Abstract
Background
Osteoarthritis (OA) is one of the leading causes of disability in the world. Several genes are associated with the development of OA, and previous studies have shown adult children of individuals with OA have higher areal bone mineral density (BMD). Because childhood is an important period of growth and bone development, and body composition is known to be associated with BMD, we speculated that there may be differences in growth and bone measures among young children with a genetic predisposition to OA.
Questions/purposes
(1) Do differences exist at baseline in anthropometric and peripheral quantitative CT (pQCT) measurements between children and grandchildren of individuals with OA and controls? (2) Do children and grandchildren of individuals with OA accrue bone longitudinally at a different rate than controls?
Methods
Longitudinal anthropometric (height, weight) and bone (cortical and trabecular volumetric BMD and cross-sectional area) measurements by pQCT were obtained at baseline and 18 and 36 months on children (n = 178) and grandchildren (n = 230) of 23 individuals with hip or knee arthroplasty resulting from OA and 23 sex-matched controls (16 females each). Grandchildren (age, 8–30 years) were further categorized as growing (premenarcheal or male < 14 years, n = 99) or mature (≥ 2 years postmenarchal or males ≥ 18 years, n = 96). The remaining 35 grandchildren could not be categorized and were excluded.
Results
Mature granddaughters and grandsons of individuals with OA had greater trabecular volumetric BMD than controls (236 ± 24 and 222 ± 26 mg/cm3, respectively, for granddaughters, difference of 14 [95% confidence interval {CI}, 1-28] mg/cm3, p = 0.041 and 270 ± 22 and 248 ± 30 mg/cm3, respectively, for grandsons, difference of 22 [95% CI, 1-42] mg/cm3, p = 0.040). Greater trabecular volumetric BMD was observed in daughters of individuals with OA compared with daughters of controls (228 ± 28 and 212 ± 33 mg/cm3, respectively, difference of 18 [95% CI, 3-30] mg/cm3, respectively [p = 0.021]). Growing granddaughters and grandsons of controls had greater decreases in cortical volumetric BMD than grandchildren of individuals with OA (time-by-group [T*G] based on mixed model [± standard error] -9.7 ± 4.3 versus -0.8 ± 4.4 mg/cm3/year, respectively, for granddaughters, difference of 9.0 [95% CI, 2.4-15.5] mg/cm3/year, p = 0.007 and -6.8 ± 3.3 versus 4.5 ± 3.4 mg/cm3/year, respectively, for grandsons, difference of 11.3 [95% CI, 4.3-18.3] mg/cm3/year, p = 0.002). Cortical volumetric BMD was maintained in sons of individuals with OA, but decreased in sons of controls (-0.0 ± 1.5 versus -4.3 ± 1.0 mg/cm3/year, respectively, difference of 4.3 [95% CI, 0.7-7.8] mg/cm3/year, p = 0.019 [T*G]). There was a greater apparent decrease in cross-sectional area among daughters of individuals with OA than in controls (-4.6 ± 0.9 versus -1.7 ± 0.9 mm2/year, respectively, difference of -2.9 [95% CI, -5.3 to -0.6] mm2/year, p = 0.015 [T*G]).
Conclusions
Several anthropometric and bone differences exist between children and grandchildren of individuals with OA and controls. If these differences are confirmed in additional studies, it would be important to identify the mechanism so that preventive measures could be developed and implemented to slow or reduce OA development.
Clinical Relevance
Differences in growth and bone development may lead to increased loads on cartilage that may predispose offspring to the development of OA. If these differences are confirmed in additional studies, it would be important to identify the mechanism so that preventive measures could be developed and implemented to slow or reduce OA development.
Introduction
Osteoarthritis (OA) affects 27 million adults in the United States [18] and is one of the leading causes of disability throughout the world [34, 35] with an annual cost of more than USD 42 billion associated with hip and knee arthroplasties [21]. The higher prevalence of radiographic versus symptomatic OA indicates that joint degradation occurs before individuals become symptomatic [7, 18].
