Abstract
This review summarizes evidence developed at the University of Iowa concerning the management and outcomes of developmental dysplasia of the hip beginning with the observations and analyses of Dr Arthur Steindler in the early 1900s. The strong evidence-based practice tradition established by Steindler 100 years ago continues as we critically evaluate our procedures and patient outcomes, only altering approaches when warranted by strong personal and research evidence. Our practice continues to be conservative in that we strive to produce the best environment possible for the hip to develop on its own and operate only when less invasive methods have failed.
Introduction
The University of Iowa’s contributions to the management and study of developmental dysplasia of the hip (DDH) span more than 100 years and include multiple landmark publications. Our continuous cycle of evidence review, application to practice, and outcomes assessment began in 1915 when Dr Arthur Steindler became the first chairman of the Department of Orthopaedic Surgery at the University of Iowa. His meticulous recordkeeping system and loyal patients have contributed a wealth of knowledge to support our treatment of DDH and other pediatric orthopaedic conditions. Additionally, anatomic studies of normal hips and the pathoanatomy of DDH conducted by Steindler’s pupil, and the senior author’s (SLW) mentor, Dr Ignacio V. Ponseti, improved our understanding of the biologic mechanisms responsible for what we observe in our patients.
Steindler encouraged evidence-based practice (EBP) long before EBP became the expected approach. He integrated practice and evaluation by recording pre- and posttreatment observations, cataloguing imaging studies, and following children to adulthood with serial assessments using the most “suitable and fair” [39] outcome measures of the day. If the outcomes were good, the protocol was continued. If not, he sought potential patient and treatment risk factors by reviewing his own work and the literature, only altering the protocol in the face of compelling evidence. Each major change in the treatment protocol was assessed and the cycle continued. Over the last 40 years we have continued Professor Steindler’s research cycle, seeking the best possible clinical results using the least intervention possible to obtain the desired effects.
Early Foundations
Arthur Steindler was born in the Austro-Hungarian Empire in 1878. After his medical studies in Vienna, he practiced at the University Outpatient Department for Orthopaedic Surgery directed by Adolf Lorenz. Lorenz developed eczema secondary to carbolic acid exposure, effectively ending his operative career. As a result, he practiced so-called “bloodless” manipulative treatments for many childhood disorders including DDH. Although not the first to develop such techniques [38], Lorenz was certainly the most famous and soon developed a worldwide reputation. In 1902 to 1903, Lorenz lectured and treated patients in the United States. He worked with John Ridlon, who in turn mentored Steindler when he immigrated to the United States in 1907 [5]. Steindler eventually moved to Iowa in 1913. Steindler was greatly influenced by Lorenz, Ridlon, and Vittorio Putti of the Rizzoli Institute, who also had an interest in the nonoperative treatment of DDH.
Lorenz treated DDH with traction to stretch the soft tissues, manipulation to reduce the femoral head, and a plaster cast to retain the reduction. Both reduction and retention are necessary to allow the hip “unhindered expression to its intrinsically normal growth tendencies” [17] to deepen the acetabulum and stabilize the hip. Steindler credited Putti with recognizing that better results came with early diagnosis (unfortunately rare at the time) and for advocating “abduction treatment.” For children < 1 year of age, Putti placed the hips in 45° abduction with internal rotation, thus directly aligning the femoral head with the acetabulum [30].
In 1935, Steindler et al. [39] published their first clinical series of patients with DDH. This seminal paper laid the groundwork for future DDH research at Iowa. He reported on his experience from 1915 to 1933 treating 501 hips in 387 patients. Of these, 319 hips were treated by “bloodless reduction.” His protocol included bloodless reductions in children aged < 5 years with bilateral dislocations or up to age 6 years for those with unilateral dislocation. Steindler restrained from “strenuous and over forceful reduction” [39, p 302] to prevent immediate damage to the femoral head, but “more so [to prevent] the post-reduction degenerative changes, such as osteochondritis deformans, coxa plana, osteo-arthritis and other deformities…as results of the reduction trauma” [39, p 302]. Postreduction, the hip was casted in the Lorenz “first” position (90° flexion, 90° abduction) for 3 months followed by the Lange “second” position (extension, abduction, and maximum internal rotation) for another 3 months. He reported standardized clinical and radiographic results stratified by the age at treatment and by length of followup. Good early clinical results deteriorated to fair or poor beyond 5-year followup in 25% of the patients. He astutely noted that “a painless, stable hip ten years after reduction is not an absolutely definite criterion (of success), and some of the older cases (15 years) of congenital dislocations…which have never been treated may show a painless and stable hip” [39, p 303]. In this series, 44 hips were treated openly (rare in those days) and Steindler et al. noted obstacles to reduction including capsular adhesions and constrictions, pelvifemoral contractures, acetabular tissues, and head and neck deformities. Less than satisfied with these outcomes, they concluded “…the extreme optimism concerning results in the closed method, as is shown by some German and Italian authors, does not seem to be entirely justified” [39, p 305]. Applying the fundamental tenet of all pediatric outcomes research to DDH, they stated: “…[the] results of definitive significance are given only by patients who, after reduction in childhood, are grown up to manhood and womanhood” [39].
