Abstract
Background
Primary shoulder hemiarthroplasty is used to address a range of glenohumeral disorders, including fracture, arthritis, avascular necrosis, and capsulorrhaphy arthropathy; some patients with hemiarthroplasties undergo revision surgery for persistent pain or residual shoulder dysfunction. The literature does not clarify the features of the hemiarthroplasties having repeat surgery in a way that can guide surgeons’ efforts to minimize the need for revision. To help address this gap, we analyzed the characteristics of patients from our region for whom we performed surgical revision of a prior humeral hemiarthroplasty
Questions/Purposes
(1) What are the common characteristics of shoulder hemiarthroplasties having a revision? (2) What are the common characteristics of the subset of revised shoulder hemiarthroplasties that were performed for fracture? (3) What are characteristics of the subset of all revised hemiarthroplasties that were associated with glenoid bone erosion?
Methods
Data for 983 patients for whom we performed a surgical revision of any type of shoulder arthroplasty between January 1991 and January 2017 were identified in our longitudinally maintained institutional arthroplasty revision database. In each case, revision had been elected by shared patient and surgeon decision-making after consideration of the disorder, degree of compromised comfort and function, treatment alternatives, and the risks of surgery. Of these 983 patients, 359 (37%) had a revision of a prior primary hemiarthroplasty; these patients were the subjects of this investigation. In this group of patients, we investigated the patient demographics, shoulder characteristics, prerevision radiographic findings, and findings at revision surgery. No patients were excluded. The patients having revision of primary hemiarthroplasties had severe loss of self-assessed shoulder comfort and function, with Simple Shoulder Test (SST) scores averaging 2.2 ± 2.2 of the maximum score of 12. The majority of these patients (81%) were women. The medical records of these 359 patients were abstracted to determine the diagnosis for the index primary hemiarthroplasty, clinical characteristics before surgery, and findings at surgical revision. One hundred twelve of the arthroplasties had been performed for fracture-related diagnoses; a subgroup analysis was performed on these patients. Two hundred seventy-three of the 359 patients (76%) had plain radiographs performed within 3 months before revision surgery that were adequate for assessing the radiographic characteristics of the glenoid, humerus, humeral component, and glenohumeral relationships; a subgroup analysis was performed on these patients. The degree of glenoid erosion was measured by a single observer in accordance with established criteria: Grade 1 is no erosion, Grade 2 is erosion limited to subchondral bone, Grade 3 is moderate erosion with medialization, and Grade 4 is medialization beyond the coracoid base. Some patients were included in both of these subgroups.
Results
Common characteristics of the revised hemiarthroplasties included female sex (81%), rotator cuff (89 of 359; 25%) or subscapularis (81 of 359; 23%) failure, problems related to prior fracture (154 of 359; 43%), glenoid erosion 125 of 359; 35%), and component malposition (89 of 359; 25%). Hemiarthroplasties performed for fracture-related problems often were associated with tuberosity malunion or nonunion (58 of 79; 73%) and decentering of the humeral component on the glenoid surface (45 of 71; 63%). Major erosion of the bony glenoid (Grade 3 or 4) was more common in decentered hemiarthroplasties (42 of 102; 41%) than for centered hemiarthroplasties (36 of 146; 25%) (Fisher’s exact p = 0.008) and more common for hemiarthroplasties positioned in valgus (28 of 50; 56%) than for those positioned in neutral or varus (40 of 188; 21%) (Fishers’ exact p < 0.0001).
Conclusions
These findings suggest that some revisions of primary hemiarthroplasties may be avoided by surgical techniques directed at centering the prosthetic humeral articular surface on the glenoid concavity using proper humeral component positioning and soft tissue balance, by avoiding valgus positioning of the humeral component, and by managing glenoid disorders with a primary glenohumeral arthroplasty rather than a hemiarthroplasty alone. When durable security of the subscapularis, rotator cuff, and tuberosities is in question, the surgeon may consider a reverse total shoulder arthroplasty.
Level of Evidence
Level III, therapeutic study
Introduction
Primary shoulder hemiarthroplasty is a commonly used procedure for the treatment of various shoulder disorders [9, 11, 12, 14, 17, 18, 26, 28, 32, 33, 35, 42, 45]. In the treatment of a proximal humeral fracture, primary hemiarthroplasty is considered when displaced fracture fragments cannot be treated with internal fixation or when there is concern regarding head collapse. In the treatment of shoulder arthritis, capsulorrhaphy arthropathy, or avascular necrosis, a primary hemiarthroplasty may be performed if there is minimal glenoid disorder, if the shoulder is too tight to admit a glenoid component, if there is insufficient bone stock to support a prosthetic glenoid, if the patient wishes to avoid the risks and limitations associated with a glenoid component, or if the surgeon is not comfortable with performing another type of shoulder arthroplasty. In some cases, hemiarthroplasty may not provide the comfort and function desired by the patient. Unsatisfactory outcomes can be associated with pain and stiffness, rotator cuff or tuberosity failure, weakness, component loosening, instability, or erosion of the bony glenoid surface—any of which may cause sufficient symptoms to indicate revision surgery. Much of the available information regarding surgical revisions comes from published case series of hemiarthroplasties from the practices of experienced surgeons [2, 3, 7, 19-21, 25, 27, 30, 34, 37, 43, 50]. These series point to the problem of glenoid bone erosion after hemiarthroplasty, but do not provide information regarding the factors associated with it. These publications also point to the difficulty in using hemiarthroplasty in the management of fracture-related problems, but do not provide detailed information regarding the characteristics of shoulders having revision of a primary hemiarthroplasty for fracture. Given the widespread use of hemiarthroplasty [46], it is important to identify the characteristics of revised hemiarthroplasties to help guide efforts to improve the clinical outcomes of this procedure and to minimize the need for hemiarthroplasty revision. To complement publications reporting outcomes of case series of primary hemiarthroplasties performed at individual centers, we studied patients having revisions at a regional referral center of some of the hemiarthroplasties performed by the surgeons in our region. In that the characteristics of the population of hemiarthroplasties from which the revisions were drawn are not known, such an investigation cannot provide odds or risk ratios for different possibly contributing factors; however, it can reveal the demographic, clinical, radiographic and surgical characteristics typical of shoulders having surgical revision of a primary hemiarthroplasty.
