Abstract
Aim
Correction of metabolic acidosis in patients with chronic kidney disease has been associated with improvement in thyroid function. We examined whether changes in bicarbonate were associated with changes in thyroid function in patients with end-stage renal disease receiving conventional or more frequent hemodialysis.
Methods
In the Frequent Hemodialysis Network Trials, the relationship between changes in serum bicarbonate, free triiodothyronine (FT3) and free thyroxine (FT4) was examined among 147 and 48 patients with endogenous thyroid function who received conventional (3x/week) or more frequent (6x/week) hemodialysis (Daily Trial) or who received conventional or more frequent nocturnal hemodialysis (Nocturnal Trial). Equilibrated normalized protein catabolic rate (enPCR) was examined to account for nutritional factors affecting both acid load and thyroid function.
Results
Increasing dialysis frequency was associated with increased bicarbonate level. Baseline bicarbonate level was not associated with baseline FT3 and FT4. Change in bicarbonate level was not associated with changes in FT3 and FT4 in the Daily Trial nor for FT4 in the Nocturnal Trial (r≤0.14, P>0.21). While, a significant correlation between change in serum bicarbonate and change in FT3 (r=0.44, P=0.02) was observed in the Nocturnal Trial; findings were no longer significant after adjusting for change in enPCR (r=0.37, P=0.08). For participants with baseline bicarbonate <23mMol/L, no association between change in bicarbonate and change in thyroid indices were seen in the Daily Trial; for the Nocturnal Trial, findings were also not significant for change in FT3 and the association between change in bicarbonate and change in FT4 (r=0.54, P=0.03) was no longer significant after adjusting for enPCR (r=0.45, P=0.11).
Conclusions
Changes in bicarbonate were not associated with changes in thyroid hormone levels after adjusting for enPCR, as a marker of nutritional status. Future studies should examine whether improvement in acid base status improves thyroid function in hemodialysis patients with evidence of thyroid hypofunction.
Keywords: metabolic acidosis, bicarbonate, thyroid function, hemodialysis, hypothyroidism, enPCR
Introduction
Chronic kidney disease (CKD) is associated with alterations in metabolic and endocrine functions 1, including an increased prevalence of hypothyroidism.2,3 The mechanisms underlying these associations are not completely clear, since several factors, not primarily related to intrinsic thyroidal illness, may contribute to the reduced thyroid function.2 In this light, Koo and colleagues reported an association between low free 3,5,3′-triiodo-L-thyronine (FT3) and both inflammation and malnutrition in hemodialysis patients.4 What was of interest was that serum bicarbonate concentration in patients with low serum T3 (< 0.8 ng/ml) was significantly lower than in those with normal thyroid function, despite reduced protein catabolic rate (PCR), a contributor of net acid load.4 These findings suggest a contributing or possibly dominant role of metabolic acidosis in altering thyroid function. Strengthening this association, two randomized control studies examining the effect of correction of metabolic acidosis, either by increasing sodium bicarbonate delivery through dialysis dose5 or pharmacologic treatment in patients with CKD not requiring dialysis6, found an improvement in thyroid function, supporting the hypothesis that metabolic acidosis may play a reversible role in establishing thyroid dysfunction in CKD.