During his residency, Ponseti reviewed the subset of Steindler’s cohort (n = 103 hips) with > 4 years of followup (range, 4-23 years) after bloodless reduction alone [26]. A positive correlation between the width of the medial wall of the acetabulum and the age at diagnosis was found along with variation in the “U shadow” (now called the tear figure or teardrop) in late-diagnosed patients. More than 50% of hips had residual dysplasia (Severin III [35] or greater), and only 46% were clinically asymptomatic. He also found a high incidence of “epiphysitis” (fragmentation of the femoral head, now known as aseptic necrosis [AVN] or proximal femoral growth disturbance [PFGD]). Ponseti attributed epiphysitis to undue pressure on the femoral head, occurring when high dislocations were not treated with prereduction traction, or when casted in > 70° abduction, both of which force the head of the femur against the acetabulum.
In response to these two critical reviews, Steindler changed his postreduction casting protocol in 1942 by decreasing immobilization to 5 months: 3 months in the Waldenström position (90° flexion, 60°-70° abduction) to prevent overstrain on the adductors and pressure on the femoral head followed by 2 months in the Lange position to reduce femoral anteversion. After cast removal, he used “controlled functional treatment,” constraining hips through an abduction bar full-time for 2 to 6 months (full extension, 50°-60° abduction, and < 75° external rotation) and then part-time for 3 years. Concerned that weightbearing before development of a deepened acetabulum would lead to subluxation, he limited walking to < 6 hours per day during the first year out of the cast.
In 1959, Ponseti (now in charge of the pediatric hip service) evaluated the impact of Steindler’s changes in 64 patients treated initially before the age of 4 years (range of followup, 8-16 years) [29]. Patients < 1 year old with a positive Ortolani sign (“preluxation,” as Dr Ponseti would have described it) were treated with an abduction brace and/or a Frejka pillow alone; dislocations were treated with closed reduction. Of these, 95% were classified as Severin I or II at followup. Noting this, Ponseti stated the “problems of (DDH) must be solved in the nurseries and not in the operating rooms” [29, p 840]. Of the 1- to 2-year-old patients treated with closed reduction, 78% had good anatomic results (Severin I or II) compared with 57% of those treated between 2 and 4 years of age when “… the opportunity for the normal development of a hip joint has passed” [29, p 842]. Additionally, more older patients developed epiphysitis and/or underwent open reduction or osteotomy as a result of recurrent subluxation.
In light of this review, Ponseti continued his current treatment for young preluxed or subluxed hips. However, the results in dislocations, especially epiphysitis, merited adjustments. Concluding (as did Trueta, Salter, and others) that epiphysitis was the result of pressure on the cartilaginous femoral head, he decreased the degrees of abduction in the first cast, used 2 to 3 weeks of prereduction traction for high dislocations, and added subcutaneous adductor tenotomy when “muscle appeared excessively shortened.” The addition of adductor tenotomy (between 1944 and 1959) was the first move away from bloodless closed reduction. Ponseti continued to monitor his and Steindler’s patients closely and adjusted his methods based on his own observations and on the research of others. By 1966, he eliminated the Lange position and the abduction bar, instead casting in the Waldenström position for 4 months, and then maintained that position with an abduction brace full-time with gradual weaning over a period of 2 to 3 years [27].
Subsequent clinical evaluations of these protocols were reported by Ishii and Ponseti [11], Lindstrom et al. [18], and Malvitz and Weinstein [20]. We are currently analyzing radiographic and self-reported functional and quality-of-life data as well as the incidence of THA or other procedures in 120 patients at a mean of 43 years after closed reduction. Other studies have combined the Steindler/Ponseti data set with patients of the senior author (SLW) treated by closed and open reductions [1, 7, 13], reflecting almost 100 years of DDH treatment at this institution.
Normal Hip Growth and Development and Abnormal Pathoanatomy
Research at our institution goes well beyond case series. In 1978, Ponseti published a now classic paper on normal growth and development of the hip [28] and defined the pathologic anatomy seen in DDH [10, 28]. We now recognize the majority of pathology in DDH is on the acetabular side. Femoral-side changes are secondary to persistent anteversion and pressure on the femoral head cartilage resulting from articulation with the peripheral acetabulum or ilium in the subluxated or dislocated position. Acetabular development is limited by the amount of prereduction cartilage damage secondary to pressure from the dislocated femoral head and/or neck pushing against the peripheral acetabular cartilage.