A possible explanation for this could be that the development of OA is a lifelong process that manifests itself in middle to late adulthood. Previous studies have shown greater areal bone mineral density (BMD) in individuals with OA than in control subjects [3, 6, 9, 11, 24, 31]. In addition to greater areal BMD, smaller femoral neck width has been reported in individuals with OA [6]. Both of these findings are similar to what was previously reported in growing grandsons and in growing and mature granddaughters of individuals with OA [31]. Additionally, body mass index (BMI) has been reported to be greater in patients with knee [15, 26] and hip [16] OA than in control subjects. In a biomechanical modeling study, obesity and greater subchondral areal BMD were positively associated with greater predicted loads at the knee in children [19]. The combination of higher bone density and smaller bones may create more stress on the joint resulting in an environment that may perpetuate joint damage owing to overload and inadequate adaptation. However, one also must consider that a decrease in subchondral density could place greater loads on articular cartilage. Both of these mechanisms are outlined as “True Overloads” in Frost’s Utah Paradigm of Skeletal Physiology [8]. Another plausible explanation could be that the process leading to bone density changes also results in cartilage degradation. Regardless, it appears that an optimal subchondral bone condition may exist and alterations may cause a disruption in articular cartilage.
Studies by Spector et al. [32] and Neame et al. [23] showed that the proportion of OA risk related to genetic factors ranges from 39% to 65% . In a previous cross-sectional study aimed at investigating whether OA may cluster in families, Neame et al. [23] reported greater hip and spine areal BMD in individuals with OA than in controls with no differences in weight or height. Specker et al. [31] also found greater hip and spine areal BMD in growing grandsons, mature granddaughters, and daughters of individuals with OA compared with offspring of controls. Female offspring of individuals with OA also had greater femoral neck and spine bone mineral content at all ages compared with female offspring of controls [31]. These findings are in agreement with a previous study of greater peak bone mass in daughters of women with OA compared with control subjects [22]. The combination of these findings supports the possibility that skeletal changes early in life may lead to increased risk of OA later in life. Because childhood is an important period of growth and bone development, and body composition is known to be associated with BMD, we speculated that there may be differences in growth and bone measures early in life between offspring of individuals with OA and controls.
The objective of this study was to answer the following questions: (1) Do differences exist at baseline in anthropometric and peripheral quantitative CT (pQCT) measurements between children and grandchildren of individuals with hip or knee arthroplasty resulting from OA and controls? (2) Do children and grandchildren of individuals with OA accrue bone longitudinally at a different rate than control subjects?
Patients and Methods
The South Dakota Rural Bone Health Study is a population-based longitudinal study among rural and nonrural populations aged 20 to 66 years that includes 585 Hutterites [30]. The Hutterites are an Anabaptist religious group of European descent that believes in communal living. Their lifestyle is primarily self-sufficient and agriculturally based. Owing to their communal lifestyle, many potential confounding factors may be reduced. For example, traditional meals are eaten by all members in a common dining hall and all women rotate through similar work schedules. The organizational structure and lifestyle of most colonies are similar [12]. The study population is described in greater detail in a previous study [30]. During the followup period of the South Dakota Rural Bone Health Study, an additional 628 Hutterites (465 individuals, age 8-19 years; 121 individuals, age 20-66 years; and 42 individuals, age > 66 years) were recruited and followed using the same protocol. All Hutterites included in this analysis resided in 17 colonies in eastern South Dakota that were included in the South Dakota Rural Bone Health Study. This was done because all visits were conducted in a mobile research unit and distance was an issue, especially when approximately half of the visits were completed during winter months. It is estimated that there currently are approximately 50 Hutterite colonies in eastern South Dakota with an average of 15 families per colony [12]. A Schmiedeleut Hutterite Family Record is available and allowed us to link children and grandchildren to the individuals with hip or knee replacement attributable to OA or to control subjects [28]. The study was approved by the South Dakota State University institutional review board and informed consent was obtained.
Our study focuses on the children and grandchildren of all Hutterites who had total hip or knee replacement for OA at baseline or any time during the 3-year South Dakota Rural Bone Health Study and the children and grandchildren of colony-, sex-, and age-matched controls. All individuals had to provide consent to participate in the study. Joint replacements resulting from a medical diagnosis of OA were confirmed by review of medical records. All 23 individuals (16 females) with joint replacements resulting from OA (referred to as individuals with OA) were identified and colony-, sex-, and age-matched controls were obtained by identifying a study participant closest in age to the individual with OA who resided in the same colony and who did not have a parent or grandparent in common with the individual with OA. Among individuals with OA and control subjects, 178 children and 267 grandchildren who were study participants were identified in an initial report [31]. There was some overlap among grandchildren of individuals with OA and control subjects, which resulted in the possibility that some grandchildren could be counted twice. Overlaps were resolved so that each grandchild was counted only once (Fig. 1). There were 178 unique children, of whom 168 (94.3%) completed at least one followup visit. Of the 267 grandchildren, eight were grandchildren of two individuals with OA and four were grandchildren of two controls. Each of these grandchildren were included only once. In addition, 25 were grandchildren of an individual with OA and a control subject, resulting in 230 unique grandchildren. The 25 participants who were grandchildren of an individual with OA and a control subject were analyzed as a grandchild of an individual with OA and not of a control. The age range for grandchildren was large (range, 8-30 years), and they were further categorized as growing or mature based on baseline menarcheal status or age (pubertal staging was not done). Females were considered to be growing if they were premenarcheal, and grandsons were considered growing if they were younger than 15 years at baseline (total n = 99). Granddaughters who were at least 2 years postmenarchal and grandsons 18 years or older at baseline were considered as mature or not growing (total n = 96). Of the 230 unique grandchildren, 35 could not be categorized as growing or mature and were excluded from the current analyses, resulting in a total of 195 grandchildren who were included with 182 [93.3%] completing at least one followup.
