging concessions, extractive industries (timber, minerals, and petroleum), large- and small-scale agriculture, and infrastructure (for example, transport and power generation).
Proactive national and regional integrated land-use planning that includes conservation as a vital element is essential to maintaining Central Africa’s biodiversity, ecological integrity, and functionality (5). One way to flag areas for economic development while minimizing negative impacts on great apes and other wildlife would be to choose sites that are far from PAs or other intact forests, where forest has already been lost or degraded, and human density is relatively high. Many of the factors highlighted in this study as detrimental to great apes also result in declines of other species (12, 13).
A good example of national land-use planning taking wildlife conservation into account is that being pioneered in Gabon. The Gabonese government is developing national strategic planning documents (www.pnatgabon.ga/) whereby, for example, oil palm and rubber plantations will be developed not only where agricultural and social conditions are appropriate but also where conservation values will not be compromised (34). In this particular case, the areas most suitable for oil palm are along a major road that crosses central and southwest Gabon—not only are the biophysical characteristics of the land appropriate for the crop (and transportation facilitated by the well-maintained road) but also the forests on either side of this national access route have already been defaunated by heavy hunting over decades.
We have shown that where no guards are present, proximity to roads negatively affects great apes; therefore, regional and national spatial road planning should minimize proximity to PAs and to large remote tracts of forest (25). It is unlikely that areas outside PAs and responsibly managed concessions will be protected by guards, so at a national and regional level, avoidance of road creation in areas suitable for great apes and other large wildlife will be key.
At the concession scale, to ensure that the best possible conditions for great apes are maintained within individual priority landscapes, timber companies should implement reduced-impact logging practices and adopt strict wildlife protection policies in their concessions. These practices are crucial in areas with the largest populations of great apes, such as a concession in northern Congo, shown by our analysis to harbor one-fifth of all western lowland gorillas. Our study underlines that two of the most important variables determining great ape density are the presence of guards (as a proxy for effective law enforcement) and maintenance of habitat quality. These are critical factors for industry to undertake not only during prospection and exploitation but also after they cease activities.
Adoption of species-specific approaches would ensure that both gorillas and chimpanzees are conserved in timber concessions. Here, land-use planning at the concession scale should take into account the maintenance of particular habitat types. A precautionary approach should be adopted, because most extractive industries and other land uses in this region reduce canopy cover in mixed-species forest, which is the habitat preferred by chimpanzees. It is also important to consider the extent of particular habitat types within a specified area, because great apes have seasonal preferences for some vegetation types (for example, monodominant forests and swamp habitats). Negative impacts on chimpanzees could be avoided through controlled harvesting of particular tree species and mitigation of the disturbance associated with exploitation. If logging companies follow the High Conservation Value (HCV) approach (an FSC forest management designation), this requires strict control over harvesting levels of tree species known to be important for great apes. HCV also calls for reforestation along abandoned roads. Road building in this region causes high levels of initial deforestation. However, most of these roads are temporary (35), so logging companies should replant important great ape food trees along closed routes. Further detailed recommendations that take into account the socioecology of gorillas and chimpanzees can be found in the studies of Morgan and Sanz (25) and Morgan et al. (36, 37).
We recommend that all industrial development projects, whether public or private sector, adopt international best practice for identifying, planning, and mitigating impacts on priority biodiversity. By any definition, Critically Endangered gorillas and Endangered chimpanzees are biodiversity priorities. International best practice for development projects and their investment partners, such as international financial institutions, are based on the International Finance Corporation’s Performance Standard 6 and associated Guidance Notes (and similar standards such as the World Bank’s Environmental and Social Safeguards) and the Equator Principles. These are relevant for all sectors, including linear infrastructure, forestry and other extractive industries, agro-industry, and hydropower. Identifying risks to wildlife and prioritizing actions to avoid negative impacts will ensure that conservation is an integral part of national development.
Future prospects
Great apes play key ecological roles in forest ecosystems—without large-bodied seed dispersers, the forest will eventually fail to regenerate, with disastrous long-term consequences (38). Given their low reproductive rates, conserving great apes and maintaining their ecological function is challenging; population declines can result from even small increases in mortality rates, whether disease-induced or caused by poaching. The rapid decline of gorillas compared to chimpanzees shown by our study was mirrored in Grauer’s gorilla and eastern chimpanzee populations in DRC (87 and 22% decreases in density, respectively), and was similarly attributed to differences in their socioecology, with chimpanzees being harder to hunt and gorillas easier to kill with guns (16).
With the vast majority of unprotected forests being opened up to selective logging, and degradation caused by multiple rotations becoming the norm, it is vital that we step up our efforts to conserve great apes. Natural resource management, including conservation, has to go hand-in-hand with economic expansion, although some land uses are clearly incompatible with conservation (for example, oil palm plantations and open-cast mines). Integrated land-use planning throughout the range of gorillas and chimpanzees is, therefore, essential for the maintenance of large tracts of suitable great ape habitat with both low human influence and as much intact, high-canopy forest as possible. Planning to maintain connectivity between these large, high-quality areas is also key.
