Abstract
Background
Measuring alpha-defensin concentrations in synovial fluid may help to diagnose periprosthetic joint infection (PJI). There are two commercially available methods for measuring alpha-defensin in synovial fluid: the enzyme-linked immunosorbent assay-based Synovasure® alpha-defensin immunoassay, which gives a numeric readout within 24 hours, and the Synovasure lateral flow test, which gives a binary readout within 20 minutes. There is no compilation of the existing literature to support the use of one of these two tests over the other.
Questions/purposes
Does the immunoassay or the lateral flow test have better diagnostic value (sensitivity and specificity) in diagnosing PJI?
Methods
We followed PRISMA guidelines and identified all studies on alpha-defensin concentration in synovial fluid as a PJI diagnostic marker, indexed to April 14, 2017, in PubMed, JSTOR, Google Scholar, and OVID databases. The search retrieved 1578 records. All prospective and retrospective studies on alpha-defensin as a PJI marker (PJI classified according to the criteria of the Musculoskeletal Infection Society) after THA or TKA were included in the analysis. All studies used only one of the two commercially available test methods, but none of them was comparative. After excluding studies with overlapping patient populations, four studies investigating the alpha-defensin immunoassay and three investigating the lateral flow test remained. Alpha-defensin immunoassay studies included 482 joints and lateral flow test studies included 119. The quality of the trials was assessed according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. The heterogeneity among studies was evaluated by the I2 index, indicating that the heterogeneity of the included studies was low. Pooled sensitivity, specificity, positive and negative likelihood ratios, and receiver operating curves were calculated for each method and compared with each other.
Results
The alpha-defensin immunoassay had superior overall diagnostic value compared with the lateral flow test (area under the curve, 0.98 versus 0.75) with higher sensitivity (96% [90%-98%] versus 71% [55%-83%], p < 0.001), but no difference in specificity with the numbers available (96% [93%-97%] versus 90% [81%-95%], p = 0.060).
Conclusions
Measurement of alpha-defensin in synovial fluid is a valuable complement to existing diagnostic criteria, and the immunoassay test detects PJI more accurately than the lateral flow test. The lateral flow test has lower sensitivity, making it difficult to rule out infection, but its relatively high specificity combined with the advantage of a quick response time can make it useful to rule in infection perioperatively.
Level of Evidence
Level III, diagnostic study.
Introduction
Alpha-defensins are antimicrobial peptides released by neutrophils in response to pathogens in synovial fluid. They play a central role in immune defense against pathogens, mainly by disrupting the structure of bacterial cell membranes, but they are also active against fungi and some enveloped viruses [1]. Alpha-defensins can be measured in synovial fluid and have been proposed as an indicator for periprosthetic joint infection (PJI) [6]. The two commercially available methods for measuring alpha-defensin in synovial fluid are the enzyme-linked immunosorbent assay-based Synovasure® alpha-defensin immunoassay (Zimmer Biomet, Warsaw, IN, USA), which gives a readout within 24 hours, and the Synovasure lateral flow test (Zimmer Biomet), which gives a binary perioperative readout within minutes. Immunoassays use the ability of antibodies to recognize and bind to specific antigens (in this case alpha-defensin), and they measure fluorescence intensity when a fluorescent dye is linked to the antibody. According to the manufacturer, the Synovasure alpha-defensin immunoassay has a sensitivity of 97% (95% confidence interval [CI], 86%–100%) and specificity of 96% (95% CI, 91%–99%) and was optimized to operate at a cutoff value of 5.2 mg/L of alpha-defensin (White Paper Synovasure alpha-defensin; CD Diagnostics, Claymont, DE, USA). The alpha-defensin immunoassay has also the ability and the advantage of measuring C-reactive protein (CRP) in synovial fluid simultaneously, which is optimized to operate at a cutoff of 3 mg/L (White Paper Synovasure alpha-defensin; CD Diagnostics). In contrast, the lateral flow test is a paper-based platform that detects the presence or absence of alpha-defensin in three drops of a diluted aspirate placed on a test device [10].