Fig. 1.
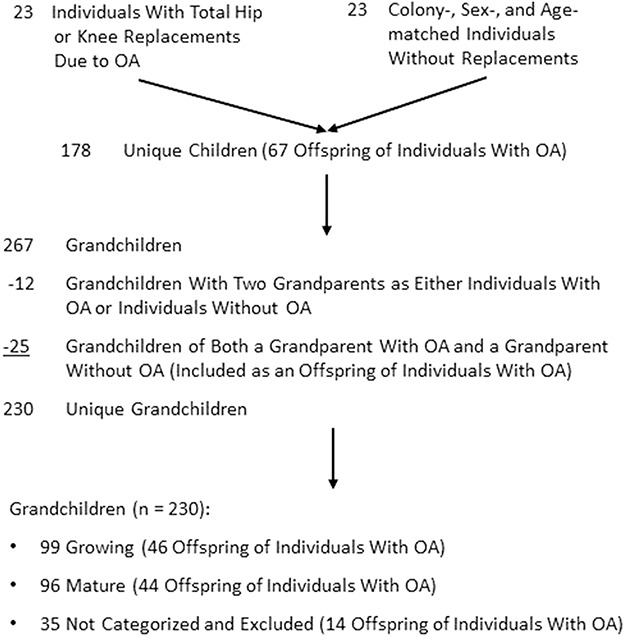
The flow diagram is shown for individuals with hip or knee replacements resulting from OA, controls, and their offspring by generation and growth status.
Anthropometrics, body composition, and bone measurements were taken at baseline and at 18- and 36-month followups. Additionally, questionnaires regarding health history and medication use were administered at each visit. Height and weight were measured using a portable stadiometer (seca, Chino, CA, USA) and a digital scale (seca). Height without shoes was measured in duplicate to the nearest 0.5 cm and repeated if the duplicate measures differed by greater than 0.5 cm. Weight, wearing surgical scrub pants and a t-shirt, was measured to the nearest 0.1 kg.
Peripheral quantitative CT measurements of the left radius were obtained using a XCT 2000 (Norland/Stratec Medizintechnic GmbH, Pforzheim, Germany). Arm length was measured from the proximal olecranon process to the distal ulnar styloid process. A scout view was obtained to identify the distal end of the radius or the most distal end of the growth plate in children when indicated and slice images were obtained at 4% and 20% of the measured arm length from the distal end using a voxel size of 0.4 mm and scan speed of 30 mm/second with a one-block rotation. The slices were analyzed using ContMode2, Peel Mode 2 and a threshold of 400 mg/cm3 to obtain trabecular density (4% site only) [1]. Cortical bone was identified using CortMode 3 (automatic threshold) at the 4% distal site and a density threshold of 710 mg/cm3 at the 20% distal site. Cortical thicknesses and periosteal and endosteal circumferences were calculated using the circular ring procedure [20]. Coefficients of variation from duplicate scans after repositioning in 35 (19 male) individuals aged 13 to 75 years old were previously determined to be 2.6% for total cross-sectional area at the 20% distal site, 2.4% for trabecular volumetric bone mineral density at the 4% site, and 0.5% for cortical volumetric BMD at the 20% site.
Statistical Analysis
All analyses were stratified by sex, generation, and growth status. Baseline differences between children and grandchildren of individuals with OA and control subjects in each stratum were compared using Student’s t-tests and mean difference with 95% confidence intervals (CIs) are provided. Linear mixed model analyses were used to determine whether longitudinal changes with time differed between offspring of individuals with OA and controls (tested as the time-by-group [T*G] interaction). Longitudinal models for weight included age and height, whereas height models included only age. Models for cortical and trabecular volumetric BMD and total cross-sectional area included age, weight, and height as covariates and annualized changes and differences in change were determined based on beta coefficients from the mixed models. Beta coefficients and standard errors are provided (see Tables, Supplemental Digital Content 1 and Supplemental Digital Content 2). Analyses were performed using STATA® Version 12 (StataCorp, College Station, TX, USA).
