Abstract
Cerebral tissue oxygen saturation (SctO2) reflects cerebral perfusion and tissue oxygen consumption, which decline in some patients with heart failure with reduced ejection fraction (HFrEF) or stroke, especially during exercise. Its physiologic basis and clinical significance remain unclear. We aimed to investigate the association of SctO2 with oxygen transport physiology and known prognostic factors during both rest and exercise in patients with HFrEF or stroke. Thirty-four HFrEF patients, 26 stroke patients, and 17 healthy controls performed an incremental cardiopulmonary exercise test using a bicycle ergometer. Integrated near-infrared spectroscopy and automatic gas analysis were used to measure cerebral tissue oxygenation and cardiac and ventilatory parameters. We found that SctO2 (rest; peak) were significantly lower in the HFrEF (66.3±13.3%; 63.4±13.8%,) than in the stroke (72.1±4.2%; 72.7±4.5%) and control (73.1±2.8%; 72±3.2%) groups. In the HFrEF group, SctO2 at rest (SctO2rest) and peak SctO2 (SctO2peak) were linearly correlated with brain natriuretic peptide (BNP), peak oxygen consumption (), and oxygen uptake efficiency slope (r between -0.561 and 0.677, p < 0.001). Stepwise linear regression showed that SctO2rest was determined by partial pressure of end-tidal carbon dioxide at rest (PETCO2rest), hemoglobin, and mean arterial pressure at rest (MAPrest) (adjusted R = 0.681, p < 0.05), while SctO2peak was mainly affected by peak carbon dioxide production () (adjusted R = 0.653, p < 0.05) in patients with HFrEF. In conclusion, the study delineates the relationship of cerebral saturation and parameters associated with oxygen delivery. Moreover, SctO2peak and SctO2rest are correlated with some well-recognized prognostic factors in HFrEF, suggesting its potential prognostic value.
Introduction
Cerebral desaturation may occur in patients with heart failure with reduced ejection fraction (HFrEF) or stroke [1, 2]. The former is due to insufficient cardiac output and dead space ventilation. The latter could be due to disruption of cerebral blood flow (CBF) from vascular abnormalities and neurologic deficit-related respiratory muscle weakness [3–5]. The brain utilizes 20% of the total oxygen consumption at rest [6]. Cerebral oxygenation is determined by arterial oxygen concentration, CBF, and cerebral tissue oxygen consumption, which represent oxygen supply and demand [7]. Insufficient hemoglobin (Hb) concentration, oxygen desaturation, and disruption of cerebral perfusion may result in decreased oxygen supply and thus, cerebral desaturation.
There is evidence that cerebral tissue deoxygenation may limit exercise performance in healthy people. Nielsen et al. demonstrated that when compared with 20% oxygen, 30% inspired oxygen concentration increased exercise performance by decreasing the extent of cerebral desaturation during strenuous exercise; meanwhile, muscle oxygenation was not changed [8]. This study concluded that an elevated inspiratory oxygen fraction increases exercise performance by maintaining cerebral oxygenation rather than through any effect on the working muscles [8]. It is likely that diminished cerebral perfusion may also limit exercise performance in patients with HFrEF. Our previous study revealed that cerebral hypoperfusion is associated with hyperventilation and diminished aerobic capacity in patients with HFrEF [9]. Additionally, frontal cortex dysfunction may impair executive function, resulting in central inhibition [1]. Conversely, poor physical fitness in patients with HFrEF was found to be associated with pathologic change of brain structure, including decreased grey matter volume and cortical thickness, while patients with good physical fitness preserve cerebral structure [10]. Therefore, it is a reasonable conjecture that cerebral tissue oxygen saturation (SctO2) in the frontal lobe has a close relationship with exercise performance in patients with HFrEF or stroke, which has not been explored yet.
To go further, peak oxygen consumption () is a well-recognized significant prognostic factor in patients with HFrEF [11]. If SctO2 is related to exercise capacity, its potential prognostic value deserves investigation. In this study, the link between known prognostic factors and SctO2 during both rest and peak exercise were explored.
Methodologically, SctO2 was measured at the bilateral frontal region during the maximal incremental cardiopulmonary exercise test (CPET), in patients with systolic HFrEF, uni-hemisphere stroke, and healthy participants. Plasma level of brain natriuretic peptide (BNP) and complete blood cell count were collected in HFrEF and stroke groups. Physical parameters that determine SctO2 during rest and exercise were investigated. We hypothesized that SctO2 at rest and exercise have a close relationship with and are correlated with some known prognostic factors.
Materials and methods
Participants
This is a case-controlled cross-sectional design. Thirty-four stable HFrEF patients, 26 first-time uni-hemisphere ischemic stroke patients with hemiparesis, and 17 healthy controls were enrolled by convenience sampling in Linkou Chang Gung Memorial Hospital, a tertiary medical center. The enrolled stroke patients were at least 3 months post onset. All patients with HFrEF had a left ventricular ejection fraction ≤ 40% and a disease duration ≥ 3 months. Healthy controls were recruited by convenience sampling. Most of them were colleagues or caregivers of the hospitalized patients. The exclusion criteria included contraindications to stress exercise testing [12], or inability to ride a bike due to musculoskeletal problems or neurologic deficits including muscle strength less than 4 based on Medical Research Council scale. Patients with HFrEF with moderate to severe carotid artery stenosis or diseases that might affect ventilation, such as chronic obstructive pulmonary disease and pulmonary hypertension, were also excluded. In the stroke group, those with ventilation disorders were also excluded. In the healthy control group, those with any cardiovascular or respiratory diseases in the medical record were excluded. Written informed consent was obtained from every subject before the experiment. The study protocol was performed in accordance with the Declaration of Helsinki and approved by the ethics committee in Chang Gung Memorial Hospital, Linkou, Taiwan. Venous blood was sampled in the morning to determine BNP and complete blood cell count in the HFrEF and stroke groups.
Cardiopulmonary exercise testing
All participants received symptom-limited incremental CPET with upright position on a calibrated bicycle ergometer (Ergoselect 150P, Germany). CPET started with 2 min of rest and 1 min warm-up at a work rate of 10 W followed by a ramp increase of 10 W/min until exhaustion. Heart rate was calculated from the R-R interval recorded on a 12-lead electrocardiogram. Blood pressure was measured automatically every 2 min (Tango, SunTech Medical, UK). Gas analysis was measured breath by breath using a microprocessor-controlled system (MasterScreen CPX, Cardinal-health Germany). The was achieved as the examinee failed to keep the cadence above 50/min despite strong encouragement. Mean arterial pressure (MAP) was calculated by the following equation: MAP = [(2 x diastolic) + systolic] / 3. Arterial oxygen saturation was measured by finger pulse oximetry (model 9500, Nonin Onyx, Plymouth, Minnesota).