The alpha-defensin lateral flow test has a reported positive agreement of 100% (59 of 59) and negative agreement of 96% (175 of 183) with the immunoassay (Synovasure Alpha Defensin Lateral Flow Test Kit), but other evaluations give varying levels of sensitivity and specificity for the two methods, indicating that agreement between the two may not be quite as high as previously proposed [9]. The lateral flow test has the advantage of immediate availability of results, and it would be of high clinical value to know whether the rapidly available result of the lateral flow test is comparable with the results from the alpha-defensin immunoassay in terms of sensitivity and specificity. Because the available evidence on these two tests has not yet been investigated, we designed the present meta-analysis.
Therefore, we asked: (1) Does the immunoassay or the lateral flow test have better diagnostic value (sensitivity and specificity) in diagnosing PJI?
Materials and Methods
Search Strategy and Criteria
We searched all studies indexed in PubMed, JSTOR, Google Scholar, and the OVID database to April 14, 2017, using the search terms arthroplasty, alpha defensin, PJI, Synovasure, and lateral flow test (Table 1). Published abstracts and conference proceedings on the relevant topics were not identified.
Table 1.
Search algorithm used in PubMed, JSTOR, Google Scholar, and the OVID database to discover included studies in this meta-analysis

Studies of patients in all age groups with a diagnosis of PJI in the hip or knee were included. No language restrictions were applied, but all available studies were written in English. The only nonreviewed literature that was finally included, as a reference, was the Synovasure alpha-defensin white paper (White Paper Synovasure alpha-defensin; CD Diagnostics).
Included studies investigated the diagnostic value of alpha-defensin measured either by the Synovasure alpha-defensin immunoassay or by the lateral flow test. We applied no restriction to the underlying diagnostic PJI algorithm, but all included studies classified PJI according to the Musculoskeletal Infection Society (MSIS) criteria. Both prospective and retrospective study designs were included, and all studies evaluated only one of the test methods; thus, none was comparative. All studies included patients with total joint arthroplasty of the hip or knee, except one study by Sigmund et al. [14] in which one arthroplasty of the elbow, one of the shoulder, and one total femoral replacement were included. Our search resulted in 1578 records. Titles and abstracts were screened by two independent reviewers (HE, JN) to identify useful articles, and 17 original articles matched the purpose of this study. Of these, four were reviews and therefore excluded. Thirteen studies were thus found suitable for full-text assessment (Fig. 1). The reference lists of these studies were screened for additional records eligible for inclusion in this study, but no additional original articles were identified [11]. One group of authors published several studies that potentially included overlapping patient populations, but only one of these studies was included [5]. This study was chosen among others as a result of the cutoff value for alpha-defensin that was defined before initiation of the study (to 5.2 mg/L).
Fig. 1.

The flowchart shows the search strategy and the number of identified studies on alpha-defensin concentration in synovial fluid as a PJI diagnostic marker, assessed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Four of the seven included studies investigated the alpha-defensin immunoassay, which together contained 482 joints: (1) a prospective diagnostic study by Deirmengian et al. [5] (level of evidence: II) included 149 patients and joints with clinical signs of PJI in the hip or knee. This study included patients with systemic inflammatory disease and ongoing antibiotic treatment. The cutoff value for the alpha-defensin test in this study was 5.5 mg/L, and the test showed a sensitivity of 97% (95% CI, 86%–100%) and specificity of 96% (95% CI, 90%–99%). (2) A retrospective diagnostic study performed by Bingham et al. [2] (level of evidence: III) included 57 patients (61 joints) with clinical signs of PJI in the hip or knee. In this study, the cutoff value for a positive alpha-defensin test was 7.72 mg/L. The test showed a sensitivity of 100% (95% CI, 79%–100%) and specificity of 95% (95% CI, 83%–99%). (3) A retrospective diagnostic study by Frangiamore et al. [7] (level of evidence: III) included 102 patients (116 joints) scheduled for revision surgery of the hip or knee resulting from PJI. The cutoff value for a positive alpha-defensin test was 5.2 mg/L. The test showed a sensitivity of 100% (95% CI, 86%–100%) and specificity of 98% (95% CI, 90%–100%). (4) Bonanzinga et al. [3] performed a prospective diagnostic study (level of evidence: I) including 156 patients and joints. The test showed a sensitivity of 97% (95% CI, 92%–99%) and specificity of 97% (95% CI, 92%–99%).