We asked: (1) What are the common characteristics of shoulder hemiarthroplasties having a revision? (2) What are the common characteristics of the subset of revised shoulder hemiarthroplasties that were performed for fracture? (3) What are characteristics of the subset of all revised hemiarthroplasties that were associated with glenoid bone erosion?
Methods
In our practice, the indications for revision of a prior arthroplasty are (1) shoulder comfort and function that are unsatisfactory to the patient, (2) a mechanical problem that potentially could be addressed by revision surgery, (3) sufficient patient physical and emotional health for repeat surgery, and (4) consent by the patient to proceed with revision surgery after discussion with the surgeon of the risks and alternatives. Between January 1991 and January 2017, 983 patients had surgical revision at our center for shoulder arthroplasties performed by surgeons in our region. In each of these cases, revision was elected by shared patient-surgeon decision-making after consideration of the disorder, degree of compromised comfort and function, treatment alternatives, and risks of surgery. Of these 983 patients, 359 (37%) patients had previously undergone primary hemiarthroplasty; these patients were the subjects of this investigation. This retrospective observational study was approved by our institutional review board (#STUDY00001281).
The intent of this study was to identify the characteristics of shoulders with primary hemiarthroplasties undergoing surgical revision. It was not intended to evaluate the rate of hemiarthroplasty revision, the technique of revision, or the outcomes of surgical revision. Surgical revision was considered when a potentially treatable mechanical cause of unsatisfactory shoulder comfort and function could be identified and when the patient desired to proceed after a thorough discussion of the risks and alternatives. Surgical revision was not considered if a treatable mechanical cause of shoulder dysfunction could not be identified, if the patient was not understanding and accepting of the risks, or if the patient was not in sufficiently robust emotional and physical health to undergo a major surgical revision.
The medical records of these patients were reviewed to collect demographic information including age, sex, date of index arthroplasty, diagnosis at index surgery, prerevision Simple Shoulder Test (SST) scores, date of revision arthroplasty, and findings at revision surgery. The SST was used as a validated tool enabling patients to self-report their shoulder’s loss of comfort and function before revision surgery [23].
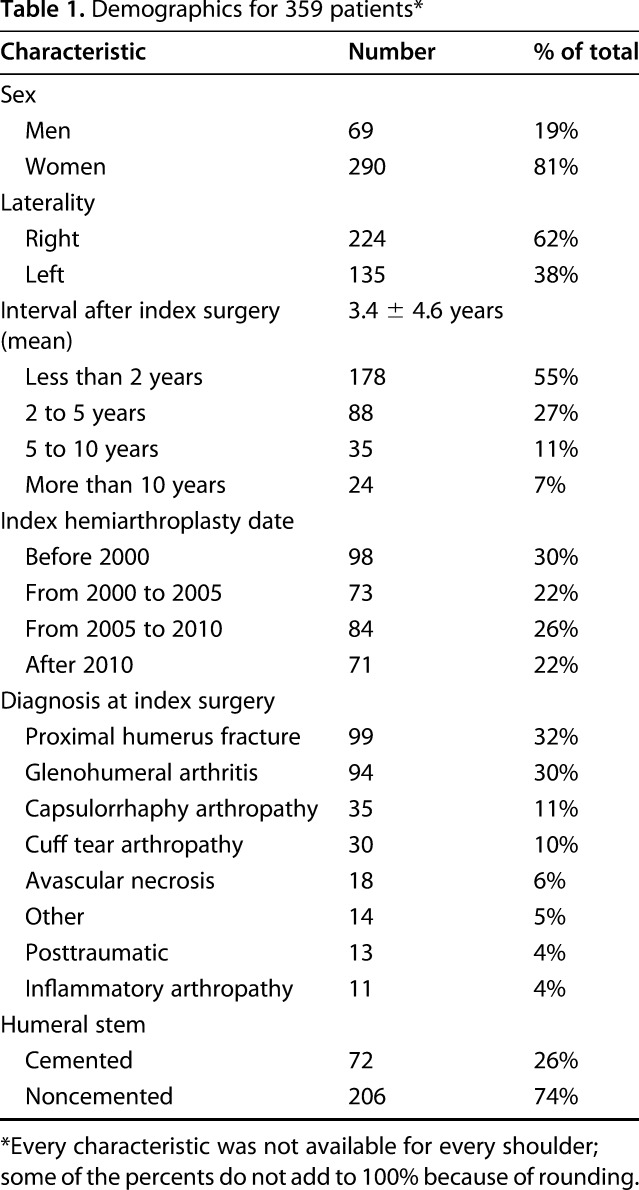
Of the 359 patients who underwent revision arthroplasty, 290 (81%) were women and 69 (19%) were men (Table 1). These patients had very low self-assessed shoulder comfort and function, with average SST scores of 2.2 ± 2.2; the maximum score on the SST is 12. The average age of the patients at index surgery was 56.9 ± 13.5 years (range, 17-87 years), and the average age at revision surgery was 60.4 ± 12.7 years (range, 22-88 years). The average interval from index arthroplasty to revision was 3.4 ± 4.6 years. The majority (55%) presented earlier than 2 years from the date of the index hemiarthroplasty. The most common diagnoses for the index hemiarthroplasty were proximal humeral fracture, malunion, or nonunion, glenohumeral arthritis, capsulorrhaphy arthropathy, cuff tear arthropathy, and avascular necrosis (Table 1).