Increased dialysis frequency, implemented as duration of dialysis per week, and intensity, through longer (nocturnal) and more frequent (every-other-day) dialysis schedules, have been reported to increase serum bicarbonate concentration in both retrospective and prospective studies.7,8 The Frequent Hemodialysis Network (FHN) Trials were randomized prospective studies aimed at evaluating the effect of increasing dialysis frequency on multiple outcomes.9, 10 Among FHN Trial participants with endogenous thyroid function (excluding 11% with presumed hypothyroidism), more frequent in-center hemodialysis (Daily and Nocturnal Trials) did not result in significant changes in thyroid hormone indices over 12 months, although relationships between change in serum bicarbonate and changes in thyroid function were not investigated.11
In this study, we examined the relationship between changes in serum bicarbonate concentration and changes in FT3 and free thyroxine (FT4) levels over 12 months, as well as the effect of baseline acid base status, in patients randomized to receive conventional versus more frequent hemodialysis in the Daily and Nocturnal FHN Trials who had evidence of endogenous thyroid function. Because acid load is also associated with dietary protein intake (which is associated with nutritional status), we also examined changes in equilibrated normalized protein catabolic rate (enPCR), which was reported to increase in the FHN Nocturnal Trial.10
Materials and Methods
The FHN Daily Trial was a multicenter, randomized trial, prospectively comparing six times per week vs three times per week, in-center hemodialysis.9,12,13 The FHN Nocturnal Trial was a prospective, multicenter, randomized trial of six times per week nocturnal at-home hemodialysis vs three times per week.9,12,13 The inclusion and exclusion criteria and data collection procedures have been previously described.9
Study population
The FHN Trials were approved by the Institutional Review Board at each participating study site, with informed consent obtained from each patient.10 Patients with end-stage renal disease maintained on hemodialysis were included in the study. Major exclusion criteria were represented by age <13 years in the daily group or age <18 years in the nocturnal group, residual kidney function >3 ml/min/35L in the daily group, or mean of creatinine and urea clearance >10 ml/min/1.73m2 in the nocturnal group.9 As previously described11, we excluded participants receiving thyroid hormone therapy and those with thyroid stimulating hormone (TSH) (uIU/ml) >8 who were presumed to have treated or untreated primary hypothyroidism, to avoid the effects of underlying hypothyroidism and/or treatment on thyroid hormone levels where changes in thyroid function in response to changes in serum bicarbonate level might not be evident. Patients receiving antithyroid drugs and those with suppressed TSH (uIU/ml) ≤0.01 were also excluded.11 Patient’s demographic characteristics, including age, gender, weight, height, body mass index (BMI; in kilograms per meter2), dialysis vintage, and Kt/V were also collected.
Characteristics of the Daily and Nocturnal Trials
The characteristics of intervention, control and adherence for the FHN Trials have been previously described.9,10,12 In the Daily Trial, study participants were randomly assigned to hemodialysis treatment either six times per week (n=125, with a target equilibrated Kt/Vurea of 0.9 and session length of 1.5–2.75 hours) or three times per week (n=120, continued their usual minimum target equilibrated Kt/Vurea of 1.1, with each session length of 2.5 and 4.0 hours).9,10,12 In the Nocturnal Trial, study participants were randomly assigned to hemodialysis treatment either six times per week (n=45, with a standardized Kt/Vurea ≥4.0/week and a session length ≥ 6 hours) or three times per week (n= 42, with a standardized Kt/Vurea of > 2.0 per week and a session length ≥2.5 hours).9,10,12
Outcome measures
As previously described11, blood samples in both Trials were collected at the participants’ dialysis units during the dialysis visit prior to initiation of hemodialysis session, centrifuged, and refrigerated and sent for laboratory analyses locally or serum was frozen and stored at −70°C at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Biorepository.11 Serum bicarbonate levels at baseline and 12 months in the FHN study were derived from local laboratory measurements coordinated by individual dialysis units. Analyses for thyroid function indices were subsequently conducted at the University of California, Davis from aliquoted frozen specimens stored at the NIDDK Biorepository.11 Serum FT3, FT4 and TSH levels were measured in duplicate by enzyme-linked immunoassay (ALPCO Diagnostics, Salem, NH, U.S.A.).11