Results
Baseline Differences
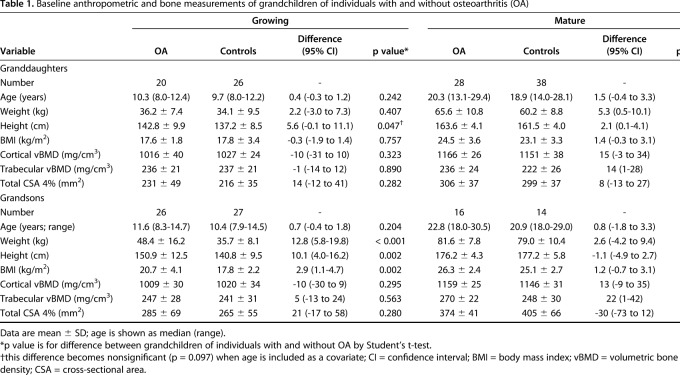
There were no differences in baseline age between grandchildren and children of individuals with OA and controls (Table 1). Growing grandsons and mature granddaughters of individuals with OA were heavier than controls (48.4 ± 16.2 and 35.7 ± 8.1 kg, respectively, for growing grandsons, difference of 12.8 [95% CI, 5.8-19.8] kg, p < 0.001 and 65.6 ± 10.8 and 60.2 ± 8.8 kg, respectively, for mature granddaughters, difference of 5.3 [95% CI, 0.5-10.1] kg, p = 0.031) and taller than controls (150.9 ± 12.5 and 140.8 ± 9.5 cm, respectively, for growing grandsons, difference of 10.1 [95% CI, 4.0-16.2] cm, p = 0.002 and 163.6 ± 4.1 and 161.5 ± 4.0 cm, respectively, for mature granddaughters, difference of 2.1 [95% CI, 0.1-4.1] cm, p = 0.045). Although there were differences in height between growing granddaughters of individuals with OA and controls, including age as a covariate resulted in no difference between groups.
Table 1.
Baseline anthropometric and bone measurements of grandchildren of individuals with and without osteoarthritis (OA)
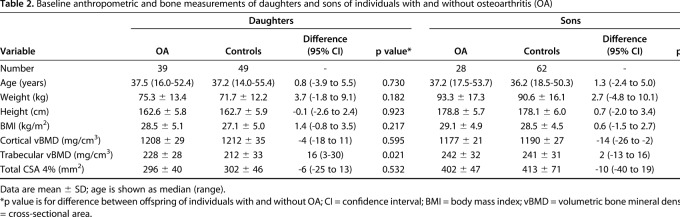
Trabecular and cortical volumetric BMD and cross-sectional area did not differ at baseline between individuals with OA and control subjects in growing grandchildren, but trabecular volumetric BMD was higher in mature granddaughters and grandsons of individuals with OA compared with controls (236 ± 24 and 222 ± 26 mg/cm3, respectively, for mature granddaughters, difference of 14 [95% CI, 1-28] mg/cm3, p = 0.041 and 270 ± 22 and 248 ± 30 mg/cm3, respectively, for mature grandsons, difference of 22 [95% CI, 1-42] mg/cm3, p = 0.040). A higher trabecular volumetric BMD also was observed in the daughters of individuals with OA compared with control subjects (228 ± 28 and 212 ± 33 mg/cm3, respectively, difference of 16 [95% CI, 3-30] mg/cm3, p = 0.021), but not among the sons (Table 2). A lower cortical volumetric BMD was observed in sons of individuals with OA compared with sons of controls (Table 2).
Table 2.
Baseline anthropometric and bone measurements of daughters and sons of individuals with and without osteoarthritis (OA)
Differential Rates of Growth
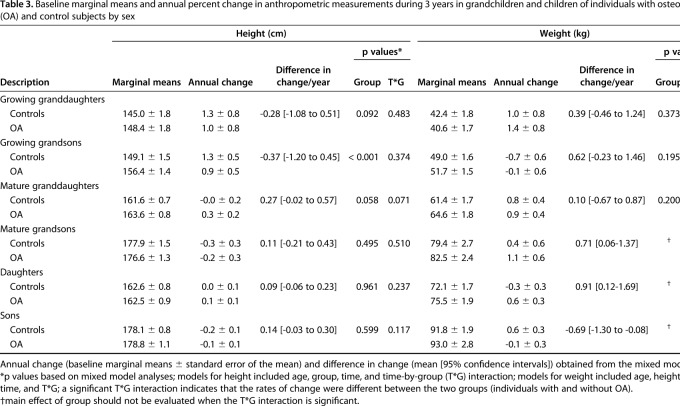
There were no differences in the rate of change in height in either the male or female grandchildren or children of individuals with OA and control subjects (Table 3). However, mature grandsons and sons and daughters of individuals with OA gained more weight per year than controls (Table 3).
