Abstract
Background
Attended polysomnography (PSG) is the standard diagnostic test for sleep apnea (SA). However, due to internight variability in SA, a single night PSG may not accurately reflect the true severity of SA. Although internight variability is a well-known phenomenon, its root causes have not been fully elucidated. The objective of this study was to determine factors associated with internight variability in the apnea-hypopnea index (AHI) and its magnitude in the home environment.
Methods
Each participant had a full overnight PSG simultaneous with a validated portable sleep apnea monitoring device (BresoDx®) followed by two overnight home tests using the portable monitor only. Patients were stratified into those with variable AHI and consistent AHI (AHI difference ≥10 or <10 between any 2 nights, respectively). Demographics, sleepiness, sleep test variable, and supine-predominant SA (supine-SA) were examined for any association with variable AHI.
Results
Forty patients completed the protocol. The correlation between PSG and simultaneous BresoDx derived AHIs was 93.4%. Inter-class correlation between the three nights’ AHIs was 89.2%. Over two-thirds (67.5%) of patients had consistent AHIs across the three nights while 32.5% had variable AHI. AHI variability was significantly associated with supine-SA (p=0.0014) and correlated with first night’s AHI (r=0.664, p<0.001). None of the other variable, including BMI, sleepiness, gender, or test duration were associated with internight variability.
Conclusion
Although portable monitoring was highly reproducible over three nights in the majority of participants, one third had a variable AHI. Supine-SA and high AHI on the first night were predictors of high internight variability.
Keywords: Supine Position, Sleep Apnea Syndromes, Sleep Monitoring
INTRODUCTION
Sleep apnea (SA) is a respiratory sleep disorder caused by repetitive partial or complete cessation of respiration. It had a prevalence of approximately 7%, as shown two decades ago 1, which has risen by approximately one third since then owing largely to an increasingly sedentary lifestyle and rising rates of obesity2. SA is a serious condition that is associated with an elevated risk of motor vehicle accidents due to sleepiness3,4, and increased risk of developing hypertension, heart failure, and stroke5,6. Attended polysomnography (PSG) is the standard reference test for the diagnosis of sleep apnea. PSG provides an assessment of SA as the frequency of apneas and hypopneas per hour of sleep (the apnea-hypopnea index or AHI).
The high internight variability in SA severity is a well-documented phenomenon. PSG studies have shown a clinically significant internight difference in AHI by 10 events per hour or greater in 18%7 to 32%8 of patients. This has implications on SA management especially in symptomatic patients with negative results on one night. Factors associated with internight variability in AHI have not been fully elucidated. Sleep position, for example, plays a role in aggravating SA, such that more time spent supine will increase the AHI and vice versa9,10, which might be a potential explanation of internight variability.
Furthermore, most of the studies in this field were limited to two nights and were performed in a sleep laboratory, which might not fully reflect the home environment. Portable home monitors have increased access to diagnosis and facilitated the performance of multiple-night testing and in a more natural home environment. This has the potential to reveal further insights into the true magnitude of the variability of the AHI and factors related to it. Accordingly, the goal of this study was to determine factors related to variability in the AHI over three nights.
METHODS
Subjects
Subjects were consecutive individuals at least 18 years of age referred for PSG due to a history suggestive of SA including at least two of the following symptoms, a history of loud habitual snoring, restless sleep, morning headaches or excessive daytime sleepiness. Exclusion criteria were short recording time of less than 3.5 hours or a sleep efficiency less than 30% on the PSG. The protocol was approved by the Research Ethics Board of the Toronto Rehabilitation Institute, University Health Network and subjects provided written informed consent prior to participation.