Ventilatory efficiency
Ventilation and carbon dioxide consumption () responses, acquired from the initiation of exercise to peak values, were used to calculate the minute ventilation (VE)- slope using least-squares linear regression (y = mx + b, m = slope), where a more horizontal slope suggests better ventilation efficiency [13]. The oxygen uptake efficiency slope (OUES) was derived from the slope of a natural logarithm plot of VE vs. . The OUES is an estimation of the efficiency of ventilation with respect to , with greater slope indicating higher oxygen uptake efficiency [14].
Cerebral oximetry
SctO2 was monitored using the FORE-SIGHT system (CAS Medical Systems, Inc., Branford, CT). The scanning frequency was 100 Hz and was averaged in one second for the value at rest and peak exercise. The SctO2 value at rest was picked up when it reached a stable baseline during two-minute pretest before the exercise started as sitting upright on the cycle ergometry. The FORE-SIGHT device is a spatially resolved near-infrared cerebral oximeter that measures the absolute value of SctO2. Four continuous near-infrared (bandwidth < 1 nm) wave-length (690 nm, 780 nm, 805 nm, and 850 nm) of lights penetrated the brain from the prism of sensors (3.625” x 1.5”). Reflected light was then sampled by detectors on the sensor. Four wave-lengths were employed to enhance measurement accuracy of oxyhemoglobin and deoxyhemoglobin levels by compensating for wavelength-dependent scattering losses and by eliminating interference from other background light absorbers (such as skin pigmentation and fluid) [15, 16]. Both resting and peak SctO2 were measured in the upright sitting posture on the stationary bike. Sensors were placed on the forehead bilaterally. The average value from bilateral forehead was analyzed along with the data from each side. They were used to represent tissue oxygenation at the frontal lobe. The validity has been established and approved by FDA in monitoring during cardiovascular surgery [16]. It has also been applied to measure cerebral oxygenation during exercise in patients with HFrEF [9, 17–19].
Echocardiography
Echocardiography was performed in HFrEF and stroke groups by a cardiologist using Vivid E90 (GE Healthcare, Milwaukee, WI) equipped with a 2.5-MHz transducer. Left ventricular ejection fraction, left ventricular end-diastolic, and end-systolic volumes were quantitated by M-mode and two-dimensional methods [20].
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. Continuous data were expressed as mean ± standard deviation. Three-group comparison was performed by ANOVA with Scheffe post hoc test in the continuous variables, and Chi-Squared test in the categorical ones. SctO2 during rest and peak exercise in the three groups were compared by two-way repeated measure ANOVA with Scheffe post hoc test. Chi-Squared test was also applied to analyze the asymmetric pattern of SctO2. Pearson correlation was used to analyze SctO2 versus other physical parameters, including Hb, BNP, and all the cardio-respiratory parameters listed in Table 1. The four variables with the highest correlation coefficients (all p < 0.05) were initially put into the linear stepwise univariate regression model to select the major variables associated with SctO2. Delta R-squared value was calculated to determine the change in R-squared value as adding another variable into the model. All probability values were two-tailed and the significance threshold was set at 0.05.
Table 1. Cardio-respiratory parameters in the incremental stress exercise testing.
| HFrEF (n = 34) | Stroke (n = 26) | Control (n = 17) | P value | ||
|---|---|---|---|---|---|
| Cardiac parameters | |||||
| HRpeak | beats/min | 124±19† | 133±19‡ | 151±20 | <0.001 |
| SBPpeak | mmHg | 138±28†* | 172.6±23 | 192±18 | <0.001 |
| MAPrest | mmHg | 88±15†* | 99±13 | 103±13 | 0.001 |
| MAPpeak | mmHg | 98±16†* | 116±14 | 126±8 | <0.001 |
| Respiratory parameters | |||||
| BFpeak | breaths/min | 30±8 | 34±11 | 37±6 | 0.083 |
| VEpeak | L/min | 40±11† | 41.2±12.5‡ | 56.5±15.1 | <0.001 |
| Vtpeak | L/min | 1.4±0.5 | 1.3±0.4 | 1.5±0.3 | 0.29 |
| ml/min/kg | 13.4±5.7† | 16±4‡ | 20.2±4.5 | 0.001 | |
| ml/min/kg | 15.8±6.5† | 19±5.7‡ | 23.3±9.3 | 0.009 | |
| RERpeak | - | 1.26±0.58 | 1.18±0.14 | 1.14±0.34 | 0.659 |
| nadir | - | 29±5.8† | 26.4±5 | 24.8±2.5 | 0.032 |
| slope | - | 34.3±6.5†* | 29.7±4.2 | 26.6±1.7 | <0.001 |
| PETO2peak | mmHg | 119.4±7†* | 115.3±4 | 113.7±4.5 | 0.003 |
| PETCO2rest | mmHg | 30.5±7.7 | 33.9±4.5 | 37±2.2 | 0.003 |
| OUES | - | 460±125†* | 591±176‡ | 784±195 | <0.001 |
Values are means ± SD; HFrEF, heart failure with reduced ejection fraction; Peak, peak exercise; HR, heart rate; SBP, systolic blood pressure; MAP, mean arterial pressure; BF, breathing frequency; VE, minute ventilation; Vt, tidal volume; VO2, O2 consumption; , CO2 production; RER, respiratory exchange ratio; PETO2 and PETCO2, end-tidal partial pressures of O2 and CO2; OUES, oxygen uptake efficiency slope
†: p < 0.05, HFrEF vs. control
‡: p < 0.05, stroke vs. control
*: p < 0.05, HFrEF vs. stroke; ANOVA with Scheffe post hoc test
Results
No significant differences in age, gender, height, weight, and body mass index were shown between the three groups (Table 2). Plasma BNP and Hb were 974±966 (pg/mL) and 13.1±2.6 (g/dL) in the HFrEF group; 469±393 (pg/mL) and 14.6±1.7 (g/dL) in the stroke group. and OUES were 13.4±5.7 (ml/min/kg) and 460±125 in the HFrEF group; 16±4 (ml/min/kg) and 591±176 in the stroke group; 20.2±4.5 and 784±195 in the control group. Mean arterial pressure (MAP) at rest and peak were 88±15 (mmHg) and 98±16 (mmHg) in the HFrEF group; 99±13 (mmHg) and 116±14 (mmHg) in the stroke group; 103±13 (mmHg) and 126±8 (mmHg) in the control group (Table 1).