Three of the seven included studies investigated the lateral flow test, which together contained 119 joints: (1) a therapeutic study by Kasparek et al. [9] (level of evidence: III) included 40 patients and joints who underwent revision surgery as a result of aseptic loosening, polyethylene wear with osteolysis, suspected chronic PJI, instability, and/or stiffness. The lateral flow test showed a sensitivity of 67% (95% CI, 35%–89%) and specificity of 93% (95% CI, 75%–99%). (2) A study performed by Sigmund et al. [14] included 49 patients and joints, 47 with suspected PJI in hip or knee, one with an infected total shoulder prosthesis, and one with an infected elbow prosthesis. The lateral flow test showed a sensitivity of 69% (95% CI, 46%–92%) and specificity of 94% (95% CI, 86%–100%). (3) A study by Suda et al. [15] on the lateral flow test included 28 patients (30 joints) with suspected PJI who underwent revision surgery for PJI or aseptic loosening. In this study, the assay showed a sensitivity of 77% and specificity of 82% (no CIs given). Several authors of the included studies on the lateral flow test pointed out the small size of their cohorts and the lack of comparison with the immunoassay method.
Assessment of Study Quality
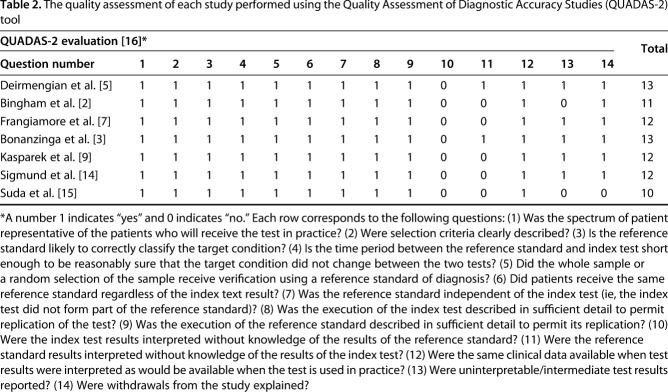
Quality assessment of each study was performed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [16] (Table 2). Maximum score is 14, and the included studies had a mean score of 12 (range, 10–13). This indicates that the studies included in this meta-analysis were generally of good quality.
Table 2.
The quality assessment of each study performed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool
Data Collection and Abstraction
Data extracted from included studies were total numbers of patients; distributions of mean age and gender; types of investigated joints; cutoff values for alpha-defensin concentrations; numbers of true-positive, false-positive, true-negative, and false-positive findings; and sensitivity and specificity including CIs. Included studies on the immunoassay reported a total of 482 joints; 112 were classified as condition-positive (with PJI according to MSIS criteria) and 370 as condition-negative. Included studies on the lateral flow test reported a total of 119 joints. Of these, 38 were classified as condition-positive and 81 as condition-negative. Data were divided into four groups each for the immunoassay and the lateral flow test: true-positive, false-positive, true-negative, and false-negative. The immunoassay identified 109 true-positives, 357 true-negatives, 13 false-positives, and three false-negatives (Table 3). The lateral flow test identified 27 true-positives, 74 true-negatives, seven false-positives , and 11 false-negatives (Table 4).
Table 3.
Predicted outcome and true condition in the Synovasure immunoassay population
Table 4.