Polysomnography
Subjects underwent overnight PSG using standard techniques and scoring criteria for sleep stages and arousals from sleep11. PSG channels were electroencephalography, chin electromyography, respiratory inductance plethysmography, nasal pressure cannulae, and pulse oximetry. Apnea was defined as a reduction in the respiratory signal, by ≥90% lasting ≥10 seconds and hypopnea as a reduction by ≥30 to 90% lasting ≥10 s and accompanied by a ≥3% desaturation or an arousal. Obstructive and central apneas and hypopneas were defined and distinguished as previously described12. PSG-derived AHI (AHIp) was evaluated using total recording time as the denominator to allow comparison with home recordings by the portable device, which did not measure sleep time. Epworth Sleepiness Scale (ESS) was used to assess patients subjective sleepiness during their clinical visit.
Portable Testing
Simultaneously with PSG, breath sounds were captured by a validated portable sleep apnea monitoring device, BresoDx® (BresoTec Inc., Toronto, Ontario, Canada). Subsequently, each subject underwent two overnight sleep tests in the home using BresoDx. BresoDx is a self-contained, open face frame unit without electrodes that is worn on the face with 93%-95% concordance with PSG13-15. It allows the patients to sleep in any position, including the prone position with the head facing to the side. AHI is calculated as the number respiratory event divided by the total recording time and the diagnosis of SA is based on an AHI ≥10 events/hr.
Internight Variability
The AHI scores obtained using BresoDx from the three nights (AHIN1, AHIN2, and AHIN3) were compared. Any pair of nights were considered consistent if the difference between their AHI’s was <10 and variable if ≥10 events/hr as practiced by other researchers in the field7,15,16. Accordingly, subjects were categorized into those with consistent, mildly variable, and highly variable AHI according to the criteria listed in Table 1.
Table 1.
Categorical classification of patients based on their AHIs across 3 nights.
| Consistent | All the nights
were consistent, e.g. AHIs of 5, 10, and 14 events/hr |
| Mildly Variable | Had 1 variable night and 2 consistent nights, e.g. AHI of 5,10, and 25 events/hr |
| Highly Variable | Had 3 variable nights, e.g. AHI of 5, 35, 20 events/hr |
AHI=apnea-hypopnea index.
Besides the categorical classification, a continuous measure of the internight variability (INV) was calculated as the intra-subject average difference among the three nights’ AHIs given by the Equation 1:
| Equation 1 |
To determine factors associated with internight variability: age, sex, sleepiness, BMI, first night AHI, and supine-predominant SA (supine-SA) were examined against INV and the presence of variable nights.
Supine-Predominant Sleep Apnea Determination
To examine the effect of body position, PSG data were used to classify patients into those with and without supine-SA. The supine AHIp is calculated as the number of apneas and hypopneas while the person is in the supine position divided by the supine sleep time. Subjects were considered to have supine-SA if: 1) the PSG overall AHI was ≥10 events/hr, 2) >10% of time was spent in the supine position and >10% of time spent in non-supine position, in order to have sufficient data in each position, 3) the supine AHIp was at least 50% greater than the non-supine AHIp, and 4) the supine AHIp was greater than the non-supine AHIp by ≥5 events/hr. Otherwise subjects were said to have non-supine-predominant SA (non-supine-SA).
Statistical Analyses
Data normality was examined using Shapiro-Wilk parametric hypothesis test. Statistical difference between the 3-night AHI scores including PSG were determined using Kruskal-Wallis test-a nonparametric version of one-way ANOVA. The mean and standard deviation for normally distributed data, or median and median absolute deviation (MAD) for non-normally distributed data, were calculated for each group. The Intra-class correlation coefficient was calculated to examine the reproducibility of AHI scores across the 3 nights. Fisher exact test was used to determine the association between categorical variables: supine-SA and variable AHI.
RESULTS
Forty subjects completed the three-night protocol, whose PSG characteristics are presented in Table 2. The median ± MAD interval between tests was 12±11 days.
Table 2.
Subject characteristics according to PSG charts.
| Male: Female | 26:14 |
|---|---|
| Age (mean± SD) | 54.7±16.4 years |
| Body Mass Index (BMI) | 29.3±7.0 kg/m2 |
| Mean Epworth Sleepiness Scale | 7.4±3.8 |
| Obstructive SA : Central SA | 39:1 |
| Supine AHIp (Median±MAD) | 24.1±22.2 events/hr |
| Non-supine AHIp (Median±MAD) | 10.5±12.0 events/hr |
| Proportion of time spent supine | 39% |
SD=standard deviation; SA=sleep apnea; AHIp=polysomnography-derived apnea hypopnea index; MAD=median absolute deviation.