Table 3.
Baseline marginal means and annual percent change in anthropometric measurements during 3 years in grandchildren and children of individuals with osteoarthritis (OA) and control subjects by sex
There were no differences in the rates of change in cortical volumetric BMD in male or female mature grandchildren and daughters of individuals with OA and controls. However, growing granddaughters and grandsons of controls had greater decreases in cortical volumetric BMD compared with offspring of individuals with OA (-9.7 ± 4.3 and -0.8 ± 4.5 mg/cm3/year, respectively, for growing granddaughters, difference of 9.0 [95% CI, 2.4-15.5] mg/cm3/year, p = 0.007 [T*G] and -6.8 ± 3.3 and 4.5 ± 3.4 mg/cm3/year, respectively, for growing grandsons, difference of 11.3 [95% CI, 4.3-18.3] mg/cm3/year, p = 0.002 [T*G]) (Fig. 2A). Sons of individuals with OA had no change in cortical volumetric BMD, whereas sons of controls had a decrease (-0.0 ± 1.5 versus -4.3 ± 1.0 mg/cm3/year, respectively, difference of 4.3 [95% CI, 0.7-7.8] mg/cm3/year, p = 0.019 [T*G]).
Fig. 2A-C.
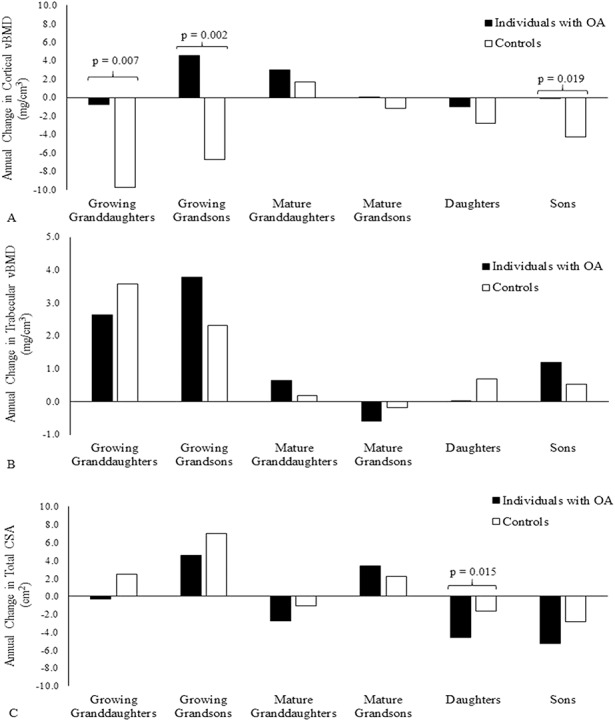
The annual changes in (A) cortical volumetric BMD (vBMD; mg/cm3/year), (B) trabecular volumetric BMD (mg/cm3/year), and (C) 4% cross-sectional area (CSA; mm2/year) during the 3-year study in children and grandchildren of individuals with OA and control subjects are shown. Probability values were obtained from T*G interactions from mixed models analyses. Covariates included were age, height, and weight.
There were no differences in rates of change in trabecular volumetric BMD for any of the groups (Fig. 2B), and total cross-sectional area decreased slightly more in daughters of individuals with OA compared with controls (-4.6 ± 0.9 versus -1.7 ± 0.9 mm3/year, respectively, difference of -2.9 [95% CI, -5.3 to -0.6] mm3/year, p = 0.015 [T*G]) (Fig. 2C).
Discussion
Studies aimed at improving our understanding of the etiology of OA are important in the long-term goal of preventing OA from occurring. As one of the leading causes of disability nationwide, the effect of OA on the population and its role in diminishing individual quality of life make prevention a critical area for study [34]. Our study is important because it focuses on younger individuals who may have a genetic predisposition for OA rather than focusing on individuals who already have OA. Identification of musculoskeletal characteristics that are different in an at-risk population may allow for future development of methods to modify or limit the effect these characteristics might have on OA development. The decrease in cortical volumetric BMD observed among growing grandchildren of controls may be a result of more rapid intracortical remodeling leading to increased porosity of cortical bone [29]. However, growing granddaughters of individuals with OA had less of a decrease in cortical volumetric BMD and growing grandsons gained cortical volumetric BMD, suggesting a possible decrease in intracortical remodeling among offspring of individuals with OA. Additionally, we found that the differences between groups appeared to be sex-specific and this leads us to theorize that the etiology of OA also may be sex-specific, which is consistent with the increased risk among females compared with males.