In addition, increasing the number of well-equipped and highly trained guards, developing effective anti-poaching strategies with a strong judiciary process that holds poachers and traffickers accountable, and deterring people from eating great apes will be critical for the long-term survival of large gorilla and chimpanzee populations. Conservation advocacy, education, and training to promote individual behavioral change will minimize the risks of disease transmission between humans and great apes and other wildlife, and will reduce demand for tropical forest resources, be they bushmeat, hardwoods, ivory, or traditional medicines (perceived medicinal properties of animal body parts).
For human development in Central Africa to progress with minimal environmental damage and maximum conservation benefit requires a significant ramping up of national, regional, and global political will and financial commitments to Endangered species conservation. Given that gorillas are more numerous and chimpanzees are more ecologically resilient than expected, and that large areas of ecologically functional great ape habitat remain, we are hopeful that robust conservation policies, well-managed parks, and responsible industrial practices can stop their declines and provide for secure and thriving populations.
MATERIALS AND METHODS
To inform a regional workshop on conservation planning for great apes convened in 2013 (1), we collected and standardized the largest survey data set ever assembled for western lowland gorillas and central chimpanzees. This included 82 foot surveys of great ape nests at 59 sites between 2003 and 2013, totaling 8700 km walked and 61,000 person-days of fieldwork. The surveys were carried out in PAs, their buffer zones, and many logging concessions across the Central African humid tropical forest region, in Cameroon, CAR, Congo, Equatorial Guinea, and Gabon. This gave us a data set of more than 20,000 nests (7521 chimpanzee and 12,524 gorilla nests). During analyses of the survey data, we (i) modeled which factors—both environmental and human—were most likely to drive gorilla and chimpanzee distribution; (ii) used the resulting top-ranked models, which included the important environmental and human variables, to predict nest density surfaces (maps of nest density) for each taxon across their entire geographic range; (iii) applied a model of nest decay based on rainfall (10) to the nest density surfaces to obtain maps of gorilla and chimpanzee density and distribution across their range; and, on the basis of the animal density maps, (iv) estimated great ape abundance in each of the six countries in their range.
To identify the most important drivers of gorilla and chimpanzee distribution and population changes over time, site-specific survey data were combined with regionally available covariate data. Table 2 presents the covariates used to represent known or suspected drivers of chimpanzee and gorilla density and distribution. Forested habitat in the region falls into three main management categories: PAs, logging concessions, and “common land.” However, these categories can be misleading. Some PAs have been logged in the very recent past (several in the period 2002 up to 2010), some common land is still intact, and some logging concessions still contain intact forest (for now). PAs are sometimes no more than “paper parks” without any guards, whereas others have an effective guard force. Similarly, some logging concessions are not protected and hunting within them is uncontrolled, whereas concessions certified by the FSC (https://ic.fsc.org/en) operate anti-poaching patrols. A quarter of all survey sites were guarded, but were not PAs. Other sites had no guards despite being PAs. For this reason, rather than using management category (logging concession or PA) as a variable that assumes a degree of habitat quality and/or protection from poachers, we used canopy height to capture habitat quality, as detailed by Zhuravleva et al. (39), and type (high-canopy and lower-canopy terra firma forest, swamp forest, and savanna) (40).
The set of covariates used included the following:Time (year of survey); the presence or absence of guards (Guards) as a proxy for conservation management (that is, reflecting the true level of protection); habitat suitability as reflected by Canopy height, Slope, and Elevation; hunting as reflected by the HII, distance to the nearest road (Distance to road and HPD), and whether or not consuming gorilla or chimpanzee meat is considered taboo (Eat gorilla or Eat chimpanzee); whether or not an outbreak of EVD has occurred in the previous 20 years (Ebola); variation in management potentially associated with level of Transparency (Corruption Perception Index) or Country; and geographic location (Latitude and Longitude).
Study design
Data collection and standardization
Field protocols followed standardized methods to survey and monitor great apes counting night nests as signs of their presence (7). All weaned great apes make a nest to sleep in every night (41). The time taken for the average nest to decay at each site can be monitored and, since the 1980s, estimation of great ape population size has most often been done by converting nest density to animal density (7). Thus, the density, distribution, and abundance of gorillas and chimpanzees were assessed using nest counts from 82 surveys at 59 sites; 21 of these sites were surveyed more than once (see table S2 for the number of surveys and the allocation of total effort per country—no surveys were conducted in Angola).
