Abstract
Background
Half of surgically-created arteriovenous fistulas (AVFs) require additional intervention to effectively support hemodialysis. Postoperative care and complications may affect clinical maturation.
Study Design
Hemodialysis Fistula Maturation (HFM) Study, a 7-center prospective cohort study.
Setting & Participants
491 patients with single-stage AVFs who had neither thrombosis nor AVF intervention before a 6-week postoperative ultrasound examination, and who required maintenance hemodialysis.
Predictors
Postoperative care processes and complications.
Outcomes
Attempted cannulation, successful cannulation, and unassisted and overall clinical maturation as defined by the HFM Study criteria.
Results
AVF cannulation was attempted in 443 of 491 (90.2%) participants, and was eventually successful in 430 of these 443 (97.1%) participants. 263 of these 430 (61.2%) reached unassisted and 118 (27.4%) reached assisted AVF maturation (overall maturation, 381/430 [88.6%]). Attempted cannulation was less likely in patients of surgeons with policies for routine 2-week versus later than 2-week first postoperative visits (OR, 0.21; 95% CI, 0.06–0.70), routine second post-operative follow-up visits (OR, 0.39; 95% CI, 0.15–0.97), and routine clinical postoperative ultrasound (OR, 0.28; 95% CI, 0.14–0.55). Attempted cannulation was also less likely among patients undergoing procedures to assist maturation (OR, 0.51; 95% CI, 0.27–0.98). Unassisted maturation was more likely for patients treated in facilities with access coordinators (OR, 1.91; 95% CI, 1.17–3.12), but less likely after pre-cannulation non-study ultrasounds (OR, 0.42 [95% CI, 0.26–0.68]/ultrasound) and initial unsuccessful cannulation attempts (OR per each additional attempt, 0.90 [95% CI, 0.83–0.98]). Overall maturation was less likely with infiltration before successful cannulation (OR, 0.44; 95% CI, 0.22–0.89). Among participants receiving maintenance hemodialysis before AVF surgery, unassisted and overall maturation were less likely with longer intervals from surgery to initial cannulation (ORs for each additional month of 0.81 [95% CI, 0.76–0.88] and 0.93 [95% CI, 0.89–0.98], respectively), and from initial to successful cannulation (ORs for each additional week of 0.87 [95% CI, 0.81–0.94] and 0.88 [95% CI, 0.83–0.94], respectively).
Limitations
Surgeons’ management policies were assessed only by questionnaire at study onset. Most participants received upper arm AVFs, planned two-stage AVFs were excluded, and maturation time windows were imposed. Some care processes may have been missed and the observational design limits causal attribution.
Conclusions
Multiple processes of care and complications are associated with AVF maturation outcomes.
Index words: Vascular access, arteriovenous fistula (AVF), arteriovenous access, fistula maturation, patency, hemodialysis, end-stage renal disease, process-of-care, cannulation
An arteriovenous fistula (AVF) is clinically mature if it can be reproducibly cannulated with two large-bore needles and provide sufficient blood flow for adequate hemodialysis. Progressive postoperative increases in vessel diameter and blood flow1 are necessary, but insufficient, for clinical AVF maturation. Numerous care processes or complications may delay and/or prevent successful cannulation and/or maturation. Surgeons or nephrologists may be reluctant for less-experienced dialysis staff to attempt cannulation. Attempted cannulations may cause infiltrations, impeding successful AVF use. New AVFs may require diagnostic tests and/or surgical or percutaneous interventions to attain clinical maturation. Extended delays may lead to abandonment. AVF management without a dedicated vascular access coordinator may lower the chance of clinical maturation. Nevertheless, only a few single-center studies have investigated relationships between care processes and clinical maturation 2–6.
The Hemodialysis Fistula Maturation (HFM) Study was a 7-center prospective cohort study of patients undergoing creation of new, single-stage AVFs,7 for which care processes constituted a protocol-specified investigational domain. Notable variations in care processes within and across centers were identified before patient enrollment. We examined the associations of AVF management care processes and complications with three critical events in achieving maturation: attempted cannulation, successful cannulation, and clinical maturation.
Methods
Population, Access Management, and Data Collection
The HFM Study enrolled 602 patients with chronic kidney disease undergoing creation of a planned single-stage upper-extremity AVF7 March 2010 through August 2013. Participants provided written informed consent approved by the clinical center Institutional Review Boards (see Item S1 for IRB numbers).
Patients underwent standardized preoperative sonographic vascular mapping, with results used by the surgeons in planning AVF surgery. The study protocol included standardized postoperative research ultrasounds at 1 day, 2 weeks, and 6 weeks. Results of these were not reported to the clinical centers except for 6-week ultrasounds at centers routinely performing them clinically. Beyond discouraging elective interventions before 6 weeks, the study did not stipulate a standard management protocol for new AVFs. Surgeons and dialysis units determined their own timing and frequency of postoperative visits and attempted AVF cannulation, use of non-study imaging studies, and type(s) and timing of interventions in non-maturing AVFs. The specific functions of access coordinators, when present, varied by unit policy. The HFM Study collected baseline patient demographics and co-morbidities; surgeon and dialysis center practice policies, including surgeon clinical and ultrasound follow-up policies; ultrasounds as above; postoperative care processes and complications related to AVF management, e.g., cannulations complicated by infiltration;7 and use of additional diagnostic tests to assess, and interventions to promote, AVF maturation. Follow-up policies were studied without tracking patients’ individual follow-up and clinical ultrasound visits, but detailed information including whether and when cannulation was attempted, whether and when the AVF was first successfully used for dialysis (by HFM Study definition), and whether attempted cannulation was complicated by an infiltration, was obtained for each dialysis session, beginning with first attempted AVF cannulation and continuing until clinical maturation failure or success, whether unassisted or assisted by intervention, was determined.
Definitions of Study Outcomes
For patients on dialysis prior to AVF creation, clinical AVF maturation was defined as use with two needles for ≥75% of hemodialysis sessions during a continuous 4-week period commencing within 9 months of AVF surgery, and including 4 consecutive sessions with mean blood pump flow >300 ml/min or, failing that, any Kt/V ≥1.4 or urea reduction ratio (URR) ≥70%. For patients initiating dialysis >9 months after AVF creation, the first dialysis session meeting the pump flow, Kt/V, or URR criterion was required to be within 4 weeks of hemodialysis initiation. Clinical maturation was subclassified as assisted if preceded by a percutaneous or surgical intervention to promote AVF maturation, and unassisted—the primary HFM Study clinical outcome—otherwise.
We focused on unassisted and overall (unassisted or assisted) clinical maturation and two related but distinct necessary intermediate outcomes: (1) the initial attempt to cannulate the AVF; and (2) successful cannulation, i.e. completion of a 2-needle dialysis session.