This study has some limitations. The relatively small number of individuals in our study population with OA is a limitation. Although a larger number of individuals with hip and knee replacements attributable to OA may have provided greater power to detect smaller differences in some of the pQCT measurements, the sample size was adequate to identify differences in several anthropometric and pQCT measures among the children and grandchildren. Another potential limitation is nonrepresentative sampling of the South Dakota Hutterite population. We included 17 of the approximate 50 colonies (approximately 34%) that exist in South Dakota. Selection of these counties was based on proximity to our facility as a result of feasibility issues in conducting the study. We feel that this would not lead to bias because of the homogenous lifestyle of the Hutterite population among colonies. In addition, owing to the complex nature of the Hutterite pedigree, we did not take into account the relatedness of the individuals in this analysis. To try to circumvent this issue, we chose our matched controls so that they did not have parents or grandparents in common with the individual who had the hip or knee replacement for whom they were matched, and all analyses were conducted after stratifying by generation. Another limitation is that the control subjects may have milder forms of OA. Because access to health care is uniform in this population, it is assumed that individuals who had undergone joint replacement had severe OA and if control subjects had OA, it would have been less severe. Theoretically, the children and grandchildren of control subjects with OA would have growth and bone measurements similar to those of children and grandchildren of individuals with hip or knee replacements owing to OA and differences would have been more difficult to detect. Thus, it is possible we underestimated the real difference that might exist between the offspring of individuals with OA and controls. Another potential limitation is that the Hutterite population is an isolated population of European descent and the ability to generalize the findings to other populations may be questioned. However, we previously conducted heritability studies on bone measures among Hutterites and found similar heritability estimates for dual-energy x-ray absorptiometry bone measurements similar to what others have reported in non-Hutterite populations [10]. In addition, a study among Hutterites found that heritability estimates for high-density lipoprotein cholesterol, triglycerides, BMI, and systolic blood pressure were similar to what other studies have reported among non-Hutterite populations [25]. Finally, the communal nature of the Hutterite lifestyle and their large family sizes allowed us to include a large number of children and grandchildren of individuals with OA and controls, whereas that may be more difficult in the general population. A final limitation is that the pQCT images were obtained at the forearm rather than the lower extremity. Although potentially it would have been beneficial to have images obtained at the tibia, the results of our study and a previously published study [31] indicate that skeletal-wide changes may be occurring in persons with OA and their offspring.
We found differences at baseline in anthropometric and pQCT bone measures between children and grandchildren of individual with OA and controls. After cessation of growth, granddaughters and grandsons of individuals with OA had greater trabecular volumetric BMD than grandchildren of controls and daughters of individuals with OA compared with controls. These findings are consistent with a previous finding that spine areal BMD is greater in individuals with hip or knee arthroplasty resulting from OA; however, we must consider that vertebrae are primarily, but not entirely, composed of trabecular bone [8]. Bennell et al. [2] reported lower subchondral trabecular volumetric BMD in several subregions in the knee in individuals with OA. Although these findings seem contrary to ours in that they showed lower trabecular volumetric BMD, whereas we found higher trabecular volumetric BMD in mature grandchildren and daughters, in the study by Bennell et al. [2], the decrease in trabecular volumetric BMD may represent a response to the disease process because individuals were studied after the onset of the disease and their results were more pronounced with advancing severity. As they suggested, a mechanism may exist whereby increased joint loads ultimately cause a loss of trabecular bone owing to thickening of the cortical plate between the articular surface and subchondral trabecular bone. Several animal and experimental models support this mechanism of decreased subchondral density early in the early stages of OA [5]. Although our results are somewhat contrary to what has been reported previously, it is important to consider that the children and grandchildren in our study were not diagnosed with OA at the time of the study. Future studies specifically targeting the subchondral region in children and grandchildren of individuals with OA and control subjects are necessary to determine if the differences we observed in the long shaft of the bone are present in the subchondral region. Further supporting the idea of joint overload is the finding that mature grandsons and daughters of individuals with OA gained more weight than controls. Frost [8] suggested that obesity developing after maturity can overload a joint, and this is supported by studies indicating that greater BMI is a risk factor for knee and hip OA [13, 14]. However, Specker et al. [31] did not find differences in BMI between individuals with and without hip or knee replacement, suggesting that some other factor may be responsible for the development of OA in this population or that the increase in weight led to the development of OA but did not persist into later life. Increased weight may help explain the greater trabecular volumetric BMD at baseline in daughters of individuals with OA because increased weight would increase loading, thus leading to greater trabecular volumetric BMD. However, controlling for weight, we still found greater trabecular volumetric BMD in daughters of individuals with OA compared with daughters of controls. The combination of greater trabecular volumetric BMD, smaller cross-sectional area, and greater weight may result in a less than favorable environment for articular cartilage [8].