Three Night AHI Correlation
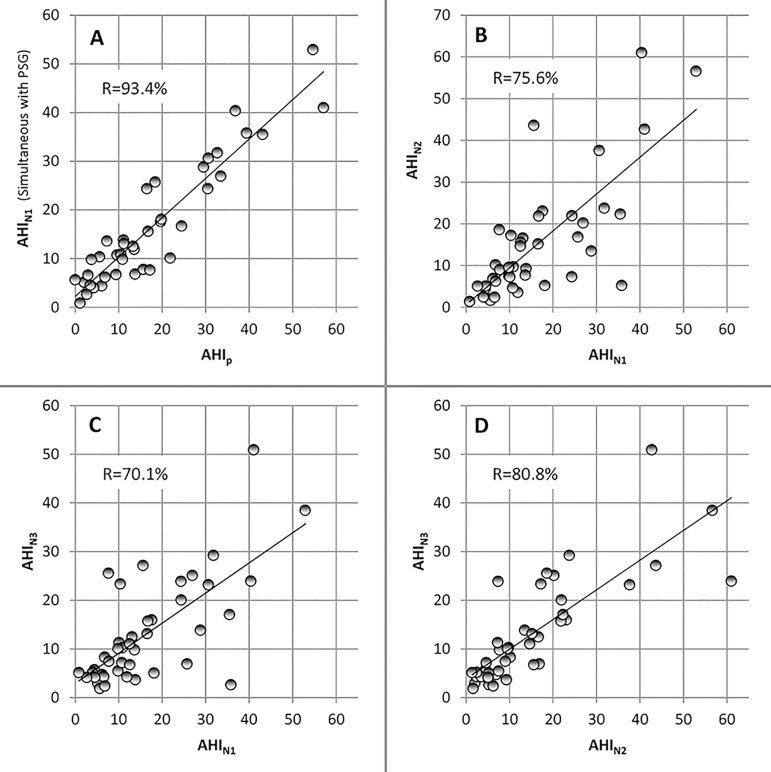
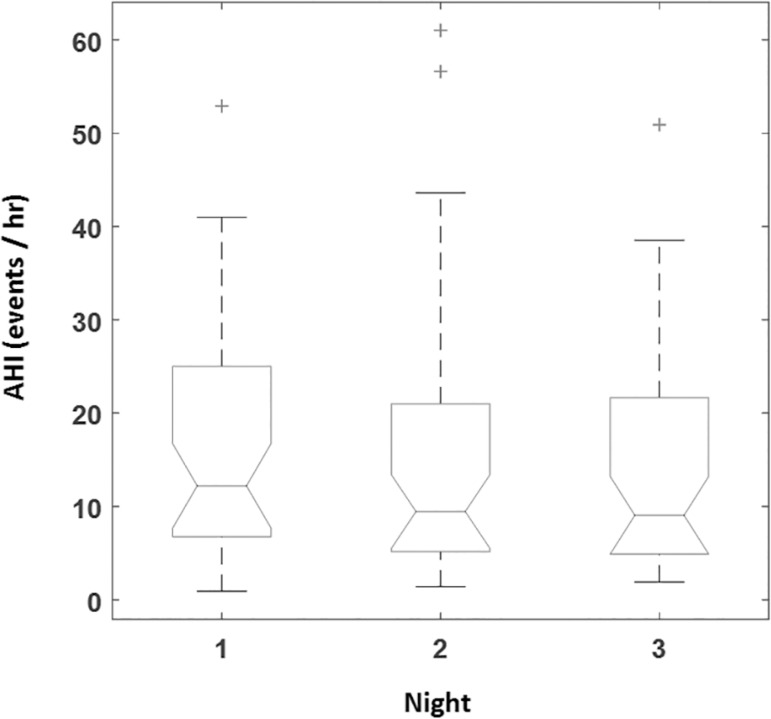
Correlation between PSG derived AHI and simultaneous BresoDx was 93.4% (Fig. 1-A) reconfirming the accuracy of the portable device14,15. Correlation between PSG and home nights was 70.6% and 75.0% respectively (p<0.001). Correlation between BresoDx-derived AHIs (AHIN1, AHIN2, and AHIN3) were 75.6, 70.1%, and 80.8% (p<0.001) as depicted in Fig. 1-B, C, and D, which were similar to, but slightly higher than, correlation between PSG and home studies. Therefore, all subsequent comparisons are between BresoDx derived AHIs only. There was no significant difference among AHIN1, AHIN2 and AHIN3 over the three nights (p=0.35, Fig. 2). The inter-class correlation coefficient among the three AHIs was 0.892, with a 95% confidence interval: 0.82- 0.94.