Table 1.
Demographics for 359 patients*
From a longitudinally maintained single-center database of shoulder arthroplasty revisions performed for patients from our region, the preoperative clinical evaluation and the surgical findings for those having revision of a primary hemiarthroplasty were abstracted to identify patient (Table 1) and shoulder characteristics (Table 2).
Table 2.
Characteristics of revised primary hemiarthroplasties#
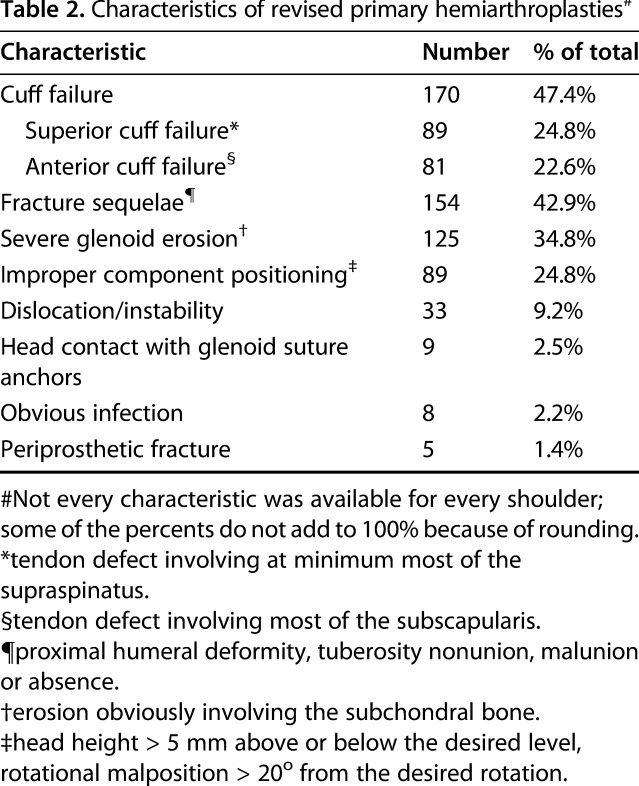
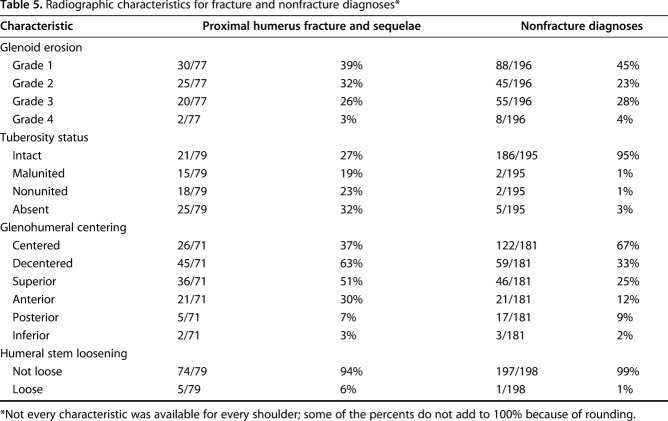
Two hundred seventy-three of the patients (76%) had radiographs performed within 3 months before revision surgery that were adequate for assessing the radiographic characteristics of the glenoid, humerus, humeral component, and glenohumeral relationships. The degree of glenoid erosion was measured on plain films by an individual observer (JEH) in accordance with the criteria described by Sperling et al. [45]. Grade 1 is no erosion, Grade 2 is erosion limited to subchondral bone, Grade 3 is moderate erosion with medialization, and Grade 4 is medialization beyond the coracoid base.
The status of the greater tuberosity was assessed as intact, malunited (>1 cm displacement from anatomic position [5]), nonunited, or absent. The angle of the neck of the humeral component with reference to the axis of the humeral canal was measured as described by Herschel et al. [20]. The humeral component was considered to be in varus if the angle was less than 40° and valgus if the angle was greater than 50°. Components between 40o and 50o are considered to be in neutral. Humeral stem radiolucencies were graded in the seven Gruen zones adapted for the shoulder [29, 38]: proximal lateral metaphysis, proximal lateral diaphysis, mid-lateral diaphysis, prosthetic tip, mid-medial diaphysis, proximal medial diaphysis, proximal medial metaphysis. A humeral component was considered loose if there was obvious subsidence [39, 48] or presence of radiolucent lines greater than 2 mm in three or more of these zones [31].
AP and superoinferior glenohumeral relationships were measured on standardized axillary and Grashey view radiographs as described previously [22]. On each of these radiographic projections, a circle was constructed congruent to the articular surface of the humeral head component. We then measured the shortest distance between this circle’s center and the perpendicular bisector of a line segment connecting the edges of the bony glenoid (anterior and posterior edges in the axillary view; superior and inferior edges in the Grashey view). This distance was divided by the diameter of the circle; this ratio was converted to a percentage. Humeral head decentering was defined as any value greater than 5%.
