ABSTRACT
Immunostimulatory antibodies against the tumor necrosis factor receptors (TNFR) are emerging as promising cancer immunotherapies. The agonism activity of such antibodies depends on crosslinking to Fc gamma RIIB receptor (FcγRIIB) to enable the antibody multimerization that drives TNFR activation. Previously, Fc engineering was used to enhance the binding of such antibodies to Fcγ receptors. Here, we report the identification of Centyrins as alternative scaffold proteins with binding affinities to homologous FcγRIIB and FcγRIIA, but not to other types of Fcγ receptors. One Centyrin, S29, was engineered at distinct positions of an anti-OX40 SF2 antibody to generate bispecific and tetravalent molecules named as mAbtyrins. Regardless of the position of S29 on the SF2 antibody, SF2-S29 mAbtyrins could bind FcγRIIB and FcγRIIA specifically while maintaining binding to OX40 receptors. In a NFκB reporter assay, attachment of S29 Centyrin molecules at the C-termini, but not the N-termini, resulted in SF2 antibodies with increased agonism owing to FcγRIIB crosslinking. The mAbtyrins also showed agonism in T-cell activation assays with immobilized FcγRIIB and FcγRIIA, but this activity was confined to mAbtyrins with S29 specifically at the C-termini of antibody heavy chains. Furthermore, regardless of the position of the molecule, S29 Centyrin could equip an otherwise Fc-silent antibody with antibody-dependent cellular phagocytosis activity without affecting the antibody's intrinsic antibody-dependent cell-meditated cytotoxicity and complement-dependent cytotoxicity. In summary, the appropriate adoption FcγRII-binding Centyrins as functional modules represents a novel strategy to engineer therapeutic antibodies with improved functionalities.
KEYWORDS: Antibody engineering; agonism; ADCP; alternative scaffold protein; Fc receptor, immunotherapy; tumor necrosis factor; OX40
Introduction
Immunotherapies have emerged as promising cancer therapeutics in recent years. One important class involves the use monoclonal antibodies directed against critical inhibitory and stimulatory receptors on components of the immune system to stimulate antitumor immunity.1,2 Success has been achieved in the clinic with antibodies against the inhibitory receptors CTLA-4 and PD-1, with recent approvals for several cancer indications. Progress is being made in the development of agonist antibodies directed against the immunostimulatory receptors, especially members of the tumor necrosis factor receptor superfamily (TNFRSF) such as OX40, CD27, 4–1BB, and GITR on T cells and CD40 on antigen-presenting cells.3-7 Activation of these receptors by the agonistic antibodies leads to the activation and proliferation of effector T cells and antigen-presenting cells for antitumor immunity. In addition, the effector functions of anti-OX40 and anti-GITR antibodies play pivotal roles in eliminating intratumoral regulatory T cells (Tregs), which negatively modulate tumor immunity.8,9
The activation of TNFRSF depends on receptor clustering that is typically mediated through interactions with their cognate oligomeric ligands. Agonistic antibodies, however, facilitate crosslinking of TNFRSF via engagement with the inhibitory FcγRIIB receptor to activate downstream signaling pathways.10-14 In several settings, the effector functions of anti-TNFRSF antibodies depend on their engagement with activating Fcγ receptors.8,9 Because human IgG antibodies have poor binding affinities to most human Fc receptors except FcγRI, Fc engineering approaches were adopted to optimize antibody-Fcγ receptor engagements. Mutations such as SELF (S267E/L328F, EU numbering) and a set of six mutations collectively known as V12 mutations were found to enhance the FcγRIIB binding affinities of IgG1 antibodies, and thus improve the agonism of several therapeutic antibodies.10,11,15-18 However, both engineering approaches affect IgG1 antibody binding to FcγRIIIA and the associated antibody-dependent cell-meditated cytotoxicity (ADCC) activity.17 Several Fc mutations have subsequently been identified that can facilitate antibody multimerization upon antigen binding.19,20 Such mutations could facilitate FcγRIIB-independent agonism enhancement with clustered anti-OX40 antibody.17,20 Furthermore, likely due to increased binding of Fcγ receptors to clustered antibodies with IgG1 Fc, the engineered anti-OX40 antibody showed an additional boost of agonism and elevated effector functions.17,20
Besides Fc engineering, we hypothesized that an alternative scaffold protein with binding affinity and specificity to Fcγ receptors could be engineered on an antibody as a functional module to provide the Fcγ binding capability. In this regard, Centyrins, a small and stable scaffold protein based upon the framework of FN3 domain from human fibronectin,21 can accommodate sequence diversities in selective strands and loops that provide the binding interfaces to other proteins. By CIS display panning, Centyrins with high affinity and specificity against multiple targets were successfully obtained.22 In this study, we report the identification of Centyrins with binding specificity to FcγRII receptors. When fused to an anti-OX40 antibody in appropriate configurations, the Centyrins enabled both agonism and antibody-dependent cellular phagocytosis (ADCP) activities. Adopting a versatile functional module represented a novel strategy to engineer anti-TNFRSF antibodies with desired functionalities.
Results
Screening for high-affinity Centyrins binding to FcγRIIB by CIS display panning
CIS-display panning was employed to identify FcγRIIB-binding Centyrins. A recombinant extracellular domain of FcγRIIB was used as the antigen in panning. The Centyrin libraries were constructed with highly diversified sequences in distinct strand and loop regions of the FN3 domain, including the BC loop, CD loop, FG loop, C strand and F strand.21,22 The pannings were performed through five successive rounds with increasing selection stringency upon each round. Multiple distinct panning approaches, including excess non-specific competitors and negative selections, were executed to select specific, high affinity binding Centyrins. Through these efforts, 140 clones were identified from the primary screenings. These clones were sequenced and evaluated for binding to recombinant FcγRIIB and FcγRIIA extracellular domains. Fifty-eight sequence-unique Centyrins were thus identified, with many showing similar binding affinities to FcγRIIB and FcγRIIA. By size-exclusion chromatography (SEC), 24 Centyrins eluted as a single protein peak with the size of a monomer.
Besides binding to recombinant FcγRII, the 58 Centyrins were tested for binding to FcγRIIB and FcγRIIA expressed on the surface of transfected Expi293F cells via flow cytometry. Promising Centyrins were engineered to the C-termini of heavy chains of an anti-OX40 antibody SF223 with the silent IgG2σ Fc.24 Such antibody-Centyrin bispecific fusion proteins were named as mAbtyrins. The mAbtyrins were assessed for binding to FcγRIIB and FcγRIIA expressed on the surface of transfected Expi293F cells. In addition, the agonistic activities of mAbtyrins were evaluated by a NF-κB reporter assay that was successfully employed previously in assessing the agonism of Fc-engineered SF2 antibodies.17 Through these screenings, 7 Centyrins that had binding affinities to both FcγRIIB and FcγRIIA and showed agonism in NF-κB reporter assay dependent upon crosslinking to FcγRIIB were identified. Among them, Centyrin S29, assessed to be the most potent in binding, was evaluated comprehensively and documented in this study.
Engineering S29 Centyrins on SF2 antibody
S29 Centyrin, with 13 residues deviation relative to the native Centyrin sequence with 89 amino acids, showed equal binding potency to recombinant FcγRIIB and FcγRIIA extracellular domains with an EC50 value of about 3 nM in an ELISA binding assay. S29 Centyrin molecules were attached at different positions of SF2 antibodies via four repeats of Gly-Gly-Gly-Gly-Ser as the linker to evaluate the effects of configurations on the activities of SF2-S29 mAbtyrin. Specifically, S29 Centyrins were engineered at the C-termini of heavy chains (SF2IgG2σ_S29_HC-C), C-termini of light chains (SF2IgG2σ_S29_LC-C), N-termini of heavy chains (SF2IgG2σ_S29_HC-N) and N-termini of light chains (SF2IgG2σ_S29_LC-N) of SF2IgG2σ, the SF2 antibody with IgG2σ Fc (Fig. 1A). S29 Centyrins were also engineered at the C-termini of heavy chains (SF2IgG1_S29_HC-C) and light chains (SF2IgG1_S29_LC-C) of SF2 antibody with IgG1 Fc (SF2IgG1). The mAbtyrins were expressed in Expi293F cells with comparable levels to corresponding antibodies, and could be efficiently purified by protein A-based affinity chromatography.
Figure 1.