Statistical analyses
Continuous variables were described as mean and standard deviation or median, as appropriate. Categorical variables were described as frequencies and percentages. Continuous variables were compared between dialysis frequency groups using the Student’s t-test. The Fisher exact or chi-square test was used to compare categorical variables. Baseline associations of serum FT3 and FT4 levels with serum bicarbonate levels were examined using Pearson’s correlation analysis. We evaluated the relationship of changes over 12 months in serum bicarbonate level with frequency of dialysis for each FHN Trial. To examine the effect of dialysis frequency (six times per week vs three times per week) on changes in serum bicarbonate, and to include all observations, even in those subjects with no 12-month measurements (e.g., due to missing end of study visit and/or missing sample), we employed a mixed-effects model with an unstructured covariance matrix, adjusting for clinical center in the Daily Trial.11 Since the question of interest was whether metabolic acidosis per se contributed to thyroid dysfunction, we additionally stratified patients based on bicarbonate levels (mMol/L) < 23 or ≥ 23 to examine whether improvement in baseline acidosis affected thyroid function. Pearson correlations were used to assess the association of change in serum bicarbonate and change in thyroid hormone levels, with findings also adjusted for changes in enPCR between baseline and Month 12. All analyses were conducted using SAS statistical software version 9.4 (SAS Statistical Institute, Cary NC) and a P value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 147 euthyroid participants were included in the Daily Trial, and 48 euthyroid participants were included in the Nocturnal Trial.11 The baseline demographic and clinical characteristics of the study participants are shown in Table 1. The mean age was 50.9 ± 14.1 years in the Daily Trial (67% male) and 53.8 ± 13.8 years in the Nocturnal Trial (62% male). As previously described, participants in the Daily Trial were more likely (68%) to have had end-stage renal disease for ≥ 2 years (P < 0.001) compared to the Nocturnal Trial (35%) but were less likely to have diabetes (42% vs 56%, P = 0.09).11 The majority of the participants in both Trials were overweight or obese (BMI ≥ 25 kg/m2; 58% in the Daily and 69% in the Nocturnal Trial) (Table 1).
Table 1.
Baseline euthyroid participant’s characteristics
| Variables | FHN Daily Trial N=147 |
FHN Nocturnal Trial N=48 |
P |
|---|---|---|---|
| Age (years) | 50.9 ± 14.1 | 53.8 ± 13.8 | 0.21 |
| Female | 48 (33%) | 18 (38%) | 0.54 |
| Diabetes Mellitus | 62 (42%) | 27 (56%) | 0.09 |
| Years since ESRD (Vintage) | <0.001 | ||
| < 2 years | 47 (32%) | 31 (65%) | |
| >= 2 years | 100 (68%) | 17 (35%) | |
| Body Mass Index (kg/m2) | 0.40 | ||
| < 18.5 | 7 (5%) | 2 (4%) | |
| 18.5–24.9 | 55 (37%) | 13 (27%) | |
| >= 25.0 | 85 (58%) | 33 (69%) | |
| Percent Body Fat (%) | 42.0 ± 8.63 | 43.7 ± 7.87 | 0.25 |
| ICW/kg | 0.27 ± 0.06 | 0.26 ± 0.07 | 0.35 |
| enPCR (g/kg/d) | 1.03 ± 0.26 | 0.95 ± 0.23 | 0.05 |
| HCO3− (Bicarbonate) (mMol/L) | 23.5 ± 3.55 | 21.9 ± 3.79 | 0.007 |
| FT3 (pg/mL) | 2.52 ± 0.80 | 2.71 ± 1.08 | 0.21 |
| FT4 (ng/dL) | 0.89 ± 0.24 | 0.94 ± 0.21 | 0.20 |
Abbreviations include: FHN, Frequent Hemodialysis Network; ESRD, end stage renal disease; ICW, intracellular water; enPCR, equilibrated normalized protein catabolic rate; FT3, free 3,5,3′-triiodo-L-thyronine; FT4, free thyroxine.
Baseline serum bicarbonate and thyroid hormone levels
Baseline bicarbonate levels (mMol/L) were significantly higher for participants in the Daily Trial compared to the Nocturnal Trial (23.5 ± 3.55 vs 21.9 ± 3.79; P = 0.007) (Table 1), but baseline values did not differ by treatment arm within each Trial (Daily Trial, P = 0.26; Nocturnal Trial, P = 0.48). In both Trials, the mean changes over 12 months in the bicarbonate levels increased significantly more in the six times per week hemodialysis group than in the three times per week hemodialysis group (Table 2). In the Daily Trial, baseline bicarbonate levels were not significantly correlated with baseline FT3 levels (r= −0.14, P = 0.09), although the correlation with FT4 levels (r = 0.16, P = 0.05) was borderline significant. In the Nocturnal Trial, no association was seen between baseline bicarbonate levels and baseline FT3 and FT4 levels.