Statistical Analysis
Measures of AVF physiological maturation (AVF flow, vein diameter) and accessibility for cannulation (vein depth) may guide care processes and decisions of whether and when to cannulate the AVF, and are prognostic for clinical maturation8,9, thereby potentially confounding by indication the relationships of some care processes to cannulation and/or maturation. To avoid this, we both restricted analysis to the cannulation-eligible subcohort of patients whose AVFs survived without assistance to the point of 6-week ultrasound examination (491 patients), and controlled analyses for 6-week ultrasound flow, diameter, and depth. We studied associations of care processes, AVF infiltrations, and interventions with decisions to attempt cannulation in this group, and/or with clinical maturation in the further subset cannulated successfully, i.e. the cannulated subcohort, as indicated in Table 1. For these analyses, we used multiply imputed10,11 race (Black vs. other) in 7 participants who omitted it, unassisted and overall AVF maturation outcomes in 6 and 10 participants, respectively, for whom they were unresolved by study closure, and 6-week AVF ultrasound measurements in 45 participants who missed this appointment or had technically unsatisfactory ultrasounds (missing measurements and/or poor image quality). We used 20 imputations and Rubin’s formula.8
Table 1.
Care Processes Examined for Attempted Cannulation in Cannulation-Eligible Subcohort, and for Unassisted and Overall Clinical Maturation in Cannulated Subcohort
| Outcomes Studied | ||
|---|---|---|
| Process or Event | Cannulation Attempt | Clinical Maturation |
| Transposed vs. non-transposed AVF | X | |
| Timing of surgeon’s earliest regular follow-up visit | X | |
| Surgeon has regular 2nd follow-up visit | X | |
| Surgeon’s routine use of post-operative ultrasound (vs. no or selectively) | X | X |
| Timing of surgeon’s earliest regular ultrasound follow-up | X | X |
| Dedicated vascular access coordinator (dichotomous: yes, and participates in scheduling referrals, evaluations, and surgery, vs. other) | X | X |
| No. of non-study ultrasounds done pre-cannulation | X | X |
| Any invasive diagnostic procedure or intervention to assist the fistula | X | X |
| Surgeon’s usual follow-up routine (0,1, ≥2 routine visits) | X | |
| Surgeon’s usual ultrasound follow-up routine (0,1, ≥2 usual ultrasounds) | X | |
| Dialysis unit of 1st cannulation attempt: No. of stations | X | |
| Dialysis unit of 1st cannulation attempt: # maintenance HD patients treated per week | X | |
| Dialysis unit of 1st cannulation attempt: responsibility for initial cannulation decisions (varies by patient, dialysis unit staff, nephrologist, surgeon, group decision) | X | |
| Dialysis unit of 1st cannulation attempt: written protocol for initial cannulation procedure | X | |
| Surgery to dialysis interval (for those not on maintenance dialysis at time of surgery) | X | |
| Surgery to 1st cannulation interval (for those on maintenance dialysis at time of surgery) | X | |
| Initial cannulator role (technician or nurse) | X | |
| Initial cannulator years of experience | X | |
| Size of the largest needle at 1st cannulation (15, 16, or 17 gauge) | X | |
| No. of cannulation attempts to achieve 1st successful cannulation | X | |
| Interval from 1st attempt to successful cannulation | X | |
| Any infiltration during cannulation attempt prior to 1st successful cannulation | X | |
| Any additional infiltrations requiring new needle placement, or interruption of dialysis or study fistula use, after successful cannulation and prior to determination of respective (unassisted or overall) maturation outcome | X | |
| Intervention attributed to stenosis or vein depth prior to determination of overall maturation outcome. | X | |
Note: See Figure 1 for subcohort composition.
HD, hemodialysis
We treated kidney transplantation without cannulation and death without transplantation or cannulation as competing risks for cannulation analyses; death as a clinical maturation failure, as in other HFM Study analyses; and used the Fine-Gray cumulative-incidence approach18 to describe times to cannulation and maturation. For patients in the cannulation-eligible subcohort and on maintenance dialysis at the time of AVF creation, we estimated and plotted cumulative incidences from AVF creation to first attempted cannulation and overall clinical maturation. For those cannulated, we similarly described times from first attempted to first successful cannulation, and from then to overall clinical maturation. These descriptive analyses involved no imputation.
We used logistic regression to examine associations of care processes with outcomes. Influences of care processes that vary between clinical centers may partially manifest as outcome differences across centers. Composition of local ESRD populations, and both measured and unmeasured clinical center correlates, may confound statistical associations of specific care processes with outcomes. We controlled for variation in patient populations and, potentially indirectly, for unmeasured pre-cannulation care processes affecting initial post-operative AVF response, using models adjusting for sex, race (Black vs. other), age (decade), diabetes, maintenance dialysis status at time of surgery, AVF location (forearm or upper arm), and blood flow, diameter and depth on 6-week ultrasound examination. We assessed associations of outcomes with each individual care process in primary models without clinical center adjustment, and then with random or fixed clinical center effects, to respectively reduce or remove confounding by other center-associated variables. The latter models control confounding at the risk of overmatching bias when variation in outcomes by center is truly attributable to care process effects. Random effects models were estimated by Laplace likelihood approximation (SAS PROC GLIMMIX), with confidence intervals (CIs) and Wald test statistics from model-based covariance estimates. We also tested interactions of care processes with AVF location, and examined the significance of residual clinical center outcome variation in models with fixed effects of clinical center and the baseline case-mix and 6-week ultrasound measures, with and without each care process.
Local study coordinators occasionally continued to follow some patients’ dialysis sessions beyond the protocol-specified time windows, sometimes long enough to confirm late satisfaction of maturation criteria. In a sensitivity analysis, we relaxed the 9-month maturation time window for determination of AVF maturation (Item S1).
Because timing of landmark events appeared strongly related to each outcome, we fit models jointly incorporating and adjusting further for several timing variables, nested by dialysis status at surgery. These joint models for cannulation decisions and overall maturation are viewed as exploratory and primarily descriptive (Item S1).