Table 2. Demographic and clinical characteristics.
| HFrEF (n = 34) | Stroke (n = 26) | Control (n = 17) | P value | ||
|---|---|---|---|---|---|
| Gender | n (M/F) | 31/3 | 20/6 | 15/2 | 0.316 |
| Age | year | 56±13 | 58±11 | 56±14 | 0.684 |
| Height | cm | 166.2±7.8 | 162.4±8.6 | 164.8±7.3 | 0.183 |
| Weight | kg | 67.2±12.5 | 66.3±10.4 | 67.0±13.9 | 0.959 |
| BMI | kg/meter2 | 24.2±3.8 | 25.1±2.6 | 24.5±3.5 | 0.636 |
| Comorbidities | |||||
| Hypertension | n (%) | 13 (38) | 17 (61) | - | 0.126 |
| Hyperlipidemia | n (%) | 5 (15) | 4 (14) | - | 1.000 |
| Smoking | n (%) | 4 (12) | 2 (7) | - | 0.678 |
| Diabetes mellitus | n (%) | 11 (32) | 5 (18) | - | 0.245 |
| Sleep apnea | n (%) | 0 (0) | 2 (7) | - | 0.207 |
| Coronary artery disease | n (%) | 4 (12) | 1 (4) | - | 0.363 |
| Atrial fibrillation | n (%) | 4 (12) | 2 (7) | - | 0.678 |
| Overweight | n (%) | 3 (9) | 0 (0) | - | 0.243 |
| Medication | |||||
| ACEI | n (%) | 2 (6) | 1 (4) | - | 1.000 |
| ARB | n (%) | 7 (20) | 5 (18) | - | 1.000 |
| B-blocker | n (%) | 17 (50)* | 6 (21) | - | <0.05 |
| Ca++ channel blocker | n (%) | 8 (24)* | 15 (54) | - | <0.05 |
| Diuretics | n (%) | 23 (68)* | 5 (18) | - | <0.05 |
| Nitrates | n (%) | 5 (15) | 8 (29) | - | 0.227 |
| Digoxin | n (%) | 12 (35)* | 3 (11) | - | <0.05 |
| Anti-arrhythmic | 1 (3) | 1 (4) | - | 1.000 | |
| Echocardiography | |||||
| LVEF | % | 32±14* | 64±10 | - | <0.05 |
| LVEDD | mm | 60±10.1* | 48±7 | - | <0.05 |
| LVESD | mm | 47±13* | 31±6 | - | <0.05 |
| Hb | g/dL | 13.1±2.6* | 14.6±1.7 | - | <0.05 |
| BNP | pg/mL | 549 (232–1605)* | 329 (182–1063) | - | <0.05 |
| Hemisphere lesion |
right: left |
11:15 |
Values except BNP are means ± SD; BNP is presented as median (25th to75th percentile)
Overweight is defined as BMI greater than or equal to 25
HFrEF, heart failure with reduced ejection fraction; M, male; F, female; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ACEI, angiotensin-converting-enzyme inhibitor; ARB, Angiotensin II receptor blocker; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; Hb, hemoglobin; BNP, B-type natriuretic peptide.
*: p < 0.05, HFrEF vs. stroke; independent t-test for continuous variables and Chi-Squared test for categorical ones
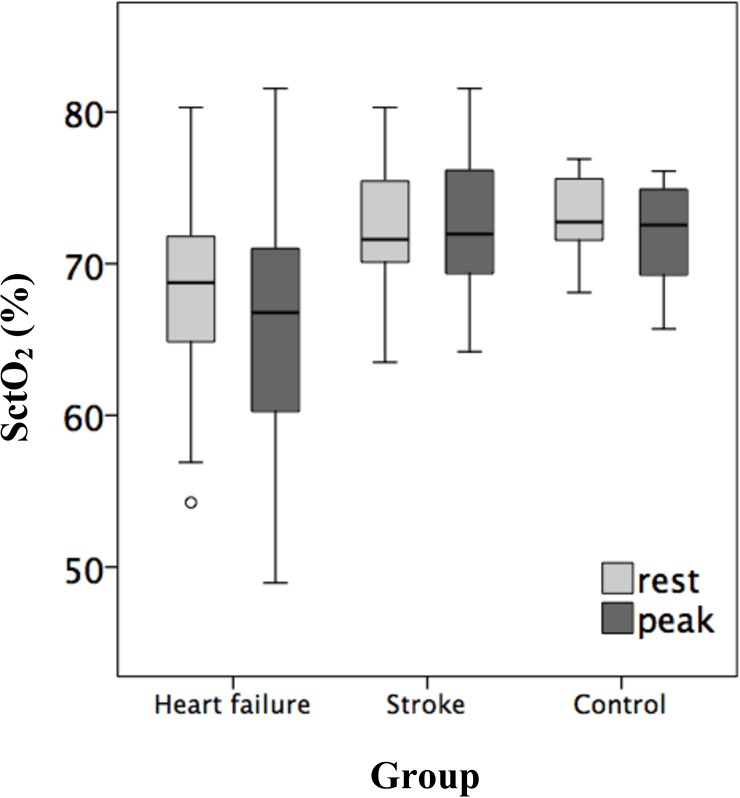
Oxygen saturation of cerebral tissue (rest; peak) were significantly lower in the HFrEF (66.3±13.3%; 63.4±13.8%) than that in the stroke (72.1±4.2%; 72.7±4.5%) and control (73.1±2.8%; 72±3.2%) groups as revealed by two-way repeated measure ANOVA, while those in the stroke group were close to the healthy control. Moreover, in the HFrEF group, SctO2 decreased significantly at peak exercise, a phenomenon not observed in the stroke or healthy control groups (Fig 1, Table 3). Additionally, all the arterial oxygen saturation among three groups were 98~100% during both rest and peak exercise.
Fig 1. Boxplots of SctO2 during rest and peak exercise among the three groups.