We found differences in longitudinal bone measures between grandchildren and children of individuals with OA and controls. The decrease in cortical volumetric BMD in growing grandchildren of controls may be a reflection of increased intracortical remodeling, which may initially lead to increased porosity of cortical bone that occurs during growth [29]. This appears to be attenuated among grandchildren of individuals with OA. One possible explanation could be an increase in the sensitivity to estrogen, which could lead to a decrease in intracortical remodeling. Khosla et al. [17] observed a positive relationship between cortical volumetric BMD and bioavailable estradiol (E2), but only among postmenopausal women with low E2 concentrations. They speculated that this positive relationship was the result of a greater sensitivity of cortical bone to estrogen because cortical bone contains ERα almost exclusively, which is known to be more sensitive to estrogen than ERβ. Variants of the ERα gene have been shown to be associated with responsiveness of bone to estrogen as well as an increased risk of OA [27]. Therefore, an increased sensitivity to estrogen in offspring of individuals with OA may have resulted in decreased remodeling among grandchildren and maintenance of cortical volumetric BMD among the sons.
The higher incidence and greater severity of OA among females that were noted previously [4, 33], and the sex differences we observed among the offspring of individuals with OA, suggest sex-specific disease etiologies in OA development. Further investigation in the role of estrogen and estrogen receptors in cartilage development might provide important information regarding the hormonal influence on development of OA.
In our study of a relatively homogenous rural population of European descent, we identified several anthropometric and bone measures that differ between children and grandchildren of individuals with OA and control subjects that may predispose them to the development of OA. These differences include greater trabecular volumetric BMD in daughters and mature granddaughters and grandsons of individuals with OA. In addition, growing grandsons and granddaughters of controls had decreases in cortical volumetric BMD that were attenuated in the grandchildren of individuals with OA. Although the relationship between our findings and cartilage development and maintenance are not well established, future studies focusing on this area should be done. As a scientific community, it is important to identify whether differences in bone characteristics are directly affecting cartilage development or if there is a physiologic process that is affecting bone and cartilage. Biomechanical studies that model the contribution of subchondral volumetric BMD to total forces exerted on the joint may help explain the observed processes. Without an understanding of the underlying mechanisms, it might be difficult to develop prevention programs aimed at preserving cartilage in at-risk individuals.
Acknowledgments
We acknowledge the Hutterite communities for their participation in this research.
Footnotes
Supported in part by a grant from the National Institutes of Health (RO1-AR47852) and the EA Martin Endowment at South Dakota State University (BS).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Anonymous. Measurement analysis. In: XCT 2000 Operator's Manual. Pforzheim, Germany: Stratec Medizintechnik; 2000:72-7.10. [Google Scholar]
- 2.Bennell KL, Creaby MW, Wrigley TV, Hunter DJ. Tibial subchondral trabecular volumetric bone density in medial knee joint osteoarthritis using peripheral quantitative computed tomography technology. Arthritis Rheum. 2008;58:2776–2785. [DOI] [PubMed] [Google Scholar]
- 3.Bergink AP, Uitterlinden AG, Van Leeuwen JP, Hofman A, Verhaar JA, Pols HA. Bone mineral density and vertebral fracture history are associated with incident and progressive radiographic knee osteoarthritis in elderly men and women: the Rotterdam Study. Bone. 2005;37:446–456. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427(Suppl):S6–15. [DOI] [PubMed] [Google Scholar]
- 5.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8:665–673. [DOI] [PubMed] [Google Scholar]
- 6.Coster MC, Rosengren BE, Karlsson C, von Schevelow T, Magnusson H, Brudin L, Karlsson MK. Bone mass and anthropometry in patients with osteoarthritis of the foot and ankle. Foot Ankle Surg. 2014;20:52–56. [DOI] [PubMed] [Google Scholar]
- 7.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 8.Frost HM. Some IO-biomechanical causes of arthroses. In: Frost HM, ed. The Utah Paradigm of Skeletal Physiology. Vol. II Athens, Greece: International Society of Musculoskeletal and Neuronal Interactions; 2004:235–274. [Google Scholar]