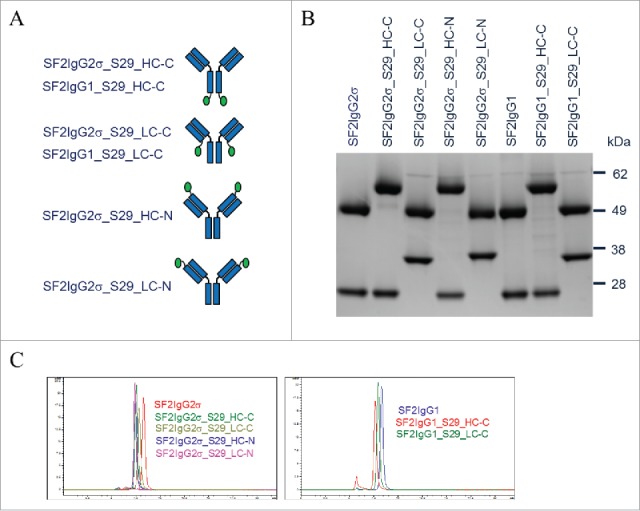
Generation of SF2 mAbtyrins. (A) Cartoon diagrams illustrating mAbtyrins employed in this study in which the S29 centyrins (green oval ball) were attached at the C-termini of heavy chains (SF2IgG2σ_S29_HC-C and SF2IgG1_S29_HC-C), C-termini of light chains (SF2IgG2σ_S29_LC-C and SF2IgG1_S29_LC-C), N-termini of heavy chains (SF2IgG2σ_S29_HC-N), or N-termini of light chains (SF2IgG2σ_S29_LC-N) of SF2 antibody. (B) Protein band image of antibodies and mAbtyrins resolved by SDS-PAGE (4–12% gel) under reduced condition. (C) SEC profiles of SF2 antibodies and mAbtyrins. The y axes are Absorbance at 280 nm (mAU) and the x axes are retention times (min).
The proper engineering of the S29 Centyrin molecules on SF2 antibody was confirmed by SDS-PAGE analysis of mAbtyrins under reducing conditions (Fig. 1B). The SF2 antibody heavy and light chain had a molecular weight of about 50 kDa and 25 kDa, respectively. The heavy and light chains of corresponding mAbtyrins with the 10 kDa S29 Centyrin attached migrated on the gel with a molecular weight around 60 kDa and 35 kDa, respectively. SEC analysis revealed that mAbtyrins were predominantly monomers in solution, just as the corresponding SF2 antibodies (Fig. 1C). However, the presence of the two fused Centyrins with a combined 20 kDa molecular weight resulted in SEC elution of mAbtyrins as monomeric protein peaks with a shorter elution time than the corresponding antibodies. The mAbtyrins with Centyrins fused at the C-termini of light chains had a slightly longer elution time relative to others, likely due to a unique conformation they adopted that favored retention on the SEC column. SDS-PAGE analysis of mAbtyrins under non-reducing conditions revealed that all the mAbtyrins migrated on the gel with major bands around 170 kDa.
S29 Centyrin facilitated mAbtyrins with binding capacity to FcγRIIB and FcγRIIA
The binding of mAbtyrins to FcγRIIB and FcγRIIA receptors expressed on transiently-transfected Expi293F cells were assessed by flow cytometry-based binding assays. The proper expression of FcγRIIB and FcγRIIA receptors were confirmed via monoclonal antibodies to FcγRIIB (2B6)25 and FcγRIIA (IV.3),26 respectively (Fig. 2). While the SF2IgG1 and SF2IgG2σ antibodies had no or poor binding to FcγRIIB, addition of S29 Centyrins enabled mAbtyrins with either IgG1 or IgG2σ Fc to bind FcγRIIB, regardless of their positions (Fig. 2A), in a manner similar to the enhancement of antibody binding to FcγRIIB enabled by the S267E/L328F or V12 mutations.15-17 The mAbtyrins with S29 Centyrins fused at the C-termini of heavy chains showed about two-fold better efficacy in binding to FcγRIIB relative to others. The S29 Centyrin molecules, when attached to SF2IgG2σ, enabled the resulting mAbtyrins to have binding capacity to FcγRIIA as well (Fig. 2B). Although SF2IgG1 antibody showed binding to FcγRIIA at higher concentrations, the S29 Centyrins attached to the C-termini greatly enhanced the potency of SF2IgG1_S29_HC-C and SF2IgG1_S29_LC-C binding to FcγRIIA (Fig. 2B). Inferred from the EC50 values of dose-response curves, all the mAbtyrins, regardless of the S29 positions, showed less than 2-fold differences in binding potency to FcγRIIB and FcγRIIA receptors. They also had slightly better potencies compared to the 2B6 and IV.3 antibodies, respectively (Fig. 2).
Figure 2.
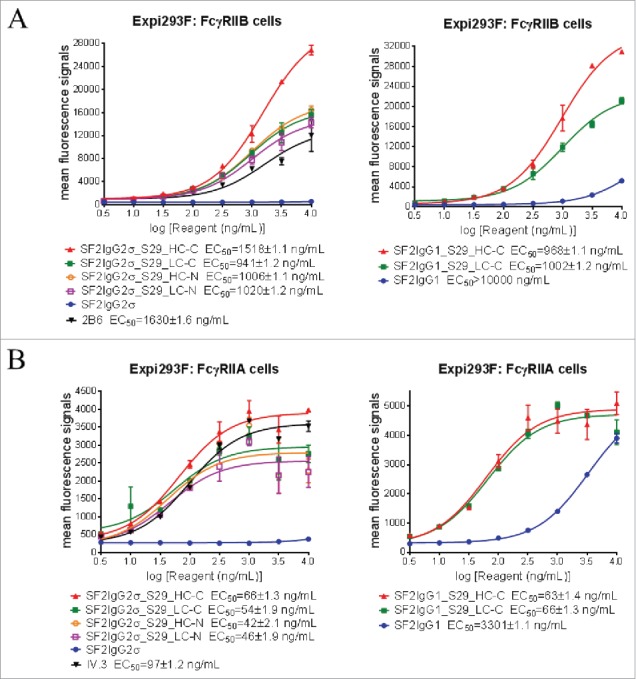
Binding properties of SF2 antibodies and mAbtyrins to FcγRII receptors expressed on cell surface. Increasing concentrations (3 to 10000 ng/mL) of SF2 antibodies and mAbtyrins were assessed for their binding to Expi293F cells transfected with human FcγRIIB receptor (A) and FcγRIIA receptor (B) by flow cytometry assays. Anti- FcγRII receptor antibodies 2B6 and IV.3 were also assessed for their binding to FcγRIIB receptor (A) and FcγRIIA receptor (B), respectively. Mean fluorescence signals were plotted against the concentrations of test agents (Data expressed as mean ± SEM, n 2).
The binding of mAbtyrins to FcγRI and FcγRIIIA receptors expressed on transiently-transfected Expi293F cells were also assessed. The expression of FcγRI and FcγRIIIA receptors were confirmed by monoclonal antibodies specific to FcγRI (10.1)27 and FcγRIIIA (3G8),28 respectively (Fig. S1). SF2IgG2σ antibody had minimal binding to either FcγRI or FcγRIIIA due to the silent nature of IgG2σ Fc.24 As expected, S29 Centyrin molecules failed to enable mAbtyrins based on SF2IgG2σ to bind either FcγRI or FcγRIIIA, no matter where they were positioned (Fig. S1). The mAbtyrins based on SF2IgG1 showed dose-dependent binding to FcγRI or FcγRIIIA, but the potencies of binding were less than 2-fold different from that of SF2IgG1 antibody (Fig. S1). These data indicated that S29 Centyrin mediated binding specifically to FcγRIIB and FcγRIIA, but not to FcγRI or FcγRIIIA.