Results
Study Participants
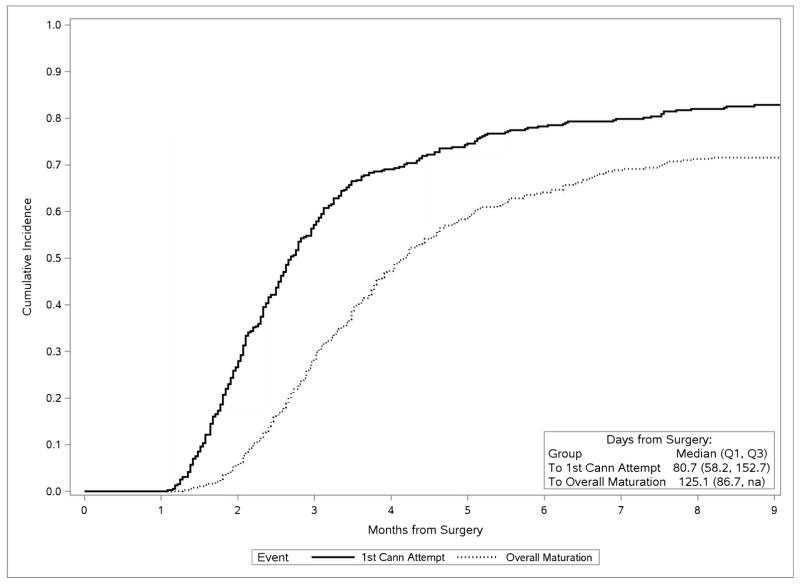
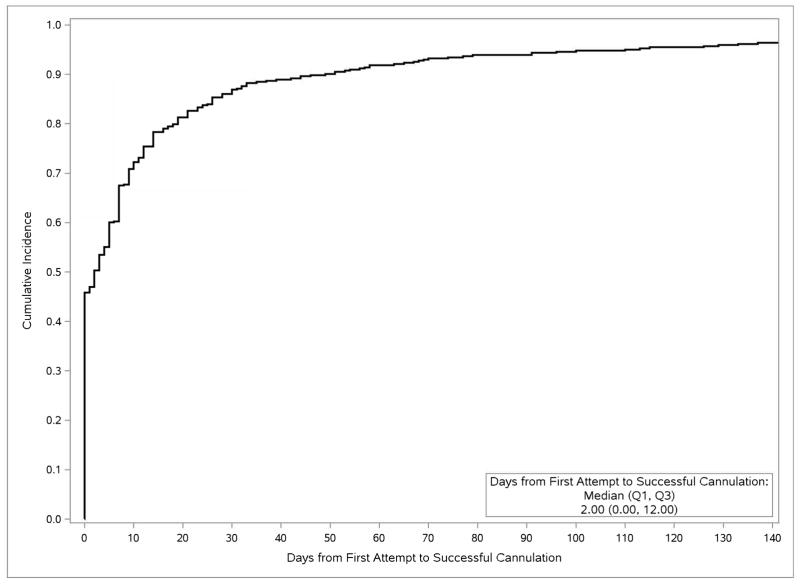
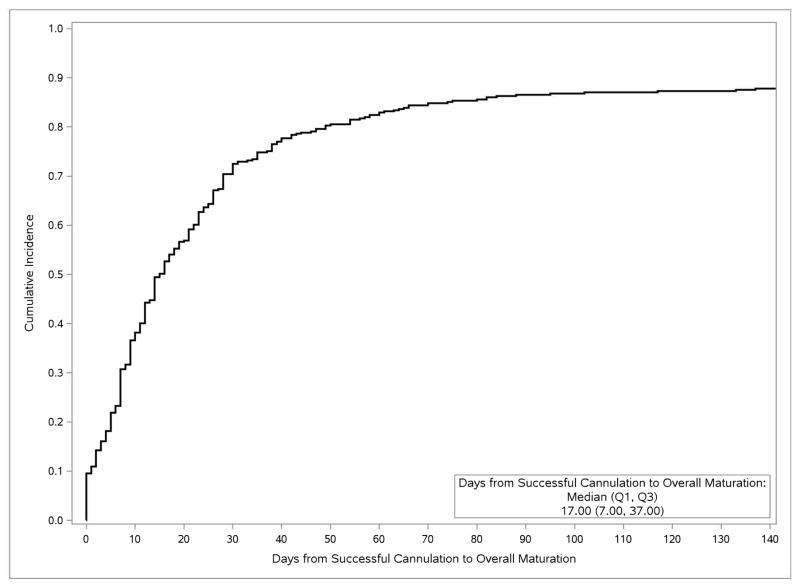
In unadjusted cumulative incidence analysis of the 387 HFM participants whose AVFs were created after initiating maintenance dialysis, the median interval from AVF creation to first cannulation attempt was 81 (interquartile range [IQR], 58–153) days, and from AVF creation to overall maturation was 125 (IQR, 87-NA [not applicable]) days (Fig 1), with, respectively, 5%, 25%, 42%, 61%, and 72% of cannulation-eligible patients achieving overall AVF maturation at 2, 3, 4, 6, and 9 months. The median interval from first attempted to first successful cannulation, among all participants ever cannulated, was 2 (IQR, 0–12) days (Fig 2a), and the median interval from first successful cannulation to overall clinical maturation among those successfully cannulated was 17 (IQR, 7–37) days (Fig 2b).
Fig 1.
Cumulative incidences of attempted AVF cannulation and overall maturation after AVF creation surgery, among members of the cannulation-eligible subcohort on dialysis at the time of surgery (N=387), censored for loss to and termination of follow-up, treating kidney transplantation as a competing risk and death as a maturation failure and a competing risk for cannulation.
Fig 2.
Cumulative incidences. Top panel, of successful cannulation after first cannulation attempt, among members of the cannulation-eligible subcohort for whom cannulation was attempted (N=443), censored for loss to and termination of follow-up, and treating kidney transplantation and death as competing risks. Bottom panel, of clinical AVF maturation after successful cannulation, in the successfully cannulated subcohort (N=430), censored for loss to and termination of follow-up, and treating death as a maturation failure and kidney transplantation as a competing risk.
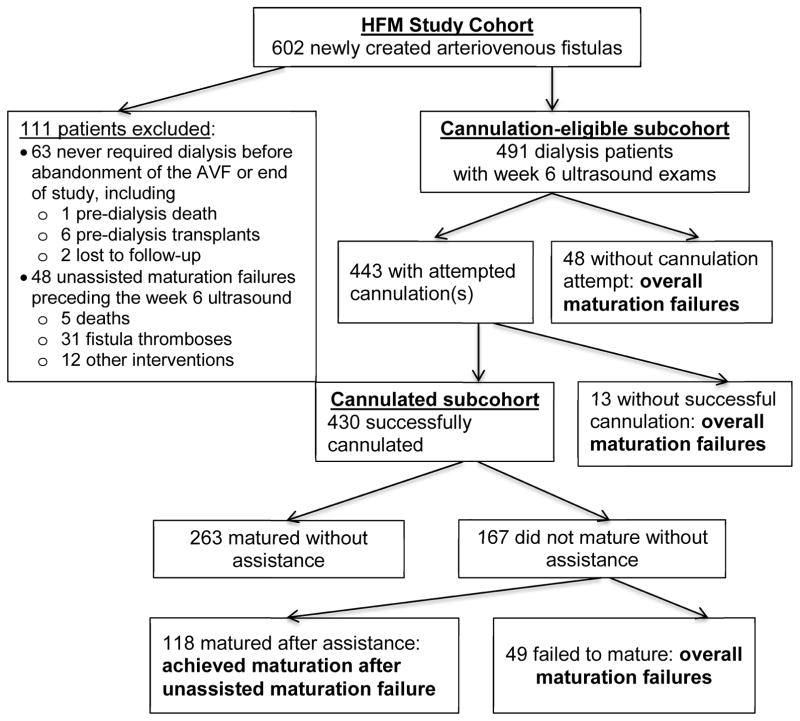
For analyses relating processes of care to cannulation and maturation, we excluded 63 otherwise cannulation-eligible participants who did not initiate maintenance hemodialysis, for whom cannulation was thus not clinically indicated during the study. The cannulation-eligible subcohort further excluded 48 additional patients due to thromboses (n=31, many very early), interventions (n=12), or deaths (n=5) before the 6-week study protocol ultrasound (Fig 3). Among the 491 remaining cannulation-eligible participants, 443 (90.2%) had at least one attempted AVF cannulation. Of these, 430 (97.1%) eventually completed at least one successful two-needle hemodialysis session (successful cannulation). Among the successfully cannulated subcohort, 381 (88.6%) achieved clinical AVF maturation, including 263 (61.2%) without and 118 (27.4%) with assistance. Unassisted, assisted, and overall AVF maturations among the full cannulation-eligible subcohort were 53.6%, 24.0%, and 77.6%, respectively.