Predicted outcome and true condition in the Synovasure lateral flow test population
Statistical Analysis
CIs for sensitivity and specificity of each study are Clopper-Pearson intervals. For both meta-analyses, we used a bivariate random-effects model to pool sensitivity, specificity, and respective 95% CIs [12]. Because there were no observed false-negatives in one immunoassay study, 0.5 was added to all values in that meta-analysis. From the estimated model, we calculated positive likelihood ratio (posLR), negative likelihood ratio (negLR), diagnostic odds ratio (DOR), and respective 95% CI using the Zwindermann and Bossuyt elaborated Monte Carlo Markov chain procedure [17]. Using the hierarchical summary receiver operating characteristic (HSROC) model of Rutter and Gatsonis, we constructed the summary ROC, the summary point, and the 95% confidence region around the summary operating point [13] and calculated the respective area under the curve (AUC; Fig. 2). Between-study heterogeneity was assessed separately for sensitivity and specificity in each study using the I2 statistic [8]. I2 takes values between 0% and 100%, and a value of 0% means that all variability in effect size estimates is the result of sampling error within studies and that heterogeneity is low. The I2 statistics for the sensitivity and specificity of the alpha-defensin immunoassay were 0% (95% CI, 0–0%) and 0% (95% CI, 0–50.9%), respectively. The I2 statistics for the sensitivity and specificity of the lateral flow test were 0% (95% CI, 0–40.2%) and 3.4% (95% CI, 0–89.9%), respectively. The observed values of I2 in these meta-analyses indicated that there was little heterogeneity present, but the CIs hint at the possibility of substantial heterogeneity. All statistical tests were two-sided and p values < 0.05 were considered statistically significant.
Fig. 2.
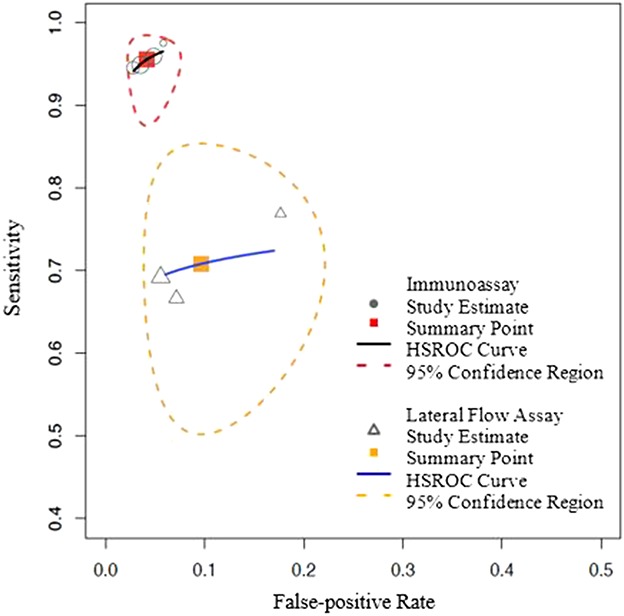
The figures show HSROC curves for both the Synovasure immunoassay and Synovasure lateral flow test, presenting the study estimates, summary point, and the 95% confidence region around the summary operating point.
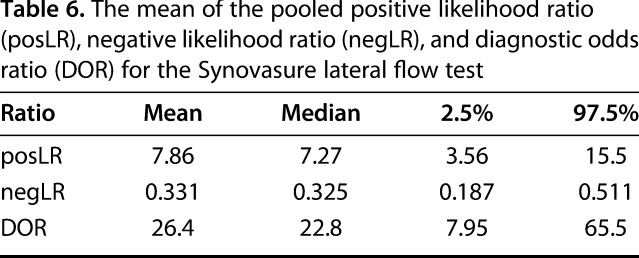
The mean of the pooled posLR for the alpha-defensin immunoassay was estimated at 24 (95% CI, 14–38), the mean of the pooled negLR was estimated at 0.05 (95% CI, 0.02–0.1), and the DOR was 574 (95% CI, 178–1400; Table 5). The mean of the pooled posLR for the lateral flow test was estimated at 8 (95% CI, 4–16), the mean of the pooled negLR was estimated at 0.03 (95% CI, 0.2–0.5), and the DOR was 26 (95% CI, 8–66; Table 6).
Table 5.