The agonism of mAbtyrins in NFκB reporter assay
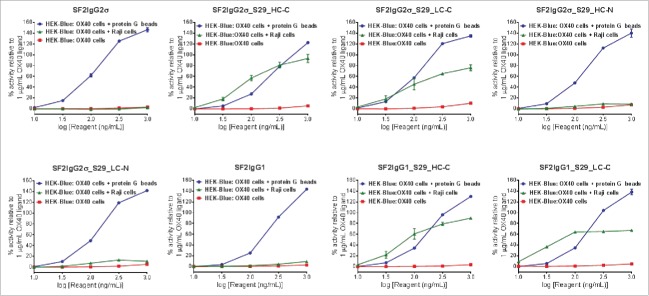
The agonistic activities of the SF2 antibody depend on crosslinking to FcγRIIB receptors.17 Since the S29 Centyrin had binding affinities to FcγRIIB receptors, the functional effects of S29 Centyrin on the agonism of mAbtyrins were evaluated by a HEK-Blue NF-κB reporter assay in which the mAbtyrins were applied to HEK-Blue: OX40 cells co-cultured with human B lymphoblastoid Raji cells that had FcγRIIB expression.29 Such an assay system was employed previously to assess increased agonism of SF2 antibody with engineered Fc in a FcγRIIB crosslinking-dependent fashion.17
Before the assay was performed, binding of mAbtyrins to Raji cells expressing FcγRIIB receptors and HEK-Blue: OX40 cells expressing OX40 receptors was evaluated by flow cytometry-based binding assays. Regardless of S29 architecture, the mAbtyrins showed binding affinities to Raji cells that was similar to SF2 antibody engineered with either S267E/L328F or V12 mutations,17 while the SF2IgG1 and SF2IgG2σ antibodies had no binding activities (Fig 3A). The mAbtyrins with S29 Centyrins fused at the C-termini of heavy chains showed about two-fold better efficacies in binding to Raji cells relative to the others. In contrast, all the mAbtyrins showed binding to HEK-Blue: OX40 cells as the SF2IgG1 and SF2IgG2σ antibodies with less than 4-fold differences in binding potency, although the mAbtyrins had lower efficacies in binding relative to the corresponding antibodies, especially for SF2IgG2σ_S29_HC-C (Fig. 3B). This indicated that the S29 Centyrins, regardless of where they were positioned, did not abrogate the binding of mAbtyrins to OX40 receptors.
Figure 3.
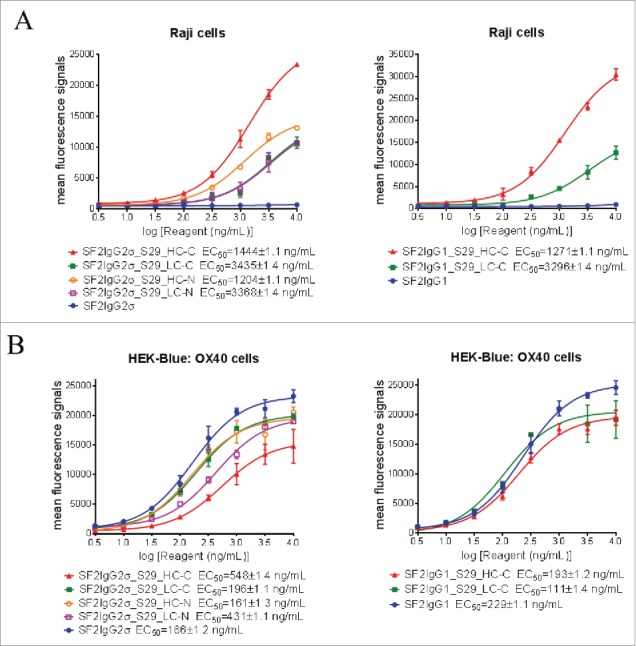
Binding of SF2 antibodies and mAbtyrins to Raji cells and HEK-Blue: OX40 cells. Increasing concentrations (3 to 10000 ng/mL) of SF2 antibodies and mAbtyrins were assessed for their binding to Raji cells (A) and HEK-Blue: OX40 cells (B) by flow cytometry assays. Anti- FcγRIIB antibody 2B6 was also assessed for its binding to FcγRIIB receptors expressed on Raji cells (A). Mean fluorescence signals were plotted against the concentrations of test agents (Data expressed as mean ± SEM, n 2).
In the HEK-Blue NF-κB reporter assay, neither SF2 antibodies nor mAbtyrins showed agonistic activity when these molecules were applied to HEK-Blue: OX40 cells in the absence of crosslinker (Fig. 4). When crosslinked to protein G beads via Fc binding, all SF2 antibodies and mAbtyrins could stimulate reporter gene expression in a dose-dependent manner to levels better than 1 μg/mL OX40 ligand, indicating that the mAbtyrins were functional agonist molecules upon the presence of crosslinking. However, with co-cultured Raji cells, the antibodies and mAbtyrins showed dramatically different agonistic properties (Fig. 4). Neither SF2IgG1 nor SF2IgG2σ antibodies showed agonism due to the inability of these respective Fc domains to bind FcγRIIB on Raji cells. The mAbtyrins in which the S29 Centyrins were positioned at the C-termini of either the heavy or light chains of SF2IgG2σ (SF2IgG2σ_S29_HC-C and SF2IgG2σ_S29_LC-C) or of SF2IgG1 (SF2IgG1_S29_HC-C and SF2IgG1_S29_LC-C) could stimulate reporter gene expression in a dose-dependent manner (Fig. 4). The maximal degrees of stimulation were in the range of 70%-90% of activities induced by 1 μg/mL OX40 ligand, while SF2 antibody engineered with either S267E/L328F or V12 mutations showed over 2-fold better efficacy relative to OX40 ligand.17 In contrast, the mAbtyrins in which the S29 Centyrins were positioned at the N-termini of SF2IgG2σ (SF2IgG2σ_S29_HC-N and SF2IgG2σ_S29_LC-N) showed very little agonism (Fig. 4), although they showed comparable binding to Raji cells and HEK-Blue: OX40 cells as those mAbtyrins with C-terminal attachments (Fig. 3). These data indicated that the S29 Centyrins could endow agonism to SF2 antibodies, regardless the IgG1 or IgG2 subtypes, by facilitating the crosslinking to FcγRIIB on Raji cells. However, this effect depended on the location of the N versus C terminal position of the Centyrin fusion on the SF2 antibodies.
Figure 4.
HEK-Blue NF-κB reporter assay for the assessment of agonism of SF2 antibodies and mAbtyrins. Increasing concentrations (10 to 1000 ng/mL) of SF2 antibodies and mAbtyrins as indicated were applied to HEK-Blue: OX40 cells and their agonistic activities were assessed by HEK-Blue NF-κB reporter assay. Similar assays were also set up to evaluate the agonism by co-culturing HEK-Blue: OX40 cells with Raji cells or in the presence of protein G beads. The agonistic activities of SF2 antibodies and mAbtyrins, normalized as percent activity relative to that driven by 1 μg/mL OX40 ligand, were plotted against the concentrations of test agents (Data expressed as mean ± SEM, n).
The agonism of mAbtyrins in T cell activation assay
The functional effects of S29 Centyrins on the agonism of SF2 mAbtyrins were also evaluated by a T-cell activation assay (TCA) in which the mAbtyrins were applied to isolated CD4+ T cells in the presence of immobilized suboptimal amount of anti-CD3 OKT3 antibody and recombinant FcγRIIB or FcγRIIA extracellular domains to provide the crosslinking activities. The activation of the T cells due to the agonism of the mAbtyrins was measured by quantitation of the induced interferon (IFN)γ and TNFα expression.
First, binding of mAbtyrins to recombinant FcγRIIB or FcγRIIA extracellular domains coated on the assay plates were evaluated by ELISA-based binding assays. Although the SF2IgG1 and SF2IgG2σ antibodies had little binding activities to FcγRIIB, the mAbtyrins, no matter where the S29 Centyrins were attached, showed binding to recombinant FcγRIIB dose-dependently (Fig. 5A). Similarly, the S29 Centyrins could endow SF2 antibodies with binding to FcγRIIA as well (Fig. 5B). The S29 Centyrins at the C-termini of light chains of SF2 (SF2IgG2σ_S29_LC-C and SF2IgG1_S29_LC-C) had less potency in binding to FcγRIIB and FcγRIIA relative to other mAbtyrins.
Figure 5.