Figure 1.
Relationships between AHIp, AHIN1, AHIN2, and AHIN3. All units are in events/hr. AHIp=polysomnography-derived apnea-hypopnea index, AHIN1=night 1 apneahypopnea index, AHIN2=night 2 apnea-hypopnea index, AHIN3=night 3 apnea-hypopnea index.
Figure 2.
Median AHI derived from BresoDx during the three nights. AHI=apneahypopnea index.
Internight Variability in AHI
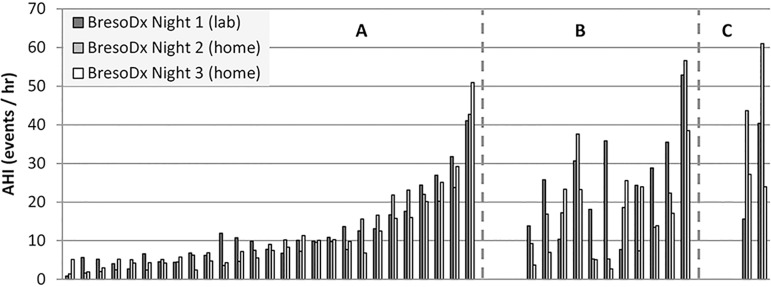
The average INV (calculated from Equation 1) for all subjects was 6.2±5.8 events/hr. When examining intra-subject AHI variability across the three nights, 27 patients (67.5%) had consistent AHIs i.e. an AHI difference between any pair nights <10 events/hr (Fig. 3-A). The remaining 13 patients (32.5%) had at least 1 pair of variable nights of whom 11 patients (27.5%) had mildly variable AHI and 2 patients (5%) had highly variable AHIs (as defined in Table 1) The distribution of variable patients is depicted in Fig. 3-B and C.
Figure 3.
Distribution of the 40 subjects into three groups according to internight variability A: consistent group (27 patients) who had AHI scores with a difference <10/hr between any pair of the three nights; B: mildly variable group (11 patients) who had two consistent nights and one variable night; and C: highly variable group (2 patients) with variable AHIs on all three nights. AHI=apnea-hypopnea index.
In order to examine the effect of internight variability on SA designation, we have quantified the number of patients who altered diagnosis from the baseline i.e. changed from SA to no-SA or vice versa using a diagnostic AHI cut-off of ≥10. In total, 14 of 40 (35%) subjects changed designation compared to the 1st night. Excluding the consistent group in whom the difference across nights was small, 5 (12.5%) subjects of the variable group changed designation from baseline. Of the latter, 4 subjects changed from SA to no-SA and only 1 subject changed from no-SA to SA.
Predictors of Internight Variability
Age, sex, sleepiness, BMI, first night AHI, and supine-SA were examined for association with variable AHI or INV. There was a significant correlation between AHI on night 1 and INV (r=0.664, p<0.001), i.e. the higher the AHI on the first night, the higher the internight variability. However, there was no correlation between INV and the ESS (r=-0.125, p=0.30), age (r=0.097, p=0.55), BMI (r=0.315, p=0.509), the average time interval between portable tests (r=-0.23, p=0.15), or test duration (r=-0.04, p=0.79). There was no relationship between the presence of variable AHI and sex (p=0.32).