The typical dysplastic hip has a ridge in the superoposterior and inferior aspects of the acetabulum. This ridge or neolimbus is composed of very cellular hyaline cartilage [6, 12, 25]. The femoral head glides over this ridge, in and out of the acetabulum, to produce the palpable sensation known as the Ortolani sign. The labrum in most patients with newborn DDH is everted. Older studies [34, 37] refer to the limbus (a hypertrophied inverted labrum) as an obstacle to reduction. Our studies suggest a true limbus is a rare pathologic structure only occurring in antenatal teratologic dislocations or after failed closed reduction where tissue had been forced into the acetabulum [12, 42]. The peripheral acetabular tissue may ossify differently in DDH than in normal hips, but remains critical to acetabular development and must be left intact during any surgical procedure.
Accessory centers of ossification in the periphery of the acetabular cartilage may appear up to 10 years after reduction [28]. Accessory centers are rare in normal hips and, if present, are not usually evident before the age of 11 years. The timing and prevalence of these ossifications in DDH presumably reflect the degree of prereduction cartilage damage. In 180 hips, 7% treated before age 1 year developed accessory ossifications compared with 30% reduced between 1 and 2 years of age and 44% reduced after the age of 2 years [28]. Postreduction radiographs should be routinely evaluated for accessory centers, because their presence indicates the continued potential for adequate acetabular remodeling.
While evaluating arthrograms of patients undergoing treatment for residual dysplasia, we observed that all femoral heads were fully covered by acetabular cartilage. The apparent acetabular dysplasia seen on radiographs in DDH is not a “deficiency” as often suggested, but a biologic failure of ossification of the acetabulum. In young patients, this failure of development is usually anterior. However, in older patients with DDH, ossification failure may be anterior, posterior, or global [22].
Biomechanical Studies of Hip Dysplasia
We next attempted to answer the question of why some hips, despite concentric reduction, develop residual dysplasia and proceed to subluxation and subsequent degenerative joint disease (DJD). We pursued a biomechanical approach through two related studies. The first [9] followed Legal’s [16] approach to estimating the effective articular contact stress using 431 serial pre- and postreduction radiographs and clinical data from 84 patients [18]. A time-dependent cumulative pressure exposure parameter, accounting for both the intensity and the length of exposure, was derived. The strongest relationship among the Severin classification, presence of DJD, and Iowa hip ratings was found when contact pressure was characterized as the number of years of pressure beyond a 2-MPa threshold. Of the 147 hips with < 10 years of overpressure, 81% were classified as Severin I/II and 90% of 21 hips with > 10 years overpressure were classified as Severin III/IV. The odds of developing overpressure in hips with PFGD were two times that of hips without a growth disturbance. This may have been the first objective estimation of intrinsic hip cartilage pressure tolerance.
This model assumed spatially uniform articular cartilage stress [21]. Using the same data set, two additional models were derived, both of which assumed nonuniform contact stress (nonuniform Legal and Brinckman models [3]) to see which assumption led to a stronger association with outcomes. The tolerance thresholds most predictive of Severin grade were 2 MPa in the Brinckman and 4.5 MPa in the nonuniform Legal model. These studies quantify the relationship between the dose (pressure over time) and response (subluxation and DJD) in hips with DDH, providing biomechanical evidence to support aggressive treatment of residual dysplasia.
Studies and Responses to Obstacles to Reduction
In the late 1970s, after noting that 60% of patients in our closed reduction series had developed PFGD (later reported in 1994 [20]), we postulated the high incidence of PFGD resulted from using the cartilaginous femoral head to overcome obstacles to reduction. This led us to compare what we saw on the arthrogram with what we observed during open reduction after failed closed reduction [12]. The arthrogram accurately diagnosed large ligamentum teres, hourglass constrictions of the capsule, and prominent transverse ligaments, but not necessarily the shape of the head, size of the acetabulum, or enlargement of the transverse acetabular ligament. The ligamentum teres cast a shadow over the femoral head, causing it to appear falsely flattened, whereas soft tissue interposition (a large pulvinar or ligamentum teres or a tight anteromedial capsule or iliopsoas tendon) caused the acetabulum to appear falsely small on the arthrogram.
We concluded that the main extraarticular obstacles to reduction were contracted and shortened adductor longus and the iliopsoas. Intraarticular obstacles, in decreasing order of importance, include the anteromedial joint capsule, enlarged ligamentum teres, and the transverse acetabular ligament. The peripheral acetabular cartilage (neolimbus) was not found to obstruct the reduction, and because it is composed of hyaline/epiphyseal cartilage (as mentioned previously), it should never be excised, instead being left in place to contribute to acetabular development.
Studies on Open Reduction
Before 1970, we had been using the anterior Smith Peterson approach, but we were not satisfied with our ability to directly visualize or approach certain obstacles to reduction. We adapted the medial approach described by Ludloff [19] by making a transverse instead of longitudinal incision (for cosmetic reasons) and leaving the capsule open instead of suturing it. We section the adductors whenever they restrict wide abduction, and we routinely section the iliopsoas tendon and excise the ligamentum teres. A large neolimbus is often encountered, appearing on the arthrogram as an inverted labrum, but as noted, this is never an obstacle to reduction and therefore not removed [12, 28]. Patients are casted in 90° to 110° flexion and 40° to 60° abduction for 10 to 12 weeks followed by an abduction brace, at first full-time and then part-time for 1 to 2 years.