Table 3. Cerebral tissue oxygen saturation in incremental exercise testing.
| HFrEF (n = 34) | Stroke (n = 26) | Control (n = 17) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Rest | Peak | Rest | Peak | Rest | Peak | |||
| SctO2 | % | 66.3±13.3 | 63.4±13.8†* | 72.1±4.2 | 72.7±4.5 | 73.1±2.8 | 72±3.2 | <0.001 |
| L't SctO2 | % | 68.1±5.9 | 65.5±7.8†* | 71.8±4.1 | 72.5±4.7 | 72.9±2.6 | 71.6±2.8 | <0.001 |
| R't SctO2 | % | 68.6±6.1 | 65.2±7.8†* | 72.6±5.1 | 73.3±7.3 | 73.3±3.1 | 72.6±3.6 | <0.001 |
Values are means ± SD; HFrEF, heart failure with reduced ejection fraction; Peak, peak exercise
L’t, Left; R’t, Right; SctO2, cerebral tissue oxygen saturation
†: p < 0.05, HFrEF vs. control
*: p < 0.05, HFrEF vs. stroke; repeated measure ANOVA with Scheffe post hoc test
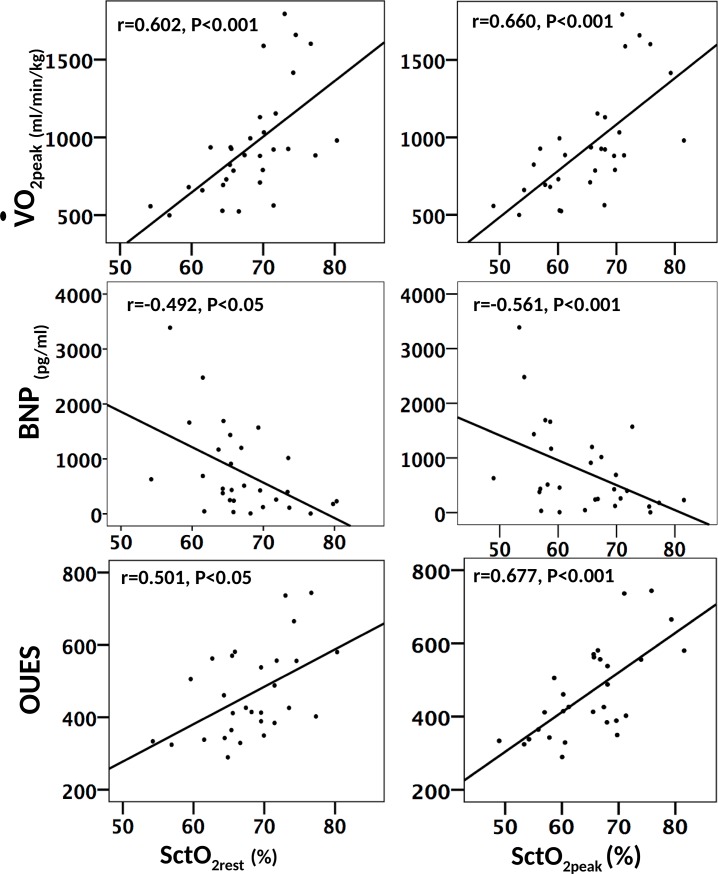
Pearson correlation was performed between SctO2 and cardio-respiratory and hematologic parameters. In the HFrEF group, SctO2rest and SctO2peak were positively correlated with (r = 0.602, p < 0.001; r = 0.660, p < 0.001) and OUES (r = 0.501, p < 0.05; r = 0.677, p < 0.001) and negatively correlated with BNP (r = -0.492, p < 0.05; r = -0.561, p < 0.001) (Fig 2). On the other hand, the change in SctO2 from rest to peak (-1.4 ± 3.3%) was not correlated with the investigated prognostic factors, including (r = -0.430), OUES (r = -0.362), BNP (r = -0.230), slope (r = -0.025), PETCO2rest (r = -0.177) and SBPpeak (r = -0.263). Additionally, no significant correlation was found between SctO2 and cardio-respiratory or hematologic parameters in the stroke and control groups.
Fig 2. Scatter plots of SctO2rest and SctO2peak against , BNP and OUES.
Linear stepwise univariate regression analysis was employed to identify the major physiologic determinants of SctO2rest and SctO2peak in patients with HFrEF. (r = 0.602), MAPrest (r = 0.597), partial pressure of end-tidal carbon dioxide at rest (PETCO2rest) (r = 0.586), Hb (r = 0.51), and were entered into the model of SctO2rest. OUES (r = 0.677), (r = 0.66), peak carbon dioxide production () (r = 0.651), and BNP (r = -0.561) were entered into the model of SctO2peak (S1 Table). It revealed that SctO2rest was determined by PETCO2rest, Hb, and MAPrest (R = 0.681, p < 0.05), while SctO2peak was by VCO2peak (R = 0.653, p < 0.05) (Tables 4 and 5). It is worth mentioning that Hb is also significantly correlated with SctO2peak (r = 0.39). However, it does not increase the predictive power significantly when it was entered after (p-value of ß was 0.052).
Table 4. Linear regression modeling of SctO2rest in HFrEF group.
| ß | t | P(ß) | R | ΔR2 | F | |
|---|---|---|---|---|---|---|
| Model 1 | 0.593 | 0.352 | 14.679* | |||
| PETCO2rest | 0.593 | 3.831 | 0.001 | |||
| Model 2 | 0.639 | 0.056 | 19.257* | |||
| PETCO2rest | 0.552 | 3.757 | 0.001 | |||
| Hb | 0.314 | 2.140 | 0.042 | |||
| Model 3 | 0.681 | 0.056 | 25.009* | |||
| PETCO2rest | 0.517 | 3.804 | 0.001 | |||
| Hb | 0.331 | 2.451 | 0.022 | |||
| MAPrest | 0.323 | 2.398 | 0.024 |
PETCO2, the end-tidal partial pressures of CO2; Hb, hemoglobin; MAP, mean arterial pressure
* p < 0.05; the p-value indicates the overall significance of the linear regression model
P(ß): p-value for ß; R and ΔR2 are adjusted values
Table 5. Linear regression modeling of SctO2peak in HFrEF group.
| ß | t | P(ß) | R | ΔR2 | F | |
|---|---|---|---|---|---|---|
| Model 1 | 0.653 | 0.426 | 13.624* | |||
| 0.678 | 3.691 | 0.002 |
: peak CO2 production
* p < 0.05; the p-value indicates the overall significance of the linear regression model
P(ß): p-value for ß; R and ΔR2 are adjusted values
The experimental finding of bilateral comparison of SctO2 in the HFrEF vs. control groups and its related discussion are appended in the supporting information (S1 Supporting information).