Most data (80%) were collected using systematic line-transect distance sampling, where perpendicular distance to each great ape nest was recorded (6). The remaining data were collected using systematic reconnaissance surveys, commonly referred to as “recces” (7), in areas known to have high hunting pressure (and consequently low wildlife density), where transect surveys would be prohibitively expensive. Survey designs were generated by Distance software (42), which allowed random placement of the sampling units. Transects and recces were generally placed systematically with random starting points and perpendicular to roads and major rivers (6). Data from recce surveys were used only when straight lines were walked, thereby ensuring minimal bias. Recce data collected by following roads or paths eroded by elephants were excluded from the analysis. If both transect and recce data existed for the same survey, then only the transect data were included.
The unit of measurement was the number of gorilla or chimpanzee nests recorded along each transect or recce segment, adjusted for distance walked and detectability. For reconnaissance data, encounter rates (nests recorded per kilometer) tend to be lower than for transects, because recces are generally conducted at a quicker walking pace and detection rates are lower. To make nest counts on transects and recces as comparable as possible, the recce segment counts were adjusted. The adjustment was made by first calculating nest encounter rate on transects with the most similar environmental characteristics to a particular recce survey. Then, the ratio of that encounter rate to the recce survey encounter rate was calculated, and finally, the original recce counts were multiplied by that ratio. Transect data were analyzed using Distance 7.0 (42) to obtain survey-specific estimates of detectability in terms of the effective strip half-width . As before, the from the most similar transect survey was used during analysis of the recce survey data.
Where gorillas and chimpanzees are sympatric, it is necessary to distinguish between nests of the two taxa to obtain species-specific results. Fresh (1 to 3 days old) or “recent” (4 to 20 days old) nests can reliably be attributed to either gorilla or chimpanzee using distinguishing characteristics such as dung, hair, or whether or not the nest was on the ground (10, 41). Additional characteristics of nests and of the immediate environment can be used post hoc to construct a predictive logistic regression model to assign taxon to the nest builder for older nests (23). These predictive models were developed when site characteristics had been recorded and the sample size of fresh and recent nests was large enough. On average, these models correctly classified more than 95% of nests in the validation subsamples of fresh and recent nests. If sample size was inadequate, but the nest characteristics were available, then a predictive model from a site with similar environmental characteristics was used to assign taxon to older nests; for the remaining sites, the taxon attributed to the nest in the field was used (table S3).
All surveys were carried out independently. Limited resources resulted in surveys targeting areas known or suspected to harbor wildlife. Although great ape populations were known to be very low in some sites, few sites thought to be completely devoid of apes were surveyed. Survey data encompassed the range of values for each of the environmental or human covariates in our analysis.
Statistical analysis
We used generalized additive mixed models because of their flexibility and ability to deal with nonlinear responses, evident in these data, as well as their capacity to deal with potential spatial or temporal correlation in the count data (43). We used a Tweedie distribution (44) that is able to deal with zero-inflated data (45), which was the case for this data set where no great ape nests were found on many transects or recce segments. For each of the two species, the Tweedie distribution was used with a log link and an iterative search to estimate the value of its power parameter (46).
Area surveyed (, where is the survey-specific effective strip half-width and li is the length of transect or recce segment i) was included as an offset term in the model; thus, in effect, gorilla or chimpanzee nest density was being modeled.
We considered the correlation between all variables and used either HII or a combination of Distance to road and HPD in any particular model to avoid severe colinearity between the variables. To avoid numerical issues when fitting the models, a number of variables were transformed from continuous into categorical variables with cutpoints giving approximately the same number of data points in each category. The categories for Canopy height used were “None” (zero) and four additional categories with cutpoints 15, 25, and 35 m. Canopy height >25 m indicated intact forest (39), which are typical of IFLs (20), as well as “hinterland forests” (47). They can include selectively logged areas where logging intensity is low, as in this region. Canopy height <15 m corresponded to areas that are heavily degraded, agricultural, or along the edges of savannas and in riverbeds (39). For Slope, the categories were “Flat,” “Medium,” “Steep,” and “Steepest” with the cutpoints at 0.5°, 2.5°, and 10°. For Elevation, the categories were “Low,” “Medium,” and “High” with cutpoints at 200 and 700 m. The remaining continuous variables—HII,Distance to road, and HPD—were normalized.
The models were fitted using the mgcv package (48) in R software (49). Thin plate regression splines were used to fit the smooth functions to the continuous variables (described below), except for Latitude and Longitude where a two-dimensional (2D) smooth function was used to account for potential spatial correlations associated with location. To investigate trends in time, models with and without a smooth function of time were contrasted (models with a tensor product of the 2D location smoother with a time effect were also considered). Models that considered human influence on gorilla or chimpanzee distribution across the entire region, as represented by Distance to road and HPD or HII, were contrasted to models with separate smooth functions conditioned on Country or Transparency or the presence or absence of guards. This allowed us to consider potential country-specific human influence or management (Guards) effects by investigating the interaction between Distance to road and HPD or HII and these other variables with either factor added as a main effect as well. Heterogeneity associated with Time (year of survey) was incorporated by using random effect terms to account for potential hierarchy in the data. Model diagnostics produced by the gam.check function within the mgcv package were considered to assess model fit. The statistical significance of the terms in the model (P values were calculated by the mgcv package, which, for parametric terms, are based on Wald tests that use the coefficients’ Bayesian covariance matrix and, for smooth terms, are based on a test statistic whose distribution is determined by the term’s unrounded estimated degrees of freedom), and the adjusted R2 values were also considered. Models were ranked using AIC weight that represents the probability that a particular model is the best model for the given data and set of candidate models (8).