Table 2.
Baseline and 12 month bicarbonate (HCO3−) levels in FHN euthyroid participants according to dialysis frequency.
| Daily Trial | 3x/week (N = 56) | 6x/week (N =62) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 month | Change | Baseline | 12 month | Change | P* | |
| HCO3− (mMol/L) | 24.0 ± 3.74 | 23.7 ± 2.97 | −0.33 ± 3.23 | 23.0 ± 3.30 | 24.2 ± 2.64 | 1.15 ± 3.32 | 0.016 |
| enPCR (g/kg/day) | 1.04 ± 0.25 | 0.99 ± 0.23 | −0.05 ± 0.24 | 1.06 ± 0.25 | 1.09 ± 0.29 | 0.03 ± 0.23 | 0.061 |
| Nocturnal Trial | 3x/week (N =23) | 6x/week (N =18) | |||||
| Baseline | 12 month | Change | Baseline | 12 month | Change | P* | |
| HCO3− (mMol/L) | 21.6 ± 3.47 | 22.1 ± 2.23 | 0.57 ± 3.62 | 21.8 ± 4.09 | 25.4 ± 3.67 | 3.61 ± 4.60 | 0.023 |
| enPCR (g/kg/day) | 0.96 ± 0.21 | 1.11 ± 0.20 | 0.15 ± 0.24 | 0.87 ± 0.19 | 1.09 ± 0.36 | 0.22 ± 0.26 | 0.381 |
Paired data with both baseline and 12 month measurements comparing changes between three times per week (3x/week) and six times per week (6x/week) hemodialysis (Means ± SD).
Abbreviations include: enPCR, equilibrated normalized protein catabolic rate
The relationship of change in serum bicarbonate levels and changes in thyroid hormone levels
The effect of hemodialysis frequency on changes in bicarbonate were examined in the 147 Daily and 48 Nocturnal participants with endogenous thyroid function, as well as in all FHN Trial participants, using mixed-effects models to account for missing values (Table 3). A positive mean change indicates that the month 12 bicarbonate value increased from baseline. The treatment effect compares the change from baseline to month 12 between the hemodialysis frequency arms. A treatment effect with a positive sign indicates there was a net increase comparing daily to three times per week hemodialysis, whereas a negative sign indicates a net decrease comparing daily to three times per week hemodialysis. Six times per week hemodialysis was associated with a significant increase in serum bicarbonate levels in both the Daily and Nocturnal Trials. These findings were evident in the overall cohort and also in the subset with evidence of endogenous thyroid function, with larger effects seen in the Nocturnal Trial.
Table 3.
The effect of dialysis frequency on bicarbonate (HCO3−) levels in the subset of euthyroid FHN participants and the overall cohort.
| Euthyroid Participants# | Overall Participants# | |||
|---|---|---|---|---|
| HCO3− estimate (95% CI) | P | HCO3− estimate (95% CI) | P | |
| DAILY Trial* | ||||
| 3x/week | 0.28 (−0.52 to 1.08) | 0.49 | 0.25 (−0.51 to 1.01) | 0.52 |
| 6x/week | 1.21 (0.43 to 1.99) | 0.003 | 1.11 (0.36 to 1.86) | 0.004 |
| Treatment Difference | 0.92 (0.04 to 1.81) | 0.04 | 0.86 (0.02 to 1.70) | 0.045 |
| NOCTURNAL Trial* | ||||
| 3x/week | 0.31 (−1.15 to 1.78) | 0.67 | 0.12 (−1.19 to 1.44) | 0.85 |
| 6x/week | 3.56 (1.97 to 5.15) | <0.001 | 3.11 (1.77 to 4.45) | <0.001 |
| Treatment Difference | 3.25 (1.46 to 5.04) | 0.001 | 2.99 (1.41 to 4.56) | <0.001 |
Mixed model analysis (for Daily Trial, adjusted for clinical center).