The mean of the pooled positive likelihood ratio (posLR), negative likelihood ratio (negLR), and diagnostic odds ratio (DOR) for the Synovasure immunoassay
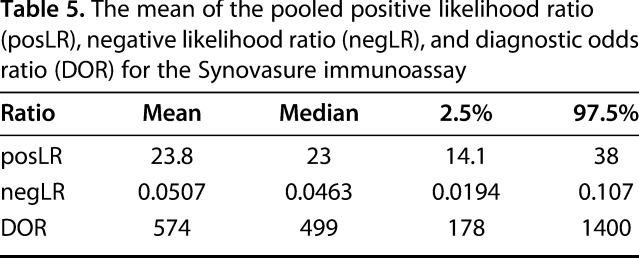
Table 6.
The mean of the pooled positive likelihood ratio (posLR), negative likelihood ratio (negLR), and diagnostic odds ratio (DOR) for the Synovasure lateral flow test
All analyses were performed using the meta and mada packages in R statistics software (Version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
The alpha-defensin immunoassay had superior overall diagnostic value compared with the lateral flow test (AUC, 0.98 versus 0.75) with higher sensitivity (96%, 95% CI, 90-98% versus 71%, 95% CI, 55-83%; p < 0.001) but no difference in specificity (96%, 95% CI, 93-97% versus 90%, 95% CI, 81-95%; p = 0.060) (Figs. 3, 4).
Fig. 3.

The figure shows the sensitivity and specificity of the Synovasure alpha-defensin immunoassay given in the included studies, including CIs.
Fig. 4.
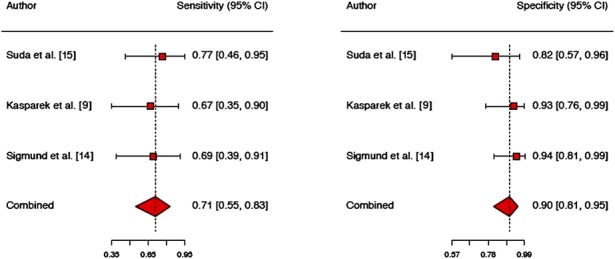
The figure shows the sensitivity and specificity of the Synovasure lateral flow test given in the included studies, including CIs.
Discussion
Measurements of alpha-defensin concentrations in synovial fluid have been proposed as a diagnostic marker of PJI with the potential to improve over the accuracy of currently available diagnostic criteria. Two methods for measuring alpha-defensin in synovial fluid are commercially available: (1) the alpha-defensin immunoassay, which gives a numeric readout within 24 hours; and (2) the lateral flow test, which gives a binary readout within minutes. Until now, the two methods have not been systematically compared, and given the difference in response times, it would be important to know whether the test with a rapidly available result is as reliable as the test that gives its readout the subsequent day. We therefore carried out a meta-analysis of the available literature describing sensitivity and specificity of either of these two tests and calculated pooled sensitivity, specificity, and the AUC separately for both tests. In summary, the alpha-defensin immunoassay has a high sensitivity and specificity in diagnosing PJI, whereas the lateral flow test has a lower sensitivity than the alpha-defensin immunoassay. As a result of its high specificity in combination with the advantage of the quick readout that could be used perioperatively, the lateral flow test may still be useful to primarily rule in a potential infection, but not to rule it out.
There are several weaknesses in this meta-analysis. With only seven studies included in the final analysis, the total number of investigated patients is relatively low, and thus the no-difference findings regarding specificity of the two tests need to be interpreted cautiously. The absence of difference regarding specificity between the two tests implies that the tests may differ in specificity, although we were unable to detect such differences at the predetermined level of statistical significance. The cutoff values in the included studies varied but the differences were small, and the cutoff values in these studies are equal to or above the cutoff limit that is defined by the manufacturer; thus, we considered that this issue did not affect our results. Our results are susceptible to spectrum bias, because diagnostic tests may have different accuracy in patients with conditions such as systemic inflammatory disease, metallosis, and prior or ongoing antibiotic treatment. We believe this not to be a major problem, because the included studies did not vary grossly in the presence of such confounders; however, there may be an element of underreporting of such issues, and this must be considered when interpreting our results.