To determine how much of the variability in the dependent variable is explained by each term in the top-ranked model, we fitted models without each term and calculated the reduction in deviance (50). To truly reflect the reduction in deviance, the calculations were done using the same smoothing parameters in the reduced model as in the full model. This approach, together with the changes to the AIC weights of the models with and without random effects, was also used to assess whether or not it was necessary to include the random effects.
Variance and percentile CIs of gorilla or chimpanzee abundance were estimated using a combination of nonparametric and parametric bootstrapping (51). A total of 999 bootstraps were conducted, during which data from sampling units within surveys assumed to be independently and identically distributed were resampled at random and with replacement. To account for the hierarchy in the original data, during each bootstrap resample, the same number of sampling units was selected as in the original data set for each of the 82 surveys with their corresponding number of transects or recce segments, thus maintaining the original structure of the data set (nonparametric component). Nest abundance estimates were obtained from these resampled data conditioned on the original model fit. Nest abundance estimates were converted to gorilla or chimpanzee abundance by applying conversion factors (described below) with associated total variance obtained by incorporating the variance associated with the conversion factors. During each bootstrap iteration, conversion factor values were generated from a normal distribution with a mean equal to the estimated value of the conversion factor and a variance equal to the squared value of the associated SE (parametric component). Estimates of gorilla or chimpanzee numbers were ordered from smallest to largest, and the 25th and 975th values were used to define the percentile CI.
All nests were included in the analysis, except for those built in raphia palms, where nests classed as “old” or “very old” were excluded (52). Nests built from palm fronds (Raphia spp., Elaeis guineensis) last much longer than other nest types; thus, older nest classes would skew the results. Nest density estimates were converted to gorilla or chimpanzee numbers using nest production and decay rates. A production rate of one nest per day for gorillas and 1.09 (SE, 0.05) for chimpanzees (weaned individuals) was used (9). The model used to predict decay rate across the forested area of WEA included the variables Species (gorilla or chimpanzee), Gilbertio (whether or not a nest was made in a Gilbertiodendron dewevrei tree), Average daily rainfall (over the lifetime of the ape nest), Nest structure (the density of the nest structure), Nest type (categories included herbaceous, tree, mixed, and minimally built nests), and Nest construction (whether the vegetation used to make the nest was broken, detached, or bent). Range-wide predictions of nest decay rate were derived from the species-specific averages of each of these variables, except for the Gilbertio variable (set to zero, as nests of this type decay more slowly and are uncommon) and rainfall (average rainfall was calculated using Global Climate Data for precipitation at a 2.5 arc min resolution). The model was assumed to asymptote for average rainfall values above 6 mm and below 3 mm per day to account for saturation at the upper extremes of daily average rainfall and other factors superseding rainfall at the lower extremes (10). The forested portion of WEA straddles the equator between 5.99°N and 5.76°S ranging across a longitude of 8.71°E to 20.49°E, where mean average daily rainfall is 4.7 mm (2.3 to 8.7 mm). Based on the decay rate model, average time to decay across the region is 107.9 days (76.5 to 164.6 days) for gorilla nests and 113.6 days (80.6 to 173.3 days) for chimpanzee nests.
The final models selected for each taxon, together with the regional layers of values for those environmental and human covariates included in the model, were used to predict range-wide nest density and distribution. Having covariate values made it possible to use the models to predict density even for Angola, which had no survey data. The taxon-specific nest density surface was converted to gorilla or chimpanzee density and distribution across their range using the nest creation rate and the time to decay. Great ape abundance for each of the six countries and across their range was estimated by aggregating individual animal density over the area of interest. To assess population changes over time, the differences in abundance using the predicted density surfaces for 2005 and 2013 were calculated. The temporal trend curve was left-truncated, as the model fit was imprecise for the first 2 years of the time series.