Our first report on this approach included 17 patients (22 hips) at an average 42 months after surgery (range, 26-36 months) [42]. Two subluxations occurred, and one underwent repeat open reduction followed by derotation osteotomy. The fraction of hips with PFGD was 10% using Salter’s criteria [31]. The acetabular index (AI) decreased quickly during the first year, and by 4 years postreduction, it averaged < 20° regardless of the age of the patient.
Finding early success, we continued this approach and later evaluated 76 patients (93 hips) at an average age of 11 years (range, 4-23 years) [23]. The fraction of hips developing PFGD [31] was 43% compared with 60% in our closed series. Of these, 24% were Type II, 14% Type III, and 3% Type IV [4].
Studies of Proximal Femoral Growth Disturbance
Many of the protocol changes over the years have been in response to unacceptably high rates of PFGD. We prefer to use the term PFGD, rather than AVN, because no pathology reports have been published documenting necrotic bone in the femoral head. Combining the Steindler/Ponseti database with more recent closed and open reductions (n = 229) [7], we reported on 29 hips in 22 patients with Type III PFGD. The disturbance was apparent 5 to 19 months after reduction. The odds of developing a Type III PFGD were three times greater in high dislocations (Tönnis Grade 4 [40]) relative to Grades 2 or 3. At skeletal maturity, 90% of the hips were Severin III/IV compared with 35% of controls. Degenerative joint disease almost certainly develops in Severin III/IV hips, and even at skeletal maturity, DJD was present in 24% of Type III hips. It follows, then, that high dislocations, even in younger patients, should be treated early and aggressively with an anterior approach, open reduction, and concurrent procedures such as femoral shortening osteotomy.
In contrast to Type III, the anatomic outcomes of hips with Type II PFGD (lateral growth arrest tilting the head into valgus) are less ominous although not as predictable [13]. We documented acetabular development over an average of 21 years (range, 10-55 years) in 48 patients (58 hips) with Type II PFGD. Lateral tilting of the epiphysis was noted between 4 and 10 years of age. Serial radiographs did not suggest any consistent, early patterns of change in the physes predicting development of growth arrest. At followup, 59% were classified as Severin I/II. By 6 to 8 years of age (frequently before the development of lateral tilt), differences in the mean acetabular angle, acetabular quotient, acetabular roof angle, and percent femoral head coverage were noted between the Severin I/II and Severin III/IV hips. The lower percentage of Severin III/IV classification seen in Type II relative to Type III PFGD (41% versus 90%) is most likely the result of the relatively late development of Type II PFGD, by which time the majority of acetabular development is complete. Thus, the relationship between the femoral head and the acetabulum may be relatively normal, resulting in a better long-term prognosis.
Animal studies in the 1980s support the changes in casting position made by Steindler and Ponseti to prevent PFGD [15, 33]. Today, hips are casted in the safer “human” position (100° flexion, 45° abduction, neutral rotation). Although traction was advocated [8, 24] and used early in our department to facilitate closed treatment and decrease the risk of PFGD, our 1997 review identified no high-level evidence supporting its use and we have since abandoned it [41].
Clinical Studies of Hip Development
Observation of hip development after reduction reveals the adequacy of initial treatment and alerts clinicians to the potential indication for secondary procedures. We developed studies to address both qualitative and quantitative measures of hip development to guide these critical treatment decisions.
In 1978, Ishii and Ponseti [11] focused on acetabular development in 32 patients (40 hips) treated with closed reduction before the age of 1 year (average followup, 15 years; range, 8–29 years). Hips were divided into those reduced before and after 6 months. The AI decreased rapidly in the first year after reduction in all hips and slowly continued for another 4 years in those reduced before 6 months, but continued until skeletal maturity in those reduced up to age 1 year. Overall, the acetabulum developed normally, resulting in an AI of < 22° in all patients; 31 (78%) were Severin I/II; eight of the Severin III/IV hips had PFGD. There were no redislocations, although in some high dislocations, severe abduction was used to retain the reduction. We now proceed to open reduction when the hip is not stable in mild abduction.
Next came a larger study of closed reductions treated between 1940 and 1968 [18]. Those with pre- and postreduction radiographs and at minimum followup to 8 years of age (n = 185 hips in 148 patients) were included. Graphing the AI over time, we found remodeling continued for at least 6 years after reduction if the head remained reduced as judged by normal centering ratios [36]. The final AI was > 24° at 8 years after reduction in 17% of hips; the femoral heads of these hips were superiorly displaced after reduction and they continued to subluxate over time. Based on these findings, we concluded that “persistence in the closed method of treatment…was ill-advised” [18, p 116] and better outcomes may have been achieved if we had proceeded instead to open reduction with removal of any obstacles blocking the acetabulum.