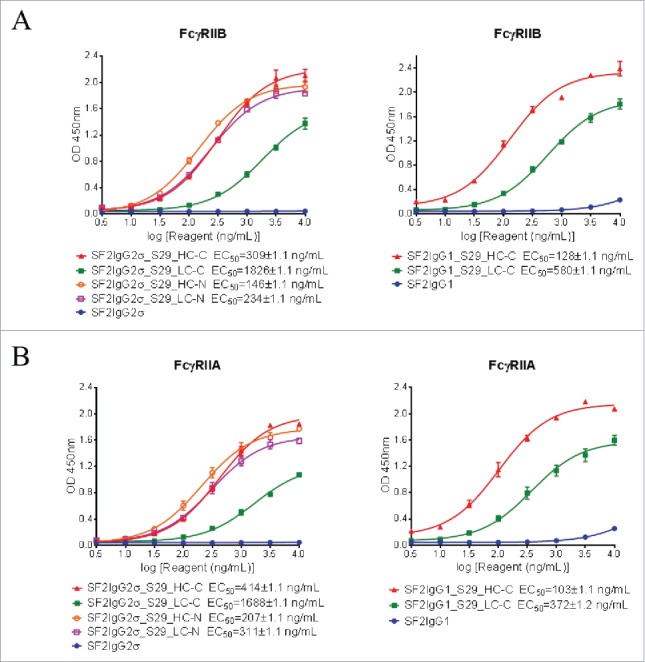
Binding of SF2 antibodies and mAbtyrins to recombinant FcγRII receptors. Increasing concentrations (3 to 10000 ng/mL) of SF2 antibodies and mAbtyrins were assessed for their binding to purified plate-bound recombinant FcγRIIB receptor (A) and FcγRIIA receptor (B) by ELISA assays. OD at 450 nm were plotted against the concentrations of test agents (Data expressed as mean ± SEM, n 2).
For the T-cell activation assay, CD4+ T cells isolated from peripheral blood mononuclear cells (PBMC) were cultured in the presence of 1 μg/mL phytohemagglutinin overnight to induce OX40 receptor expression. In the presence of suboptimal amounts of OKT3 antibody, neither SF2IgG1 nor SF2IgG2σ mAb had significant agonistic activity like the isotype control antibodies. The positive control OX40 ligand facilitated significant IFNγ or TNFα expression in a dose-dependent fashion (Fig. 6, S2). Only the mAbtyrins with the S29 Centyrins on the C-termini of heavy chains (SF2IgG2σ_S29_HC-C and SF2IgG1_S29_HC-C) could dose-dependently facilitate significant IFNγ (Fig. 6A) or TNFα (Fig. S2) expression in the presence of immobilized FcγRIIB extracellular domain. In contrast, mAbtyrins with other configurations failed to induce T cell activation (Fig. 6A, S2A), although they showed comparable binding to FcγRIIB in ELISA-based binding assays (Fig. 5A). Similarly, only SF2IgG2σ_S29_HC-C and SF2IgG1_S29_HC-C mAbtyrins could dose-dependently facilitate significant IFNγ (Fig. 6B) or TNFα (Fig. S2B) expression in the presence of immobilized FcγRIIA extracellular domain. The omission of coating either the OKT3 antibody or FcγRII receptors on the plate prior to the assay failed to support the agonistic activities of SF2IgG2σ_S29_HC-C and SF2IgG1_S29_HC-C, suggesting that the agonism activity of these mAbtyrins depended on T cell receptor activation and FcγRII receptor crosslinking. These observations indicated that the S29 Centyrins could endow agonism to SF2 antibodies, regardless the IgG1 and IgG2σ subtypes, in the T cell activation assay by facilitating the crosslinking to FcγRIIB or FcγRIIA. However, this effect was confined only to the mAbtyrins in which S29 Centyrin molecules were positioned at the C-termini of antibody heavy chains.
Figure 6.
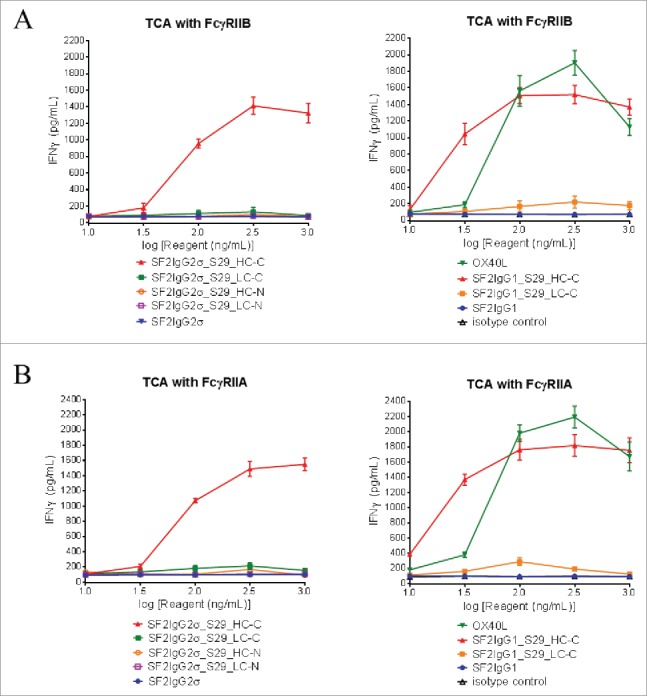
FcγRII-crosslinking dependent T cell activation assay for the assessment of agonism of SF2 antibodies and mAbtyrins. Increasing concentrations (10 to 1000 ng/mL) of testing agents as indicated were applied to CD4+ T cells in assay plate pre-coated with OKT3 antibody and recombinant FcγRIIB (A) or FcγRIIA (B) receptors. T cell activation in terms of induced expression of cytokines IFNγ were assessed. The amount of IFNγ were plotted against the concentrations of test agents (Data expressed as mean ± SEM, n of T cells from two donors).
Fc effector functions of mAbtyrins
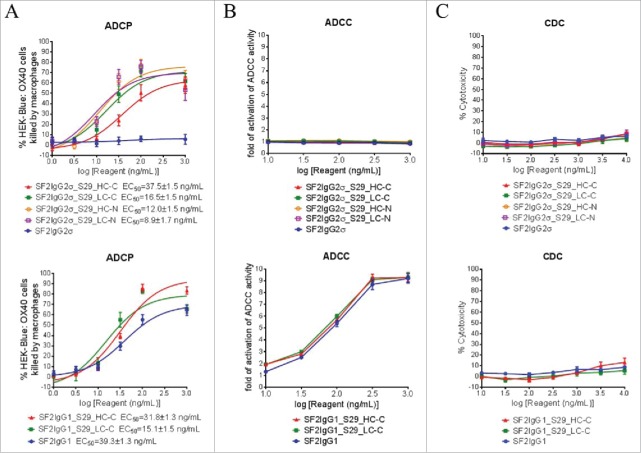
The S29 Centyrin had binding affinity to FcγRIIA, which is one of the major Fc receptors mediating ADCP.30 The ADCP activities of the mAbtyrins were thus evaluated by the phagocytosis of green fluorescent protein (GFP)-expressing HEK-Blue: OX40 cells by differentiated macrophages. While the SF2IgG2σ antibody did not show ADCP activity in this assay, the S29 Centyrins, regardless of where they were positioned, could dose-dependently endow the mAbtyrins with elevated killings of HEK-Blue: OX40 target cells by the macrophages (Fig. 7A). On the other hand, the SF2IgG1 antibody had ADCP activity and the S29 Centyrins could endow the corresponding mAbtyrins (SF2IgG1_S29_HC-C and SF2IgG1_S29_LC-C) with slightly more efficient phagocytosis (Fig. 7A). These data indicated that the S29 Centyrins could facilitate ADCP activity of SF2 antibodies regardless the IgG subtypes and the configuration of the mAbtyrins.
Figure 7.
Effector functions of SF2 antibodies and mAbtyrins. (A) ADCP activities of SF2 antibodies and mAbtyrins. Increasing concentrations (1 to1000 ng/mL) of SF2 antibodies and mAbtyrins with IgG2σ or IgG1 Fc were incubated with GFP positive HEK-Blue: OX40 cells co-cultured with differentiated macrophages and the phagocytosis of GFP positive target cells were evaluated by flow cytometry assay. The percentages of GFP positive HEK-Blue: OX40 cells eliminated, which reflected the ADCP activities, were plotted against the concentrations of test agents (Data expressed as mean ± SEM, n). (B) ADCC activities of SF2 antibodies and mAbtyrins. Increasing concentrations (10 to 1000 ng/mL) of SF2 antibodies and mAbtyrins were incubated with HEK-Blue: OX40 cells co-cultured with effectors cells and the ADCC reporter bioassays were performed. The folds of activation of ADCC activities were plotted against the concentrations of test agents (Data expressed as mean ± SEM, n ). (C) CDC activities of SF2 antibodies and mAbtyrins. Increasing concentrations (10 to10000 ng/mL) of SF2 antibodies and mAbtyrins were incubated with HEK-Blue: OX40 cells in the presence of rabbit complement. The CDC activities were quantitated by measuring LDH activity released from the cytosol of lysed HEK-Blue: OX40 cells and expressed as percent cytotoxicity relative to that lysed by Triton X-100 (Data expressed as mean ± SEM, n ).