In order to examine the effect of supine-SA on AHI variability, we included patients who had spent at least 10% of the time in the supine and non-supine positions, as described in the Methods section. Four out of 40 patients were excluded because they didn’t meet this criterion. The distribution of the included 36 subjects is presented in Table 3. There was a significant association between supine-SA and variable AHI (p=0.0014).
Table 3.
Distribution of subjects according to internight variability in AHI.
| Variable AHI | Consistent AHI | |
|---|---|---|
| Supine SA | 10 (27.8%) | 6 (16.7%) |
| Non-supine SA | 2 (5.6%) | 18 (50.0%) |
AHI=apnea-hypopnea index; SA=sleep apnea.
DISCUSSION
This study has given rise to two main observations. First, in the majority of subjects, AHI determined by portable monitor was reproducible across three nights, as shown by a high inter-class correlation coefficient (89.2%) and a consistent AHI scores in over two thirds of patients. Second, variability in AHI was present in approximately one third of the patients and the factors significantly related to it were the presence of a high first-night AHI and supine-SA, as determined by PSG. To our knowledge, this is the first study to demonstrate a statistically significant relationship between supine-predominant SA and internight variability in AHI.
Variability in the AHI performed on two nights during an attended sleep laboratory PSG is well-documented with relatively low between night correlations of 0.4417 to 0.7718. Similar results have been described for unattended portable home tests19. Earlier studies stratified subjects into those with and without internight variability in AHI based on a difference between the two nights of ≥10 events/hr7,17. The proportion of subjects who exhibited internight variability between two nights using PSG varied between 18%7 and 35%8 in most studies. In the present study, the proportion of subjects who exhibited internight variability is well within the range reported by previous PSG-based studies.
In this study population, the concordance between the portable monitor, BresoDx, and simultaneous PSG was excellent, which further confirms its validity as a portable monitor in the subsequent unattended nights. Over the three-night study period the AHIs were consistent for the majority of those tested and the population medians didn’t differ significantly. Yet, internight variability was present in 32.5% between any two nights. Within the variable group 11 (27.5%) were mildly variable, while a minority of 2 subjects (5%) were highly variable, according to the criteria in Table 1. This suggests that most subjects with variable AHIs can still have consistent nights, in which the AHI on two of the three nights does not vary between them, and that these two nights might be the true indicator of their severity. A small proportion of patients (12.5%) changed designation from the 1st to subsequent nights mostly from SA to no-SA, with an AHI difference >10 events/hr between nights. This suggests that a single in-laboratory PSG might not fully represent the true SA severity in those patients and that multiple night testing could provide a more reliable basis for clinical management of SA.
Although supine sleep position is a well-known cause of elevated AHI, its effect on internight AHI variability has not been reported in literature. In this study, the impact of sleep position was assessed based on the PSG study. This showed that sleep position was responsible for the variability in the AHI in 83.3% of those with variable AHIs, while 75% of those with consistent AHIs did not have supine-SA. This significant association is likely due to the differences in duration spent supine between nights. Unfortunately, we could not examine this possibility in the home because body position sensing was not available via BresoDx during the course of this study. Yet, since supine-SA designation was based on in-lab PSG, which the gold standard for SA diagnosis, and since the relation was highly statistically significant (p=0.0014), these are powerful indicators of the association between supine-SA and internight variability.
Another predictor of internight variability was the first night’s AHI. There was a significant correlation between the first night’s AHI and internight variability. Those with more severe SA tend to have greater differences between their home and PSG-derived AHIs than those with mild or no SA. This observation is consistent with previous studies such as Bliwise et al.7 who found that subjects with high internight variability had an average AHI of 37.7±19.3 compared to 7.4±9.1 events/hr in the consistent group. Le Bon et al.20 also showed that a higher AHI on a first night was associated with an increase in AHI of ≥20 events/hr on a second night. Therefore, in clinical settings, a subject with low AHI on PSG (especially <10) is likely to maintain low AHI but not vice versa. Our data didn’t show an association between internight variability in AHI and sex, age, sleepiness, or BMI, which agrees with the findings of Prasad et al.21.