Discussion
To the best of our knowledge, the current investigation is the first to determine the potential prognostic value of SctO2 and its possible physiologic basis in patients with HFrEF. Significant experimental findings were as follows: I. SctO2 of the HFrEF group were significantly decreased compared with healthy control and stroke groups. II. Patients with HFrEF not only had diminished cerebral oxygenation at rest, but also showed further reduced oxygenation during incremental exercise testing, which was not observed in the stroke and healthy groups. III. Importantly, both SctO2rest and SctO2peak of the HFrEF group (especially SctO2peak) were correlated with , BNP, and OUES in moderate degree, which are well-established prognostic markers. IV. In the HFrEF cohort, linear regression analysis showed that SctO2rest was primarily determined by PETCO2rest, Hb and MAPrest, while SctO2peak was affected primarily by . V. The numerical value of SctO2 did not differ between the stroke and healthy control groups. Its association with other investigated biomarkers was not obvious in these two groups either.
Low cerebral oxygenation in patients with HFrEF
CBF is reduced in patients with mild to severe HFrEF even in resting state, as confirmed through 133XE radionuclide angiography injection method, and transcranial and extracranial Doppler ultrasonography [21–23]. During dynamic exercise, cerebral perfusion can be sacrificed to active skeletal muscle in patients with HFrEF. Hellstrom et al. demonstrated that healthy subjects had a 20% increase in mean middle cerebral artery blood velocity (MCA Vmean) when performing one-legged exercise and this increase was maintained when performing two-legged exercise. However, in patients with HFrEF, MCA Vmean was not increased during one-legged exercise and was significantly decreased during two-legged exercise [24]. For stroke patients, Robertson et al. reported that regional CBF was reduced after low-intensity exercise but increased after moderate-intensity exercise [25]. Similarly, the present investigation found that SctO2 was lower in patients with HFrEF during resting state and decreased significantly from rest to peak exercise only in the HFrEF group. It is very possible that cerebral autoregulation fails to compensate CBF in face of low systemic BP commonly seen in HFrEF patients, leading to reduced SctO2 [26]. The reason why our data did not demonstrate cerebral oxygenation reduction in the stroke group could be that only patients with mild severity of stroke were recruited. Their muscle strength in the hemiparetic limbs were 4 or 5- in manual muscle testing. Thus, the data pattern was similar in the stroke and healthy control groups. Meanwhile, the potential influence of medications should be considered. Calcium channel blockers (CCB) elevate CBF[27], while beta-blockers attenuate the increase in cardiac output, CBF and cerebral oxygenation during exercise [28, 29]. In our study, significantly more patients took CCB in the stroke group, while more patients took beta-blockers in the HFrEF group. Although these medications may augment cerebral deoxygenation in HFrEF patients, the prescription was in line with the current treatment guidelines and reflects the real-world situation [30, 31].
Potential prognostic value of SctO2 in HFrEF
Our data showed that in patients with HFrEF, both SctO2rest and SctO2peak (especially SctO2peak) were correlated with , BNP, and OUES in moderate degree. BNP has been recognized as a prognostic and diagnostic factor for HFrEF and is used to assess the severity of HFrEF [32, 33]. provides objective assessment for cardio-pulmonary fitness and is one of the most significant short-term and long-term prognostic factors for patients with HFrEF [34, 35]. OUES measures oxygen uptake efficiency in relation to ventilation during an incremental exercise test and has been shown to be a prognostic marker, which is even more predictive than in some of the HFrEF studies [14, 36].
Koike et al. found that in patients with coronary artery disease, the change of cerebral oxyhemoglobin (O2Hb) from rest to peak exercise is prognostic for cardiovascular morbidity [37]. However, our results showed that the difference between SctO2rest and SctO2peak were not associated with the known prognostic factors investigated. Instead, it was the absolute value during peak or rest that might be related to prognosis. An explanation is that O2Hb and SctO2 are essentially different. Decrease of O2Hb from rest to peak exercise may result from the reduction of CBF or increased oxygen consumption of the tissue. However, SctO2 is decreased only when O2Hb has a relatively larger reduction than deoxyhemoglobin [24]. Probably, it is the reduction of CBF that is primarily associated with prognosis.
Determinants of SctO2rest in HFrEF: PETCO2rest, Hb, and MAPrest
The linear stepwise regression model indicated that SctO2rest of the HFrEF group was primarily determined by PETCO2rest, Hb, and MAPrest. The explanatory power was 46% (R2 = 0.464) in this model. PETCO2 is very close to partial pressure of arterial carbon dioxide (PaCO2) during rest since those with ventilation problems were excluded in the HFrEF group [38–40]. Therefore, our experimental findings of PETCO2 during rest may be interpreted as PaCO2. PaCO2 was previously demonstrated to be positively linearly correlated to CBF from 15 to 60 mmHg [41]. Our data of PETCO2 in the HFrEF group and the control group were 30.5±7.7 and 37±2.2 mmHg, respectively. Therefore, the reduced PETCO2 in the HFrEF group may affect SctO2rest due to decreased CBF. In addition, low PETCO2 is also a significant predictor of cardiac-related events in patients with HFrEF [42].
Cerebral oxygenation is also influenced by arterial oxygen concentration [43]. Anemia leads to a decreased oxygen supply to the cerebral tissue. It is also associated with an increased mortality rate in patients with HFrEF [44, 45]. In healthy subjects, the disadvantage of anemia is compensated by increased cardiac output, plasma volume, reduced systemic vascular resistance, and widened arteriovenous oxygen gradient. However, these compensatory mechanisms are impaired in patients with HFrEF [46, 47].
MAP also influences cerebral oxygenation based on our results. Herholz et al. adopted 133Xe clearance technique and demonstrated that the linear increase in CBF resulted not only from PaCO2 but also MAP [41]. Rifai et al. also found a positive correlation between SctO2 and MAP in patients with HFrEF at rest [48]. Higher MAP may be associated with higher cerebral perfusion [49]. As perfusion into the tissue vascular bed is increased, Hb density in the tissue increases and thereby, SctO2 is less reduced. Accordingly, MAPrest, plays a role in determining CBF and thereby SctO2rest. Meanwhile, MAP was inversely related to the total and cardiovascular mortality [50], which as well indicated potential prognostic value of SctO2. Nonetheless, MAP was not correlated with SctO2peak. Previous investigations confirmed that CBF does not increase or even decline at peak exercise even though MAP rises in patients with HFrEF [9, 51]. In other words, MAP does not reflect CBF during exercise in patients with HFrEF. In summary, PETCO2rest, Hb, and MAPrest are important determinants of SctO2rest, as well as prognostic markers in patients with HFrEF.