The ADCC activities of the mAbtyrins were also evaluated by a FcγRIIIA-mediated ADCC reporter bioassay.31 The SF2IgG2σ antibody did not have ADCC activity in this assay due to the silent Fc. The mAbtyrins based on SF2IgG2σ were also silent in ADCC (Fig. 7B) since the S29 Centyrin did not have binding activity to FcγRIIIA (Fig. S1B). Likewise, the SF2IgG1_S29_HC-C and SF2IgG1_S29_LC-C mAbtyrins showed similar ADCC activity as SF2IgG1 (Fig. 7B).
The complement-dependent cytotoxicity (CDC) activities of the mAbtyrins were determined by a rabbit complement-mediated cell killing assay.17 Neither the SF2IgG2σ nor SF2IgG1 antibody had significant CDC activity towards HEK-Blue: OX40 target cells up to 10000 ng/mL. As shown in Fig. 7C, all the tested mAbtyrins remained silent in CDC assays.
Discussion
In order to improve the antitumor immunity of agonistic antibodies directed against immunostimulatory TNFRSFs, Fc engineering approaches have been adopted to optimize their binding to selective Fcγ receptors to enhance antibody functions.15,16 In this study, we evaluated a novel strategy by developing Centyrins as alternative scaffold proteins with binding affinity and specificity to FcγRII receptors. Such Centyrins can serve as functional modules to be engineered on antibodies, and the resulting mAbtyrins gained agonistic and ADCP activities owing to the engagements to FcγRII receptors by the Centyrin module.
Although our initial intention was to identify Centyrins with specific binding to FcγRIIB, all the Centyrins identified through the panning showed affinities to both FcγRIIB and FcγRIIA. This is likely due to the high sequence similarity between FcγRIIB and FcγRIIA, with a difference in only 10 amino acids scattered in their extracellular domains of 180 amino acids.32 S29 Centyrin showed FcγRII binding activities in various assay formats, recognizing not only recombinant FcγRII receptor extracellular domains, but also receptors expressed on the surface of transfected Expi293F and Raji cells. S29 had specificity in binding, with affinity to FcγRII but not to FcγRI or FcγRIIIA receptors. S29 showed high affinity bindings to both FcγRII receptors with potencies at least comparable to antibodies directed to FcγRIIB (2B6) and FcγRIIA (IV.3). When the S29 Centyrin was engineered onto SF2 antibody, the mAbtyrin kept the specificity and affinity to FcγRIIB and FcγRIIA regardless the IgG subtypes or where on the antibody S29 were engineered.
Regardless the fusion position, S29 did not significantly affect the mAbtyrins in binding to the OX40 receptors. As a result, in the presence of protein G beads as the crosslinker, all the mAbtyrins showed agonism comparable to the corresponding SF2 antibodies in NFκB reporter assays. All the mAbtyrins constructed on IgG1 Fc, but not on the silent IgG2σ Fc, showed ADCC and ADCP effector functions expected by the nature of the IgG subtypes, indicating that the presence and positions of S29 Centyrins did not affect antibody effector functions. These observations revealed that both the antibody portion and the Centyrin portion of the mAbtyrin could function independently without interfering with each other under our engineering scheme. This could be attributed by the fact that both the antibody and the Centyrin intrinsically can fold into highly stable three-dimensional structures.21,33,34 Moreover, the linker used to engineer the mAbtyrin, consisting of four repeats of Gly-Gly-Gly-Gly-Ser, could be long enough to separate these two parts as independent functional modules.
By the NFκB reporter assay, the mAbtyrins showed agonism in the presence of Raji cells. The agonism enhancements were observed on mAbtyrins with the silent IgG2σ Fc and similar enhancement were observed regardless of IgG subtypes, suggesting that the agonism were mediated by the S29 Centyrins interacting with the Raji cells. Similar agonism enhancements were observed on SF2 antibodies engineered with S267E/L328F and V12 mutations, which facilitated the antibody binding to FcγRIIB.17 Consistently, our binding assays revealed that S29 Centyrin molecules could mediate binding to FcγRIIB comparable to that for Fc engineering mutations directed to FcγRIIB. In contrast, SF2 antibody fused with Centyrins lacking binding affinity to FcγRIIB could not mediate agonism in a negative control NFκB reporter assay. To further demonstrate that the agonism was mediated by crosslinking with FcγRIIB expressed on Raji cells, we also performed similar NFκB reporter assays with pre-blocking of the FcγRIIB receptors with excess 2B6 antibody.32 Although no significant effect was observed for S29 mAbtyrins, likely due to the comparable affinity of S29 Centyrin and 2B6 antibody in binding to FcγRIIB, abrogation of the Raji cell-dependent agonism enhancement were observed for mAbtyrins constructed with two other Centyrins with lower potencies relative to 2B6 in binding to FcγRIIB (data not shown). Therefore, such agonism was mediated by Centyrins crosslinking to FcγRIIB.
Interestingly, the agonism enhancements in the reporter assays were observed only on mAbtyrins with S29 attached at the C-termini of SF2 antibody heavy or light chains, despite the fact that the mAbtyrins with N-terminal attachment had comparable binding to Raji cells and HEK-Blue: OX40 cells. These observations suggested that the functional activation of the OX40 receptors in the reporter assay depended on proper architectures of the mAbtyrins. Although the S29 Centyrins and SF2 antibodies were capable of binding to their respective targets individually, there could be physical hindrances for the mAbtyrins with N-terminal attachment that prevented the optimal interactions with both OX40 receptors and FcγRIIB needed for functional activation.
The mAbtyrins demonstrated dose-dependent agonism in T cell activation assays as well as the NFκB reporter assay. These mAbtyrins were co-stimulatory since no agonism enhancement was observed in the absence of suboptimal anti-CD3 antibody to stimulate T cell receptor-CD3 complex.35 The agonism relied on crosslinking to immobilized FcγRII receptors by S29 Centyrin molecules, so similar activities were observed for the mAbtyrins with either IgG1 or IgG2σ Fc. S29 had binding affinities to either FcγRIIB or FcγRIIA, and thus immobilization of either of them could support the agonism. Although FcγRIIB receptors were demonstrated as the major Fcγ receptor,10–12 agonism of anti-TNFR antibodies could be mediated by the crosslinking to other FcγR, such as FcγRIIA, depending on FcγR availability in the target environments.36 In this regard, the binding of S29 to both FcγRIIB and FcγRIIA could be an advantage, considering that the mAbtyrins may have agonism in a broader circumstance when cells expressing any one of these receptors could be present. Interestingly, only the mAbtyrins comprising S29 at the C-termini of heavy chains of SF2 antibodies showed agonism in the T cell activation assay, while those with S29 at the C-termini of light chains barely had activities. This could be explained by the lower affinities of SF2IgG1_S29_LC-C and SF2IgG2σ_S29_LC-C to FcγRII receptors. Alternatively, in contrast to the reporter assay, co-stimulating OX40 receptors in the more complex T cell activation assay might have a more stringent requirement for the mAbtyrins to be in a specific configuration to function properly.
Besides agonism, S29 enabled the SF2IgG2σ antibody, which was silent in ADCP, to be potent in phagocytosis activity. However, S29 did not affect the ADCC or CDC effector functions. These observations were consistent with S29 binding specificity to Fcγ receptors, with affinity to FcγRIIA mediating ADCP, but not to FcγRIIIA, which mediated ADCC.25 Interestingly, all the mAbtyrins with S29 at different positions showed comparable ADCP activities, indicating that ADCP activity may be less dependent on mAbtyrin architecture, in contrast to the agonism enhancement. Moreover, mAbtyrins with S29 at the N-termini could be developed to enhance ADCP exclusively without affecting agonism and other effector functions.