The answers to Question 1 were derived from all 359 cases. The answers to Question 2 were derived from the 112 of the 359 revisions that were performed after primary hemiarthroplasty for fracture or fracture sequelae. The answers to Question 3 were derived from the 273 of the 359 revisions with preoperative radiographs adequate for analysis of glenoid erosion. Some shoulders were included in the analysis for Questions 2 and 3.
Statistical Analysis
Descriptive statistics were used to describe the characteristics of these shoulders; means and SDs were presented for continuous variables, and frequencies were tabulated for categorical variables. Fishers’ exact test was used to test the relationship of major (Grades 3 and 4) glenoid erosion with humeral centering in the glenoid and valgus positioning.
Results
Common Characteristics of Revised Primary Hemiarthroplasties
Of the 359 patients, the most common characteristics of the revised hemiarthroplasties included superior or anterior cuff failure (170 of 359 patients; 47%), persistent fracture sequelae, including tuberosity malunion and nonunion (154 of 359 patients; 43%), and glenoid erosion (125 of 359 patients; 35%) (Table 2). Other features included improper component positioning (89 of 359 patients; 25%), instability (33 of 359 patients; 9%), head contact with suture anchors (nine of 359 patients; 3%), obvious infection (8 of 359 patients; 2%), and periprosthetic fracture (five of 359 patients; 1%). Some shoulders had more than one of these features. Of the 273 patients with acceptable radiographs, glenoid erosion was present in 155 of 273 patients (57%): Grade 2 in 70 of 273 patients (26%), Grade 3 in 75 of 273 patients (28%), and Grade 4 in 10 of 273 patients (3%) (Table 3; Fig. 1). One hundred four of the 273 patients (41%) showed glenohumeral decentering, with the most common direction of decentering being superior (82 of 273 patients; 30%) (Fig. 2). Malunion, nonunion, or absent tuberosities was present in 67 of 273 patients (25%). Humeral component loosening was present in six of 273 patients (2%).
Table 3.
Radiographic characteristics of failed primary hemiarthroplasties*
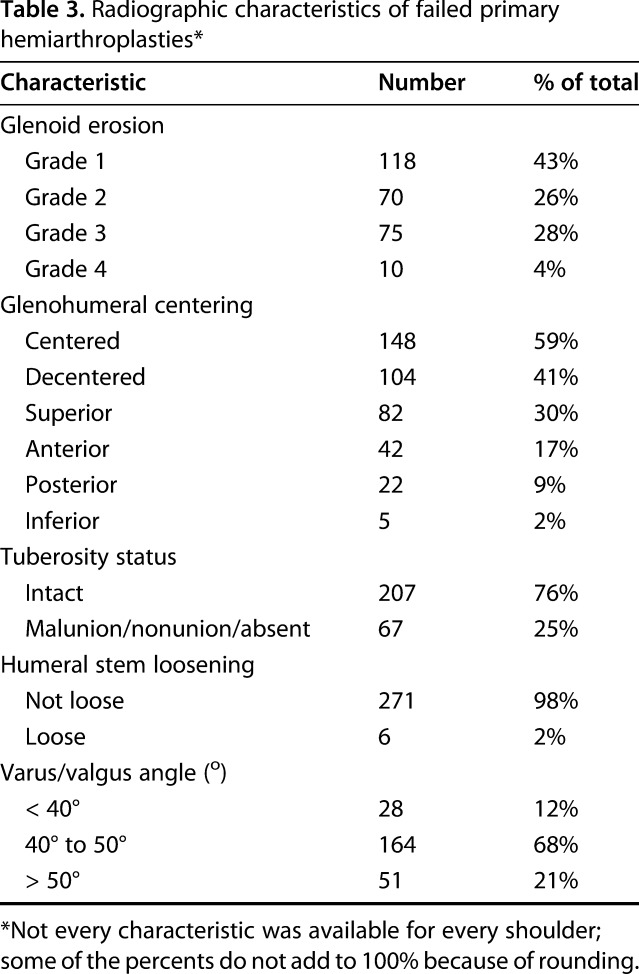
Fig. 1.
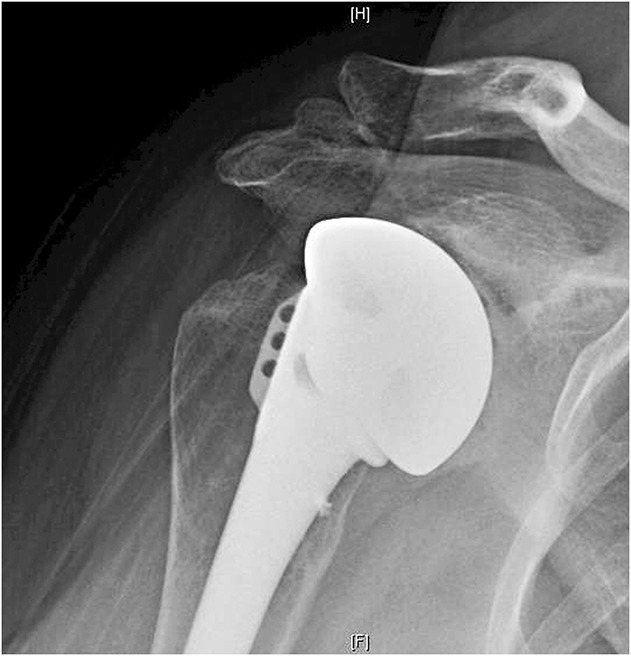
Grade 3 glenoid erosion associated with superior malposition of the humeral head is shown.
Fig. 2.