Fig 3.
Analysis subcohorts and maturation-related outcomes (N=602).
Table 2 summarizes baseline demographic, clinical characteristics, follow-up policies, and AVF outcomes, by clinical center in the cannulation-eligible subcohort. There was substantial variation among the 7 clinical centers (A–G) in each care process included in Table 2; subsequent analyses focus on associations of care processes and AVF outcomes.
Table 2.
Baseline case-mix factors, 6-week ultrasound AVF measurements, pre-cannulation care factors, and cannulation in cannulation-eligible subcohort, overall and by clinical center
| Clinical center | ||||||||
|---|---|---|---|---|---|---|---|---|
| All | A | B | C | D | E | F | G | |
| Demographic or clinical factor | ||||||||
| Age at AVF surgery (y) | 55.0±13.8 | 55.8±12.1 | 51.1±11.2 | 55.5±13.7 | 55.1±11.3 | 49.9±13.1 | 56.9±14.9 | 58.2±15.0 |
| Female (%) | 28.5 | 35.6 | 30.0 | 21.5 | 24.1 | 22.2 | 26.1 | 33.9 |
| Black (%) | 44.4 | 57.8 | 86.7 | 12.7 | 13.8 | 47.8 | 52.2 | 46.5 |
| History of Diabetes (%) | 60.9 | 63.3 | 50.0 | 64.6 | 72.4 | 61.1 | 56.5 | 58.3 |
| Dialysis initiated before AVF surgery (%) | 70.1 | 72.2 | 80.0 | 57.0 | 44.8 | 75.6 | 78.3 | 73.2 |
| Forearm AVF (%) | 21.4 | 21.1 | 30.0 | 29.1 | 27.6 | 31.1 | 15.2 | 8.7 |
| Transposed vein AVF (%) | 31.4 | 26.7 | 46.7 | 19.0 | 10.3 | 36.7 | 13.0 | 46.5 |
| Week 6 Ultrasound measures | ||||||||
| AVF blood flow (ml/min) | 1031±613 | 846±483 | 788±495 | 1059± 725 | 873±521 | 1097 ± 666 | 1101±528 | 1166±611 |
| Minimal AVF diameter (cm) | 0.52±0.15 | 0.51±0.15 | 0.49±0.15 | 0.56±0.16 | 0.58±0.15 | 0.52±0.15 | 0.53±0.16 | 0.52±0.14 |
| AVF depth (cm) | 0.45±0.22 | 0.39±0.23 | 0.45±0.24 | 0.44±0.24 | 0.47±0.21 | 0.43±0.19 | 0.41±0.22 | 0.51±0.22 |
| Process of care factors | ||||||||
| Pts whose surgeon has routine 2-wk surgical f/u policy (%) | 77.0 | 8.3 | 0.0 | 100.0 | 77.8 | 90.9 | 0.0 | 100.0 |
| Pts whose surgeon has routine 2nd surgical f/u visit (%) | 77.3 | 58.8 | 0.0 | 67.1 | 60.7 | 90.0 | 100.0 | 100.0 |
| Pts whose surgeon has routine postoperative ultrasound policy (%) | 71.5 | 30.0 | 100.0 | 100.0 | 10.3 | 94.5 | 0.0 | 100.0 |
| Pts whose surgeon routinely obtains earlier vs. 6-wk ultrasound f/u (%) | 25.9 | 92.6 | 0.0 | 63.4 | 66.7 | 66.7 | -- | 0.0 |
| Pts with 1 non-study ultrasound f/u (%) | 17 | 12 | 17 | 15 | 21 | 4 | 4 | 35 |
| Pts with ≥2 non-study ultrasound f/u (%) | 4 | 1 | 0 | 2 | 14 | 3 | 2 | 8 |
| Pts undergoing invasive diagnostic or rescue intervention (%) | 29.7 | 17.8 | 26.7 | 22.8 | 24.1 | 16.7 | 63.0 | 41.7 |
| Pts at dialysis units with active vascular access coordinators (%) | 39.5 | 57.3 | 0.0 | 18.6 | 3.4 | 78.9 | 36.4 | 28.7 |
| AVF clinical outcomes | ||||||||
| Attempted AVF cannulation (%) | 90.2 | 94.4 | 90.0 | 96.2 | 93.1 | 94.4 | 91.3 | 79.5 |
| Successful/attempted AVF cannulation (%) | 97.1 | 96.5 | 100.0 | 94.7 | 100.0 | 98.8 | 100.0 | 95.0 |
| Unassisted maturation/successful cannulation (%)1 | 61.4 | 61.0 | 59.3 | 65.3 | 63.0 | 77.4 | 38.1 | 55.2 |
| Overall maturation/successful cannulation (%)1 | 88.8 | 84.2 | 92.6 | 93.1 | 85.2 | 96.4 | 81.0 | 86.5 |
Note: Cannulation-eligible subcohort n = 491. Unless otherwise indicated, values are given as mean ± standard deviation.