Supplementary Material
Acknowledgments
We are very grateful for the collaboration of all conservation partners working in the region. The research authorities, forestry, and wildlife departments from Cameroon, CAR, Congo, Equatorial Guinea, and Gabon are thanked for granting permissions. The Central Africa roads data set came from Global Forest Watch supplemented with local knowledge for some recently-built road segments. Our thanks go to these individuals who assisted in the field or in organization of surveys: E. Ambassa, A. Bezangoye, F. Kiminou, K. M. Pambou, A. Mounguengui, F. Nzolani, and F. Princée. We thank T. Maschler of World Resources Institute (WRI) for pointing us in the direction of useful data in Global Forest Watch and elsewhere. We sincerely thank A. Rylands and two anonymous reviewers for their constructive suggestions and editorial assistance. Funding: Field surveys (data collection) were funded by the following agencies: Agence Française de Développement; Arcus Foundation award no. 1202-01; Convention on International Trade in Endangered Species of Wild Fauna and Flora Monitoring of the Illegal Killing of Elephants; Columbus Zoo and Aquarium; Conservation International and Margot Marsh Biodiversity Foundation; European Union (E.U.) Agreement EuropeAid/128320/C/ACT/Multi; E.U. award no. FED/2013/332-377; E.U. Espèces Phares; Fondation Odzala-Kokoua; Foundation for Environment and Development in Cameroon; Global Environment Facility Composante 2 Projet GEF/PARC: Renforcement des capacités pour la gestion des parcs nationaux et de la biodiversité; Jane Goodall Institute; Liz Claiborne Art Ortenberg Foundation; Max Planck Institute for Evolutionary Anthropology; Programme de Conservation et Utilisation Rationale des Ecosystèmes Forestiers en Afrique Centrale; Spain-UNEP Lifeweb; The Aspinall Foundation; Total (Gabon); United Nations Educational, Scientific and Cultural Organisation’s Central Africa World Heritage Forest Initiative; U.S. Agency for International Development’s Central Africa Regional Program for the Environment agreement nos. 623-A-00-06-00065, 623-A-00-06-00066, 623-A-00-06-00068, 623-A-00-06-00069, and 623-A-00-06-00071; U.S. Fish and Wildlife Service award nos. F12AP00553, F12AP01126, F13AC00558, 96200-0-G071, 96200-1-G196, 96200-9-G111, 96200-9-G179, 96200-9-G247, 98210-6-G137, 98210-6-G147, 98210-7-G233, 98210-7-G289, 98210-7-G290, 98210-7-G297, 98210-7-G299, 98210-8-G529, 98210-8-G651, Afe-0856, and GA-0412; Wildlife Conservation Society; World Bank Group Award No. Project P114077; World Wide Fund for Nature Germany, Netherlands, USA. Some logistical support was provided by Congolaise Industrielle du Bois, Decolvenaere, Rougier, Société d’Exploitations Forestières et Agricoles du Cameroun, and Alpi Pietro et Fils Cameroun. The authors did not receive funds directly from grants—the organizations they work for did, as part of ongoing conservation activities in PAs and surrounding buffer zones. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service or any other funders. Author contributions: S.S. and F.M. designed the study, formulated the hypotheses, and created the figures. S.S., F.M., S.B., E.J.S., R.A., G.A., A.A., R.D.A., P.C.B., M.B., A.B., B.B.d.S., P.R.B., N.B., T.B., G.C., M.E.A., C.I.-N., C.F.I., M.K., H.S.K., S.L., B.M., C.M., G.-A.F.M., R.M., V.M., P.M., G.M., B.S.M., M.M., C.N., A.P., H.J.R., T.R., H.R., A.T., H.V., A.V., and Y.W. contributed to data acquisition or analysis. S.S., F.M., E.A.W., S.B., E.J.S., T.B., A.T.C.F., D.G., K.J.J., H.S.K., D.B.M., M.M., A.P., H.J.R., T.R., H.R., C.M.S., A.T., and D.S.W. participated in the writing and/or critical revision of the manuscript. P.D.W., F.E.M., B.F., R.F., T.N., Z.N., and L.P. provided support. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All survey data are archived with the I.D. “WEA_multi_sites_2003_01_01_Strindberg_et_al” as a single data set in the IUCN Species Survival Commission (SSC) Ape Populations, Environments and Surveys (A.P.E.S.) database on the IUCN SSC A.P.E.S. Portal (http://apesportal.eva.mpg.de/). To access the data set, (i) use the URL http://apesportal.eva.mpg.de/database/archiveTable; (ii) in the “Datasets” search window, enter “WEA”; (iii) select the data set and click “Request data”; (iv) enter name, email address, and submit the request.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/eaar2964/DC1
fig. S1. Percentage variance explained by different variables for (A) western lowland gorillas and (B) central chimpanzees.
fig. S2. Estimated conditional dependence of chimpanzee nest density on proximity to roads.
table S1. Parameter estimates of the top-ranked model used to predict (A) western lowland gorilla and (B) central chimpanzee densities across their range.
table S2. Survey effort for the 82 survey sites included in the analysis.
table S3. Details of the 82 survey sites included in the analysis.
REFERENCES AND NOTES
- 1.IUCN, Regional Action Plan for the Conservation of Western Lowland Gorillas and Central Chimpanzees 2015-2025 (IUCN SSC Primate Specialist Group, 2014).