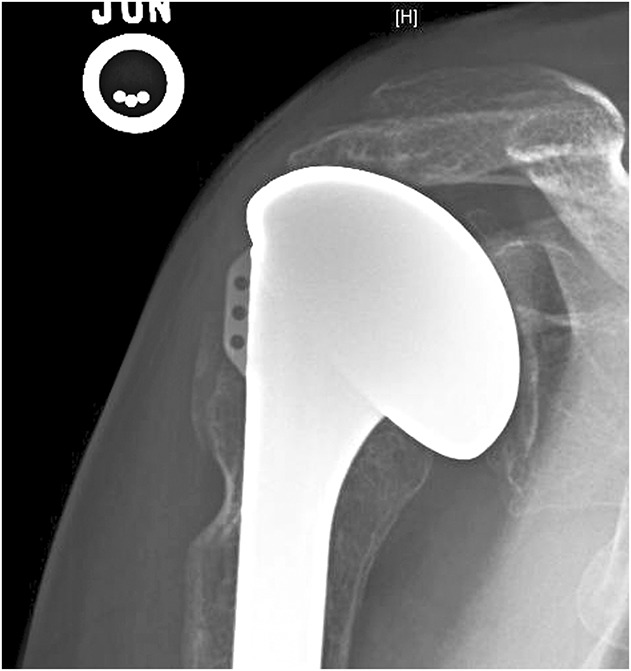
Superior malcentering of the humeral component associated with rotator cuff failure is shown.
Features of Revised Hemiarthroplasties Initially Performed for Fracture
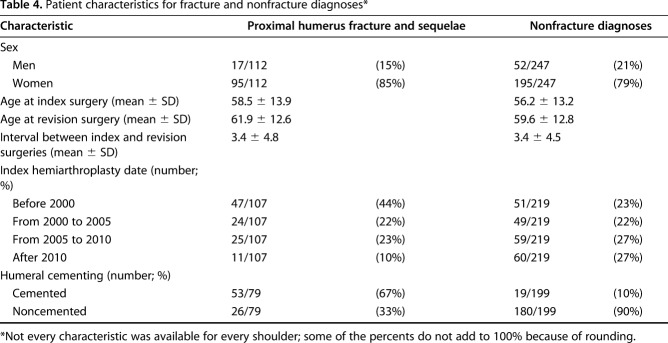
Revised primary arthroplasties performed for fracture-related diagnoses were predominantly in women. Revision typically was performed 3 years after the index procedure; the humeral component was commonly cemented (Table 4). These shoulders frequently showed failure of the tuberosity and superior or anterior decentering (Table 5).
Table 4.
Patient characteristics for fracture and nonfracture diagnoses*
Table 5.
Radiographic characteristics for fracture and nonfracture diagnoses*
Features of revised hemiarthroplasties with glenoid bone erosion
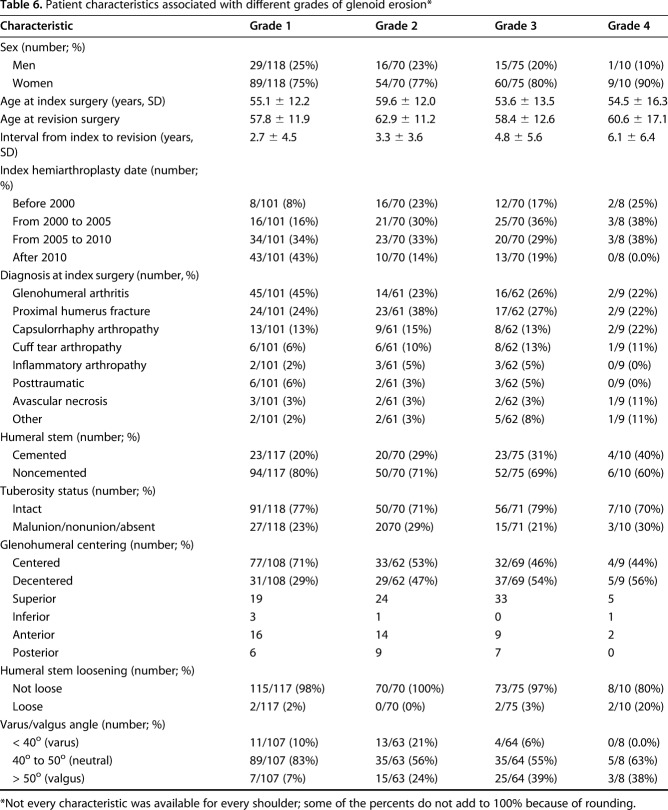
Most of the characteristics of revised primary hemiarthroplasties were not strongly associated with glenoid erosion (Table 6). However, major erosion of the bony glenoid (Grade 3 or 4) was more common in decentered hemiarthroplasties (42 of 102; 41%) than for centered hemiarthroplasties (36 of 146; 25%) (Fisher’s exact p = 0.008)) and more common for hemiarthroplasties positioned in valgus (28 of 50; 56%) than for those positioned in neutral or varus (40 of 188; 21%) (Fishers’ exact p < 0.0001). Major erosion was seen in 23% (18 of 77) of revised hemiarthroplasties performed for arthritis and 29% (19 of 66) of revised hemiarthroplasties performed for fracture (Fisher’s exact p = 0.566).
Table 6.
Patient characteristics associated with different grades of glenoid erosion*
Discussion
Hemiarthroplasty is a commonly performed procedure for shoulder disorders, including glenohumeral arthritis, avascular necrosis, capsulorrhaphy arthropathy, avascular necrosis, and proximal humeral fractures [11, 12, 14, 17, 24, 26, 28, 44, 45]. While often successful, this procedure can result in shoulders that are painful, stiff, weak or unstable [10, 41, 49]. These outcomes can cause the patient to consider revision surgery. As indicated by their SST scores at the time of presentation for revision surgery, patients who underwent revision of their hemiarthroplasties had very poor shoulder comfort and function. Our goal was to review a large number of patients from our region having revision surgery to seek modifiable factors that may enhance the outcome for primary hemiarthroplasty.