As demonstrated by in vitro assays, the mAbtyrin composed of S29 Centyrin and the SF2 antibody had enhanced agonism and ADCP, but the in vivo activity of the mAbtyrin remained to be explored. The binding property of S29 Centyrin to FcγRII receptor is analogous to the SELF mutations that also enhanced antibody binding to both FcγRIIB and FcγRIIA.16 Albeit with enhanced FcγRII receptor binding, no immune complex-mediated side effects were reported for mice dosed with anti-CD40 antibodies with the SELF mutations,18 implying less safety concerns for likewise engineering approaches. However, the SELF mutations facilitated much less agonism enhancement and antitumor immunity for anti-CD40 antibodies in the in vivo setting than the V11 mutations, which selectively enhanced FcγRIIB binding, although by in vitro assays both sets of mutations conferred anti-CD40 antibodies with enhanced agonism.18 This suggested that selective FcγRIIB engagement is needed for the agonistic activity of anti-CD40 mAbs, while the engagement of FcγRIIA may inhibit such activity. At this time, however, obtaining Centyrins that bind exclusively to FcγRIIB is challenging. An alternative approach worth exploring is the engineering of the Fab domain of the 2B6 antibody, which binds FcγRIIB specifically,32 as a single-chain variable fragment on anti-CD40 antibodies to mimic the effects of V11 mutations. It is worth noting that the in vivo requirement for FcγRII engagement for anti-CD40 antibody is different from those for anti-OX40 and anti-GITR antibodies, whose antitumor activities depend on the engagement of activating Fcγ receptors rather than the inhibitory FcγRIIB receptor.8,9 Both OX40 and GITR receptors are highly expressed on intratumoral Tregs, which negatively modulate antitumor immune responses. The selective elimination of Tregs in an activating FcγR-dependent manner was proved to be essential for the antitumor activity of anti-OX40 and anti-GITR antibodies,8,9 although FcγRIIB dependent activation of effector T cells might also play a role, especially in lymph nodes.37 In this regard, the dual engagement of both FcγRIIA and FcγRIIB by S29 Centyrin might offer a distinct advantage to facilitate the antitumor activity of anti-OX40 and anti-GITR antibodies.
Compared to traditional Fc engineering approaches, the utility of FcγRII-binding Centyrins to engineer SF2 antibody offers several advantages. A prominent feature of Centyrins is their specificity in binding and functionality. In this regard, the mAbtyrin approach provides only the intended functionalities, which in this case included agonism and ADCP enhancement due to the specificity of S29 to FcγRII receptors, without affecting other Fc effector functions such as ADCC and CDC. In contrast, although Fc engineering provided the intended activity enhancement, these were often accompanied with certain liabilities. Specifically, the S267E/L328F and V12 Fc mutations could enhance the agonism of SF2 antibodies due to improved FcγRIIB engagement, but both engineering approaches affected ADCC owing to disrupted binding to FcγRIIIA.15-17 On the other hand, Fc mutations that facilitated antibody multimerization could also enhance the agonism of SF2 antibody, but such multimerized antibodies also had elevation in multiple effector functions.17,20 Another important feature of Centyrins is that they are modular in nature. As a small functional module, they can be engineered onto any antibodies, regardless their IgG subtypes, to provide the intended functionality in a plug-and-play format. Centyrins can also be engineered as bivalent functional modules, which has apparent advantage over a bispecific antibody that targets FcγRIIB with monovalent activity.38 However, appropriate configurations would need to be evaluated for proper function. In this case, S29 attached at the N-termini of SF2 antibodies enabled only the ADCP activity, while those at the C-termini of antibody heavy chains facilitated both agonism as well as ADCP activities.
In summary, FcγRII-binding Centyrins could be used as a novel strategy to improve therapeutic antibodies whose activities depend on the engagements of FcγRII receptors, including immunostimulatory anti-TNFRSF antibodies such as SF2. Besides therapeutic activities, other aspects of mAbtyrins, including the pharmacokinetics, developability, manufacturability and immunogenicity, must be rigorously evaluated. Nonetheless, use of an alternative scaffold protein to generate multi-specific biologics should be a valuable addition to the toolbox for the engineering of therapeutic antibodies.
Materials and methods
Selection of high-affinity Centyrins binding to FcγRIIB by CIS display panning
A recombinant extracellular domain of FcγRIIB (R&D Systems, Minneapolis, MN, catalog number 1875-CD) was biotinylated using the SureLINK Biotin Kit (Seracare, Milford, MA) as the antigen to pan against Centyrin libraries diversified in distinct strand and loop regions of the FN3 domain.21 The selection of specific Centyrins was done essentially as previously described.22 Multiple distinct panning approaches, including excess non-specific competitors and negative selections, were executed and the pannings were performed through five successive rounds using the PCR-amplified positive clones for subsequent rounds. Individual clones from outputs at rounds three and five were screened for binding to recombinant FcγRIIB and FcγRIIA extracellular domains by ELISA as previously described.22
Positive binders, defined as having 5-fold luminescence signal above the albumin control signal, were sequenced and cloned. Their binding affinities to recombinant FcγRIIB and FcγRIIA were confirmed by ELISA. The aggregation propensities of the positive binders were assessed by SEC over a TSKgel G3SW column (Tosoh Bioscience, King of Prussia, PA) with a flow rate of 1 mL/min using Dulbecco's phosphate-buffered saline (DPBS) pH 7.2 buffer and absorbance measurements at 280 nm.
Engineering S29 Centyrin on the SF2 antibody
Plasmids encoding the heavy chain (HC) and light chain (LC) of a humanized anti-OX40 antibody SF223 were constructed for the expression of SF2 antibody with either human IgG1 Fc (SF2IgG1) or IgG2σ Fc (SF2IgG2σ). Mabtyrin constructs (Fig. 1A) were made by Genewiz (South Plainfield, NJ) to attach S29 Centyrin molecules at different positions of SF2 antibodies via four repeats of Gly-Gly-Gly-Gly-Ser as the linker in order to express the following mAbtyrins described in this study:
SF2IgG2σ_S29_HC-C: SF2IgG2σ antibody with S29 attached at the C-termini of heavy chain;
SF2IgG2σ_S29_LC-C: SF2IgG2σ antibody with S29 attached at the C-termini of light chain;
SF2IgG2σ_S29_HC-N: SF2IgG2σ antibody with S29 attached at the N-termini of heavy chain;
SF2IgG2σ_S29_LC-N: SF2IgG2σ antibody with S29 attached at the N-termini of light chain;
SF2IgG1_S29_HC-C: SF2IgG1 antibody with S29 attached at the C-termini of heavy chain;
SF2IgG1_S29_LC-C: SF2IgG1 antibody with S29 attached at the C-termini of light chain;
Expression and Purification of SF2 antibodies and mAbtyrins
Plasmids encoding the HC and LC of SF2 antibodies and mAbtyrins were co-transfected at a 1:3 (HC: LC) molar ratio into Expi293F cells by Expifectmine293 transfection kit (Thermo Scientific, San Jose, CA). Cells were spun down five days post transfection and the supernatant were passed through a 0.2 µm filter. The titer of antibody and mAbtyrin expressed was quantified using Octet (ForteBio, Menlo Park, CA). Antibody and mAbtyrin purification were carried out by affinity chromatography over MabSelect SuRe column followed by a desalting column (GE Healthcare Life Sciences, Pittsburgh, PA). Protein concentration was determined by UV absorbance at 280 nm. Quality was assessed by SEC as noted earlier and SDS-PAGE (4-12% gel, Thermo Scientific, San Jose, CA) of reduced and non-reduced samples.
Flow Cytometry based binding assays
Plasmids expressing human FcγRI (NM_000566), FcγRIIA (NM_021642), FcγRIIB (NM_004001), and FcγRIIIA (NM_000569) (Origene Technologies, Rockville, MD) were transiently transfected into Expi293F cells by Expifectmine293 transfection kit (Thermo Scientific, San Jose, CA). Flow cytometry assays were performed 48 h after transfection. To confirm the expression of transfected Fcγ receptors, their specific antibodies 10.1 for FcγRI (BD Pharmingen, San Jose, CA, catalog number 555525), IV.3 for FcγRIIA (StemCell Technologies, Vancouver, Canada, catalog number 60012), 2B6 for FcγRIIB (in house preparation), and 3G8 for FcγRIIIA (BD Pharmingen, San Jose, CA, catalog number 555403)) were employed as positive controls in binding assays. Raji cells (ATCC: CCL-86) and HEK-Blue: OX40 cells17 were also employed to test the binding of SF2 antibodies and mAbtyrins to FcγRIIB and OX40 receptors, respectively.