Our study has a number of limitations. The number of subjects was relatively small, which could be anticipated in a multiple night study requiring patients to undergo several overnight tests, although statistical analysis via repeated measures helped counteract this. Additionally, the BresoDx device used in this study was unable to determine body position in the home. Therefore, the classification of subjects into supine-predominant SA and non-supine-predominant was based solely on PSG. Subsequent versions of the portable monitor are furnished with a position sensor, which can be deployed in future home test studies. A major strength of our study is the reliability of our AHI from the BresoDx as it is determined by a computer-based algorithm, which is not subject to inter or intra-rated scoring differences.
In conclusion, this study illustrates that approximately one-third of SA patients have high internight variability in their AHI that is associated with supine predominant-SA and a high AHI on the first night. This association is yet to be further confirmed by recording of body position in the home, which could be investigated in future studies. Finally, portable monitoring offers a reliable method to perform multiple-night testing in individuals whose first night AHI does not explain their clinical presentation.
ACKNOWLEDGMENTS
We would like to thank the Toronto Rehabilitation Institute (TRI) sleep laboratory technicians for their support in data collection; in particular Fiona Rankin and Wen-Hou Tseng and Maryam Patel for her support in paper submission. This project has been supported by the TRI, the Ontario Ministry of Research and Innovation, MaRS Innovation, Ontario Centres of Excellence, J&J Inc, the Ontario Brain Institute via FedDev, and NSERC scholarship to Dr Alshaer. Dr Bradley is supported by the Clifford Nordal Chair in Sleep Apnea and Rehabilitation Research.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. http://dx.doi.org/10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. http://dx.doi.org/10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward KL, Hillman DR, James A, Bremner AP, Simpson L, Cooper MN, et al. Excessive daytime sleepiness increases the risk of motor vehicle crash in obstructive sleep apnea. J Clin Sleep Med. 2013;9(10):1013–1021. doi: 10.5664/jcsm.3072. http://dx.doi.org/10.5664/jcsm.3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep. 1997;20(8):608–613. doi: 10.1093/sleep/20.8.608. http://dx.doi.org/10.1093/sleep/20.8.608 [DOI] [PubMed] [Google Scholar]
- 5.Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107(12):1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. http://dx.doi.org/10.1161/01.CIR.0000061757.12581.15 [DOI] [PubMed] [Google Scholar]
- 6.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. http://dx.doi.org/10.1164/rccm.200505-702OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliwise DL, Benkert RE, Ingham RH. Factors associated with nightly variability in sleep-disordered breathing in the elderly. Chest. 1991;100(4):973–976. doi: 10.1378/chest.100.4.973. http://dx.doi.org/10.1378/chest.100.4.973 [DOI] [PubMed] [Google Scholar]
- 8.Chediak AD, Acevedo-Crespo JC, Seiden DJ, Kim HH, Kiel MH. Nightly variability in the indices of sleep-disordered breathing in men being evaluated for impotence with consecutive night polysomnograms. Sleep. 1996;19(7):589–592. doi: 10.1093/sleep/19.7.589. http://dx.doi.org/10.1093/sleep/19.7.589 [DOI] [PubMed] [Google Scholar]