AVF, arteriovenous fistula; Pt, patient; f/u, follow-up
A small fraction of maturation outcomes were missing and multiply imputed. Results are averaged over these multiple imputations
Factors Associated With AVF Cannulation
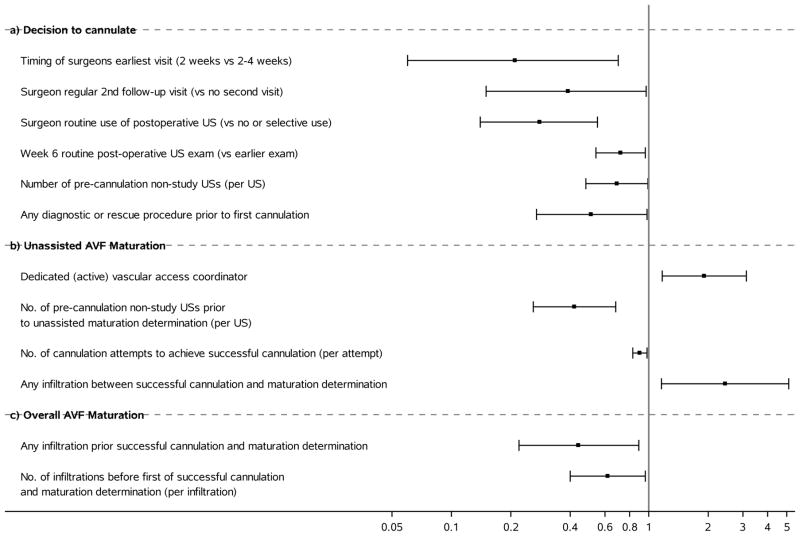
The percentages of cannulation-eligible patients for whom AVF cannulation was attempted varied from 79.5% to 96.2% among centers, with all but one ≥90% (Table 2). After controlling for baseline case-mix factors and 6-week ultrasound measures, cannulation was statistically significantly less likely in patients whose surgeons had routine protocols requiring an early (2-week) postoperative visit (odds ratio [OR], 0.21; 95% CI, 0.06–0.70), multiple follow-up visits (OR, 0.39; 95% CI, 0.15–0.97), any routine non-study postoperative ultrasound (OR, 0.28; 95% CI, 0.14–0.55), or a 6-week vs. earlier routine ultrasound follow-up (OR, 0.72; 95% CI, 0.54–0.96); who underwent more non-study ultrasounds (OR, 0.69 [95% CI, 0.48–0.99] per examination); or who underwent any pre-cannulation invasive diagnostic procedure or rescue intervention (OR, 0.51; 95% CI, 0.27–0.98) (Fig 4a; Table 3). These factors, which did not significantly interact with AVF location, largely reflect surgeon policies that tended to be similar within the same center; consequently, their associations with attempted cannulation became nonsignificant after further adjustment for clinical center (Item S1). Attempted cannulation was not statistically significantly associated with having a transposed AVF, the presence of a dedicated vascular access coordinator, or the surgeon’s routine number of postoperative ultrasounds (Table 3).
Fig 4.
Forest plots of statistically significant estimated odds ratios, with 95% confidence intervals, relating AVF care processes and complications to attempted cannulation and maturation. a) attempted cannulation (N=491); b) unassisted clinical maturation (N=430); c) overall clinical maturation (N=430).
Table 3.
Associations of process of care factors and AVF complications/procedures with attempt to cannulate the AVF in cannulation eligible subcohort, adjusted for basic demographic, case-mix, and ultrasound factors
| OR (95% CI) | P | ||
|---|---|---|---|
| Process of care factors | |||
| Timing of surgeon’s earliest visit, 2 wk vs 2–4 wk | 0.21 (0.06–0.70) | 0.01 | |
| Surgeon has regular 2nd follow-up visit, vs no second visit | 0.39 | 0.15–0.97 | 0.04 |
| Surgeon’s routine use of postoperative ultrasound, vs. no or selective use | 0.28 | 0.14–0.55 | < 0.001 |
| Week 6 vs. earlier routine post-operative ultrasound examination | 0.72 | 0.54–0.96 | 0.03 |
| Transposed AVF | 0.55 | 0.27–1.10 | 0.09 |
| Surgeon’s routine no. of post-operative ultrasounds, 1 vs. ≥2 | 1.18 | 0.75–1.85 | 0.5 |
| Dedicated (active) vascular access coordinator | 1.24 | 0.64–2.39 | 0.5 |
| AVF complications/procedures | |||
| No. of pre-cannulation non-study ultrasounds, vs none | 0.69 | 0.48–0.99 | 0.04 |
| Invasive diagnostic or rescue procedure prior to first cannulation, vs none | 0.51 | 0.27–0.98 | 0.04 |
Note: Cannulation eligible subcohort n=491. Adjusted for age, black race, female sex, dialysis status at surgery, diabetes, AVF location, as well as AVF flow, diameter, and depth at week 6. Measurements from missed US examinations, occasional missing observations for race, and unresolved maturation outcomes were multiply imputed.
AVF, arteriovenous fistula; CI, confidence interval; OR, odds ratio
Cannulation, once attempted, ultimately supported a full dialysis session for 97.1% of patients (Table 2); we did not further investigate the minimal variation in cannulation success.
Factors Associated With Unassisted and Overall AVF Maturation
Among successfully cannulated AVFs, unassisted AVF maturation varied 2-fold, from 38.1% to 77.4%, across clinical centers (Table 2). After adjusting for baseline case-mix and 6-week ultrasound covariates, unassisted maturation was statistically significantly more frequent when the surgical facility or dialysis unit was staffed with an active vascular access coordinator (OR, 1.91; 95% CI, 1.17–3.12). Non-study ultrasound prior to attempting cannulation (OR, 0.42; 95% CI, 0.26–0.68), and more attempts prior to successful cannulation (OR per each additional attempt, 0.90 [95% CI, 0.83–0.98]) were significantly associated with less frequent unassisted maturation while, unexpectedly, an AVF infiltration after successful cannulation was associated with unassisted maturation success (OR, 2.44; 95% CI, 1.16–5.12) (Fig 4b). There were no significant interactions between AVF location and these factors, nor was unassisted maturation significantly associated with other factors related to dialysis unit size or cannulation protocols (Table 4).
Table 4.
Associations of process of care factors and AVF complications/procedures with unassisted maturation in cannulated subcohort, adjusted for basic demographic, case-mix, and ultrasound factors
| OR (95% CI) | P | ||
|---|---|---|---|
| Process of care factors | |||
| Dedicated (active) vascular access coordinator, vs none | 1.91 (1.17–3.12) | 0.01 | |
| No. of pre-cannulation non-study ultrasounds prior to unassisted maturation determination, vs none | 0.42 | 0.26–0.68 | <0.001 |
| No. of cannulation attempts to achieve successful cannulation, per attempt (vs none) | 0.90 | 0.83–0.98 | 0.02 |
| Transposed AVF | 1.45 | 0.84–2.44 | 0.2 |
| Surgeon’s routine use of postoperative ultrasound, vs. no or selective use | 0.68 | 0.42–1.09 | 0.1 |
| Initial cannulator experience | 1.06 | 0.53–2.50 | 0.9 |
| Size of largest needle in first cannulation attempt, per increase in gauge | 0.55 | 0.21–1.45 | 0.2 |
| Dialysis unit, written protocol for initial cannulation procedure | 1.59 | 0.90–2.78 | 0.1 |
| Dialysis unit, no. of stations | 1.01 | 0.98–1.04 | 0.6 |
| Dialysis unit weekly maintenance HD patients, per 25 patients | 1.05 | 0.98–1.12 | 0.2 |
| AVF complications/procedures | |||
| Any infiltration between successful cannulation and maturation determination, vs none | 2.44 | 1.16–5.12 | 0.01 |
| Any infiltration prior to successful cannulation and maturation determination | 1.30 | 0.76–2.17 | 0.3 |
| No. of infiltrations prior to successful cannulation and maturation determination | 0.83 | 0.57–1.19 | 0.3 |
Note: Cannulated subcohort n=430. Adjusted for age, black race, female sex, dialysis status at surgery, diabetes, AVF location, as well as AVF flow, diameter, and depth at week 6. Measurements from missed ultrasound examinations, occasional missing observations for race, and unresolved maturation outcomes were multiply imputed.