- 2.IUCN, IUCN Red List of Threatened Species. 2017.3; http://www.iucnredlist.org/.
- 3.Wich S. A., Garcia-Ulloa J., Kühl H. S., Humle T., Lee J. S. H., Koh L. P., Will oil palm’s homecoming spell doom for Africa’s great apes? Curr. Biol. 24, 1659–1663 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Laurance W. F.,Sloan S.,Weng L.,Sayer J. A., Estimating the environmental costs of Africa’s massive “development corridors”. Curr. Biol. 25, 3202–3208 (2015). [DOI] [PubMed] [Google Scholar]
- 5.J. Mackinnon, C. Aveling, R. C. D. Olivier, M. Murray, C. Paolini, Larger Than Elephants: Inputs for the Design of a Wildlife Conservation Strategy for Africa. A Regional Analysis (Publications Office of the European Union, 2016).
- 6.S. T. Buckland, D. R. Anderson, K. P. Burnham, J. L. Laake, D. Borchers, L. Thomas, Distance Sampling: Estimating Abundance of Biological Populations (Oxford Univ. Press, ed. 2, 2001). [Google Scholar]
- 7.H. Kühl, F. Maisels, M. Ancrenaz, E. A. Williamson, Best Practice Guidelines for Surveys and Monitoring of Great Ape Populations (IUCN SSC Primate Specialist Group, 2008).
- 8.K. P. Burnham, D. R. Anderson, Model Selection and Inference: A Practical Information-Theoretic Approach (Springer, ed. 3, 2002). [Google Scholar]
- 9.Morgan D., Sanz C., Onononga J. R., Strindberg S., Ape abundance and habitat use in the Goualougo Triangle, Republic of Congo. Int. J. Primatol. 27, 147–179 (2006). [Google Scholar]
- 10.Morgan D., Sanz C., Onononga J. R., Strindberg S., Factors influencing the survival of sympatric gorilla (Gorilla gorilla gorilla) and chimpanzee (Pan troglodytes troglodytes) nests. Int. J. Primatol. 37, 718–737 (2016). [Google Scholar]
- 11.World Bank, World Development Indicators 1960-2017 (World Bank, 2017); http://data.worldbank.org/data-catalog/world-development-indicators.
- 12.Abernethy K. A., Maisels F., White L. J. T., Environmental issues in Central Africa. Annu. Rev. Environ. Resour. 41, 1–33 (2016). [Google Scholar]
- 13.Maisels F., Strindberg S., Blake S., Wittemyer G., Hart J., Williamson E. A., Aba’a R., Abitsi G., Ambahe R. D., Amsini F., Bakabana P. C., Hicks T. C., Bayogo R. E., Bechem M., Beyers R. L., Bezangoye A. N., Boundja P., Bout N., Akou M. E., Bene L. B., Fosso B., Greengrass E., Grossmann F., Ikamba-Nkulu C., Ilambu O., Inogwabini B.-I., Iyenguet F., Kiminou F., Kokangoye M., Kujirakwinja D., Latour S., Liengola I., Mackaya Q., Madidi J., Madzoke B., Makoumbou C., Malanda G.-A., Malonga R., Mbani O., Mbendzo V. A., Ambassa E., Ekinde A., Mihindou Y., Morgan B. J., Motsaba P., Moukala G., Mounguengui A., Mowawa B. S., Ndzai C., Nixon S., Nkumu P., Nzolani F., Pintea L., Plumptre A., Rainey H., Bokoto de Semboli B., Serckx A., Stokes E., Turkalo A., Vanleeuwe H., Vosper A., Warren Y., Devastating decline of forest elephants in Central Africa. PLOS ONE 8, e59469 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulsen J. R., Clark C. J., Bolker B. M., Decoupling the effects of logging and hunting on an Afrotropical animal community. Ecol. Appl. 21, 1819–1836 (2011). [DOI] [PubMed] [Google Scholar]
- 15.E. A. Williamson, B. M. Rawson, S. M. Cheyne, E. Meijaard, S. A. Wich, in State of the Apes: Extractive Industries and Ape Conservation, Arcus Foundation, Ed. (Cambridge Univ. Press, 2014), pp. 65–99. [Google Scholar]
- 16.A. J. Plumptre, S. Nixon, R. Critchlow, G. Vieilledent, R. Nishuli, A. Kirkby, E. A. Williamson, J. S. Hall, D. Kujirakwinja, Status of Grauer’s Gorilla and Chimpanzees in Eastern Democratic Republic of Congo: Historical and Current Distribution and Abundance (Wildlife Conservation Society, Fauna & Flora International and Institut Congolais pour la Conservation de la Nature, 2015).