As noted by Ponseti in 1944, the longer the dislocation exists, the wider the U shadow (teardrop figure) and the acetabular floor [26]. In the mid-1990s, using lead markers and a cadaver pelvis, we defined the anatomic components of the teardrop and conducted a three-dimensional analysis under varying degrees of pelvic rotation [32]. This was followed by a study of 45 patients treated with closed reduction after the age of 6 months and followed to skeletal maturity [2]. The teardrops of dysplastic hips (Severin IV) were more likely to be V-shaped than U-shaped and were wider at the superior aspect than in normal controls. The acetabular floor thickness (AFT) progressively widened over time and was wider at 10 years of age in the dislocated hips, confirming Ponseti’s observations.
Noting the lack of evidence-based indications for secondary procedures, we created statistical models predicting the Severin classification at maturity after closed or open reduction to (1) find early predictors of residual dysplasia; and (2) generate guidance for the use and timing of secondary procedures [1]. We first established the validity of the Severin classification at skeletal maturity as a surrogate outcome for longer term risk of DJD by determining that a Severin III or IV hip at skeletal maturity had double the odds of undergoing THA. Working backward, we then created models predicting the Severin classification at skeletal maturity. As suspected, older age at reduction was highly associated with the risk of developing a Severin III/IV hip. The probability of a Severin III/IV outcome increases from 20% when the hip is reduced at 7 months, to 35% at 18 months, and 50% at 26 months. A strong relationship also exists between the early AI and the AFT and the Severin classification at maturity. The AI no longer improved after 4 to 5 years postreduction in Severin III/IV hips compared with 6 years in Severin I/II hips. Additionally, a difference between the mean AI in the Severin I/II and Severin III/IV hips was found as early as 1 year after reduction, indicating different rates of acetabular development. Using the AI as a prognostic test for joint dysplasia (Severin III/IV), it would be positive if the AI was > 32° at 2 years, > 28° at 4 years, or > 26° at 6 years after reduction (Fig. 1). The diagnostic accuracy of these models ranged from 75% to 84%, becoming more accurate with increasing age.
Fig. 1.
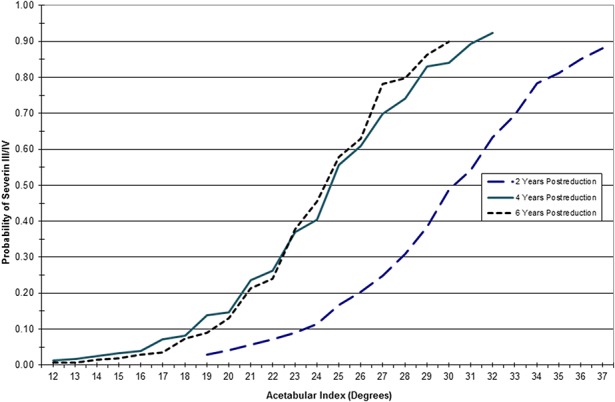
Graph shows the AI at 2, 4, and 6 years postreduction and the probability of developing a Severin III/IV hip.
Long-term Followup of Closed Reductions
We are fortunate to have access to a large number of patients willing to participate in serial studies of their disease and treatment outcomes. The latest published functional and radiographic review of patients treated from 1938 to 1969 included 119 patients (152 hips) at an average 31 years of age (range, 16-56 years) [20]. All had been treated with closed reduction initially or after failed treatment with an abduction splint. Pre- and postreduction radiographs were evaluated. The main endpoints of interest included the Severin classification and the presence of DJD and PFGD. Function was assessed using the Iowa Hip Rating (IHR) [14]. The IHR summarizes pain, function, gait, motion, and deformity and is scored from 0 to 100 points, with 100 indicating absence of hip disability.
The average IHR was 91; 75% of hips were rated as excellent (90-100 points) and 13% as poor (≤ 69 points). Most patients were pain-free. Of hips reduced before the age of 6 months, 91% had an excellent IHR compared with 76% reduced after this. Decreased functional ratings were highly related to acetabular dysplasia (measured by the center-edge angle, acetabular angle, and percent coverage of the femoral head) as well as having a nonspherical femoral head.
PFGD was noted in 36% of hips reduced before 6 months of age compared with 64% of those treated later. That said, the very young femoral head cartilage may be particularly vulnerable. Of those with any PFGD, we observed a higher prevalence of Type III (64%) in the < 6-month treatment group compared with 27% in those treated at a later age. Degenerative changes were noted more often in hips with a growth disturbance than without (53% versus 28%), although the prevalence of severe DJD was the same (16% versus 15%).
Our goal of a concentric reduction was met in 82% of hips (defined by normal lateral and superior centering ratios immediately postreduction) [36]. Concentric reduction was associated with lower rates of PFGD, better anatomic results (Severin I/II), lower rates of DJD, and higher functional ratings than less centered hips. Reductions accompanied by adductor tenotomy were also associated with a lower risk of DJD. These results support the following chain of events: an uncentered femoral head results from difficult or unsuccessful reduction. The cartilage of the head and the acetabulum can be damaged before, during, or after reduction. Damage to the head leads to PFGD; damage to the acetabulum leads to failure of ossification; both contribute to residual dysplasia and an increase in contact stress over time, resulting ultimately in the development of DJD [9, 21].