AVF, arteriovenous fistula; CI, confidence interval; OR, odds ratio; HD, hemodialysis
Overall AVF maturation varied across clinical centers less than unassisted AVF maturation, from 81.0% to 96.4%, (Table 2). Among successfully cannulated participants, after adjustment for baseline case-mix and 6-week ultrasound covariates, overall maturation was less frequent if there were any AVF infiltrations (OR, 0.44; 95% CI, 0.22–0.89) or more infiltrations before successful cannulation (OR, 0.62 [95% CI, 0.40–0.96] per infiltration) (Fig 4c). These infiltration effects did not significantly interact with AVF location. Overall AVF maturation was not significantly associated with other factors related to dialysis unit size or cannulation protocols (Table 5).
Table 5.
Associations of process of care factors and AVF complications/procedures with overall maturation in cannulated subcohort, adjusted for basic demographic, case-mix, and ultrasound factors
| Process of care factors | OR (95% CI) | P | |
|---|---|---|---|
| Transposed AVF | 1.97 (0.87–4.42) | 0.1 | |
| Surgeon usual routine of postoperative visits, ≥2 vs 1 | 1.34 | 0.63–2.83 | 0.4 |
| Surgeon use of postoperative ultrasound, vs no or selective use | 0.84 | 0.42–1.70 | 0.6 |
| Dedicated (active) vascular access coordinator | 0.87 | 0.43–1.78 | 0.7 |
| No. of pre-cannulation non-study ultrasounds before maturation determination, per 1 greater | 1.03 | 0.66–1.60 | 0.9 |
| Initial cannulator, nurse vs. technician | 1.05 | 0.51–2.16 | 0.9 |
| Size of largest needle in first cannulation attempt, per each increase in gauge | 0.69 | 0.18–2.68 | 0.6 |
| Dialysis unit has written protocol for initial cannulation procedure | 0.80 | 0.31–2.08 | 0.7 |
| Dialysis unit no. of stations, per 1 greater | 1.00 | 0.80–1.24 | 0.9 |
| Dialysis unit: weekly maintenance HD patients, per 25 patients greater | 1.00 | 0.91–1.10 | 0.9 |
| AVF complications/procedures | |||
| Any infiltration prior to successful cannulation and maturation determination, vs none | 0.44 | 0.22–0.89 | 0.02 |
| No. of infiltrations preceding successful cannulation and maturation determination, vs none | 0.62 | 0.40–0.96 | 0.03 |
| Any infiltration between successful cannulation and maturation determination | 0.75 | 0.34–1.67 | 0.5 |
| No. of cannulation attempts to achieve successful cannulation, per attempt | 0.94 | 0.87–1.02 | 0.2 |
| Invasive diagnostic or intervention procedure | 0.49 | 0.23–1.01 | 0.05 |
| Intervention for stenosis or vein depth p/to overall maturation | 0.79 | 0.39–1.63 | 0.5 |
Note: Cannulated subcohort n=430. Adjusted for age, black race, female sex, dialysis status at surgery, diabetes, AVF location, as well as AVF flow, diameter, and depth at week 6. Measurements from missed ultrasound examinations, occasional missing observations for race, and unresolved maturation outcomes were multiply imputed.
AVF, arteriovenous fistula; CI, confidence interval; OR, odds ratio; HD, hemodialysis
Associations of Time to Intermediate Events With Unassisted and Overall AVF Maturation
Among patients on hemodialysis at AVF creation, longer interval from AVF creation to first cannulation was associated with less frequent unassisted and overall AVF maturation (ORs of 0.81 [95% CI, 0.76–0.88] and 0.93 [95% CI, 0.89–0.98] per month, respectively) (Table 6). Similarly, longer interval from first attempted to first successful cannulation was associated with less frequent AVF maturation (ORs of 0.87 [95% CI, 0.81–0.94] and 0.88 [95% CI, 0.83–0.94] per week for unassisted and assisted, respectively).
Table 6.
Associations of time to first attempted cannulation and first successful cannulation with unassisted and overall AVF maturation in cannulated subcohort, adjusted for basic demographic, case-mix, and ultrasound factors
| OR (95% CI) | P | |
|---|---|---|
| Months from surgery to first attempted cannulation, patients on dialysis at time of AVF surgery | ||
| Unassisted AVF maturation, per additional month | 0.81 (0.76–0.88) | < 0.001 |
| Overall AVF maturation, per additional month | 0.93 (0.89–0.98) | 0.003 |
| Weeks from 1st to successful cannulation, cannulated subcohort | ||
| Unassisted AVF maturation, per additional week | 0.87 (0.81–0.94) | <0.001 |
| Overall AVF maturation, per additional week | 0.88 (0.83–0.94) | <0.001 |
Note: Adjusted for age, black race, female sex, dialysis status at surgery, diabetes, AVF location, as well as AVF flow, diameter, and depth at week 6. Measurements from missed ultrasound examinations, occasional missing observations for race, and unresolved maturation outcomes were multiply imputed.
AVF, arteriovenous fistula; CI, confidence interval; OR, odds ratio
Clinical Center Variation in AVF Maturation
Effects of further adjustment for clinical center on the preceding results were modest. After adjustment for baseline case-mix and 6-week ultrasound covariates, clinical center effects were highly statistically significant for unassisted AVF maturation, but nonsignificant for overall maturation. In exploratory models for either AVF maturation end point that incorporated the several timing variables and individual other care processes, the timing effects were most persistent in significantly predicting the maturation outcome, with or without adjustment for clinical center (Item S1).
Discussion
We analyzed associations of care processes, early infiltrations, and/or early interventions with attempted cannulation and clinical maturation in a large, multi-center prospective cohort study whose centers employed varying care processes and exhibited notable inter-center variation in AVF outcomes, even after adjustment for baseline case-mix and 6-week ultrasound measurements. Several findings are noteworthy. First, more routine intensive postoperative follow-up (earlier and/or more frequent surgical visits and routine [but not earlier] use of ultrasound) was unexpectedly associated with lower likelihood of attempted AVF cannulation. Second, dedicated vascular access coordinators were associated with more frequent unassisted AVF maturation. Third, even one AVF infiltration was associated with substantially less frequent overall AVF maturation. Finally, longer times from AVF creation to first attempted cannulation, and from then to first successful cannulation, were associated with less frequent unassisted and overall AVF maturation. While the latter is unsurprising, the former is unexpected and unsupportive of very early AVF creation. These findings are pertinent to patients on or initiating maintenance hemodialysis within roughly one year of surgery whose AVFs survive ≥6 weeks.