Determinants of SctO2peak in HFrEF:
The stepwise linear regression showed that the most major determinant of SctO2peak in the HFrEF group is (R = 0.653; R2 = 0.426). There are two explanations: first, and have high collinearity. Therefore, low suggests low oxygen delivery to the peripheral tissue, including brain and thus leads to decreased SctO2 [43]. Second, patients with HFrEF with lower (thus, lower ) tend to have lower PaCO2 due to ventilation-perfusion mismatch [43], which results in cerebral vasoconstriction [52]. This could explain why SctO2peak in the HFrEF group is primarily determined by rather than .
SctO2 in stroke
The numerical value of SctO2 in the stroke and healthy control groups were not different based on the present data. Also, it was not associated with the investigated known prognostic factors. It can be explained by the mild severity in the included stroke patients. Their muscle strength in the hemiparetic limbs were 4 or 5- in manual muscle testing. In consideration of ergometer-riding being the modality of exercise testing, those with poorer muscle strength were not adequate to be included because maximal effort cannot be reached due to the neurologic deficits. In Table 3, the respiratory exchange ratio of the stroke group was not different from that of the control group (1.18±0.14 vs. 1.14±0.34), indicating that maximal exertion was nearly approached in the stroke group. The data showed that although patients with mild ischemic stroke were less fit (lower VO2peak, HRpeak and VEpeak) than the control group, SctO2 during rest or peak were not lower. Further study on stroke patients with higher severity and significant cerebrovascular stenosis is needed.
Limitations of NIRS cerebral oximetry
Some limitations concerning NIRS cerebral oximetry need to be taken into consideration. First, SctO2 was measured without differentiating vascular bed as being arterial, capillary, or venous. Since it is estimated that more than 70% of the Hb in the brain is in venous bed, the measured SctO2 may reflect larger proportion of venous saturation [53]. Second, extracranial contamination and melanin may absorb light and thus attenuate the signal, though all the participants are Asian. [54, 55].
Study limitation
A longitudinal study is needed to confirm the prognostic value of SctO2. Moreover, PETCO2 rather than PaCO2 was measured in the current study though patients with ventilation disorder were already excluded. However, in patients with advanced HFrEF, a certain degree of increased dead space ventilation may be present; thus, PaCO2 might be slightly higher than PETCO2, even at rest [43]. Additionally, lack of direct CO and CBF measurement and absence of echocardiographic data, Hb, and BNP in the control group limit precise interpretation and analysis. Also, it may influence the SctO2 value in the stroke group to have the NIRS sensors placed on the fixed positions regardless of whether the infarction area was right underneath the sensor.
Conclusion
Cerebral oxygenation was reduced in patients with HFrEF compared with healthy controls. Moreover, cerebral oxygenation, especially at peak exercise, is correlated with , BNP, and OUES, which are well-recognized prognostic factors. Cerebral oxygenation during rest is determined mainly by PETCO2, Hb, and MAPrest, while at peak exercise, is primarily affected by in patients with HFrEF. These findings provided the physiologic basis of cerebral oxygenation and its potential prognostic value in patients with HFrEF, and may have clinical value in the future.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
Support from the staff of the Cardiac Rehabilitation Center in Chang Gung Memorial Hospital is appreciated.
Abbreviations
- BNP
brain natriuretic peptide
- CBF
cerebral blood flow
- CO
cardiac output
- CPET
cardiopulmonary exercise test
- Hb
hemoglobin
- HFrEF
heart failure with reduced ejection fraction
- MAP
mean arterial pressure
- MCA Vmean
mean middle cerebral artery blood velocity
- O2Hb
oxyhemoglobin
- OUES
oxygen uptake efficiency slope
- PaCO2
partial pressure of arterial carbon dioxide
- PETCO2
partial pressure of end-tidal carbon dioxide
- SctO2
cerebral tissue oxygen saturation
carbon dioxide production
- VE
minute ventilation
oxygen consumption
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors would like to thank the Chang Gung Medical Research Program (CMRPG3G0601) and Ministry of Science and Technology, ROC (NMRPG3E6091/2) for the financial support.
References
- 1.Ferdinand P, Roffe C. Hypoxia after stroke: a review of experimental and clinical evidence. Experimental & translational stroke medicine. 2016;8:9 Epub 2016/12/17. doi: 10.1186/s13231-016-0023-0 ; PubMed Central PMCID: PMCPMC5143450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brassard P, Gustafsson F. Exercise Intolerance in Heart Failure: Did We Forget the Brain? The Canadian journal of cardiology. 2016;32(4):475–84. Epub 2016/02/15. doi: 10.1016/j.cjca.2015.12.021 . [DOI] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. The New England journal of medicine. 2005;353(19):2034–41. Epub 2005/11/12. doi: 10.1056/NEJMoa043104 . [DOI] [PubMed] [Google Scholar]
- 4.Ezeugwu VE, Olaogun M, Mbada CE, Adedoyin R. Comparative lung function performance of stroke survivors and age-matched and sex-matched controls. Physiother Res Int. 2013;18(4):212–9. Epub 2013/01/30. doi: 10.1002/pri.1547 . [DOI] [PubMed] [Google Scholar]
- 5.Hu X, De Silva TM, Chen J, Faraci FM. Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circulation research. 2017;120(3):449–71. Epub 2017/02/06. doi: 10.1161/CIRCRESAHA.116.308427 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoiland RL, Bain AR, Rieger MG, Bailey DM, Ainslie PN. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310(5):R398–413. Epub 2015/12/18. doi: 10.1152/ajpregu.00270.2015 ; PubMed Central PMCID: PMCPMC4796739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog Neurobiol. 2000;61(4):397–414. Epub 2000/03/23. . [DOI] [PubMed] [Google Scholar]