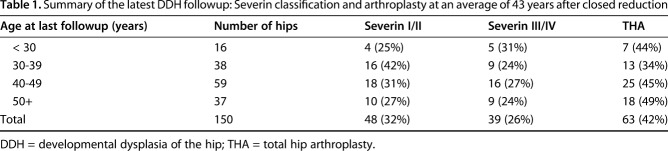
We have recently begun analysis of 120 patients 10 years after the last followup by Malvitz and Weinstein [20], now at an average of 43 years after closed reduction (range, 19-69 years). Overall, 32% (of 150 hips) were classified as Severin I/II and 43% have undergone arthroplasty (Table 1). Of the 63 arthroplasties, 68% were performed in hips initially treated at ≥ 18 months of age. Note that only 10% of hips had any acetabular or femoral procedures after the initial closed reduction. In light of these results, we conclude that most patients with late-diagnosed DDH can be managed successfully well into adulthood after minimal intervention.
Table 1.
Summary of the latest DDH followup: Severin classification and arthroplasty at an average of 43 years after closed reduction
Current Treatment Protocols
The protocols and research of Steindler, Ponseti, and now of our own patients over the past 40 years form the basis for our current treatment of DDH. The majority of children with DDH diagnosed < 1 year of age can be successfully treated by closed means: (1) a Pavlik harness for those < 6 months; and (2) closed reduction in the operating room when the hip remains unreduced despite treatment with a Pavlik harness or for children between 6 and 12 months of age when the harness has a low likelihood of success. Closed reduction is routinely accompanied by adductor tenotomy and release of the extraarticular obstacles (adductor longus and the iliopsoas) by sectioning through an anteromedial approach. If anatomic reduction is obtained and documented by arthrogram, a cast is applied in the “human position” to the ankle for the involved hip and above the knee for the “noninvolved hip.” After 12 months of age, the chance of successful closed treatment steadily decreases. Thus, the closer the child is to 18 months of age, the more likely open reduction will be required, which we do through the anteromedial approach. Older children and those with high dislocations are treated using the anterior approach. Our series suggest that > 70% of hips treated with open reduction alone will undergo satisfactory acetabular remodeling, resulting in a Severin I or II classification; hence, no concurrent secondary procedures are included [1, 23]. We follow children every 3 to 6 months watching for qualitative improvement in the teardrop, the acetabular development through measures of the AI and the AFT, and the appearance of accessory centers of ossification in the acetabular cartilage.
At 24 months of age, we are more likely to accompany open reduction with an acetabular procedure, femoral shortening, and occasionally anteversion correction, because the probability of persistent dysplasia in this age group is approximately 50% [1]. Here, we accept the fact that we may be overtreating some hips, but believe the high probability of residual dysplasia and early development of DJD justify additional surgery. There is good evidence femoral anteversion will correct spontaneously after reduction, and therefore, we do not routinely add procedures on the femoral side. We never use traction in advance of closed or open treatment, but in high dislocations, we consider adding femoral shortening, with anteversion correction, if we feel that pulling the femoral head down appropriately would be difficult even with open treatment. In these patients, we add shortening in the hopes of decreasing the risk of PFGD.
Steindler and Ponseti emphasized continual vigilance knowing that a certain number of hips, despite excellent early results, may still have biologic failure of acetabular development. As Steindler et al. observed, “We are dealing with a congenital deformity which has a strong tendency to persist” [39]. Our data suggest the first 2 to 3 years postreduction are critical to normalization of the hip. If the AI is not decreasing into the normal range (eg, < 32° at 24 months and 28° at 48 months), we intervene with an acetabular osteotomy (most frequently a Pemberton or Salter) to prevent or delay the development of DJD.
We have continued the strong evidence-based practice tradition Arthur Steindler established 100 years ago by continually evaluating our patients and changing treatment only when warranted by strong personal and research evidence. Our practice continues to be conservative in that we strive to produce the best environment possible for the hip to develop on its own and only operate when less invasive methods have failed. This has resulted in no additional surgery beyond closed reduction for 90% of patients at an average 43-year followup, most of whom (who haven't undergone arthroplasty) continue to function at a high level and with little pain.
Acknowledgments
We thank the generous patients who contributed their time and medical data so that we could better understand the natural history and treatment outcomes of DDH. We also thank all of the physicians and researchers who worked before us, and beside us, to create the work summarized here. Lastly, we acknowledge and thank our mentors, Arthur Steindler and Ignacio V. Ponseti.