Flow cytometry-based binding assays were described previously.17 Briefly, 2 × 105 cells per well were seeded in 96-well plate and blocked in BSA Stain Buffer (BD Biosciences, San Jose, CA) for 30 min at 4°C. Cells were stained by test antibodies and mAbtyrins for 1.5 h followed by R-PE labeled anti-human or anti-mouse IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA catalog number 109–116–097 and 115-116-072, respectively) for 45 min at 4°C. PE signals of the stained cells were detected by Miltenyi MACSQuant flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany) and the geometric mean fluorescence signals were determined for at least 10,000 live events collected. FlowJo software (Tree Star, Ashland, OR) was used for analysis. Data was plotted as the logarithm of antibody concentration versus mean fluorescence signals. Nonlinear regression analysis was performed by GraphPad Prism 6 (GraphPad Software, La Jolla, CA) and EC50 values were calculated.
Cloning, expression and purification of extracellular domains of FcγRIIA and FcγRIIB
DNA sequences of extracellular domains of human FcγRIIA (Uniprot – AAA35827) and FcγRIIB (Uniprot – P31994) along with native signal peptides and multi-histidine C-terminal tags were synthesized and cloned into mammalian expression vectors (Genewiz, South Plainfield, NJ). Expi293F cells were transiently transfected with the plasmids using ExpiFectamine 293 transfection kits (Thermo Scientific, San Jose, CA). Cultures were harvested after 4–5 days incubation by centrifugation and filtered. Supernatants were then loaded onto HisTrap HP columns (GE Healthcare Life Sciences, Pittsburgh, PA), washed with 20 mM imidazole buffer (pH 7.5) and eluted with 300 mM imidazole buffer (pH 7.5). Fractions were combined, concentrated with Amicon Ultra 3K MWCO centrifugal filtration devices and purified (buffer exchanged) over a SRT-10C SEC 300 column (Sepax Technologies, Newark, DE) in DPBS pH 7.2 buffer. Fractions were combined, checked by SDS-PAGE, flash frozen in liquid nitrogen and stored in -80 0C freezer until use.
ELISA based binding assays
100 μL of 1 μg/mL of purified extracellular domains of FcγRIIA or FcγRIIB in DPBS pH7.2 were coated on Maxisorp 96-well plate overnight. Antibodies and mAbtyrins were added to the assay well and incubated for 2 h at room temperature with shaking. After washing the plates four times, the binding of the antibodies and mAbtyrins to immobilized FcγRIIA or FcγRIIB were detected by a horseradish peroxidase-conjugated anti-human IgG(γ) secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, catalog number 109-036-097) and quantitated by ELISA using TMB substrate (Biolegend, San Diego, CA). Data was plotted as the logarithm of antibody concentration versus absorbance at 450 nm. Nonlinear regression analysis was performed by GraphPad Prism 6 (GraphPad Software, La Jolla, CA) and EC50 values of binding were calculated.
HEK-Blue NF-κB reporter assay
The stable HEK-Blue reporter cell line expressing human OX40 (HEK-Blue: OX40) and the HEK-Blue NF-κB reporter assay used to study the agonistic activity of SF2 antibody were described previously.17 Briefly, the OX40 ligand or SF2 antibodies and mAbtyrins were applied to 1 × 105 HEK-Blue: OX40 cells re-suspended in 200 μl culture media in each well of the 96-well assay plate. To test the crosslinking effect, either 1 μL of protein G magnetic beads (Thermo Scientific, San Jose, CA) or 1 × 105 Raji cell was added in the same assay well. After incubation at 37°C overnight, the agonistic activities of the molecules were evaluated by the quantification of the induced secreted alkaline phosphatase reporter gene expression using Quanti-Blue detection kit (Invivogen, San Diego, CA). The agonistic activities of SF2 antibodies and mAbtyrins were normalized as percent activity relative to that induced by 1 μg/mL OX40 ligand.
T cell activation assay
Human CD4-positive T cells were isolated from PBMCs by a CD4+ T cell negative selection kit (StemCell Technologies, Vancouver, Canada). OX40 expression was induced by culturing the isolated T cells in the presence of 1 μg/mL phytohemagglutinin (Sigma-Aldrich, St. Louis, MO) overnight. One day before the assay, 100 μL of 30 ng/mL anti-human CD3 OKT3 antibody35 and 1 μg/mL of purified extracellular domains of FcγRIIB or FcγRIIA in DPBS were coated in U-bottomed 96-well tissue culture plates. On the assay day, the coating solution in the plate was aspirated and 150 μL RPMI media was added to block the plate for 30 min. The cultured T cells were washed three times by RPMI culture media and between 25,000 to 50,000 CD4-positive T cells were seeded in each well in the assay plate. Testing antibodies or mAbtyrins were added to the cells and the plates were incubated for 3 days at 37°C. The increased IFNγ and TNFα levels in the cell culture media, which reflects T cell activation, were quantitated by human IFNγ and TNFα ELISA detection kits, respectively (Biolegend, San Diego, CA).
ADCC assay
The ADCC activities of SF2 antibodies and mAbtyrins were evaluated by an ADCC reporter bioassay (Promega, Madison, WI) as described previously.17 Briefly, 25,000 HEK-Blue: OX40 cells per well plated in 96-well plate overnight were mixed with the engineered effector cells in which the activation of FcγRIIIA receptor lead to the expression of a luciferase reporter. SF2 antibodies and mAbtyrins were added to the cells and incubated at 37°C for 6 h. Then, Bio-Glo luciferase reagent was added and the luciferase signals were quantitated by Envision (PerkinElmer, Akron, OH). The ADCC activities of SF2 antibodies and mAbtyrins were expressed as fold of activation of luciferase signals over that without testing antibody added.
ADCP assay
The ADCP assay for SF2 antibodies was described previously.17 Briefly, differentiated macrophages were mixed with GFP-expressing HEK-Blue: OX40 cells (8: 1 ratio) in 96-well U-bottom plates. The test antibodies and mAbtyrins were added and the phagocytosis of target cells was carried out for 24 hours. The macrophages were stained with anti-CD11b and anti-CD14 antibodies (BD Biosciences, San Jose, CA, catalog number 555385 and 555395, respectively) coupled to Alexa Fluor 647 (Thermo Scientific, San Jose, CA). GFP-positive HEK-Blue: OX40 target cells and Alexa647 positive macrophages were identified by flow cytometry using Miltenyi MACSQuant flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany). Significant numbers of GFP+, CD11b+, CD14+ cells were observed during the 24-hour incubation time, indicating the phagocytosis of GFP-positive HEK-Blue: OX40 target cell by macrophages. The data were analyzed using FlowJo software (Tree Star, Ashland, OR) and ADCP-mediated cell killing was determined by measuring the reduction in GFP fluorescence using the following equation: Percentage of target cells killed = ((Percentage of GFP+, CD11b−, CD14− cells with the lowest concentration of antibody) − (Percentage of GFP+, CD11b−, CD14− cells with the test concentration of antibody))/(Percentage of GFP+, CD11b−, CD14− cells with the lowest concentration of antibody) × 100.
CDC assay
The CDC activities of SF2 antibodies and mAbtyrins were evaluated by a complement-mediated cell killing assay. Briefly, 1 × 105 HEK-Blue: OX40 cells were incubated with 6.7% (v/v) rabbit complement (Cedar Lane Labs, Burlington, Canada, catalog number CL3010) and testing antibodies and mAbtyrins for one hour. The lactate dehydrogenase activity released from the cytosol of lysed HEK-Blue: OX40 cells into the supernatant were measured by a Cytotoxicity Detection Kit (Roche Diagnostics, Indianapolis, IN). The complement-mediated cytotoxicity was expressed as percent cytotoxicity relative to that lysed by 0.67% (v/v) Triton X-100.
Supplementary Material
Abbreviations
- ADCC
antibody-dependent cell-meditated cytotoxicity
- ADCP
antibody-dependent cellular phagocytosis
- BSA
bovine serum albumin
- CDC
complement-dependent cytotoxicity
- ELISA
enzyme-linked immunosorbent assay
- DPBS
Dulbecco's phosphate-buffered saline
- FcγRII
Fc gamma RII receptor
- HC
heavy chain
- LC
light chain
- PBMC
peripheral blood mononuclear cell
- R-PE
R-phycoerythrin
- SEC
size-exclusion chromatography
- TCA
T-cell activation assay
- TMB
tetramethylbenzidine
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- TNFRSF
tumor necrosis factor receptor superfamily
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Steven Jacobs, Randy Brezski, Bill Strohl, Stephen Jarantow, Jose Pardinas, Matthew Bunce, Adam Zwolak, Susan Tam, Shixue Shen and Gordon Powers for their contributions to this work and technical supports. We thank Mark Salvati and Gary Gilliland for helpful comments during the manuscript review.