- 8.Nielsen HB, Boushel R, Madsen P, Secher NH. Cerebral desaturation during exercise reversed by O2 supplementation. The American journal of physiology. 1999;277(3 Pt 2):H1045–52. Epub 1999/09/14. . [DOI] [PubMed] [Google Scholar]
- 9.Fu TC, Wang CH, Hsu CC, Cherng WJ, Huang SC, Wang JS. Suppression of cerebral hemodynamics is associated with reduced functional capacity in patients with heart failure. American journal of physiology Heart and circulatory physiology. 2011;300(4):H1545–55. Epub 2011/02/01. doi: 10.1152/ajpheart.00867.2010 ; PubMed Central PMCID: PMCPmc3283169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, et al. Poorer physical fitness is associated with reduced structural brain integrity in heart failure. Journal of the neurological sciences. 2013;328(1–2):51–7. Epub 2013/03/27. doi: 10.1016/j.jns.2013.02.015 ; PubMed Central PMCID: PMCPmc3625509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers J, Arena R, Cahalin LP, Labate V, Guazzi M. Cardiopulmonary Exercise Testing in Heart Failure. Current problems in cardiology. 2015;40(8):322–72. Epub 2015/06/23. doi: 10.1016/j.cpcardiol.2015.01.009 . [DOI] [PubMed] [Google Scholar]
- 12.Medicine DRJKEGLMMACoS. ACSM's guidelines for exercise testing and prescription 10th ed: Philadelphia: Wolters Kluwer; 2018. [Google Scholar]
- 13.Shen Y, Zhang X, Ma W, Song H, Gong Z, Wang Q, et al. VE/VCO(2) slope and its prognostic value in patients with chronic heart failure. Experimental and Therapeutic Medicine. 2015;9(4):1407–12. doi: 10.3892/etm.2015.2267 ; PubMed Central PMCID: PMCPMC4353809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alba AC, Adamson MW, MacIsaac J, Lalonde SD, Chan WS, Delgado DH, et al. The Added Value of Exercise Variables in Heart Failure Prognosis. Journal of cardiac failure. 2016;22(7):492–7. Epub 2016/02/05. doi: 10.1016/j.cardfail.2016.01.012 . [DOI] [PubMed] [Google Scholar]
- 15.Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biological psychiatry. 2002;52(7):679–93. Epub 2002/10/10. . [DOI] [PubMed] [Google Scholar]
- 16.Fischer GW. Recent advances in application of cerebral oximetry in adult cardiovascular surgery. Seminars in cardiothoracic and vascular anesthesia. 2008;12(1):60–9. Epub 2008/04/10. doi: 10.1177/1089253208316443 . [DOI] [PubMed] [Google Scholar]
- 17.Scheeren TW, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. Journal of clinical monitoring and computing. 2012;26(4):279–87. Epub 2012/04/03. doi: 10.1007/s10877-012-9348-y ; PubMed Central PMCID: PMCPMC3391360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu TC, Wang CH, Lin PS, Hsu CC, Cherng WJ, Huang SC, et al. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. International journal of cardiology. 2013;167(1):41–50. Epub 2011/12/27. doi: 10.1016/j.ijcard.2011.11.086 . [DOI] [PubMed] [Google Scholar]
- 19.Fu TC, Yang NI, Wang CH, Cherng WJ, Chou SL, Pan TL, et al. Aerobic Interval Training Elicits Different Hemodynamic Adaptations Between Heart Failure Patients with Preserved and Reduced Ejection Fraction. Am J Phys Med Rehabil. 2016;95(1):15–27. Epub 2015/06/09. doi: 10.1097/PHM.0000000000000312 . [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2015;28(1):1–39.e14. Epub 2015/01/07. doi: 10.1016/j.echo.2014.10.003 . [DOI] [PubMed] [Google Scholar]
- 21.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32(11):2530–3. Epub 2001/11/03. . [DOI] [PubMed] [Google Scholar]
- 22.Vogels RL, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Transcranial Doppler blood flow assessment in patients with mild heart failure: correlates with neuroimaging and cognitive performance. Congestive heart failure (Greenwich, Conn). 2008;14(2):61–5. Epub 2008/04/11. . [DOI] [PubMed] [Google Scholar]
- 23.Fraser KS, Heckman GA, McKelvie RS, Harkness K, Middleton LE, Hughson RL. Cerebral hypoperfusion is exaggerated with an upright posture in heart failure: impact of depressed cardiac output. JACC Heart failure. 2015;3(2):168–75. Epub 2014/12/30. doi: 10.1016/j.jchf.2014.07.017 . [DOI] [PubMed] [Google Scholar]
- 24.Hellstrom G MB, Wahlgren NG, Gordon A, Sylven C & Saltin B. Physical exercise may impair cerebral perfusion in patients with chronic heart failure. Cardiol Elder. 1996;4(5–6):191–4. [Google Scholar]
- 25.Robertson AD, Crane DE, Rajab AS, Swardfager W, Marzolini S, Shirzadi Z, et al. Exercise intensity modulates the change in cerebral blood flow following aerobic exercise in chronic stroke. Experimental brain research. 2015;233(8):2467–75. Epub 2015/05/25. doi: 10.1007/s00221-015-4317-6 . [DOI] [PubMed] [Google Scholar]
- 26.Rosen SD, Murphy K, Leff AP, Cunningham V, Wise RJ, Adams L, et al. Is central nervous system processing altered in patients with heart failure? European heart journal. 2004;25(11):952–62. Epub 2004/06/03. doi: 10.1016/j.ehj.2004.03.025 . [DOI] [PubMed] [Google Scholar]
- 27.Gelmers HJ. Effect of calcium antagonists on the cerebral circulation. The American journal of cardiology. 1987;59(3):173b–6b. Epub 1987/01/30. . [DOI] [PubMed] [Google Scholar]
- 28.Seifert T, Rasmussen P, Secher NH, Nielsen HB. Cerebral oxygenation decreases during exercise in humans with beta-adrenergic blockade. Acta physiologica (Oxford, England). 2009;196(3):295–302. Epub 2008/12/05. doi: 10.1111/j.1748-1716.2008.01946.x . [DOI] [PubMed] [Google Scholar]
- 29.Ide K, Boushel R, Sorensen HM, Fernandes A, Cai Y, Pott F, et al. Middle cerebral artery blood velocity during exercise with beta-1 adrenergic and unilateral stellate ganglion blockade in humans. Acta physiologica Scandinavica. 2000;170(1):33–8. Epub 2000/09/06. doi: 10.1046/j.1365-201x.2000.00757.x . [DOI] [PubMed] [Google Scholar]
- 30.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). Jama. 2014;311(5):507–20. Epub 2013/12/20. doi: 10.1001/jama.2013.284427 . [DOI] [PubMed] [Google Scholar]
- 31.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Journal of cardiac failure. 2017;23(8):628–51. Epub 2017/05/04. doi: 10.1016/j.cardfail.2017.04.014 . [DOI] [PubMed] [Google Scholar]