The results of this study should be viewed in light of some important limitations. First, this was a retrospective study of patients presenting for revision surgery to a regional shoulder center; therefore, our results may be different from those of studies of other patient groups. Specifically, the patients in this series were selected for revision because the surgeons in our center believed that there was a surgically manageable mechanical issue with the shoulder and that the patient was a good candidate for revision surgery. In that each shoulder was revised only after surgeon-patient shared decision-making, it is quite possible that different selections would be reached in other practices. While this study focused on patients having primary hemiarthroplasties who underwent revision surgery, it is likely that a substantial number of patients having primary hemiarthroplasties with poor clinical outcomes do not undergo revision surgery because of patient or surgeon disinclination for a second procedure; information regarding these hemiarthroplasties could not be included in our study [15]. Second, because we do not have the data on the population from which these patients were drawn, the study does not allow us to report the rates of revision or the relative risk associated with the characteristics of the patient or shoulder. Third, we did not have the opportunity to evaluate the glenoid bone quality, greater tuberosity integrity, and glenohumeral relationships before or immediately after the primary hemiarthroplasty [20]. Fourth, 24% (86 of 359) of our patients lacked prerevision radiographs of adequate quality for analysis; we do not, however, have reason to believe that this loss was selective in a way that would bias the results. Fifth, while some designs of humeral prostheses might be more prone to problems of component positioning, tuberosity stability, or glenoid erosion, we were unable to document the effect of different component designs on the nature of hemiarthroplasty revision.
Despite these limitations, this study suggests that there are at least three issues that surgeons can consider in striving for improved outcomes from primary shoulder arthroplasty: the problem of cuff failure, the special features of arthroplasty for fractures, and the problem of glenoid erosion.
Many of the revised hemiarthroplasties in this series were associated with rotator cuff deficiency noted at the time of revision. It cannot be determined with certainty whether these cuff deficiencies existed at the time of the primary hemiarthroplasty or developed subsequently owing to tissue quality rendered tenuous by subsequent age-related degeneration or injury. However, in performing a hemiarthroplasty the surgeon is in a position to directly assess the quality of the cuff tendons and their attachment to the greater tuberosity. When the cuff appears frail at the time of the primary procedure, the surgeon may consider an alternative procedure, such as a reverse total shoulder arthroplasty [50].
As observed by previous authors, a large proportion of unsatisfactory hemiarthroplasties were performed initially for fracture-related diagnoses [4, 7, 16, 36]; such cases often showed nonunited or malunited tuberosities, glenohumeral decentering, and humeral loosening. These observations indicate the difficulty of prosthetic reconstruction for a fractured proximal humerus. In this setting, implantation of the body of a hemiarthroplasty prosthesis can limit the amount of bone available for tuberosity fixation and bone-to-bone healing. The loss of the tuberosities removes important support for the humeral component and the landmarks for humeral component positioning [6, 7]. Proximal humeral fractures often are sustained by elderly women with poor bone quality [8]. Tuberosity healing problems have been associated with superior migration, stiffness, and pain [7]. Unless the tuberosities can be secured in contact with solid proximal humeral bone, the surgeon may decide to immobilize the shoulder in the hope of improving the chances for healing; this delay in mobilization may further compromise the functional outcome. These challenges of anatomic prosthetic reconstruction suggest consideration of the reverse total shoulder arthroplasty, rather than hemiarthroplasty, in the treatment of proximal humeral fractures that cannot be securely managed with internal fixation [1, 13, 40, 47].
Erosion of the glenoid bone was a prominent feature among the revised hemiarthroplasties. The presence and severity of glenoid erosion was associated with humeral head decentering and valgus positioning of the humeral component, but not with the diagnosis before the index hemiarthroplasty. Decentering may contribute to glenoid erosion by giving rise to eccentric bone loading with diminished contact area to support the humeral head force, resulting in increased glenoid bone wear. Decentering may result from implantation of a humeral hemiarthroplasty prosthesis in a shoulder with uncorrected posterior glenoid erosion—a common finding in osteoarthritis and in capsulorrhaphy arthropathy [25]. Decentering also may result from technical factors, such as inadequate soft tissue balancing or humeral component malpositioning. At the time of surgery, centering can be optimized by managing glenoid surface biconcavity, by properly orienting the humeral component, by assuring cuff integrity, by soft tissue balancing, and by the use of humeral sided balancing measures, such as an eccentric humeral head component [22]. Valgus positioning of the humeral component in the humeral shaft was associated with increased glenoid erosion as reported by Herschel et al. [20]. A valgus component position may give rise to locally increased joint pressure when the arm is in an adducted position where the inferior corner of the humeral head is pressed into the glenoid bone surface. Surgical attention to centering the humeral component in the medullary canal may help minimize valgus positioning, especially in fracture cases or in cases where a short humeral component stem is used.
Conclusions
The results of this study suggest that surgical attention to the challenge of cuff integrity and to achieving proper and secure humeral component positioning with centering of the humeral head in the glenoid are important for successful shoulder hemiarthroplasty. Some revisions of primary hemiarthroplasties may be avoided by surgical techniques directed at centering the prosthetic humeral articular surface on the glenoid concavity using proper humeral component positioning and soft tissue balance, by avoiding valgus positioning of the humeral component, and by managing glenoid disorders with a primary glenohumeral arthroplasty rather than a hemiarthroplasty alone. When durable security of the subscapularis, rotator cuff, and tuberosities is in question, the surgeon may consider a reverse total shoulder arthroplasty.