Pros and Cons of the Two Investigated Tests
This meta-analysis indicates that measuring alpha-defensin in synovial fluid has a place in strengthening the diagnosis of PJI. The lateral flow test has the advantage of a quick response time, and its specificity is within the range of the immunoassay, although our meta-analysis may have been underpowered to detect smaller differences in specificity than those found between the two tests. However, the sensitivity of the lateral flow test was lower than that of the immunoassay, raising doubts on the reliability of a negative lateral flow test result. The broad CIs on estimates of specificity and sensitivity in this study could perhaps be narrowed down by including larger numbers of patients in future studies, and head-to-head trials of the two tests should be performed. Measuring alpha-defensin in synovial fluid provides a valuable addition to the diagnostic armamentarium used in the context of PJI, and of the two commercially available methods to measure this parameter, the immunoassay with its overnight response time has the best diagnostic accuracy. The lateral flow test seems inferior to the immunoassay in terms of sensitivity, but approximately equal in specificity. The alpha-defensin immunoassay has the advantage of measuring CRP in synovial fluid simultaneously, optimized for a cutoff at 3 mg/L, and this increases the precision in diagnosing PJI. This application of a CRP threshold was shown to correctly reverse false-positive results to true-negative, but it does not erroneously reverse any true-positive results. Given the rapid availability of the lateral flow test result, this method could have its place in ruling in a suspected PJI intraoperatively, but it would not be reliable in ruling it out. A combination of diagnostic criteria such as those suggested by the MSIS will have to be applied to achieve the highest possible accuracy in the evaluation of patients with PJI.
Footnotes
One of the authors (SL) received advisors’ fees in the amount of less than USD 10,000 as a member of the board of infection management for Zimmer Biomet EMEA (Winterthur, Switzerland). One of the authors (NPH) received institutional support in the amount of USD 10,000 to USD 100,000 from Zimmer Biomet for an unrelated research project.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
This work was performed at Uppsala University Hospital, Uppsala, Sweden.
References
- 1.Barrera GJ, Sanchez G, Gonzalez JE. Trefoil factor 3 isolated from human breast milk downregulates cytokines (IL8 and IL6) and promotes human beta defensin (hBD2 and hBD4) expression in intestinal epithelial cells HT-29. Bosn J Basic Med Sci. 2012;12:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472:4006–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonanzinga T, Zahar A, Dütsch M, Lausmann C, Kendoff D, Gehrke T. How reliable is the alpha-defensin immunoassay test for diagnosing periprosthetic joint infection? A prospective study. Clin Orthop Relat Res. 2017;475:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deirmengian C, Hallab N, Tarabishy A, Della Valle C, Jacobs JJ, Lonner J, Booth RE. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468:2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:1439–1445. [DOI] [PubMed] [Google Scholar]
- 6.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31:456–460. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 9.Kasparek MF, Kasparek M, Boettner F, Faschingbauer M, Hahne J, Dominkus M. Intraoperative diagnosis of periprosthetic joint infection using a novel alpha-defensin lateral flow assay. J Arthroplasty. 2016;31:2871–2874. [DOI] [PubMed] [Google Scholar]
- 10.Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. [DOI] [PubMed] [Google Scholar]
- 13.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865–2884. [DOI] [PubMed] [Google Scholar]
- 14.Sigmund IK, Holinka J, Gamper J, Staats K, Böhler C, Kubista B, Windhager R. Qualitative α-defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty. Bone Joint J. 2017;99:66–72. [DOI] [PubMed] [Google Scholar]
- 15.Suda AJ, Tinelli M, Beisemann ND, Weil Y, Khoury A, Bischel OE. Diagnosis of periprosthetic joint infection using alpha-defensin test or multiplex-PCR: ideal diagnostic test still not found. Int Orthop. 2017;41:1307–1313. [DOI] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 17.Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med. 2008;27:687–697. [DOI] [PubMed] [Google Scholar]