- 32.Heil B, Tang WH. Biomarkers: Their potential in the diagnosis and treatment of heart failure. Cleveland Clinic journal of medicine. 2015;82(12 Suppl 2):S28–35. Epub 2015/12/24. doi: 10.3949/ccjm.82.s2.05 . [DOI] [PubMed] [Google Scholar]
- 33.Maisel A, Mueller C, Adams K Jr., Anker SD, Aspromonte N, Cleland JG, et al. State of the art: using natriuretic peptide levels in clinical practice. European journal of heart failure. 2008;10(9):824–39. Epub 2008/09/02. doi: 10.1016/j.ejheart.2008.07.014 . [DOI] [PubMed] [Google Scholar]
- 34.Pilote L, Silberberg J, Lisbona R, Sniderman A. Prognosis in patients with low left ventricular ejection fraction after myocardial infarction. Importance of exercise capacity. Circulation. 1989;80(6):1636–41. Epub 1989/12/01. . [DOI] [PubMed] [Google Scholar]
- 35.Cattadori G, Agostoni P, Corra U, Sinagra G, Veglia F, Salvioni E, et al. Heart failure and anemia: Effects on prognostic variables. European journal of internal medicine. 2017;37:56–63. Epub 2016/10/04. doi: 10.1016/j.ejim.2016.09.011 . [DOI] [PubMed] [Google Scholar]
- 36.Mezzani A, Agostoni P, Cohen-Solal A, Corra U, Jegier A, Kouidi E, et al. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. European journal of cardiovascular prevention and rehabilitation: official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2009;16(3):249–67. Epub 2009/05/15. doi: 10.1097/HJR.0b013e32832914c8 . [DOI] [PubMed] [Google Scholar]
- 37.Koike A, Nagayama O, Hoshimoto-Iwamoto M, Suzuki T, Tajima A, Uejima T, et al. Clinical significance of cerebral oxygenation during exercise in patients with coronary artery disease. Circulation journal: official journal of the Japanese Circulation Society. 2008;72(11):1852–8. Epub 2008/10/04. . [DOI] [PubMed] [Google Scholar]
- 38.Wahba RW, Tessler MJ. Misleading end-tidal CO2 tensions. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 1996;43(8):862–6. Epub 1996/08/01. doi: 10.1007/BF03013040 . [DOI] [PubMed] [Google Scholar]
- 39.Bhavani-Shankar K, Moseley H, Kumar AY, Delph Y. Capnometry and anaesthesia. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 1992;39(6):617–32. Epub 1992/07/01. doi: 10.1007/BF03008330 . [DOI] [PubMed] [Google Scholar]
- 40.McSwain SD, Hamel DS, Smith PB, Gentile MA, Srinivasan S, Meliones JN, et al. End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respiratory care. 2010;55(3):288–93. Epub 2010/03/04. ; PubMed Central PMCID: PMCPMC2837928. [PMC free article] [PubMed] [Google Scholar]
- 41.Herholz K, Buskies W, Rist M, Pawlik G, Hollmann W, Heiss WD. Regional cerebral blood flow in man at rest and during exercise. Journal of neurology. 1987;234(1):9–13. Epub 1987/01/01. . [DOI] [PubMed] [Google Scholar]
- 42.Arena R, Guazzi M, Myers J. Prognostic value of end-tidal carbon dioxide during exercise testing in heart failure. International journal of cardiology. 2007;117(1):103–8. Epub 2006/07/18. doi: 10.1016/j.ijcard.2006.04.058 . [DOI] [PubMed] [Google Scholar]
- 43.Karlman Wasserman JEH, Sue Darryl Y., Stringer William W. and Whipp Brian J. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications 5th ed: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 44.Wang JS, Fu TC, Wang CH, Chou SL, Liu MH, Cherng WJ. Exertional periodic breathing potentiates erythrocyte rheological dysfunction by elevating pro-inflammatory status in patients with anemic heart failure. International journal of cardiology. 2013;167(4):1289–97. Epub 2012/04/24. doi: 10.1016/j.ijcard.2012.03.170 . [DOI] [PubMed] [Google Scholar]
- 45.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. Journal of the American College of Cardiology. 2008;52(10):818–27. Epub 2008/08/30. doi: 10.1016/j.jacc.2008.04.061 . [DOI] [PubMed] [Google Scholar]
- 46.Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. American heart journal. 1972;83(3):415–26. Epub 1972/03/01. . [DOI] [PubMed] [Google Scholar]
- 47.Mancini DM, Kunavarapu C. Effect of erythropoietin on exercise capacity in anemic patients with advanced heart failure. Kidney international Supplement. 2003;(87):S48–52. Epub 2003/10/09. . [DOI] [PubMed] [Google Scholar]
- 48.Rifai L, Winters J, Friedman E, Silver MA. Initial description of cerebral oximetry measurement in heart failure patients. Congestive heart failure (Greenwich, Conn). 2012;18(2):85–90. Epub 2012/03/22. doi: 10.1111/j.1751-7133.2011.00284.x . [DOI] [PubMed] [Google Scholar]
- 49.Pott F, Knudsen L, Nowak M, Nielsen HB, Hanel B, Secher NH. Middle cerebral artery blood velocity during rowing. Acta physiologica Scandinavica. 1997;160(3):251–5. Epub 1997/07/01. doi: 10.1046/j.1365-201X.1997.00144.x . [DOI] [PubMed] [Google Scholar]
- 50.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. Journal of the American College of Cardiology. 1999;33(4):951–8. Epub 1999/03/26. . [DOI] [PubMed] [Google Scholar]
- 51.Oliveira MF, Alencar MC, Arbex F, Souza A, Sperandio P, Medina L, et al. Effects of heart failure on cerebral blood flow in COPD: Rest and exercise. Respiratory physiology & neurobiology. 2016;221:41–8. Epub 2015/11/04. doi: 10.1016/j.resp.2015.10.005 . [DOI] [PubMed] [Google Scholar]
- 52.Harper AM, Glass HI. Effect of alterations in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. J Neurol Neurosurg Psychiatry. 1965;28(5):449–52. Epub 1965/10/01. ; PubMed Central PMCID: PMCPMC495935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wahr JA, Tremper KK, Samra S, Delpy DT. Near-infrared spectroscopy: theory and applications. Journal of cardiothoracic and vascular anesthesia. 1996;10(3):406–18. Epub 1996/04/01. . [DOI] [PubMed] [Google Scholar]
- 54.Greenberg S, Shear T, Murphy G. Extracranial Contamination of Near-Infrared Spectroscopy Devices. Anesthesia & Analgesia. 2017;124(1):356–8. doi: 10.1213/ane.0000000000001290 00000539-201701000-00039. [DOI] [PubMed] [Google Scholar]
- 55.Jones S, Chiesa ST, Chaturvedi N, Hughes AD. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery research. 2016;16:25–33. Epub 2016/12/13. doi: 10.1016/j.artres.2016.09.001 ; PubMed Central PMCID: PMCPMC5134760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.