Footnotes
A note from the Editor-in-Chief: We are pleased to present to readers of Clinical Orthopaedics and Related Research® the winner of this year’s Nicolas Andry Award. This award, presented by the Association of Bone and Joint Surgeons®, highlights “…a body of musculoskeletal research that has significantly contributed to orthopaedic knowledge, and has been conducted and published over an extended period of time.” The reader will note at once that the format of this paper deviates from the rest of the Journal, which focuses on original research; Andry award papers often tell the story of a program, and we believe this is an important one. We welcome reader feedback on all of our articles, including award papers; please send your comments to eic@clinorthop.org.
One or more of the authors (SLW, LAD) have received funding for this work from the Estate of Herb and Nancy Townsend of Wilton, Iowa, and the Joan and Phill Berger Charitable Fund.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Albinana J, Dolan LA, Spratt KF, Morcuende J, Meyer MD, Weinstein SL. Acetabular dysplasia after treatment for developmental dysplasia of the hip—implications for secondary procedures. J Bone Joint Surg Br. 2004;86:876–886. [DOI] [PubMed] [Google Scholar]
- 2.Albinana J, Morcuende JA, Weinstein SL. The teardrop in congenital dislocation of the hip diagnosed late. A quantitative study. J Bone Joint Surg Am. 1996;78:1048–1055. [DOI] [PubMed] [Google Scholar]
- 3.Brinckman P, Frobin W, Hierholzer E. Stress on the articular surface of the hip joint in healthy adults and person with idiopathic osteoarthrosis of the hip joint. J Biomech. 1981;14:149–156. [DOI] [PubMed] [Google Scholar]
- 4.Bucholz R, Ogden J. Patterns of ischemic necrosis of the proximal femur in nonoperative treated congenital hip disease. In: Sixth Open Scientific Meeting of the Hip Society. St Louis, MO, USA: CV Mosby; 1978:43–64. [Google Scholar]
- 5.Buckwalter JA. The Vienna heritage of Iowa orthopaedics. Iowa Orthop J. 2003;23:108–122. [PMC free article] [PubMed] [Google Scholar]
- 6.Cashman JP, Round J, Taylor G, Clarke NM. The natural history of developmental dysplasia of the hip after early supervised treatment in the Pavlik harness. A prospective, longitudinal follow-up. J Bone Joint Surg Br. 2002;84:418–425. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez CA, Dolan LA, Weinstein SL, Morcuende JA. Natural history of type III growth disturbance after treatment of developmental dislocation of the hip. Iowa Orthop J. 2008;28:27–35. [PMC free article] [PubMed] [Google Scholar]
- 8.Fish DN, Herzenberg JE, Hensinger RN. Current practice in use of prereduction traction for congenital dislocation of the hip. J Pediatr Orthop. 1991;11:149–153. [DOI] [PubMed] [Google Scholar]
- 9.Hadley NA, Brown TD, Weinstein SL. The effects of contact pressure elevations and aseptic necrosis on the long-term outcome of congenital hip dislocation. J Orthop Res. 1990;8:504–513. [DOI] [PubMed] [Google Scholar]
- 10.Ippolito E, Ishii Y, Ponseti IV. Histologic, histochemical, and ultrastructural studies of the hip joint capsule and ligamentum teres in congenital dislocation of the hip. Clin Orthop Relat Res. 1980;146:246–258. [PubMed] [Google Scholar]
- 11.Ishii Y, Ponseti IV. Long-term results of closed reduction of complete congenital dislocation of the hip in children under one year of age. Clin Orthop Relat Res. 1978;137:167–174. [PubMed] [Google Scholar]
- 12.Ishii Y, Weinstein SL, Ponseti IV. Correlation between arthrograms and operative findings in congenital dislocation of the hip. Clin Orthop Relat Res. 1980;153:138–145. [PubMed] [Google Scholar]
- 13.Kim HW, Morcuende JA, Dolan LA, Weinstein SL. Acetabular development in developmental dysplasia of the hip complicated by lateral growth disturbance of the capital femoral epiphysis. J Bone Joint Surg Am. 2000;82:1692–1700. [DOI] [PubMed] [Google Scholar]
- 14.Larson CB. Rating scale for hip disabilities. Clin Orthop Relat Res. 1963;31:85–93. [PubMed] [Google Scholar]
- 15.Law EG, Heistad DD, Marcus ML, Mickelson MR. Effect of hip position on blood flow to the femur in puppies. J Pediatr Orthop. 1982;2:133–137. [DOI] [PubMed] [Google Scholar]
- 16.Legal H. Introduction to the biomechanics of the hip. In: Tönnis D, ed. Congenital Dysplasia and Dislocation of the Hip. Berlin, Germany: Springer-Verlag; 1987. [Google Scholar]
- 17.Lesky E. The Vienna Medical School of the 19th Century. Baltimore, MD, USA: Johns Hopkins University Press; 1967. [Google Scholar]