Why might closer surgeon postoperative follow-up be associated with less chance of attempting cannulation? Surgeons with such practices may ask dialysis units to postpone cannulating the AVF due to doubts about its maturity, maturation potential, or dialysis staff skill in cannulating new AVFs. Likewise, routine postoperative ultrasounds when clinical assessment would suffice may lead to additional and sometimes unnecessary diagnostic studies or interventions that postpone initial AVF cannulation, and ultimately to decisions not to cannulate AVFs that might otherwise have been cannulated. Without such guidance from the surgeon, the dialysis staff may attempt cannulation more often.
Unassisted maturation was more frequent with a dedicated vascular access coordinator. Dialysis staff may not always regularly evaluate whether an AVF is ready to cannulate. Access coordinators may accelerate attempted cannulation by closely monitoring the AVF and prompting dialysis staff to proceed expeditiously without unnecessary tests or interventions. Finally, with an access coordinator present, the surgeon may be less likely to micromanage AVF imaging and interventions. The presence of an access coordinator favored attempted cannulation, but non-significantly and less strongly than unassisted maturation. Perhaps the surgeon often determined the initial decision to cannulate, with subsequent cannulation attempts driven primarily by the access coordinator.
In exploratory joint analyses of several components of the interval from surgery to successful cannulation, among patients on hemodialysis at surgery, each additional month from AVF creation to first attempted cannulation was associated with 19% lower odds of unassisted and 7% lower odds of overall AVF maturation. Similarly, each additional week from first attempt to first successful cannulation was associated with 13% lower odds of unassisted and 12% lower odds of overall AVF maturation. Although our study could quantify the intervals to attempted and successful cannulation, it was difficult to delineate the specific contributions of individual factors to extended intervals. A delay in attempted cannulation could reflect excessive caution by the surgeon, lack of confidence by dialysis staff performing cannulations, the need for interventions to promote maturation, or the waiting time from scheduling to performing an intervention, reflecting both the operator’s schedule and patient adherence to appointments. Likewise, a delay from first attempt to successful cannulation may be due to infiltration, the time required for the resulting hematoma to resolve, and any indicated interventions. Our analyses do not clearly distinguish the relative contributions of these factors to extended intervals preceding attempted cannulation or successful cannulation.
Interventions needed to promote AVF maturation may delay attempted cannulation. Single-center studies have observed that 35%–50% of new AVFs require intervention prior to successful use for hemodialysis2,12–14. In one, the median durations of catheter dependence following AVF creation were 159 and 99 days, respectively, a 2-month delay, in patients whose AVFs did and did not require intervention prior to successful cannulation14. In our study, 30% of patients required intervention, with increased intervals from successful cannulation to clinical maturation. Our observations are consistent with a single-center study finding that patients catheter dependent for > 6 months of hemodialysis transitioned to permanent vascular accesses at one-third the rate of those with less extended catheter dependence15. The HFM Study protocol specified that clinical AVF maturation occurred only if accomplished within 9 months of AVF creation (in patients already on dialysis). Although AVFs occasionally clinically mature later, most clinicians abandon immature AVFs by then.
The Dialysis Outcomes and Practice Patterns Study (DOPPS) highlighted international variability in AVF creation-to-cannulation intervals (median days16: Germany, 41; Japan, 25; United States, 98; fractions cannulated within one month17: Europe, 50%; Japan, 74%; United States, 2%). The median HFM Study creation-to-cannulation interval was 81 days, less than DOPPS reported in the United States, perhaps due to restricting to single-stage AVFs. What timing of initial cannulation is optimal? Earlier cannulation may shorten catheter dependence, but could increase risk of AVF failure. Our data pertain only to cannulation beyond 6 weeks. Previous observational studies of earlier cannulation have reached conflicting conclusions. A US study, Culp et al (n=118), reported 60% lower thrombosis risk for AVFs initially cannulated after >1 vs. ≤1 month18. Similarly, a larger (n=535) Italian study associated cannulation within 1 month with a higher primary AVF failure rate.19 Two larger DOPPS analyses observed doubled failure rate of AVFs cannulated within 2 weeks vs. later, but stable failure rate beyond 2 weeks (n=3,674), and comparable failure rates between dialysis units with policies to attempt cannulation within 1 month of creation vs. later17. The NKF-KDOQI (National Kidney Foundation–Kidney Disease Outcomes Quality Initiative) guideline recommends attempting cannulation 4–6 weeks after AVF creation20. The question of when to cannulate warrants a definitive randomized clinical trial21.
Needle infiltration is one concern about early AVF cannulation. In our study, even one infiltration before successful cannulation was associated with 56% lower odds of overall AVF maturation. Similarly, a single-center US study reported that AVF infiltration resulted in frequent imaging and interventions, was followed by AVF thrombosis in 26% of cases, and was associated with a median 3-month prolongation of catheter dependence22. Note that infiltration may reflect a poorly matured AVF without directly causing maturation failure.
Our study’s strengths include its multi-center prospective cohort design, relatively large sample, standardized data collection, and analysis controlling for case-mix factors and ultrasound measurements related to physiological maturation of the AVF. We also recognize several limitations. First, each surgeon’s AVF management policies were assessed by questionnaire only at study onset. Changes over time and departures from policies may have obscured relationships between care processes and outcomes. Second, three-fourths of HFM participants received upper arm AVFs. (However, we found no significant interactions between processes of care and AVF location). Third, patients with planned two-stage AVFs, for whom care processes may differ, were excluded. Fourth, some hemodialysis units may use care processes we did not document or analyze. Fifth, findings regarding cannulation attempts were greatly influenced by one center and findings at another reflect the experience of a single surgeon. More generally, some care processes varied primarily between rather than within clinical centers, although adjustment for clinical center to control such confounding only modestly affected results (Item S1). Sixth, our study protocol required limited time windows for overall clinical maturation, although this also appears to have only modestly influenced our results (see Methods and Item S1). Seventh, although seemingly unlikely, excluding patients never on maintenance dialysis could have introduced selection bias.
Finally, there is no unique explanation for our findings; the HFM Study’s observational design limits causal attribution. Cautious practitioners may perform more imaging studies and access interventions, and delay attempting cannulation, which all may contribute to AVF maturation failures. Alternatively, more imaging studies, interventions, cannulation-associated infiltrations, and other delays may result from problems with new AVFs not captured by the 6-week ultrasound measurements of vein diameter, blood flow, and depth, e.g., insufficient length for cannulation, position in the upper extremity (possibly impeding cannulation), and tortuosity (promoting infiltration during cannulation).