Mean change from baseline within three times per week (3x/week) and six times per week (6x/week) hemodialysis and treatment effect (95% confidence Interval)
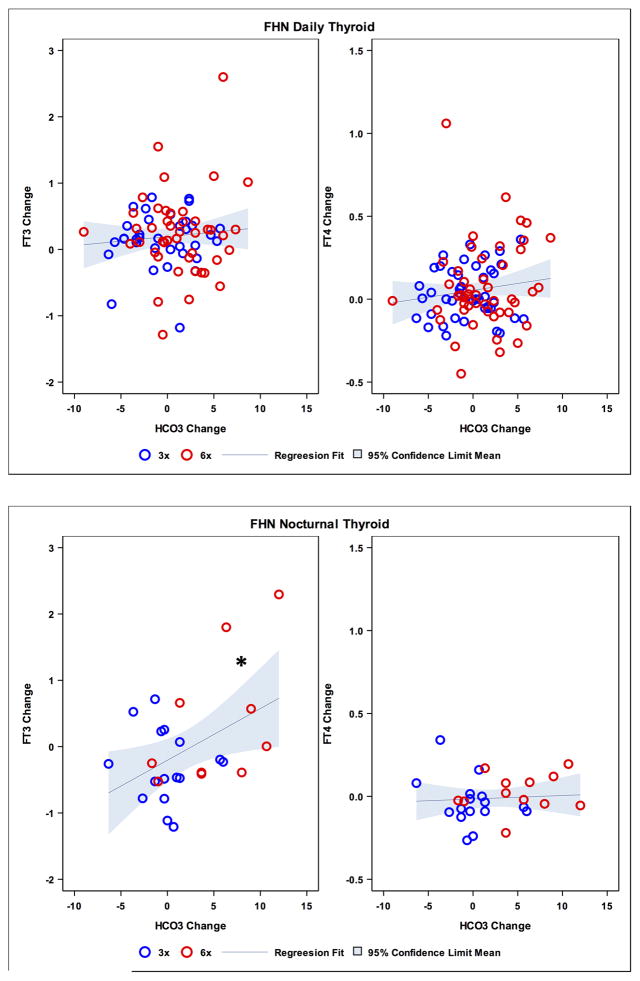
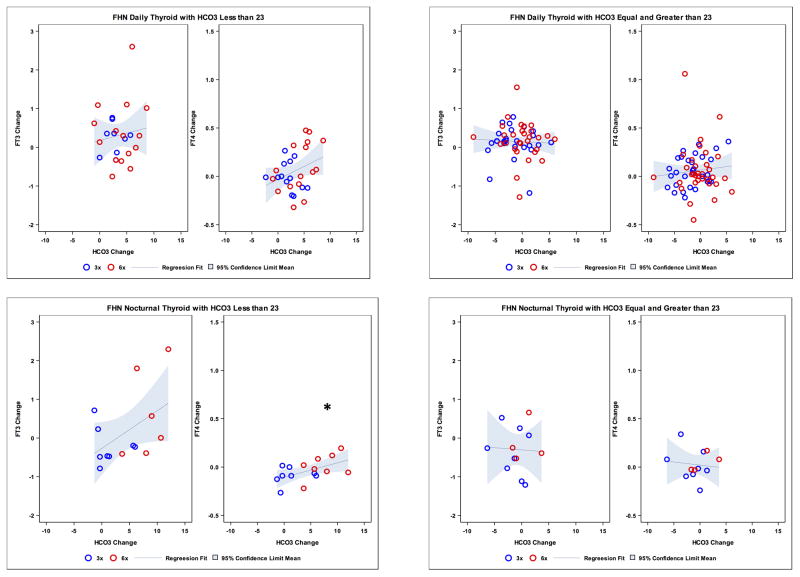
Taking into account the variation of bicarbonate levels over time with dialysis treatment, we further examined whether there was an association between change in serum bicarbonate and change in thyroid hormone levels. In the Daily Trial, overall changes in FT3 and FT4 concentration were not significantly associated with changes in bicarbonate (r = 0.09, P = 0.44 and r = 0.13, P = 0.21, respectively; Figure 1 upper panel), and no relationships were seen after adjusting for changes in enPCR. When restricting analyses to those with baseline metabolic acidosis (bicarbonate level < 23 mMol/L, in the Daily Trial), changes in FT3 and FT4 levels were not significantly associated with changes in bicarbonate (r = 0.13, P = 0.54 and r = 0.33, P = 0.08, respectively; Figure 2, upper panel), and no associations were seen after adjusting for changes in enPCR. In the Nocturnal Trial, the change in FT3 concentration was significantly associated with change in bicarbonate (r = 0.44, P = 0.02), but change in FT4 concentration was not (r = 0.07, p = 0.73; Figure 1, lower panel). However, these relationships were not significant after adjusting for enPCR. Additional stratification in this Nocturnal subset based on baseline bicarbonate level < 23 mMol/L showed no association between change in FT3 and change in bicarbonate (r = 0.48, P = 0.08), while an association was seen between change in FT4 and change in bicarbonate concentration (r = 0.54, P = 0.03; Figure 2, lower panel); however the FT4 relationship was no longer significant after adjusting for change in enPCR (r = 0.45, P = 0.11). Changes in enPCR were not significant in the Daily Trial, but a significant increase in the Nocturnal Trial was noted for each treatment group (three times per week and six times per week) and overall (P < 0.001) with the overall mean enPCR increasing by 0.18 g/kg/day in the Nocturnal Trial.
Figure 1.
Scatterplot of changes in bicarbonate (HCO3−) levels (mMol/L) and changes in free 3,5,3′-triiodo-L-thyronine (FT3) (pg/mL) and free thyroxine (FT4) (ng/dL) levels in Daily Trial (upper panel). Scatterplot of changes in bicarbonate (HCO3−) levels (mMol/L) and changes in FT3 (pg/mL) and FT4 (ng/dL) levels in Nocturnal Trial (lower panel). FHN, Frequent Hemodialysis Network.