- 18.Lindstrom JR, Ponseti IV, Wenger DR. Acetabular development after reduction in congenital dislocation of the hip. J Bone Joint Surg Am. 1979;61:112–118. [PubMed] [Google Scholar]
- 19.Ludloff K. The open reduction of the congenital hip dislocation by an anterior incision. Am J Orthop Surg. 1913;10:438–454. [Google Scholar]
- 20.Malvitz TA, Weinstein SL. Closed reduction for congenital dysplasia of the hip. Functional and radiographic results after an average of thirty years. J Bone Joint Surg Am. 1994;76:1777–1792. [DOI] [PubMed] [Google Scholar]
- 21.Maxian TA, Brown TD, Weinstein SL. Chronic stress tolerance levels for human articular cartilage: two nonuniform contact models applied to long-term follow-up of CDH. J Biomech. 1995;28:159–166. [DOI] [PubMed] [Google Scholar]
- 22.Millis MB, Murphy SB. Use of computed tomographic reconstruction in planning osteotomies of the hip. Clin Orthop Relat Res. 1992;274:154–159. [PubMed] [Google Scholar]
- 23.Morcuende JA, Meyer MD, Dolan LA, Weinstein SL. Long-term outcome after open reduction through an anteromedial approach for congenital dislocation of the hip. J Bone Joint Surg Am. 1997;79:810–817. [DOI] [PubMed] [Google Scholar]
- 24.Morel G. The treatment of congenital dislocation and subluxation of the hip in the older child. Acta Chir Scand. 1975;46:364–399. [PubMed] [Google Scholar]
- 25.Ortolani M. Congenital hip dysplasia in the light of early and very early diagnosis. Clin Orthop Relat Res. 1976;119:6–10. [PubMed] [Google Scholar]
- 26.Ponseti I. Causes of failure in the treatment of congenital dislocation of the hip. J Bone Joint Surg Am. 1944;26:775–792. [Google Scholar]
- 27.Ponseti IV. Non-surgical treatment of congenital dislocation of the hip. J Bone Joint Surg Am. 1966;48:1392–1403. [PubMed] [Google Scholar]
- 28.Ponseti IV. Morphology of the acetabulum in congenital dislocation of the hip. Gross, histological and roentgenographic studies. J Bone Joint Surg Am. 1978;60:586–599. [PubMed] [Google Scholar]
- 29.Ponseti IV, Frigerio ER. Results of treatment of congenital dislocation of the hip. J Bone Joint Surg Am. 1959;41:823–846 passim. [PubMed] [Google Scholar]
- 30.Putti V. Early treatment of congenital dislocation of the hip. J Bone Joint Surg Am. 1929;11:798–809. [Google Scholar]
- 31.Salter RB, Kostuik J, Dallas S. Avascular necrosis of the femoral head as a complication of treatment for congenital dislocation of the hip in young children: a clinical and experimental investigation. Can J Surg. 1969;12:44–61. [PubMed] [Google Scholar]
- 32.Samani DJ, Weinstein SL. The pelvic tear-figure—a 3-dimensional analysis of the anatomy and effects of rotation. J Pediatr Orthop. 1994;14:650–659. [PubMed] [Google Scholar]
- 33.Schoenecker PL, Lesker PA, Ogata K. A dynamic canine model of experimental hip dysplasia. Gross and histological pathology, and the effect of position of immobilization on capital femoral epiphyseal blood flow. J Bone Joint Surg Am. 1984;66:1281–1288. [PubMed] [Google Scholar]
- 34.Severin E. Contribution to the knowledge of congenital dislocation of the hip joint. Late results of closed reduction and arthrographic studies of recent cases. Acta Chir Scand. 1941;Supplementum 63. [Google Scholar]
- 35.Severin E. Congenital dislocation of the hip; development of the joint after closed reduction. J Bone Joint Surg Am. 1950;32:507–518. [PubMed] [Google Scholar]
- 36.Smith WS, Badgley CE, Orwig JB, Harper JM. Correlation of postreduction roentgenograms and thirty-one-year follow-up in congenital dislocation of the hip. J Bone Joint Surg Am. 1968;50:1081–1098. [PubMed] [Google Scholar]
- 37.Somerville EW. Open reduction in congenital dislocation of the hip. J Bone Joint Surg Br. 1953;35:363–371. [DOI] [PubMed] [Google Scholar]
- 38.Steindler A. The development of present-day knowledge of congenital dislocation of the hip. J Bone Joint Surg Am. 1948;30:525. [PubMed] [Google Scholar]
- 39.Steindler A, Kulowski J, Freund E. Congenital dislocation of the hip. Statistical analysis. JAMA. 1935;104:302–307. [Google Scholar]
- 40.Tönnis D. General radiography of the hip joint. In: Tönnis D, ed. Congenital Dysplasia and Dislocation of the Hip in Children and Adults. New York, NY, USA: Springer; 1987:100–142. [Google Scholar]
- 41.Weinstein SL. Traction in developmental dislocation of the hip. Is its use justified? Clin Orthop Relat Res. 1997;338:79–85. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein SL, Ponseti IV. Congenital dislocation of the hip. Open reduction through a medial approach. J Bone Joint Surg Am. 1979;61:119–124. [PubMed] [Google Scholar]