In conclusion, we identified vascular access care processes, AVF complications and AVF procedures associated with clinical maturation outcomes after controlling for patient case-mix and 6-week ultrasound measurements. Additional studies, possibly including randomized trials, are needed to reach a definitive causal interpretation of these associations.
Supplementary Material
Acknowledgments
Support: The HFM Study was funded by grants U01DK082218, U01DK082222, U01DK082232, U01DK082236, U01DK082240, U01DK082179, and U01DK082189 from the NIDDK.
Footnotes
Authors’ Contributions: Research idea and study design: MA, GJB, AKC, HIF, TG, PBI, JWK; data acquisition: MA, CEA, GJB, AKC, LMD, AF, JH, TSH, JSK, MR, MLR, PR-C, MAV; data analysis/interpretation: MA, GJB, AKC, LMD, TG, PBI, HIF, JSK; statistical analysis: TG, PBI, MR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: Dr Allon is a Consultant for CorMedix. Dr Cheung is a member of the Data and Safety Monitoring Board for a trial on vascular graft co-sponsored by Humacyte, Inc. and the National Heart, Lung, and Blood Institute as well as a member of the Clinical Events Committee and Data Safety and Monitoring Board for the Novel Endovascular Access Trial sponsored by TVA Medical Inc. Dr Dember is a member of the Data Monitoring Committee for a vascular access trials sponsored by Proteon Therapeutics. Dr Roy-Chaudhury is a consultant or advisory board member for Bard Peripheral Vascular, CorMedix Inc., WL Gore & Associates Inc., Humacyte Inc., Medtronic, and TVA Medical Inc. Dr Vazquez is a member of the Managing Committee for the University of Texas Southwestern Health Systems–DVA Dialysis Joint Venture. The other authors declare that they have no other relevant financial interests.
Peer Review: Received _______. Evaluated by 3 external peer reviewers and a statistician, with direct editorial input from an Acting Editor-in-Chief (Editorial Board Member Kevan R. Polkinghorne, PhD). Accepted in revised form ________. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Item S1: Appendix.
Supplementary Material Descriptive Text for Online Delivery
Supplementary Item S1 (PDF). Appendix.
Disclaimer: The HFM Study was conceived by NIDDK, funded by cooperative agreements, and developed collaboratively by the investigators and the NIDDK project officer, as well as the external expert panel. Decisions to publish are made by majority vote of a Publications Committee (which includes all PIs), and on which the NIDDK project officer has one vote.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robbin ML, Greene T, Cheung AK, et al. Arteriovenous fistula development in the first 6 weeks after creation. Radiology. 2016;279(2):620–629. doi: 10.1148/radiol.2015150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asif A, Cherla G, Merrill D, Cipleu CD, Briones P, Pennell P. Conversion of tunneled hemodialysis catheter-consigned patients to arteriovenous fistula. Kidney Int. 2005;67(6):2399–2406. doi: 10.1111/j.1523-1755.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 3.McLafferty R, Pryor R, Johnson C, Ramsey D, Hodgson K. Outcome of a comprehensive follow-up program to enhance maturation of autogenous arteriovenous hemodialysis access. J Vasc Surg. 2007;45(5):981–985. doi: 10.1016/j.jvs.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Flu H, Breslau P, Krol-van Straaten J, Hamming J, JWL The effect of implementation of an optimized care protocol on the outcome of arteriovenous hemodialysis access surgery. J Vasc Surg. 2008;48(3):659–668. doi: 10.1016/j.jvs.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Kiaii M, MacRae J. A dedicated vascular access program can improve arteriovenous fistula rates without increasing catheters. J Vasc Access. 2008;9(4):254–259. [PubMed] [Google Scholar]
- 6.Blessios G, Park J, Barone K. Effect of a rapid clinical protocol to the conversion from central venous hemodialysis catheter to arteriovenous access. J Vasc Access. 2016;17(2):124–130. doi: 10.5301/jva.5000489. [DOI] [PubMed] [Google Scholar]
- 7.Dember LM, Imrey PB, Beck GJ, et al. Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis. 2014;63(1):104–112. doi: 10.1053/j.ajkd.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbin ML, Chamberlain NE, Lockhart ME, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology. 2002;225(1):59–64. doi: 10.1148/radiol.2251011367. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Robbin ML, Lockhart ME, Allon M. Clinically immature arteriovenous hemodialysis fistulas: effect of US on salvage. Radiology. 2008;246(1):299–305. doi: 10.1148/radiol.2463061942. [DOI] [PubMed] [Google Scholar]
- 10.Rubin D. Imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–489. [Google Scholar]
- 11.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Software. 2011;45(3):1–67. [Google Scholar]
- 12.Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol. 2006;17(5):807–813. doi: 10.1097/01.RVI.0000217928.43396.35. [DOI] [PubMed] [Google Scholar]
- 13.Lee T, Ullah A, Allon M, et al. Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol. 2011;6(3):575–581. doi: 10.2215/CJN.06630810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harms JC, Rangarajan S, Young CJ, Barker-Finkel J, Allon M. Outcomes of arteriovenous fistulas and grafts with and without intervention prior to successful use. J Vasc Surg. 2016;64(1):155–162. doi: 10.1016/j.jvs.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: Reasons and subsequent outcomes. Am J Kidney Dis. 2005;46(3):501–508. doi: 10.1053/j.ajkd.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Rayner HC, Pisoni Rl, Gillespie BW, et al. Creation, cannulation, and survival of arteriovenous fistulae: Data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;63(1):323–330. doi: 10.1046/j.1523-1755.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 17.Saran R, Dykstra DM, Pisoni RL, et al. Timing of first cannulation and vascular access failure in hemodialysis: an analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant. 2004;19(9):2334–2340. doi: 10.1093/ndt/gfh363. [DOI] [PubMed] [Google Scholar]
- 18.Culp K, Flanigan M, Taylor L, Robinson M. Vascular access thrombosis in new hemodialysis patients. Am J Kidney Dis. 1995;26(2):341–346. doi: 10.1016/0272-6386(95)90655-x. [DOI] [PubMed] [Google Scholar]
- 19.Ravani P, Brunori G, Mandolfo S, et al. Cardiovascular comorbidity and late referral impact arteriovenous fistula survival: A prospective multicenter study. J Am Soc Nephrol. 2004;15(1):204–209. doi: 10.1097/01.asn.0000103870.31606.90. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for vascular access 2006. Am J Kidney Dis. 2006;48(suppl 1):S176–S322. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Saran R, Pison R, Young E. Timing of first cannulation of arteriovenous fistula: are we waiting too long? Nephrol Dial Transplant. 2005;20(4):688–690. doi: 10.1093/ndt/gfh730. [DOI] [PubMed] [Google Scholar]
- 22.Lee T, Barker J, Allon M. Needle infiltration of arteriovenous fistulas in hemodialysis: Risk factors and consequences. Am J Kidney Dis. 2006;47(6):1020–1026. doi: 10.1053/j.ajkd.2006.02.181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.