- 9.Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum. 2002;46:92–99. [DOI] [PubMed] [Google Scholar]
- 10.Havill LM, Mahaney MC, Binkley TL, Specker BL. Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res. 2007;22:737–746. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC, Lethbridge-Cejku M, Tobin JD. Bone mineral density and osteoarthritis: data from the Baltimore Longitudinal Study of Aging. Osteoarthritis Cartilage. 2004;12(Suppl A):S45–48. [DOI] [PubMed] [Google Scholar]
- 12.Hostetler JA. History and relevance of the Hutterite population for genetic studies. Am J Med Genet. 1985;22:453–462. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, Rong J, Wang Y, Hu F, Bao C, Li X, Zhao Y. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2011;78:150–155. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, Zhao Y, Wang C. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2012;79:291–297. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson MK, Magnusson H, Coster M, Karlsson C, Rosengren BE. Patients with knee osteoarthritis have a phenotype with higher bone mass, higher fat mass, and lower lean body mass. Clin Orthop Relat Res. 2015;473:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson MK, Magnusson H, Coster MC, Vonschewelov T, Karlsson C, Rosengren BE. Patients with hip osteoarthritis have a phenotype with high bone mass and low lean body mass. Clin Orthop Relat Res. 2014;472:1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khosla S, Riggs BL, Robb RA, Camp JJ, Achenbach SJ, Oberg AL, Rouleau PA, Melton LJ., III Relationship of volumetric bone density and structural properties at different skeletal sites to sex steroids levels in women. J Clin Endocrinol Metab. 2005;90:5096–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerner ZF, Board WJ, Browning RC. Pediatric obesity and walking duration increase medial tibiofemoral compartment contact forces. J Orthop Res. 2016;34:97–105. [DOI] [PubMed] [Google Scholar]
- 20.Louis O, Willnecker J, Soykens S, Van den Winkel P, Osteaux M. Cortical thickness assessed by peripheral quantitative computed tomorgraphy: accuracy evaluated on radius specimens. Osteoporos Int. 1995;5:446–449. [DOI] [PubMed] [Google Scholar]
- 21.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective: a population-based review of the fourth most common cause of hospitalization in US adults. Orthop Nurs. 2012;31:85–91. [DOI] [PubMed] [Google Scholar]
- 22.Naganathan V, Zochling J, March L, Sambrook PN. Peak bone mass is increased in the hip in daughters of women with osteoarthritis. Bone. 2002;30:287–292. [DOI] [PubMed] [Google Scholar]
- 23.Neame RL, Muir K, Doherty S, Doherty M. Genetic risk of knee osteoarthritis: a sibling study. Ann Rheum Dis. 2004;63:1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nevitt MC, Zhang Y, Javaid MK, Neogi T, Curtis JR, Niu J, McCulloch CE, Segal NA, Felson DT. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann Rheum Dis. 2010;69:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ober C, Abney M, McPeek MS. The genetic dissection of complex traits in a founder population. Am J Hum Genet. 2001;69:1068–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reijman M, Pols HA, Bergink AP, Hazes JM, Belo JN, Lievense AM, Bierma-Zeinstra SM. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren Y, Tan B, Yan P, You Y, Wu Y, Wang Y. Association between polymorphisms in the estrogen receptor alpha gene and osteoarthritis susceptibility: a meta-analysis. BMC Musculoskelet Disord. 2015;16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmiedeleut Family Record. Compiled by: Gross D. High Bluff, Manitoba, Canada: Sommerfeld Printshop (Sommerfeld Hutterite Colony); 1996. [Google Scholar]
- 29.Schoenau E, Neu CM, Rauch F, Manz F. Gender-specific pubertal changes in volumetric cortical bone mineral density at the proximal radius. Bone. 2002;31:110–113. [DOI] [PubMed] [Google Scholar]
- 30.Specker B, Binkley T, Fahrenwald N. Rural versus nonrural differences in BMC, volumetric BMD, and bone size: a population-based cross-sectional study. Bone. 2004;35:1389–1398. [DOI] [PubMed] [Google Scholar]
- 31.Specker BL, Wey HE, Binkley TL, Beare TM, Smith EP, Rauch F. Higher BMC and areal BMD in children and grandchildren of individuals with hip or knee replacement. Bone. 2010;46:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ. 1996;312:940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. [DOI] [PubMed] [Google Scholar]
- 34.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Available at: http://www.who.int/whr/2002/en/whr02_en.pdf?ua=1. Accessed December 13, 2017.