*p = 0.02
Figure 2.
Upper panel: Scatterplot of changes in bicarbonate (HCO3−) levels (mMol/L) and changes in 3,5,3′-triiodo-L-thyronine (FT3) (pg/mL) and free thyroxine (FT4) (ng/dL) levels in Daily Dialysis Trial according to HCO3− less than 23 (left side) and equal and greater than 23 (right side). Lower panel: Scatterplot of changes in bicarbonate (HCO3−) levels (mMol/L) and changes in FT3 (pg/mL) and FT4 (ng/dL) levels in Nocturnal Dialysis Trial according to HCO3− less than 23 (left side) and equal and greater than 23 (right side). FHN, Frequent Hemodialysis Network.
*P = 0.03
Discussion
Hypothyroidism is one of the most common endocrine abnormalities accompanying chronic renal failure.2,3,14,15 Baseline acid-base status varies among patients with dialysis dependent kidney failure and, as expected, acid-base homeostasis is responsive to the intensity of renal replacement therapy.16,17 In the present study, we examined chronic hemodialysis patients with endogenous thyroid function (lack of thyroid hormone supplementation and/or overtly elevated thyroid stimulating hormone levels) to more specifically analyze the relationship of serum bicarbonate with thyroid function within this clinical subset. We found that while changes in serum bicarbonate concentration were not correlated with changes in thyroid hormone levels in the Daily Trial, the observed associations between change in FT3 and FT4 and changes in serum bicarbonate observed in the Nocturnal Trial (albeit not consistent) were not independent of changes in enPCR. These findings suggest that the observed relationships between acid base status and thyroid function in the Nocturnal Trial, if present, may be modulated by changes in protein-calorie malnutrition, as reflected in enPCR as a marker for dietary protein intake, since dietary protein contributes to acid load. We also noted that compared to the Nocturnal Trial, participants in the Daily Trial had higher serum bicarbonate levels at study initiation and demonstrated no relationship between changes in thyroid hormones and changes in bicarbonate concentration, with or without adjusting for enPCR.