- 17.Murai M., Ruffler H., Berlemont A., Campbell G., Esono F., Agbor A., Mbomio D., Ebana A., Nze A., Kühl H. S., Priority areas for large mammal conservation in Equatorial Guinea. PLOS ONE 8, e75024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sesink Clee P. R., Abwe E. E., Ambahe R. D., Anthony N. M., Fotso R., Locatelli S., Maisels F., Mitchell M. W., Morgan B. J., Pokempner A. A., Gonder M. K., Chimpanzee population structure in Cameroon and Nigeria is associated with habitat variation that may be lost under climate change. BMC Evol. Biol. 15, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.J. L. Sunderland-Groves, F. Maisels, Large mammals of Takamanda Forest Reserve, Cameroon, in Takamanda. The Biodiversity of an African Rainforest. J. A. Comiskey, T. C. H. Sunderland, J. L. Sunderland-Groves, Eds. (Smithsonian Institution, 2003), pp. 111–127. [Google Scholar]
- 20.Potapov P., Hansen M. C., Laestadius L., Turubanova S., Yaroshenko A., Thies C., Smith W., Zhuravleva I., Komarova A., Minnemeyer S., Esipova E., The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 3, e1600821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molinario G., Hansen M. C., Potapov P. V., Tyukavina A., Stehman S., Barker B., Humber M., Quantification of land cover and land use within the rural complex of the Democratic Republic of Congo. Environ. Res. Lett. 12, 104001 (2017). [Google Scholar]
- 22.Rogers M. E., Abernethy K. A., Bermejo M., Cipolletta C., Doran D., McFarland K., Nishihara T., Remis M., Tutin C. E. G., Western gorilla diet: A synthesis from six sites. Am. J. Primatol. 64, 173–192 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Sanz C., Morgan D., Strindberg S., Onononga J. R., Distinguishing between the nests of sympatric chimpanzees and gorillas. J. Appl. Ecol. 44, 263–272 (2007). [Google Scholar]
- 24.Wilson M. L., Boesch C., Fruth B., Furuichi T., Gilby I. C., Hashimoto C., Hobaiter C. L., Hohmann G., Itoh N., Koops K., Lloyd J. N., Matsuzawa T., Mitani J. C., Mjungu D. C., Morgan D., Muller M. N., Mundry R., Nakamura M., Pruetz J., Pusey A. E., Riedel J., Sanz C., Schel A. M., Simmons N., Waller M., Watts D. P., White F., Wittig R. M., Zuberbüehler K., Wrangham R. W., Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417 (2014). [DOI] [PubMed] [Google Scholar]
- 25.D. Morgan, C. Sanz, Best Practice Guidelines for Reducing the Impact of Commercial Logging on Great Apes in Western Equatorial Africa (IUCN SSC Primate Specialist Group, 2007).
- 26.Zimmerman B. L., Kormos C. F., Prospects for sustainable logging in tropical forests. BioScience 62, 479–487 (2012). [Google Scholar]
- 27.Laurance W. F., Clements G. R., Sloan S., O’Connell C. S., Mueller N. D., Goosem M., Venter O., Edwards D. P., Phalan B., Balmford A., Van Der Ree R., Arrea I. B., A global strategy for road building. Nature 513, 229–232 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Bermejo M., Rodríguez-Teijeiro J. D., Illera G., Barroso A., Vilà C., Walsh P. D., Ebola outbreak killed 5000 gorillas. Science 314, 1564 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Caillaud D., Levréro F., Cristescu R., Gatti S., Dewas M., Douadi M., Gautier-Hion A., Raymond M., Ménard N., Gorilla susceptibility to Ebola virus: The cost of sociality. Curr. Biol. 16, R489–R491 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Genton C., Cristescu R., Gatti S., Levrero F., Bigot E., Caillaud D., Pierre J.-S., Menard N., Recovery potential of a western lowland gorilla population following a major Ebola outbreak: Results from a ten year study. PLOS ONE 7, e37106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan S. J., Walsh P. D., Consequences of non-intervention for infectious disease in African great apes. PLOS ONE 6, e29030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leendertz S. A. J., Wich S. A., Ancrenaz M., Bergl R. A., Gonder M. K., Humle T., Leendertz F. H., Ebola in great apes—Current knowledge, possibilities for vaccination, and implications for conservation and human health. Mamm. Rev. 47, 98–111 (2017). [Google Scholar]
- 33.K. V. Gilardi, T. R. Gillespie, F. H. Leendertz, E. J. Macfie, D. Travis, C. Whittier, E. A. Williamson, Best Practice Guidelines for Health Monitoring and Disease Control in Great Ape Populations (IUCN SSC Primate Specialist Group, 2015).