References
- 1.Dempke WCM, Fenchel K, Uciechowski P, Dale SP. Second- and third-generation drugs for immuno-oncology treatment-The more the better? Eur J Cancer. 2017;74:55–72. doi: 10.1016/j.ejca.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voo KS, Bover L, Harline ML, Vien LT, Facchinetti V, Arima K, Kwak LW, Liu YJ. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J Immunol. 2013;191:3641–50. doi: 10.4049/jimmunol.1202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He LZ, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, Pilsmaker CD, Round SM, Tutt A, Glennie MJ, et al.. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191:4174–83. doi: 10.4049/jimmunol.1300409. [DOI] [PubMed] [Google Scholar]
- 5.Murillo O, Arina A, Hervas-Stubbs S, Gupta A, McCluskey B, Dubrot J, Palazón A, Azpilikueta A, Ochoa MC, Alfaro C, et al.. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin Cancer Res. 2008;14:6895–906. doi: 10.1158/1078-0432.CCR-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P, L'Italien L, Hodges D, Schebye XM. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J Immunol. 2007;179:7365–75. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
- 7.Hunter TB, Alsarraj M, Gladue RP, Bedian V, Antonia SJ. An agonist antibody specific for CD40 induces dendritic cell maturation and promotes autologous anti-tumour T-cell responses in an in vitro mixed autologous tumour cell/lymph node cell model. Scand J Immunol 2007; 65:479–86. doi: 10.1111/j.1365-3083.2007.01927.x. [DOI] [PubMed] [Google Scholar]
- 8.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–93. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–80. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–4. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, Ravetch JV. Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcgamma receptor engagement. Proc Natl Acad Sci U S A. 2012;109:10966–71. doi: 10.1073/pnas.1208698109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White AL, Chan HT, Roghanian A, French RR, Mockridge CI, Tutt AL, Dixon SV, Ajona D, Verbeek JS, Al-Shamkhani A, et al.. Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187:1754–63. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Ravetch JV. Antitumor activities of agonistic anti-TNFR antibodies require differential FcgammaRIIB coengagement in vivo. Proc Natl Acad Sci U S A. 2013;110:19501–6. doi: 10.1073/pnas.1319502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, et al.. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer cell. 2011;19:101–13. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Chu SY, Vostiar I, Karki S, Moore GL, Lazar GA, Pong E, Joyce PF, Szymkowski DE, Desjarlais JR. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Mol Immunol. 2008; 45:3926–33. doi: 10.1016/j.molimm.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Mimoto F, Katada H, Kadono S, Igawa T, Kuramochi T, Muraoka M, Wada Y, Haraya K, Miyazaki T, Hattori K. Engineered antibody Fc variant with selectively enhanced FcgammaRIIb binding over both FcgammaRIIa(R131) and FcgammaRIIa(H131). Protein Eng Des Sel. 2013;26:589–98. doi: 10.1093/protein/gzt022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Goldberg MV, Chiu ML. Fc Engineering Approaches to Enhance the Agonism and Effector Functions of an Anti-OX40 Antibody. J Biol Chem. 2016;291:27134–46. doi: 10.1074/jbc.M116.757773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahan R, Barnhart BC, Li F, Yamniuk AP, Korman AJ, Ravetch JV. Therapeutic activity of agonistic, human anti-CD40 monoclonal antibodies requires selective FcγR engagement. Cancer cell. 2016;29:820–31. doi: 10.1016/j.ccell.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, et al.. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–3. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang D, Armstrong AA, Tam SH, McCarthy SG, Luo J, Gilliland GL, Chiu ML. Functional optimization of agonistic antibodies to OX40 receptor with novel Fc mutations to promote antibody multimerization. mAbs. 2017;9:1129–42. doi: 10.1080/19420862.2017.1358838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs SA, Diem MD, Luo J, Teplyakov A, Obmolova G, Malia T, Gilliland GL, O'Neil KT. Design of novel FN3 domains with high stability by a consensus sequence approach. Protein Eng Des Sel. 2012;25:107–17. doi: 10.1093/protein/gzr064. [DOI] [PubMed] [Google Scholar]
- 22.Diem MD, Hyun L, Yi F, Hippensteel R, Kuhar E, Lowenstein C, Swift EJ, O'Neil KT, Jacobs SA. Selection of high-affinity Centyrin FN3 domains from a simple library diversified at a combination of strand and loop positions. Protein Eng Des Sel. 2014;27:419–29. doi: 10.1093/protein/gzu016. [DOI] [PubMed] [Google Scholar]
- 23.Simons PJ, Boon L, Luo J, Brezski RJ, Goldberg M. Humanized anti-CD134 (OX40) antibodies and uses thereof. U.S. Patent 2014/0377284. [Google Scholar]
- 24.Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ, Strohl WR. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods. 2014;65:114–26. doi: 10.1016/j.ymeth.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 26.Joulia R, Gaudenzio N, Rodrigues M, Lopez J, Blanchard N, Valitutti S, Espinosa E. Mast cells form antibody-dependent degranulatory synapse for dedicated secretion and defence. Nat Commun. 2015;6:6174. doi: 10.1038/ncomms7174. [DOI] [PubMed] [Google Scholar]
- 27.Dougherty GJ, Selvendran Y, Murdoch S, Palmer DG, Hogg N. The human mononuclear phagocyte high-affinity Fc receptor, FcRI, defined by a monoclonal antibody, 10.1. Eur J Immunol. 1987;17:1453–9. doi: 10.1002/eji.1830171011. [DOI] [PubMed] [Google Scholar]
- 28.He YY, He XJ, Guo PF, Du MR, Shao J, Li MQ, Li DJ. The decidual stromal cells-secreted CCL2 induces and maintains decidual leukocytes into Th2 bias in human early pregnancy. Clin Immunol. 2012;145:161–73. doi: 10.1016/j.clim.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Rankin CT, Veri MC, Gorlatov S, Tuaillon N, Burke S, Huang L, Inzunza HD, Li H, Thomas S, Johnson S, et al.. CD32B, the human inhibitory Fc-gamma receptor IIB, as a target for monoclonal antibody therapy of B-cell lymphoma. Blood. 2006;108:2384–91. doi: 10.1182/blood-2006-05-020602. [DOI] [PubMed] [Google Scholar]
- 30.Indik ZK, Park JG, Hunter S, Schreiber AD. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995;86:4389–99. [PubMed] [Google Scholar]
- 31.Cheng ZJ, Garvin D, Paguio A, Moravec R, Engel L, Fan F, Surowy T. Development of a robust reporter-based ADCC assay with frozen, thaw-and-use cells to measure Fc effector function of therapeutic antibodies. J Immunol Methods. 2014;414:69–81. doi: 10.1016/j.jim.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, Stein KE, Bonvini E, Koenig S. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121:392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber R, Deisenhofer J, Colman PM, Matsushima M, Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976;264:415–20. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- 34.Gronenborn AM, Filpula DR, Essig NZ, Achari A, Whitlow M, Wingfield PT, Clore GM. A novel, highly stable fold of the immunoglobulin binding domain of streptococcal protein G. Science. 1991;253:657–61. doi: 10.1126/science.1871600. [DOI] [PubMed] [Google Scholar]
- 35.Kung P, Goldstein G, Reinherz EL, Schlossman SF. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206:347–9. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- 36.White AL, Dou L, Chan HT, Field VL, Mockridge CI, Moss K, Williams EL, Booth SG, French RR, Potter EA, et al.. Fcgamma receptor dependency of agonistic CD40 antibody in lymphoma therapy can be overcome through antibody multimerization. J Immunol 2014;193:1828–35. doi: 10.4049/jimmunol.1303204. [DOI] [PubMed] [Google Scholar]
- 37.Stewart R, Hammond SA, Oberst M, Wilkinson RW. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J for ImmunoTherapy of Cancer. 2014;2:29–38. doi: 10.1186/s40425-014-0029-x. [DOI] [Google Scholar]
- 38.Jackman J, Chen Y, Huang A, Moffat B, Scheer JM, Leong SR, Lee WP, Zhang J, Sharma N, Lu Y, et al.. Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling. J Biol Chem. 2010;285:20850–9. doi: 10.1074/jbc.M110.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.