A previous study reported that an increase in serum bicarbonate, achieved through oral bicarbonate supplementation, resulted in improvement in both FT3 and FT4 levels in predialysis patients with metabolic acidosis.6 However, this investigation was not accompanied by significant changes in protein catabolic rate, so that the effect of a change in bicarbonate could be evaluated as a single parameter. 6 Moreover, a study conducted in healthy individuals18 after induction of metabolic acidosis describes a mechanism at the level of the thyroid, rather than a central (hypothalamo-pituitary axis) inhibitory effect.19 After correction of metabolic acidosis, thyroid function improved progressively, with the circulating hormones reaching normal levels.5 Similarly, in children with new onset of type 1 diabetes, decreased serum FT3 levels and FT3/FT4 ratio resolved coordinately with resolution of the metabolic acidosis,20 although the potential role of improving acidosis and effects on sick euthyroid status cannot be excluded.
Our study has several limitations, including the small number of participants in the Nocturnal Trial, particularly when stratifying for the presence of metabolic acidosis, which may limit the findings obtained in this cohort. We also selected patients with endogenous thyroid function, eliminating those with overt hypo- or hyperthyroidism, thus restricting our observations to thyroid hormone variations within euthyroid subjects where circulating free thyroid hormone levels tended to be in the lower range. Subjects in the Nocturnal Trial experienced a significant increase in enPCR during the trial,10 which obscured the relationship between changes in bicarbonate and changes in thyroid hormone levels. While dialysis frequency was not associated with significant changes in nutritional parameters,10 we cannot exclude the possibility that modification of protein catabolism may explain at least in part our findings. Thyroid function is potently influenced by malnutrition,19 contributing to a sick euthyroid state and reduced catabolism. Levels of FT3 are also known to manifest a circadian rhythm,21 and variation in the timing of laboratory tests may have influenced our results. Finally, serum bicarbonate levels were measured in the clinical laboratories linked to the providers associated with each dialysis center and may have resulted in greater between-subject variability in bicarbonate levels, although this would be less relevant for within subject (paired measurements) comparisons.
In summary, these data derived from a large randomized trial of dialysis frequency, showed that changes in bicarbonate level were inconsistently associated with nocturnal dialysis frequency and the observed relationships did not persist after adjusting for enPCR. However, the relationship of protein-calorie malnutrition, metabolic acidosis, and thyroid homeostasis in patients receiving hemodialysis may be complex. Two partially competing processes, metabolic acidosis and protein-calorie malnutrition, may contribute to reduced thyroid hormone levels in dialysis patients. A treatment paradigm that corrects both simultaneously may fail to explore the effect of either process. Further studies should be conducted in larger clinical populations of patients receiving hemodialysis, including those with endogenous thyroid hypofunction, to determine whether changes in acid base status are independently associated with changes in thyroid function and whether there are consequent effects on patient outcome.
Acknowledgments
This work was supported by The Frequent Hemodialysis Network Trials (registered at Clinical Trials.gov NCT00264758 and NCT00271999).
Funds
This study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (U01 DK066579, U01 DK066597, U01 DK066480, U01 DK066481, and ancillary study funding R01 DK076165), and the Centers for Medicare and Medicaid Services and the National Institutes of Health Research Foundation (with contributions from Amgen, Baxter, DaVita, Dialysis Clinics, Inc., Fresenius Medical Care North America, Renal Research Institute and Satellite Healthcare).