- 34.Burton M. E. H., Poulsen J. R., Lee M. E., Medjibe V. P., Stewart C. G., Venkataraman A., White L. J. T., Reducing carbon emissions from forest conversion for oil palm agriculture in Gabon. Conserv. Lett. 10, 297–307 (2017). [Google Scholar]
- 35.Kleinschroth F., Healey J. R., Sist P., Mortier F., Gourlet-Fleury S., How persistent are the impacts of logging roads on Central African forest vegetation? J. Appl. Ecol. 53, 1127–1137 (2016). [Google Scholar]
- 36.Morgan D., Mundry R., Sanz C., Ayina C. E., Strindberg S., Lonsdorf E., Kühl H. S., African apes coexisting with logging: Comparing chimpanzee (Pan troglodytes troglodytes) and gorilla (Gorilla gorilla gorilla) resource needs and responses to forestry activities. Biol. Conserv. 218, 277–286 (2018). [Google Scholar]
- 37.D. Morgan, C. Sanz, D. Greer, T. Rayden, F. Maisels, E. A. Williamson,Great Apes and FSC: Implementing ‘Ape Friendly’ Practices in Central Africa’s Logging Concessions (IUCN SSC Primate Specialist Group, 2013).
- 38.Abernethy K. A., Coad L., Taylor G., Lee M. E., Maisels F., Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120303 ( 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuravleva I., Turubanova S., Potapov P., Hansen M., Tyukavina A., Minnemeyer S., Laporte N., Goetz S., Verbelen F., Thies C., Satellite-based primary forest degradation assessment in the Democratic Republic of the Congo, 2000–2010. Environ. Res. Lett. 8, 024034 (2013). [Google Scholar]
- 40.Mayaux P., Bartholomé E., Fritz S., Belward A., A new land-cover map of Africa for the year 2000. J. Biogeogr. 31, 861–877 (2004). [Google Scholar]
- 41.Tutin C. E. G., Parnell R. J., White L. J. T., Fernandez M., Nest building by lowland gorillas in the Lopé Reserve, Gabon: Environmental influences and implications for censusing. Int. J. Primatol. 16, 53–76 (1995). [Google Scholar]
- 42.Thomas L., Buckland S. T., Rexstad E. A., Laake J. L., Strindberg S., Hedley S. L., Bishop J. R. B., Marques T. A., Burnham K. P., Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 47, 5–14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A. F. Zuur, E. N. Ieno, N. Walker, A. A. Saveliev, G. M. Smith, Mixed Effects Models and Extensions in Ecology With R (Springer, 2009). [Google Scholar]
- 44.Jørgensen B., Exponential dispersion models. J. R. Stat. Soc. Ser. B Stat. Methodol. 49, 127–162 (1987). [Google Scholar]
- 45.Peel D., Bravington M. V., Kelly N., Wood S. N., Knuckey I., A model-based approach to designing a fishery-independent survey. J. Agric. Biol. Environ. Stat. 18, 1–21 (2012). [Google Scholar]
- 46.Miller D. L., Burt M. L., Rexstad E. A., Thomas L., Spatial models for distance sampling data: Recent developments and future directions. Methods Ecol. Evol. 4, 1001–1010 (2013). [Google Scholar]
- 47.Tyukavina A., Hansen M. C., Potapov P. V., Krylov A. M., Goetz S. J., Pan-tropical hinterland forests: Mapping minimally disturbed forests. Glob. Ecol. Biogeogr. 25, 151–163 (2016). [Google Scholar]
- 48.S. N. Wood, Generalized Additive Models: An Introduction with R (Chapman and Hall/CRC Press, 2006). [Google Scholar]
- 49.R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2015).
- 50.T. Hastie, R. Tibshirani, Generalized Additive Models (Chapman and Hall, 1990). [DOI] [PubMed] [Google Scholar]
- 51.Buckland S. T., Monte-Carlo confidence intervals. Biometrics 40, 811–817 (1984). [Google Scholar]
- 52.Rainey H. J., Iyenguet F. C., Malanda G.-A. F., Madzoke B., Dos Santos D., Stokes E. J., Maisels F., Strindberg S., Survey of Raphia swamp forest, Republic of Congo, indicates high densities of Critically Endangered western lowland gorillas Gorilla gorilla gorilla. Oryx 44, 124–132 (2010). [Google Scholar]
- 53.Verhegghen A., Mayaux P., de Wasseige C., Defourny P., Mapping Congo Basin vegetation types from 300 m and 1 km multi-sensor time series for carbon stocks and forest areas estimation. Biogeosciences 9, 5061–5079 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/eaar2964/DC1
fig. S1. Percentage variance explained by different variables for (A) western lowland gorillas and (B) central chimpanzees.
fig. S2. Estimated conditional dependence of chimpanzee nest density on proximity to roads.
table S1. Parameter estimates of the top-ranked model used to predict (A) western lowland gorilla and (B) central chimpanzee densities across their range.
table S2. Survey effort for the 82 survey sites included in the analysis.
table S3. Details of the 82 survey sites included in the analysis.