Acknowledgements
We thank Susan DeBartolo BA (Department of Orthopaedics and Sports Medicine, University of Washington) for editorial work on this manuscript.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alentorn-Geli E, Guirro P, Santana F, Torrens C. Treatment of fracture sequelae of the proximal humerus: comparison of hemiarthroplasty and reverse total shoulder arthroplasty. Arch Orthop Trauma Surg. 2014;134:1545–1550. [DOI] [PubMed] [Google Scholar]
- 2.Andres-Cano P, Galan A, Arenas J, Del Aguila B, Guerado E. Results of uncemented hemiarthroplasty as primary treatment of severe proximal humerus fractures in the elderly. Eur J Orthop Surg Traumatol. 2015;25:273–280. [DOI] [PubMed] [Google Scholar]
- 3.Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23:791–799. [DOI] [PubMed] [Google Scholar]
- 4.Bell JE, Leung BC, Spratt KF, Koval KJ, Weinstein JD, Goodman DC, Tosteson AN. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beredjiklian PK, Iannotti JP, Norris TR, Williams GR. Operative treatment of malunion of a fracture of the proximal aspect of the humerus. J Bone Joint Surg Am. 1998;80:1484–1497. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P, Chuinard C, Le Huec JC, Walch G, Trojani C. Proximal humerus fracture sequelae: impact of a new radiographic classification on arthroplasty. Clin Orthop Relat Res. 2006;442:121–130. [DOI] [PubMed] [Google Scholar]
- 7.Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11:401–412. [DOI] [PubMed] [Google Scholar]
- 8.Boileau P, Winter M, Cikes A, Han Y, Carles M, Walch G, Schwartz DG. Can surgeons predict what makes a good hemiarthroplasty for fracture? J Shoulder Elbow Surg. 2013;22:1495–1506. [DOI] [PubMed] [Google Scholar]
- 9.Bonnevialle N, Melis B, Neyton L, Favard L, Mole D, Walch G, Boileau P. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745–751. [DOI] [PubMed] [Google Scholar]
- 10.Brorson S, Salomonsson B, Jensen SL, Fenstad AM, Demir Y, Rasmussen JV. Revision after shoulder replacement for acute fracture of the proximal humerus. Acta Orthop. 2017:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:1947–1956. [DOI] [PubMed] [Google Scholar]
- 12.Clinton J, Franta AK, Lenters TR, Mounce D, Matsen FA., 3rd Nonprosthetic glenoid arthroplasty with humeral hemiarthroplasty and total shoulder arthroplasty yield similar self-assessed outcomes in the management of comparable patients with glenohumeral arthritis. J Shoulder Elbow Surg. 2007;16:534–538. [DOI] [PubMed] [Google Scholar]
- 13.Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95:2050–2055. [DOI] [PubMed] [Google Scholar]
- 14.Edwards TB, Kadakia NR, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg. 2003;12:207–213. [DOI] [PubMed] [Google Scholar]
- 15.Eichinger JK, Miller LR, Hartshorn T, Li X, Warner JJ, Higgins LD. Evaluation of satisfaction and durability after hemiarthroplasty and total shoulder arthroplasty in a cohort of patients aged 50 years or younger: an analysis of discordance of patient satisfaction and implant survival. J Shoulder Elbow Surg. 2016;25:772–780. [DOI] [PubMed] [Google Scholar]
- 16.Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:557–563. [DOI] [PubMed] [Google Scholar]
- 17.Feeley BT, Fealy S, Dines DM, Warren RF, Craig EV. Hemiarthroplasty and total shoulder arthroplasty for avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2008;17:689–694. [DOI] [PubMed] [Google Scholar]
- 18.Gadea F, Alami G, Pape G, Boileau P, Favard L. Shoulder hemiarthroplasty: outcomes and long-term survival analysis according to etiology. Orthop Traumatol Surg Res. 2012;98:659–665. [DOI] [PubMed] [Google Scholar]
- 19.Hawi N, Tauber M, Messina MJ, Habermeyer P, Martetschlager F. Anatomic stemless shoulder arthroplasty and related outcomes: a systematic review. BMC Musculoskelet Disord. 2016;17:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herschel R, Wieser K, Morrey ME, Ramos CH, Gerber C, Meyer DC. Risk factors for glenoid erosion in patients with shoulder hemiarthroplasty: an analysis of 118 cases. J Shoulder Elbow Surg. 2017;26:246–252. [DOI] [PubMed] [Google Scholar]
- 21.Hettrich CM, Weldon E, 3rd, Boorman RS, Parsons IM. 4th, Matsen FA 3rd. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg Am. 2004;86:1446–1451. [DOI] [PubMed] [Google Scholar]
- 22.Hsu JE, Gee AO, Lucas RM, Somerson JS, Warme WJ, Matsen FA., 3rd Management of intraoperative posterior decentering in shoulder arthroplasty using anteriorly eccentric humeral head components. J Shoulder Elbow Surg. 2016;25:1980–1988. [DOI] [PubMed] [Google Scholar]
- 23.Hsu JE, Russ SM, Somerson JS, Tang A, Warme WJ, Matsen FA., 3rd Is the Simple Shoulder Test a valid outcome instrument for shoulder arthroplasty? J Shoulder Elbow Surg. 2017;26:1693–1700. [DOI] [PubMed] [Google Scholar]
- 24.Leung B, Horodyski M, Struk AM, Wright TW. Functional outcome of hemiarthroplasty compared with reverse total shoulder arthroplasty in the treatment of rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21:319–323. [DOI] [PubMed] [Google Scholar]