Footnotes
Conflict of interest
All the authors declare no conflict of interest
References
- 1.Muscaritoli M, Molfino A, Bollea MR, Rossi Fanelli F. Malnutrition and wasting in renal disease. Curr Opin Clin Nutr Metab Care. 2009;12:378–83. doi: 10.1097/MCO.0b013e32832c7ae1. [DOI] [PubMed] [Google Scholar]
- 2.Rhee CM, Alexander EK, Bhan I, Brunelli SM. Hypothyroidism and mortality among dialysis patients. Clin J Am Soc Nephrol. 2013;8:593–601. doi: 10.2215/CJN.06920712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160:503–15. doi: 10.1530/EJE-08-0837. [DOI] [PubMed] [Google Scholar]
- 4.Koo HM, Kim CH, Doh FM, et al. The impact of low triiodothyronine levels on mortality is mediated by malnutrition and cardiac dysfunction in incident hemodialysis patients. Eur J Endocrinol. 2013;169:409–19. doi: 10.1530/EJE-13-0540. [DOI] [PubMed] [Google Scholar]
- 5.Wiederkehr MR, Kalogiros J, Krapf R. Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1190–97. doi: 10.1093/ndt/gfh096. [DOI] [PubMed] [Google Scholar]
- 6.Disthabanchong S, Treeruttanawanich A. Oral sodium bicarbonate improves thyroid function in predialysis chronic kidney disease. Am J Nephrol. 2010;32:549–56. doi: 10.1159/000321461. [DOI] [PubMed] [Google Scholar]
- 7.Đurić PS, Đurić Ž, Janković A, Tošić J, Popović J, Dimković N. Influence of hemodialysis duration per week on parameters of dialysis adequacy and cardiovascular morbidity. Med Pregl. 2014;67:385–91. [PubMed] [Google Scholar]
- 8.Maduell F, Arias M, Durán CE, et al. Nocturnal, every-other-day, online haemodiafiltration: an effective therapeutic alternative. Nephrol Dial Transplant. 2012;27:1619–31. doi: 10.1093/ndt/gfr491. [DOI] [PubMed] [Google Scholar]
- 9.Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–59. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 10.Kaysen GA, Greene T, Larive B, et al. The effect of frequent hemodialysis on nutrition and body composition: frequent Hemodialysis Network Trial. Kidney Int. 2012;82:90–9. doi: 10.1038/ki.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo JC, Beck GJ, Kaysen GA, et al. Thyroid function in end stage renal disease and effects of frequent hemodialysis. Hemodial Int. 2017 doi: 10.1111/hdi.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FHN Trial Group. Chertow GM, Levin NW, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daugirdas JT, Greene T, Rocco MV, et al. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013;83:949–58. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim VS, Fang VS, Katz AI, Refetoff S. Thyroid dysfunction in chronic renal failure. J Clin Invest. 1977;60:522–34. doi: 10.1172/JCI108804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutlay S, Atli T, Koseogullari O, Nergizoglu G, Duman N, Gullu S. Thyroid disorders in hemodialysis patients in an iodine-deficient community. Artif Organs. 2005;29:329–32. doi: 10.1111/j.1525-1594.2005.29055.x. [DOI] [PubMed] [Google Scholar]
- 16.Ok E, Duman S, Asci G, et al. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrol Dial Transplant. 2011;26:1287–96. doi: 10.1093/ndt/gfq724. [DOI] [PubMed] [Google Scholar]
- 17.Mastrangelo F, Alfonso L, Napoli M, DeBlasi V, Russo F, Patruno P. Dialysis with increased frequency of sessions (Lecce dialysis) Nephrol Dial Transplant. 1998;13:139–47. doi: 10.1093/ndt/13.suppl_6.139. [DOI] [PubMed] [Google Scholar]
- 18.Brüngger M, Hulter HN, Krapf R. Effect of chronic metabolic acidosis on thyroid hormone homeostasis in humans. Am J Physiol. 1997;272:F648–53. doi: 10.1152/ajprenal.1997.272.5.F648. [DOI] [PubMed] [Google Scholar]
- 19.Lim VS. Thyroid function in patients with chronic renal failure. Am J Kidney Dis. 2001;38:S80–4. doi: 10.1053/ajkd.2001.27410. [DOI] [PubMed] [Google Scholar]
- 20.Balsamo C, Zucchini S, Maltoni G, et al. Relationships between thyroid function and autoimmunity with metabolic derangement at the onset of type 1 diabetes: a cross-sectional and longitudinal study. J Endocrinol Invest. 2015;38:701–7. doi: 10.1007/s40618-015-0248-0. [DOI] [PubMed] [Google Scholar]
- 21.Russell W, Harrison RF, Smith N, et al. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab. 2008;93:2300–6. doi: 10.1210/jc.2007-2674. [DOI] [PubMed] [Google Scholar]