- 9.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7(2):110–114. doi: 10.1093/sleep/7.2.110. http://dx.doi.org/10.1093/sleep/7.2.110 [DOI] [PubMed] [Google Scholar]
- 10.Kavey NB, Blitzer A, Gidro-Frank S, Korstanje K. Sleeping position and sleep apnea syndrome. Am J Otolaryngol. 1985;6(5):373–377. doi: 10.1016/s0196-0709(85)80015-6. http://dx.doi.org/10.1016/S0196-0709(85)80015-6 [DOI] [PubMed] [Google Scholar]
- 11.Berry R, Brooks R, Gamaldo C, Harding S, Lloyd R, Marcus C, et al. Respiratory Rules for Adults. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien: American Academy of Sleep Medicine; 2015. pp. 50–56.http://dx.doi.org/10.5664/jcsm.4618 Version 2.2. [Google Scholar]
- 12.White LH, Lyons OD, Yadollahi A, Ryan CM, Bradley TD. Effect of below-the-knee compression stockings on severity of obstructive sleep apnea. Sleep Med. 2015;16(2):258–264. doi: 10.1016/j.sleep.2014.12.005. http://dx.doi.org/10.1016/j.sleep.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 13.Alshaer H, Levchenko A, Bradley TD, Pong S, Tseng WH, Fernie GR. A system for portable sleep apnea diagnosis using an embedded data capturing module. J Clin Monit Comput. 2013;27(3):303–311. doi: 10.1007/s10877-013-9435-8. http://dx.doi.org/10.1007/s10877-013-9435-8 [DOI] [PubMed] [Google Scholar]
- 14.Alshaer H, Fernie GR, Maki E, Bradley TD. Validation of an automated algorithm for detecting apneas and hypopneas by acoustic analysis of breath sounds. Sleep Med. 2013;14(6):562–571. doi: 10.1016/j.sleep.2012.12.015. http://dx.doi.org/10.1016/j.sleep.2012.12.015 [DOI] [PubMed] [Google Scholar]
- 15.Alshaer H, Fernie GR, Tseng WH, Bradley TD. Comparison of in-laboratory and home diagnosis of sleep apnea using a cordless portable acoustic device. Sleep Med. 2016;22:91–96. doi: 10.1016/j.sleep.2015.11.003. http://dx.doi.org/10.1016/j.sleep.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 16.Bittencourt LR, Suchecki D, Tufik S, Peres C, Togeiro SM, Bagnato MC, et al. The variability of the apnoea-hypopnoea index. J Sleep Res. 2001;10(3):245–251. doi: 10.1046/j.1365-2869.2001.00255.x. http://dx.doi.org/10.1046/j.1365-2869.2001.00255.x [DOI] [PubMed] [Google Scholar]
- 17.Levendowski DJ, Zack N, Rao S, Wong K, Gendreau M, Kranzler J, et al. Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath. 2009;13(2):163–167. doi: 10.1007/s11325-008-0214-6. http://dx.doi.org/10.1007/s11325-008-0214-6 [DOI] [PubMed] [Google Scholar]
- 18.Aber WR, Block AJ, Hellard DW, Webb WB. Consistency of respiratory measurements from night to night during the sleep of elderly men. Chest. 1989;96(4):747–751. doi: 10.1378/chest.96.4.747. http://dx.doi.org/10.1378/chest.96.4.747 [DOI] [PubMed] [Google Scholar]
- 19.Quan SF, Griswold ME, Iber C, Nieto FJ, Rapoport DM, Redline S, et al. Sleep Heart Health Study. Research Group Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography--the Sleep Heart Health Study. Sleep. 2002;25(8):843–849. [corrected] [PubMed] [Google Scholar]
- 20.Le Bon O, Hoffmann G, Tecco J, Staner L, Noseda A, Pelc I, et al. Mild to moderate sleep respiratory events: one negative night may not be enough. Chest. 2000;118(2):353–359. doi: 10.1378/chest.118.2.353. http://dx.doi.org/10.1378/chest.118.2.353 [DOI] [PubMed] [Google Scholar]
- 21.Prasad B, Usmani S, Steffen AD, Van Dongen HP, Pack FM, Strakovsky I, et al. Short-Term Variability in Apnea-Hypopnea Index during Extended Home Portable Monitoring. J Clin Sleep Med. 2016;12(6):855–863. doi: 10.5664/jcsm.5886. http://dx.doi.org/10.5664/jcsm.5886 [DOI] [PMC free article] [PubMed] [Google Scholar]