- 25.Levine WN, Fischer CR, Nguyen D, Flatow EL, Ahmad CS, Bigliani LU. Long-term follow-up of shoulder hemiarthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2012;94:e164. [DOI] [PubMed] [Google Scholar]
- 26.Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality-of-life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis: a prospective, randomized trial. J Bone Joint Surg Am. 2005;87:2178–2185. [DOI] [PubMed] [Google Scholar]
- 27.Lucas RM, Hsu JE, Gee AO, Neradilek MB, Matsen FA., 3rd Impaction autografting: bone-preserving, secure fixation of a standard humeral component. J Shoulder Elbow Surg. 2016;25:1787–1794. [DOI] [PubMed] [Google Scholar]
- 28.Mather RC, 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT., 3rd Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:325–334. [DOI] [PubMed] [Google Scholar]
- 29.Matsen FA, 3rd, Iannotti JP, Rockwood CA., Jr Humeral fixation by press-fitting of a tapered metaphyseal stem: a prospective radiographic study. J Bone Joint Surg Am. 2003;85-A:304–308. [DOI] [PubMed] [Google Scholar]
- 30.Matsen FA, 3rd, Russ SM, Vu PT, Hsu JE, Lucas RM, Comstock BA. What factors are predictive of patient-reported outcomes? A prospective study of 337 shoulder arthroplasties. Clin Orthop Relat Res. 2016;474:2496–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLendon PB, Schoch BS, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the pegged glenoid component in shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2017;26:1469–1476. [DOI] [PubMed] [Google Scholar]
- 32.Melis B, Bonnevialle N, Neyton L, Levigne C, Favard L, Walch G, Boileau P. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21:342–349. [DOI] [PubMed] [Google Scholar]
- 33.Pastor MF, Kaufmann M, Gettmann A, Wellmann M, Smith T. Total versus hemiarthroplasty for glenohumeral arthritis according to preoperative glenoid erosion. Orthop Rev (Pavia). 2015;7:5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puskas GJ, Meyer DC, Lebschi JA, Gerber C. Unacceptable failure of hemiarthroplasty combined with biological glenoid resurfacing in the treatment of glenohumeral arthritis in the young. J Shoulder Elbow Surg. 2015;24:1900–1907. [DOI] [PubMed] [Google Scholar]
- 35.Raiss P, Edwards TB, Deutsch A, Shah A, Bruckner T, Loew M, Boileau P, Walch G. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96:e54. [DOI] [PubMed] [Google Scholar]
- 36.Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85:1215–1223. [DOI] [PubMed] [Google Scholar]
- 37.Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA., 3rd Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20:609–615. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Sotelo J, O'Driscoll SW, Torchia ME, Cofield RH, Rowland CM. Radiographic assessment of cemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10:526–531. [DOI] [PubMed] [Google Scholar]
- 39.Schnetzke M, Coda S, Raiss P, Walch G, Loew M. Radiologic bone adaptations on a cementless short-stem shoulder prosthesis. J Shoulder Elbow Surg. 2016;25:650–657. [DOI] [PubMed] [Google Scholar]
- 40.Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures: a blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23:1419–1426. [DOI] [PubMed] [Google Scholar]
- 41.Singh JA, Sperling JW, Cofield RH. Risk factors for revision surgery after humeral head replacement: 1,431 shoulders over 3 decades. J Shoulder Elbow Surg. 2012;21:1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somerson JS, Neradilek MB, Service BC, Hsu JE, Russ SM, Matsen FA., 3rd Clinical and radiographic outcomes of the ream-and-run procedure for primary glenohumeral arthritis. J Bone Joint Surg Am. 2017;99:1291–1304. [DOI] [PubMed] [Google Scholar]
- 43.Somerson JS, Wirth MA. Self-assessed and radiographic outcomes of humeral head replacement with nonprosthetic glenoid arthroplasty. J Shoulder Elbow Surg. 2015;24:1041–1048. [DOI] [PubMed] [Google Scholar]
- 44.Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13:604–613. [DOI] [PubMed] [Google Scholar]
- 45.Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16:683–690. [DOI] [PubMed] [Google Scholar]
- 46.Trofa D, Rajaee SS, Smith EL. Nationwide trends in total shoulder arthroplasty and hemiarthroplasty for osteoarthritis. Am J Orthop. 2014;43:166–172. [PubMed] [Google Scholar]
- 47.van der Merwe M, Boyle MJ, Frampton CMA, Ball CM. Reverse shoulder arthroplasty compared with hemiarthroplasty in the treatment of acute proximal humeral fractures. J Shoulder Elbow Surg. 2017;26:1539–1545. [DOI] [PubMed] [Google Scholar]
- 48.Verborgt O, El-Abiad R, Gazielly DF. Long-term results of uncemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S13–18. [DOI] [PubMed] [Google Scholar]
- 49.Werner BC, Burrus MT, Begho I, Gwathmey FW, Brockmeier SF. Early revision within 1 year after shoulder arthroplasty: patient factors and etiology. J Shoulder Elbow Surg. 2015;24:e323–330. [DOI] [PubMed] [Google Scholar]
- 50.Young SW, Zhu M, Walker CG, Poon PC. Comparison of functional outcomes of reverse shoulder arthroplasty with those of hemiarthroplasty in the treatment of cuff-tear arthropathy: a matched-pair analysis. J Bone Joint Surg Am. 2013;95:910–915. [DOI] [PubMed] [Google Scholar]